Abstract
Tumor necrosis factor-related apoptosis inducing ligand (TRAIL) is a promising cancer therapeutic target due to its selective apoptosis-inducing effect in cancer cells. To efficiently deliver TRAIL to the tumor cells, an oncolytic adenovirus (p55-hTERT-HRE-TRAIL) carrying the TRAIL coding sequence was constructed. In the present study, we aimed to investigate the effect of p55-hTERT-HRE-TRAIL on the growth and metastasis of triple-negative breast cancer (TNBC). We observed that infection of the recombinant adenovirus resulted in expression of TRAIL and massive cell death in a TNBC cell line MDA-MB-231. This effect is much weaker in MCF-10A, which is a normal breast cell line. Administration of P55-HTERT-HRE-TRAIL significantly reduced orthotopic breast tumor growth and extended survival in a metastatic model. Our results suggest the oncolytic adenovirus armed with P55-HTERT-HRE-TRAIL, which exhibited enhanced anti-tumor activity and improved survival, is a promising candidate for virotherapy of TNBC.
Keywords: tumor necrosis factor-related apoptosis inducing ligand (TRAIL), triple-negative breast cancer (TNBC), adenovirus, virotherapy
Introduction
Breast cancer is one of the leading causes of cancer-related death among adult females in the world.1 Due to the heterogeneous feature of the breast cancer, individualized treatment is crucial for breast cancer therapy. Estrogen receptor (ER) and progesterone receptor (PR) as well as HER2 (human epidermal growth factor receptor-2) expression are routinely examined for the prediction of prognosis and selection of therapy regimen. However, one subtype of breast cancer called triple-negative breast cancer (TNBC) does not express ER, PR, or HER2 and has poor prognosis.2 TNBC patients are generally resistant to hormone-based therapy due to the absence of ER/PR expression. TNBC patients are also not sensitive to herceptin therapy since no HER2 amplification is present. Therefore, chemotherapy still represents the major regimen in TNBC therapy.2 However, relapse rate and chance of metastasis of TNBC is much higher than non-TNBC breast cancer.3 Identifying new targets or developing more efficient and specific approaches against TNBC represents one of the biggest challenges in this field.
TNF-related apoptosis inducing ligand (TRAIL) induces apoptosis through death receptor 4/5, which are expressed on the surface of target cells. Upon TRAIL binding, receptors facilitate the assembly of the death-inducing signaling complex (DISC) involving procaspase-8 or -10.4,5 Activation of procaspase-8/-10 leads to the subsequent caspase cascade activation resulting in apoptotic cell death. Multiple reports showed that TRAIL can induce cell death in a broad range of human cancer cells but not in most normal cell types indicating the value of TRAIL as a candidate for cancer therapy.6-8 Accumulating evidence suggests the following features of TRAIL as an anti-cancer molecule: (1) p53 suppressor gene is independent of TRAIL-induced cell death though TRAIL itself and its receptor are p53 targets;9 (2) combinations of TRAIL and chemotherapy generally restore tumor cell sensitivity to apoptosis;10 (3) TRAIL is naturally involved in tumor metastasis immune surveillance by natural killer cells.11 However, accumulating data also demonstrated various tumor cells can develop resistance to TRAIL12 which means combinatorial approaches may be desirable for more effective tumor therapy. Previously, most studies treated the cells with recombinant antibody of TRAIL, a pharmacological approach, to test its efficacy in tumor therapy, which is not feasible for in vivo study due to the pharmacokinetic/pharmacodynamic (PK/PD) limitations
In recent years, molecular therapies by delivering anti-cancer genes using modified adenovirus to target tissue emerged as an attractive approach in tumor therapy. Traditional replication-defective adenovirus cannot specifically target tumor cells, therefore replication selective virotherapy holds great promise for the treatment of cancer whose appealing features include tumor-selective targeting, viral self-spreading in cancer cells and no cross-resistance to current treatments.13-15 Tumor specificity can be achieved by using tumor or tissue-specific promoters, such as MUC1, PSA, or PS2, to drive adenoviral genes that are essential for replication, which allows the oncolytic adenovirus to selectively replicate in tumor cells without affecting normal tissues.16-18 hTERT (Human telomerase reverse transcriptase) promoter and hypoxia response element (HRE) promoter are also ideal promoters which can drive the expression of oncolytic adenovirus in tumor cells but not normal cells. hTERT is a catalytic subunit of telomerase. The expression of hTERT is found in more than 85% of tumor cells, whereas it is absent in most normal cells.19 Therapeutic genes under the control of the hTERT promoter will selectively express in telomerase-positive tumor cells at a high level.20 Hypoxic signaling in tumor cells induces the expression of hypoxia-inducible factor-1 (HIF-1), which binds to the hypoxia response element (HRE) and activates the transcription of target genes.21 Combining these specific promoters into dual-promoter constructs will further enhance the targeting of virus and improve the safety of the treatment.22
Here, we used hTERT promoter and HRE promoter to regulate the adenoviral E1A and E1B gene respectively (E1B-55 kD-deficient), and inserted the CMV promoter driven TRAIL expression cassette between E1A and E1B which formed the oncolytic adenovirus P55-HTERT-HRE-TRAIL. We aimed to assess the anti-tumor selectivity and therapeutic potential of P55-HTERT-HRE-TRAIL for breast cancer both in vitro and in vivo.
Results
Oncolytic activity of P55-HTERT-HRE-TRAIL in vitro
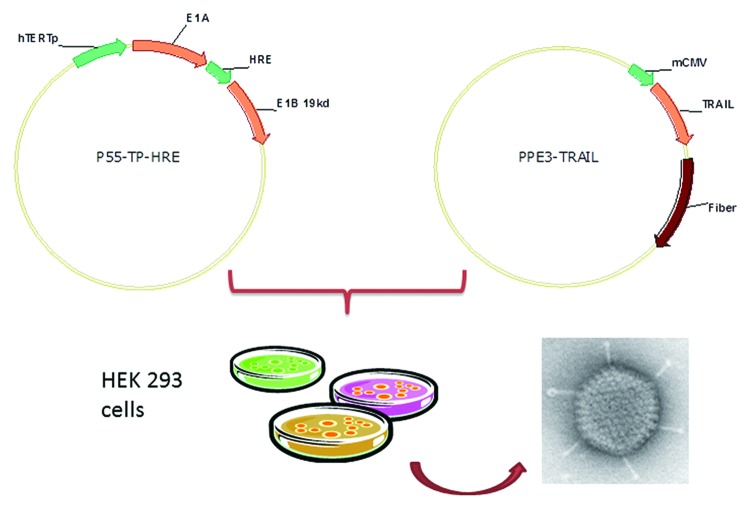
We generated an oncolytic adenovirus expressing TRAIL (P55-HTERT-HRE-TRAIL) whose replication was tightly regulated by tumor specific promoters (hTERT and HRE). The general procedures for vector construction and virus preparation were as described in the Materials and Methods and were illustrated in Figure 1. After harvest and purification of P55-HTERT-HRE-TRAIL, we first evaluated the proliferation of our recombinant virus in invasive breast tumor (MDA-MB-231) and normal breast cells (MCF10A). MDA-MB-231 is a breast cancer cell line with no expression of ER, PR, and very low level of HER2, which is generally regarded as triple negative.23 MCF10A is a non-tumorigenic human breast cell line. We first examined the production of virus in both cell lines. Infection of P55-HTERT-HRE-TRAIL in MDA-MB-231 cells for 4 d lead to a viral load of 2.3 × 105 TCID50/ml (Fig. 2A) whereas a marginal increase was observed in MCF-10A cell (1 × 103 TCID50/ml). We next measured cell viability after adenovirus infection. It is observed that, at the MOI of 10, P55-HTERT-HRE-TRAIL led to over 50% of cell death which was further elevated to over 70% at the MOI of 100 (Fig. 2B). Cell killing effect of P55-HTERT-HRE-TRAIL is significant compared with its vector control though it also induced obvious cell death (P = 0.0286, t test, Fig. 2B). On the other hand, P55-HTERT-HRE-TRAIL had only minimal effects on normal breast cell line MCF-10A (Fig. 2B). We also measured the cell viability after treating cells with recombinant TRAIL. Indeed, in accordance with previous reports,24,25 MDA-MB-231 and MCF-10A cell are similarly sensitive to TRAIL ligand (Fig. 2C). Thus, the striking difference in cell lysis between MDA-MB-231 and MCF-10A is caused mainly by the selective replication of P55-HTERT-HRE-TRAIL.
Figure 1. Construction of the oncolytic adenovirus encoding the TRAIL coding sequence (P55-HTERT-HRE-TRAIL).
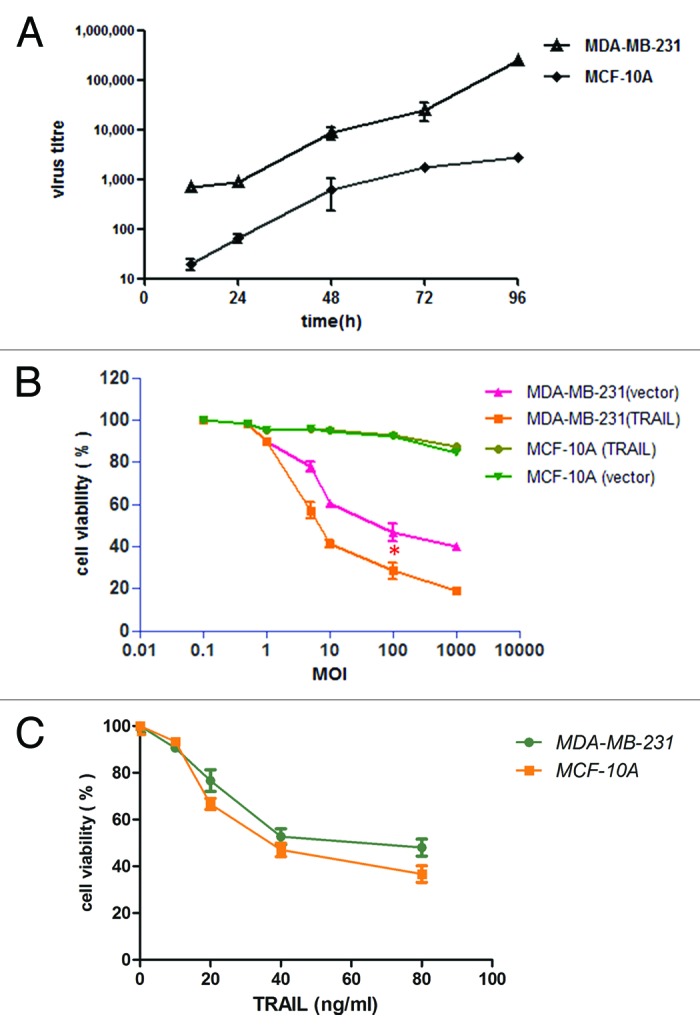
Figure 2. P55-HTERT-HRE-TRAIL induced cell death in MDA-MB-231 and but not MCF-10A cells. (A) MDA-MB-231 and MCF-10A cells were infected with P55-HTERT-HRE-TRAIL at a MOI of 5 and virus titers from the supernatant were measured by the TCID50 method at indicated time points. *Indicates the statistical significance (P < 0.05). (B) Five days after infection with P55-HTERT-HRE-TRAIL or its vector control at the indicated range of MOI, the viability of the cells was measured by MTT assay and normalized against mock infected group. (C) MDA-MB-231 and MCF-10A cells were treated with recombinant TRAIL ligand at concentrations ranging from 10 to 80 ng/ml for 24 h and cell viability was measured by MTT assay.
The expression of TRAIL in breast cancer cells and normal breast cells was quantified by ELISA and western blotting assays. As expected, 48 h after infection of P55-HTERT-HRE-TRAIL, the concentration of TRAIL protein in supernatants of infected breast cancer cells was significantly increased to 3.21 ng/ml (96 h), whereas the level of TRAIL in MCF-10A cells increased marginally (0.44 ng/ml, 96 h) (Fig. 3A). Similarly, the expression of TRAIL protein in the lysates of breast cancer cells was significantly increased, whereas the TRAIL levels in normal breast cells expressed minute amount of TRAIL 96 h after transfection (Fig. 3B). Taken together, the distinct effects of P55-HTERT-HRE-TRAIL on normal and malignant breast tumor cells confirmed its oncolytic potency and specificity.
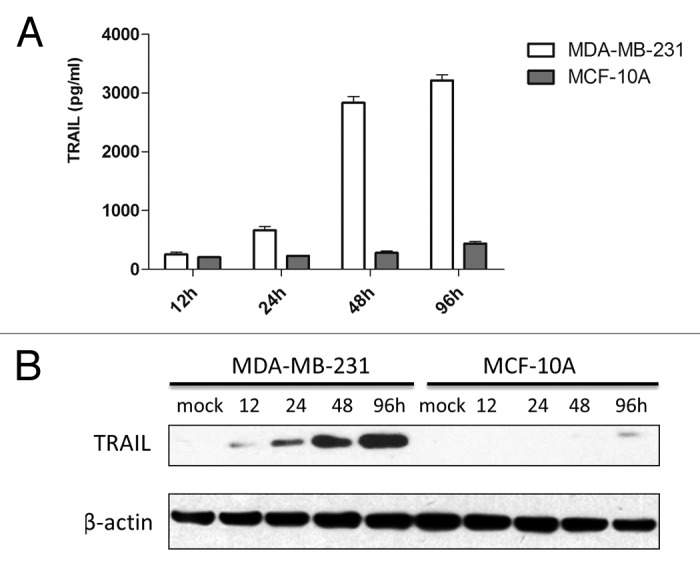
Figure 3. Expression of TRAIL in MDA-MB-231 and MCF-10A cells. (A) The concentration of TRAIL in the supernatant after infection of P55-HTERT-HRE-TRAIL, as measured by ELISA. (B) TRAIL expression was determined by western blotting, the expression of β-actin served as loading control. The quantity of TRAIL, normalized with β-actin, was estimated by densitometry.
Recent reports suggested that TRAIL can induce apoptosis in some but not all breast cancer cell lines. Triple-negative breast cancer seemed to be far more sensitive compared with cells with HER-2 amplification or ER overexpression.25 We also evaluated our recombinant virus on three non-TNBC cell lines, i.e., SK-BR-3 (HER-2 amplified), MDA-MB-453 (HER-2 amplified), and MCF-7 (ER positive). It is observed that although P55-HTERT-HRE-TRAIL can also efficiently replicate in these cells (Fig. 4A), the oncolytic potential was not enhanced by addition of the TRAIL coding region (Fig. 4B). Furthermore, in accordance with previous reports, recombinant TRAIL did not show significant apoptotic effect (Fig. 4C). These data suggest that our oncolytic virus encoding TRAIL is specific for triple-negative breast cancer.
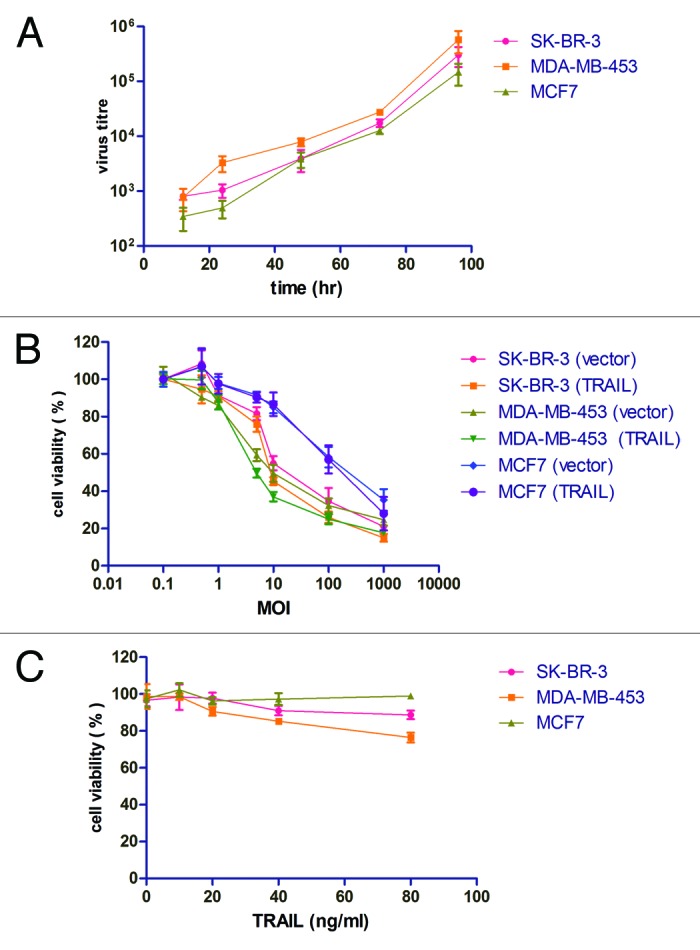
Figure 4. The effects of P55-HTERT-HRE-TRAIL and recombinant TRAIL on non-TNBC cell lines. (A) SK-BR-3, MDA-MB-453, and MCF-7 cells were infected with P55-HTERT-HRE-TRAIL at a MOI of 5 and virus titers from the supernatant were measured by the TCID50 method at indicated time points. (B) Five days after infection with P55-HTERT-HRE-TRAIL or its vector control at the indicated range of MOI, the viability of the cells was measured by MTT assay and normalized against mock infected group. (C) SK-BR-3, MDA-MB-453, and MCF-7 cells were treated with recombinant TRAIL ligand at concentrations ranging from 10 to 80 ng/ml for 24 h and cell viability was measured by MTT assay.
P55-HTERT-HRE-TRAIL inhibited orthotopic breast tumor growth and tumor metastasis in vivo
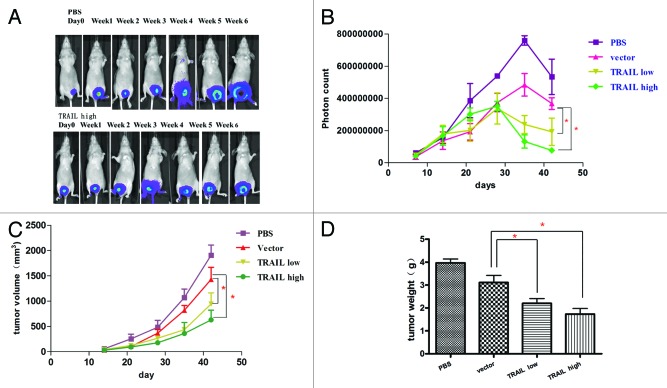
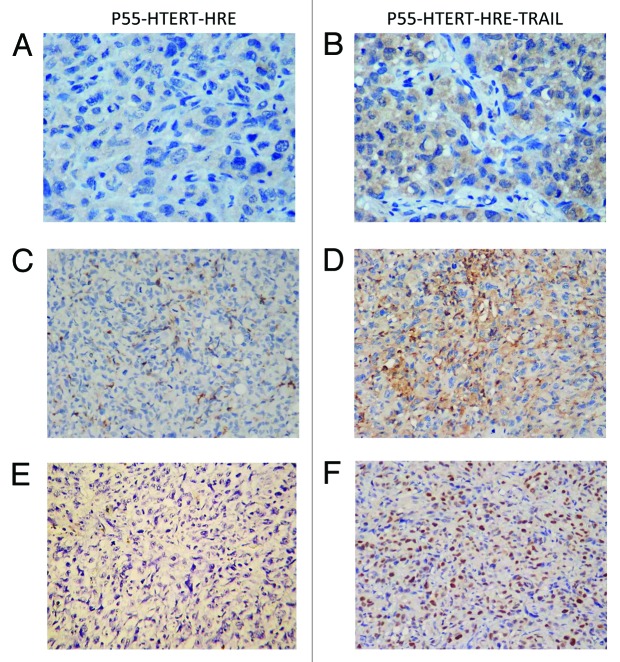
Next, we test the oncolytic effect of P55-HTERT-HRE-TRAIL virus in vivo, an orthotopic breast tumor model was established in nude mice and the growth of tumor can be visualized by live luminescence imaging. After injection of breast cancer cells, the tumors were monitored weekly with in vivo imaging system and the photon counts were recorded (Fig. 5A and B). Injection of cancer cells formed palpable tumors within 14 d (Fig. 5A and B) and tumor size peaked after 35 d (Fig. 5B). It is noticeable that P55-HTERT-HRE also reduced tumor growth compared with the PBS control, which is consistent with its oncolytic effect in vitro (Fig. 5C and D). Administration of P55-HTERT-HRE-TRAIL at either low or high dose resulted in enhanced tumor growth inhibition compared with vector group (one-way ANOVA and the Dunett multiple comparison test, P < 0.05). Terminal tumor size and volume (day 42) of TRAIL-expressing group was also markedly smaller than vector group (Fig. 5C and D). Immunohistochemistry staining showed significant increased expression of TRAIL (Fig. 6B) and Hexon (Fig. 6D) indicating efficient delivery of P55-HTERT-HRE-TRAIL to the tumor cells in vivo. As the evidence of the cell killing effect of TRAIL in vivo, TUNEL staining indicate massive apoptosis occurred in the mice infected P55-HTERT-HRE-TRAIL (Fig. 6F) but not in the mice infected by the vector control (Fig. 6E).
Figure 5. Suppression of the tumor in nude mice bearing orthotopic breast cancer by P55-HTERT-HRE-TRAIL. Log phase MDA-MB-231-luc cells were injected into the fat pad of nude mice. At 14, 16, 18, 20, and 22 d after the injection of cells, viruses were administered through intravenous injection at the dose of 2 × 108 pfu. The doses for P55-HTERT-HRE low and high group were 1 × 108 and 4 × 108 pfu respectively. Luminescent images were visualized every week (A), photon counts (B), and tumor volume (C) were also measured. Mice were sacrificed and tumor weight was measured on day 42 (D). *Indicates statistical significance (P < 0.05).
Figure 6. Infection of P55-HTERT-HRE-TRAIL induced apoptosis and enhanced TRAIL and Hexon expression. After infection with P55-HTERT-HRE (A, C, and E) or P55-HTERT-HRE-TRAIL (B, D, and F), the expression of TRAIL (A andB) and Hexon protein (C and D) were monitored by immunohistochemistry. Cell apoptosis were measured by TUNEL assay (E and F).
To assess the anti-metastatic effect of P55-HTERT-HRE-TRAIL, a metastatic model by left ventricular injection was applied in the present study. The tumor metastases were observed from day 10 by using in vivo image system every 7 d. At day 38, in vivo images showed metastatic tumors were visible in the skull, mandible, scapula, clavicle, femur, brain, lung, and liver in mice of PBS group, while P55-HTERT-HRE-TRAIL group was largely tumor-free (Fig. 7B and C). Mice in control group died within 42 d, while mice treated with P55-HTERT-HRE and P55-HTERT-HRE-TRAIL had 20% and 60% survival rate at 42 d post inoculation respectively (Fig. 7A). The survival in TRAIL group is significantly better than vector group (P = 0.0212, Mantel–Cox test)
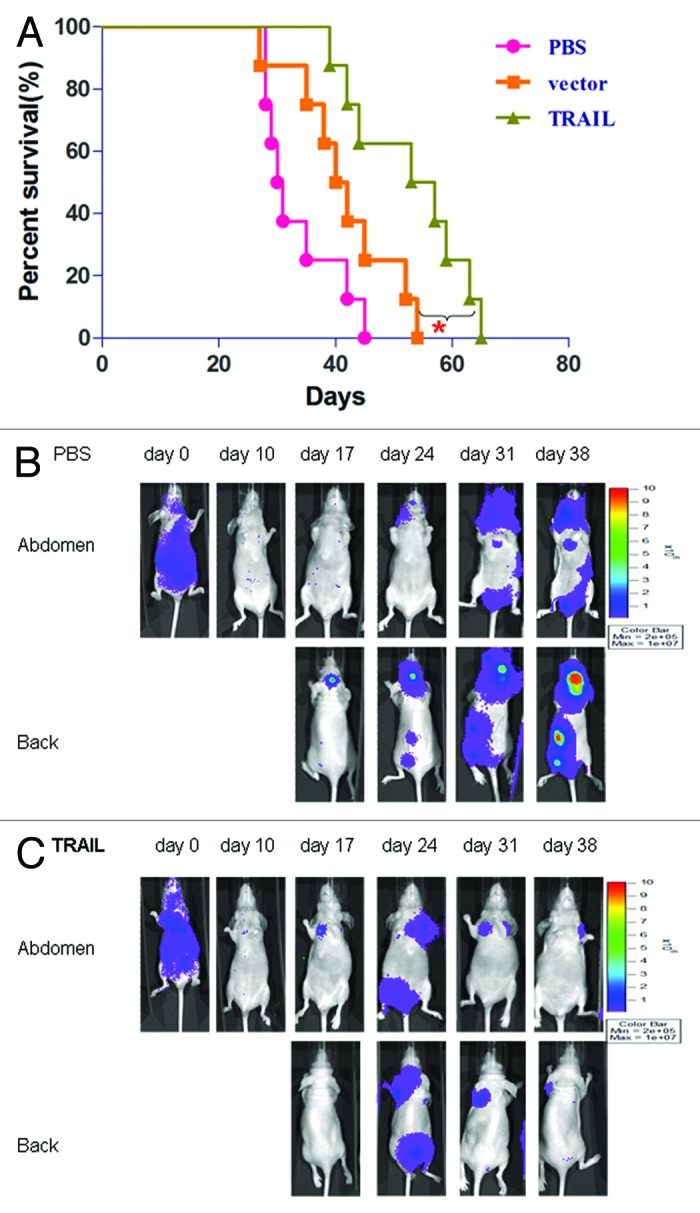
Figure 7. Inhibition of breast tumor metastasis by P55-HTERT-HRE-TRAIL. (A) Survival curves of mice in the metastatic model created by left ventricular injection of cancer cells. n = 8 for each group. In vivo imaging of the control (B) and P55-HTERT-HRE-TRAIL infected group (C) were documented in the metastatic model.
Discussion
TNBC is resistant to most effective therapies (endocrine therapies, e.g., tamoxifen, aromatase inhibitors, or HER2-directed therapy, e.g., trastuzumab) available for breast cancer. Moreover, metastatic TNBC patients usually develops visceral metastasis in lung, liver, and brain.26 Interestingly, TNBC cells are relatively sensitive to TRAIL treatment compared with HER2 or ER-positive breast tumor cells.27 Therefore, targeted oncolytic adenovirus carrying TRAIL may serve as an efficient strategy in TNBC molecular therapy.
Most of preclinical trials used recombinant human TRAIL (rhTRAIL) to induce apoptosis in multiple malignant cell lines, derived from both solid and hematologic malignancies, either alone or in combination with various chemotherapy agents or radiation.6,28-31 However, rhTRAIL has only 23–31 min half-life after administration suggesting only a short time window for the drug to reach the target tissue. Besides, rhTRAIL could not be delivered to the tumor site in a tissue-specific manner. Compared with small molecule drug or recombinant proteins, viruses have their unique properties, i.e., they can replicate and spread in addition to their carrier function to deliver anti-tumor therapeutic genes, and may be targeted specifically to tumor cells. For instance, infection of a tumor cell with Ad5-TRAIL leads to the rapid expression of TRAIL and apoptosis and TRAIL expression was observed up to seven days after an intra-tumoral injection of Ad5-TRAIL.32 So far, multiple studies have shown the TRAIL cDNA was delivered by recombinant viral vector into tumor cells.33-39
In this study, we attempted to construct an adenovirus with enhanced tumor specificity and oncolytic activity. The dual-regulated oncolytic adenovirus has better safety and specificity and thus is more suitable for clinical treatment of cancer.40 Here, we constructed a modified adenovirus plasmid (P55-HTERT-HRE-TRAIL), whose expression is driven by both the hTERT and HRE promoter. Our replication selective vector design is superior to replication defective adenoviruses as the latter type cannot specifically target cancer cells. Indeed, our results showed P55-HTERT-HRE-TRAIL can replicate in TNBC cells efficiently and induce cell death at low concentration while had minimal effects on normal cells. ELISA and western blotting assays confirmed increased TRAIL concentration in cell supernatant and in cell lysate. However, TRAIL expression was only mildly increased in normal breast cells indicating tumor cell selective expression of TRAIL, which also explains the cell death specifically in TNBC but not in normal breast cells. In addition, we observed a synergetic effect of oncolytic adenovirus and TRAIL expression as cell death inducing effect of P55-HTERT-HRE-TRAIL is markedly stronger than oncolytic adenovirus vector only, which also warrants the subsequent in vivo study. It is worth noting that our recombinant adenovirus did not show enhanced tumor cell lysis in three breast tumor cell lines that are either ER-positive or HER2 amplified. This is in accordance with previous reports indicating the TRAIL is only active in TNBC (mesenchymal) cells.25 Hence, the use of the TRAIL expressing adenovirus would only be suitable to a subset of patients with favorable phenotyping data.
To evaluate the effects of P55-HTERT-HRE-TRAIL in vivo, we established an orthotopic and a metastatic model of breast cancer by injecting TNBC cell line MDA-MB-231 harboring a luciferase gene (luc) into the recipients’ fat pads or by left-ventricular injection. In vivo results demonstrated P55-HTERT-HRE-TRAIL inhibited the orthotopic tumor growth, which is consistent with the anti-tumor effects of in vitro study. As a major coat protein in adenoviruses, expression of Hexon protein indicates the efficient delivery and replication of P55-HTERT-HRE-TRAIL in target tumor site.
In tumor metastatic model, P55-HTERT-HRE-TRAIL and P55-HTERT-HRE group showed significant higher survival rate compared with the control group. Again, P55-HTERT-HRE-TRAIL is superior to P55-HTERT-HRE in prolong the survival of the mice and reducing metastasis sites indicating the synergetic effect of the virus and TRAIL also holds true for in vivo experiment.
Micrometastases of bone marrow or other organs in breast cancer patients could lead to relapse and metastasis.41 The strategy of “gene targeting with vector” makes full use of the specific targeting tumor cell feature of oncolytic adenovirus, which has powerful replication potential and delivers the therapeutic gene to the primary and metastatic sites of tumor. The expression of therapeutic gene is amplified in combination with oncolytic effect of adenovirus demonstrating superior anti-tumor effect. Our study represents a novel approach using oncolytic adenovirus carrying TRAIL in treating TNBC tumor in vitro and in vivo and the results indicate oncolytic adenovirus-delivered TRAIL is a promising approach in TNBC therapy.
Materials and Methods
Cells and tissue culture
Human embryonic kidney 293 (HEK293) cells were purchased from Microbix Biosystems (Canada). The human breast cancer cell line MDA-MB-231 and the normal breast cell line MCF-10A were purchased from Shanghai Laboratory Animal Center of Chinese Academy of Sciences.
HEK293 and MDA-MB-231 cells were maintained in Dulbecco's minimum essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), at 37 °C, 5% CO2. MCF-10A cells were cultured in DMEM/F12 medium containing 5% horse serum, 10 ug/ml insulin, 20 ng/ml EGF, 100 ng/ml cholera toxin, and 0.5 ug/ml hydrocortisone at 37 °C. Recombinant human TRAIL was purchased from Peprotech.
Construction and preparation of the oncolytic adenovirus P55-hTERT-HRE-TRAIL
pClon9, pUC19-INS, SG502-ΔCR2, and the adenovirus backbone plasmid pPE3 were constructed by the Laboratory of Gene and Viral Therapy (Eastern Hepatobiliary Surgical Hospital, Shanghai). Restriction enzymes were purchased from New England Biolabs. The oncolytic adenovirus was kindly provided by Professor Xin-yuan Liu from the Shanghai Institutes for Biological Sciences of the Chinese Academy of Sciences. Plasmid pCLON9 was digested with XhoI and KpnI, and SG502 was digested with XbaI and SalI. The resulting 2680 bp and 1211 bp DNA fragments were ligated to create pCLON9-INS. The TRAIL expression cassette includes the human cytomegalovirus (hCMV) immediate-early promoter, the TRAIL gene and the SV40 PolyA sequence. It was extracted from ZD55-IL24 by BglII digestion and inserted into pCLON9-INS, which was digested with BamHI. The recombinant product was named pCLON9-INS-TRAIL and sent to Shanghai GeneCore Biotechnologies Co. Ltd. for sequencing. After digestion with AgeI and NotI, SG502-ΔCR2 and pCLON9-INS-TRAIL were ligated to form SG502-INS-TRAIL.
To obtain the virus, the plasmid p55-hTERT-HRE-TRAIL and type 5 adenovirus pPE3 were cotransfected into HEK293 cells with Lipofectamine 2000 (GIBCO BRL) (Fig. 1). The recombinant virus was verified by repeated PCR amplification. The correct recombinant virus, named p55-hTERT-HRE-TRAIL, was amplified in 293 cells and purified by cesium chloride density gradient centrifugation. Median tissue culture infective dose method (TCID50) was used to determine the virus titer. The titer of p55-hTERT-HER-TRAIL after amplification and purification was 2.1 × 1010 pfu/ml. The titer of p55-hTERT-HER was 2.0 × 1010 pfu/ml.
Fluorescence microscopy
MDA-MB-231 cells and MCF-10A cells were infected with p55-hTERT-HER-TRAIL at a multiplicity of infection (MOI) of 1 and observed under the fluorescence microscope. Images were taken 48 h, 72 h, and 96 h after infection.
Viral replication assay
Logarithmic phase MDA-MB-231 and MCF-10A cells were seeded at 1 × 105 cells/ml into 6-well plates. The cells were transfected with p55-hTERT-HER-TRAIL or corresponding control virus at MOI of 5 for 2 h, followed by normal culture medium. To determine titer of virus, culture supernatants were collected at 0, 12, 24, 48, 72, or 96 h after infection. The TCID50 method was used to determine titer.
Cell growth inhibition assay
MDA-MB-231 or MCF-10A cells, were adjusted to 1 × 105 cells/ml and seeded in 96-well plates with culture medium. Cells were infected with p55-hTERT-HER-TRAIL or p55-hTERT-HER at different MOI values. To measure cell proliferation, MTT experiments were performed after 5 d, as mentioned previously.42
Western blotting
Protein expression was determined by western blot analysis. Cells in 6-well-plate were washed with cold PBS and lysed with 500 μl lysis buffer (10 mM Tris-Cl, pH 7.4, 0.15 M NaCl, 5 mM EDTA, 1% Triton X100, 5 mM DTT, 0.1 mM PMSF, 5 mM ε-aminocaproic acid) per well. The cell lysates were centrifuged at 10 000 g, 4 °C for 10 min, and then the supernatants were stored at −80 °C until used for western blotting to detect the expression of TRAIL protein.
ELISA
Supernatants were collected at 12, 24, 48, and 96 h after infection. The expression of TRAIL was measured by standard ELISA assay as previously described.42
Establishment and treatment of the orthotopic breast cancer model in nude mice
Nu/nu female mice (5 to 6 weeks, 18 to 20 g) were cultivated by the Shanghai Experimental Animal Center of Chinese Academy of Sciences. All procedures were approved by the Committee on the Use and Care on Animals and done in accordance with the institution guidelines. Log phase MDA-MB-231-luc cells (Xenogen Corporation) were diluted with sterile PBS to 8 × 107 cells/ml and mixed with matrigel at a 1:1 ratio. After inhalation anesthesia, 50 μl cells were injected into the fat pad of nude mice. p55-HTERT-HRE-TRAIL viruses were administered through intravenous injection on 14, 16, 18, 20, and 22 d after cancer cells transplantation. Twelve nude mice were divided into four groups: control group injected with 100 μl saline, the p55-HTERT-HRE group injected with 2 × 108 pfu virus (100 μl), the low-dose group of p55-HTERT-HRE-TRAIL received 1 × 108 pfu virus (100 μl), and the high-dose group of p55-HTERT-HRE-TRAIL received 4 × 108 pfu (100 μl). Bioluminescence was measured weekly using an in vivo imaging system (IVIS 50, Xenogen Corporation). Tumor size was quantified with photon counts. On day 42, mice were sacrificed after anesthesia and the tumors were separated, weighed, and fixed in 4% formaldehyde. The tumor inhibition rate was calculated according to the following formula: Tumor Inhibition Rate = (mean of tumor weight in control group − mean of tumor weight in treatment group)/mean of tumor weight in control group × 100%.
Immunohistochemistry and in situ TUNEL assay
Immunohistochemical analysis of hexon (GENWAYBIO) and TRAIL (Santa Cruz, K-18) was performed on paraffin sections. Briefly, sections were deparaffinized in xylene, hydrated through graded alcohols and water. Antigen retrieval was performed by microwave treatment for 10 min in 0.01 M citrate buffer (pH 6.0). Endogenous peroxidase was inactivated with 3% hydrogen peroxide in phosphate-buffered saline (PBS) followed by incubation with the primary antibody for two hours at room temperature and then with the biotinylated secondary antibody (anti-mouse IgG) for 1 h. After incubation with streatavidin-HRP for 10 min, sections were washed and developed with DAB substrate for 3–10 min. For in situ TUNEL (Keygen Bio-Technology Development Co., Ltd.) assay, sections were deparaffinized and hydrated as described above. After proteinase K digestion, Terminal deoxynucleotidyl transferase (TdT) and dUTP-biotin was applied for 1 h at 37 °C. After washing with PBS, sections were incubated with streptavidin-HRP and developed with DAB for 10 min.
Establishment and treatment of metastatic model of breast tumor
MDA-MB-231-luc cells were adjusted to 1 × 106 cells/ml, and injected into the left heart ventricle after inhalation anesthesia. An image was taken immediately to confirm success of modeling. Viruses were intravenously administrated on day 10, 12, 14, 16, and 18 after cell injection. Twenty-four nude mice were evenly divided into three groups: control group, p55-HTERT-HRE group, and p55-HTERT-HRE-TRAIL groups. In vivo imaging of tumors was performed using IVIS 50 on day 0, 10, 17, 24, 31, and 38. The survival of mice in each group was recorded. On day 45, mice were sacrificed after anesthesia, and organs were separated, immersed immediately in d-luciferin and tested for bioluminescence ex vivo.
Statistical analysis
The experimental data are presented as mean ± SD. All statistical analyses were performed with the Statistical Product and Service Solutions 12.0 (SPSS Inc) and Prism 5 (Graphpad) software. The Student t test and one-way ANOVA analyses were employed to compare two groups and multiple groups respectively. Survival curves were plotted according to the Kaplan–Meier method and log-rank test was used to compare survival of mice receiving different therapies. Data were considered statistically significant when P < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work is supported by Grant from Shanghai Basic Science Foundation (11ZR1406800) and a young investigator fund from Shanghai Municipal Health Bureau (2010Y086).
Glossary
Abbreviations:
- TNBC
triple-negative breast cancer
- TRAIL
tumor necrosis factor-related apoptosis inducing ligand
- ER
estrogen receptor
- PR
progesterone receptor
- HER2
human epidermal growth factor receptor-2
- hTERT
human telomerase reverse transcriptase
- HRE
hypoxia response element
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/26043
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths CL, Olin JL. Triple negative breast cancer: a brief review of its characteristics and treatment options. J Pharm Pract. 2012;25:319–23. doi: 10.1177/0897190012442062. [DOI] [PubMed] [Google Scholar]
- 3.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–8. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 5.Sprick MR, Weigand MA, Rieser E, Rauch CT, Juo P, Blenis J, Krammer PH, Walczak H. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 2000;12:599–609. doi: 10.1016/S1074-7613(00)80211-3. [DOI] [PubMed] [Google Scholar]
- 6.Pollack IF, Erff M, Ashkenazi A. Direct stimulation of apoptotic signaling by soluble Apo2l/tumor necrosis factor-related apoptosis-inducing ligand leads to selective killing of glioma cells. Clin Cancer Res. 2001;7:1362–9. [PubMed] [Google Scholar]
- 7.Gazitt Y. TRAIL is a potent inducer of apoptosis in myeloma cells derived from multiple myeloma patients and is not cytotoxic to hematopoietic stem cells. Leukemia. 1999;13:1817–24. doi: 10.1038/sj.leu.2401501. [DOI] [PubMed] [Google Scholar]
- 8.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–30. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 9.Galligan L, Longley DB, McEwan M, Wilson TR, McLaughlin K, Johnston PG. Chemotherapy and TRAIL-mediated colon cancer cell death: the roles of p53, TRAIL receptors, and c-FLIP. Mol Cancer Ther. 2005;4:2026–36. doi: 10.1158/1535-7163.MCT-05-0262. [DOI] [PubMed] [Google Scholar]
- 10.Baritaki S, Huerta-Yepez S, Sakai T, Spandidos DA, Bonavida B. Chemotherapeutic drugs sensitize cancer cells to TRAIL-mediated apoptosis: up-regulation of DR5 and inhibition of Yin Yang 1. Mol Cancer Ther. 2007;6:1387–99. doi: 10.1158/1535-7163.MCT-06-0521. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 12.Khaider NG, Lane D, Matte I, Rancourt C, Piché A. Targeted ovarian cancer treatment: the TRAILs of resistance. Am J Cancer Res. 2012;2:75–92. [PMC free article] [PubMed] [Google Scholar]
- 13.Short JJ, Curiel DT. Oncolytic adenoviruses targeted to cancer stem cells. Mol Cancer Ther. 2009;8:2096–102. doi: 10.1158/1535-7163.MCT-09-0367. [DOI] [PubMed] [Google Scholar]
- 14.Wong HH, Lemoine NR, Wang Y. Oncolytic Viruses for Cancer Therapy: Overcoming the Obstacles. Viruses. 2010;2:78–106. doi: 10.3390/v2010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu XY, Gu JF. Targeting gene-virotherapy of cancer. Cell Res. 2006;16:740. doi: 10.1038/sj.cr.7310088. [DOI] [PubMed] [Google Scholar]
- 16.Hardcastle J, Kurozumi K, Chiocca EA, Kaur B. Oncolytic viruses driven by tumor-specific promoters. Curr Cancer Drug Targets. 2007;7:181–9. doi: 10.2174/156800907780058880. [DOI] [PubMed] [Google Scholar]
- 17.Lu Y. Transcriptionally regulated, prostate-targeted gene therapy for prostate cancer. Adv Drug Deliv Rev. 2009;61:572–88. doi: 10.1016/j.addr.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Chu RL, Post DE, Khuri FR, Van Meir EG. Use of replicating oncolytic adenoviruses in combination therapy for cancer. Clin Cancer Res. 2004;10:5299–312. doi: 10.1158/1078-0432.CCR-0349-03. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Jin B, Li W, Xu CX, Cui FA, Liu B, Yan YF, Liu XX, Wang XL. Targeted antitumor effect induced by hTERT promoter mediated ODC antisense adenovirus. Mol Biol Rep. 2010;37:3239–47. doi: 10.1007/s11033-009-9908-5. [DOI] [PubMed] [Google Scholar]
- 20.Kojima T, Watanabe Y, Hashimoto Y, Kuroda S, Yamasaki Y, Yano S, Ouchi M, Tazawa H, Uno F, Kagawa S, et al. In vivo biological purging for lymph node metastasis of human colorectal cancer by telomerase-specific oncolytic virotherapy. Ann Surg. 2010;251:1079–86. doi: 10.1097/SLA.0b013e3181deb69d. [DOI] [PubMed] [Google Scholar]
- 21.Binley K, Askham Z, Martin L, Spearman H, Day D, Kingsman S, Naylor S. Hypoxia-mediated tumour targeting. Gene Ther. 2003;10:540–9. doi: 10.1038/sj.gt.3301944. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Chen G, Peng L, Wang X, Yang Y, Liu C, Shi W, Su C, Wu H, Liu X, et al. Increased safety with preserved antitumoral efficacy on hepatocellular carcinoma with dual-regulated oncolytic adenovirus. Clin Cancer Res. 2006;12:6523–31. doi: 10.1158/1078-0432.CCR-06-1491. [DOI] [PubMed] [Google Scholar]
- 23.Subik K, Lee JF, Baxter L, Strzepek T, Costello D, Crowley P, Xing L, Hung MC, Bonfiglio T, Hicks DG, et al. The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by Immunohistochemical Analysis in Breast Cancer Cell Lines. Breast Cancer (Auckl) 2010;4:35–41. [PMC free article] [PubMed] [Google Scholar]
- 24.Keane MM, Ettenberg SA, Nau MM, Russell EK, Lipkowitz S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 1999;59:734–41. [PubMed] [Google Scholar]
- 25.Rahman M, Davis SR, Pumphrey JG, Bao J, Nau MM, Meltzer PS, Lipkowitz S. TRAIL induces apoptosis in triple-negative breast cancer cells with a mesenchymal phenotype. Breast Cancer Res Treat. 2009;113:217–30. doi: 10.1007/s10549-008-9924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rakha EA, Chan S. Metastatic triple-negative breast cancer. Clin Oncol (R Coll Radiol) 2011;23:587–600. doi: 10.1016/j.clon.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Rahman M, Pumphrey JG, Lipkowitz S. The TRAIL to targeted therapy of breast cancer. Adv Cancer Res. 2009;103:43–73. doi: 10.1016/S0065-230X(09)03003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–62. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL, Ross BD, Rehemtulla A. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci U S A. 2000;97:1754–9. doi: 10.1073/pnas.030545097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelley SK, Harris LA, Xie D, Deforge L, Totpal K, Bussiere J, Fox JA. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther. 2001;299:31–8. [PubMed] [Google Scholar]
- 31.Marini P, Schmid A, Jendrossek V, Faltin H, Daniel PT, Budach W, Belka C. Irradiation specifically sensitises solid tumour cell lines to TRAIL mediated apoptosis. BMC Cancer. 2005;5:5. doi: 10.1186/1471-2407-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffith TS, Broghammer EL. Suppression of tumor growth following intralesional therapy with TRAIL recombinant adenovirus. Mol Ther. 2001;4:257–66. doi: 10.1006/mthe.2001.0439. [DOI] [PubMed] [Google Scholar]
- 33.Armeanu S, Lauer UM, Smirnow I, Schenk M, Weiss TS, Gregor M, Bitzer M. Adenoviral gene transfer of tumor necrosis factor-related apoptosis-inducing ligand overcomes an impaired response of hepatoma cells but causes severe apoptosis in primary human hepatocytes. Cancer Res. 2003;63:2369–72. [PubMed] [Google Scholar]
- 34.Dong F, Wang L, Davis JJ, Hu W, Zhang L, Guo W, Teraishi F, Ji L, Fang B. Eliminating established tumor in nu/nu nude mice by a tumor necrosis factor-alpha-related apoptosis-inducing ligand-armed oncolytic adenovirus. Clin Cancer Res. 2006;12:5224–30. doi: 10.1158/1078-0432.CCR-06-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kagawa S, He C, Gu J, Koch P, Rha SJ, Roth JA, Curley SA, Stephens LC, Fang B. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001;61:3330–8. [PubMed] [Google Scholar]
- 36.Kock N, Kasmieh R, Weissleder R, Shah K. Tumor therapy mediated by lentiviral expression of shBcl-2 and S-TRAIL. Neoplasia. 2007;9:435–42. doi: 10.1593/neo.07223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lillehammer T, Engesaeter BO, Prasmickaite L, Maelandsmo GM, Fodstad O, Engebraaten O. Combined treatment with Ad-hTRAIL and DTIC or SAHA is associated with increased mitochondrial-mediated apoptosis in human melanoma cell lines. J Gene Med. 2007;9:440–51. doi: 10.1002/jgm.1036. [DOI] [PubMed] [Google Scholar]
- 38.Wenger T, Mattern J, Haas TL, Sprick MR, Walczak H, Debatin KM, Büchler MW, Herr I. Apoptosis mediated by lentiviral TRAIL transfer involves transduction-dependent and -independent effects. Cancer Gene Ther. 2007;14:316–26. doi: 10.1038/sj.cgt.7701016. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Ma H, Zhang J, Liu S, Liu Y, Zheng D. AAV-mediated TRAIL gene expression driven by hTERT promoter suppressed human hepatocellular carcinoma growth in mice. Life Sci. 2008;82:1154–61. doi: 10.1016/j.lfs.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 40.Wei N, Fan JK, Gu JF, Liu XY. Double-regulated oncolytic adenovirus-mediated interleukin-24 overexpression exhibits potent antitumor activity on gastric adenocarcinoma. Hum Gene Ther. 2010;21:855–64. doi: 10.1089/hum.2009.207. [DOI] [PubMed] [Google Scholar]
- 41.de Boer M, van Deurzen CH, van Dijck JA, Borm GF, van Diest PJ, Adang EM, Nortier JW, Rutgers EJ, Seynaeve C, Menke-Pluymers MB, et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med. 2009;361:653–63. doi: 10.1056/NEJMoa0904832. [DOI] [PubMed] [Google Scholar]
- 42.Zhu W, Wei L, Zhang H, Chen J, Qin X. Oncolytic adenovirus armed with IL-24 inhibits the growth of breast cancer in vitro and in vivo. Journal of experimental & clinical cancer research. CR (East Lansing, Mich) 2012;31:51. doi: 10.1186/1756-9966-31-51. [DOI] [PMC free article] [PubMed] [Google Scholar]