Abstract
Osteosarcoma is the most common primary bone tumor in children and adolescents. There is a critical need to find more potent drugs for patients with metastatic or recurrent disease. Berbamine (BBM) is a natural compound derived from the Berberis amurensis plants. BBM and its derivatives have been shown to have antitumor effects in several cancers. Here, we report that a novel synthetic berbamine derivative, BBMD3, inhibits cell viability and induces apoptosis of G292, KHOS, and MG-63 human osteosarcoma cells. Induction of apoptosis in these tumor cells depends on activation of caspase-3 and cleavage of poly(ADP-ribose) polymerase (PARP). Since pan-caspase inhibitor (Z-VAD-FMK) and caspase-9 inhibitor (Z-LEHD-FMK) could block the cleavage of PARP, the apoptosis induced by BBMD3 is through intrinsic signaling pathway. BBMD3 increased phosphorylation of c-Jun N-terminal kinase (JNK)/stress-activated protein kinase (SAPK), resulting in increase of phosphorylated c-Jun and total c-Fos, the major components of transcriptional factor AP-1. JNK inhibitor could partially suppress antitumor effect of BBMD3 on osteosarcoma cells. BBMD3 increased the production of reactive oxygen species (ROS) and ROS scavenger, N-acetylcysteine (NAC), could block the phosphorylation of JNK and c-Jun induced by BBMD3. BBMD3 increased the expression of the pro-apototic gene Bad, associated with apoptosis induction. Finally, BBMD3 also decreased the expression of cyclin D1 and D2, the positive cell cycle regulators, which is correlated with growth inhibition in osteosarcoma cells. Collectively, these findings indicate that BBMD3 is a potentially promising drug for the treatment of human osteosarcoma.
Keywords: berbamine derivative, osteosarcoma, apoptosis, JNK, AP-1, natural product
Introduction
Osteosarcoma is the most common pediatric bone malignancy, with a peak incidence at age of 15–19 y.1 For most patients, the etiology of osteosarcoma remains unclear. Radiation exposure, rapid bone proliferation and genetic impairments are correlated with osteosarcoma development in certain cases.2 The use of chemotherapy has improved survival in osteosarcoma patients from 11% with surgical resection alone in the 1960s to 70% by the mid-1980s.3 However, the prognosis of patients with metastasis or recurrence is still poor, with only 20% achieving a 5-y survival rate.4 Metastatic cells have specific features that render them less sensitive to conventional chemotherapy (cisplatin, doxorubicin, and high-dose methotrexate), which targets the primary osteosarcoma tumors.5,6 Therefore, it is critical to find new drugs for treatment of metastatic and recurrent osteosarcoma.
Berbamine (BBM), a natural bis-benzylisoquinoline alkaloid, is identified from the traditional Chinese medicine Berberis amurensis. BBM and its derivatives have been reported to have antitumor activities in various types of cancers, including lymphoma, myeloma, lung, and breast cancers.7-10 BBM was reported to inhibit expression of anti-apoptototic protein Bcl-2 and increase expression of pro-apoptotic protein Bax in lung and breast cancer cells.9,10 BBM also induces Fas-mediated apoptosis in heptocellular carcinoma HepG2 cells.11 The low toxicity makes BBM more promising in cancer therapeutics. Our lab examined 13 novel BBM derivatives (BBMDs), which were synthesized from natural BBM. We find that BBMD3 is most potent in this series of novel BBMDs. BBMD3 exhibited over 6-fold increase in anticancer activity compared with natural BBM in melanoma and prostate cancer cells, which was associated with inhibiting JAK2-STAT3 signaling pathway.12
The superfamily of mitogen-activated protein kinases (MAPKs) includes c-Jun N-terminal protein kinase (JNK)/stress-activated protein kinase (SAPK), p38, and extracellular signal-regulated kinase (ERK). In general, JNK and p38 are key mediators of stress and inflammation responses, while ERKs cascade is mostly induced by growth factors.13,14 JNK stress pathway participates in many different intracellular processes, including cell growth, differentiation, transformation, and apoptosis.15,16 The JNK protein kinases are encoded by three genes, which Jnk1 and Jnk2 genes are expressed ubiquitously and Jnk3 gene has a more limited pattern of expression such as brain and heart.16 JNK was originally identified by its ability to specifically phosphorylate the transcriptional factor c-Jun on its N-terminal transactivation domain at Ser63 and Ser73.17 c-Jun is a major component of activating protein 1 (AP-1), which is dimeric transcriptional factor and comprises proteins from several families.18 Though JNK/c-Jun or JNK/AP-1 pathway has dual roles in apoptosis, it is clear that activation of the JNK pathway is involved in apoptosis induced by certain death stimuli, such as UV irradiation and some drugs treatment.19-21
Here, we report that a novel synthetic berbamine derivative 3 (BBMD3) showed a strong antitumor effects on human osteosarcoma cells. BBMD3 inhibits cell viability and induces apoptosis in conventional chemotherapy-resistant osteosarcoma cells, correlated with activation of JNK/AP-1 signaling pathway.
Results
BBMD3 inhibits cell viability and induces apoptosis of human osteosarcoma cells in a time- and dose-dependent manner
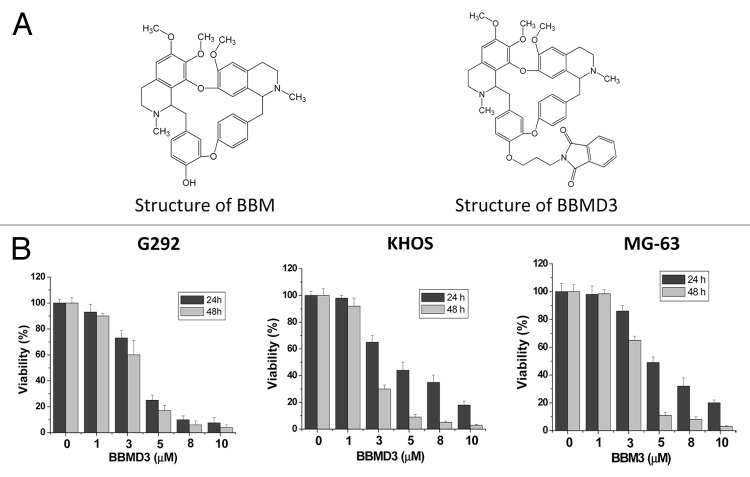
BBMD3 is a novel synthetic derivative from natural product berbamine and their structures are shown in Figure 1A. Since G292, KHOS, and MG-63 osteosarcoma cells are resistant or less sensitive to conventional chemotherapy, cisplatin, and methotrexate (data not shown), it is urgent to find new and potent drugs for osteosarcoma treatment. Thus, we tested anticancer effect of BBMD3 on these osteosarcoma cells. To investigate the effects of BBMD3 on viability of osteosarcoma cells, cells were treated with 1, 3, 5, 8, and 10 µM BBMD3 for 24 h and 48 h in culture medium containing 1% FBS. Control cells were treated with vehicle (DMSO) only. Then, cell viability was determined as described in methods. BBMD3 showed very strong inhibition of viability in G292, KHOS, and MG-63 cells with a time- and dose-dependent manner (Fig. 1B). Forty-eight hours of treatment with 10 µM BBMD3 nearly inhibited 100% of these cell viability. Since dead cells were observed after BBMD3 treatment under microscope, we further study whether the cell death induced by BBMD3 is an apoptotic process. G292, KHOS, and MG-63 cells were treated with different concentrations (0, 1, 3, 5, 10 µM) of BBMD3 for 24 h and 48 h, respectively. Then, both floating and attached cells were collected and cells were analyzed by Annexin V/propidium iodide staining followed by flow cytometry. Apoptotic cells shown in Figure 2A include both early apoptotic cells (Annexin V positive) and late apoptotic cells (Annexin V and propidium iodide double-positive). The results showed that BBMD3 induced apoptosis of G292, KHOS, and MG-63 osteosarcoma cells in a time- and dose-dependent manner. Forty-eight hours of treatment with 10 µM BBMD3 nearly killed all tumor cells, which is consistent with inhibiting results of viability.
Figure 1. BBMD3 inhibited cell viability in KHOS, G292, and MG-63 human osteosarcoma cells. (A) Structures of berbamine (BBM) and berbamine derivative 3 (BBMD3). (B) KHOS, G292, and MG-63 cells were treated with 0, 1, 3, 5, 8, and 10 µM BBMD3 for 24 h and 48 h and viability was determined by MTS assay as described in Methods. Each experiment was performed in triplicate. Each bar graph represents the mean, and the error bars represent ± SD.
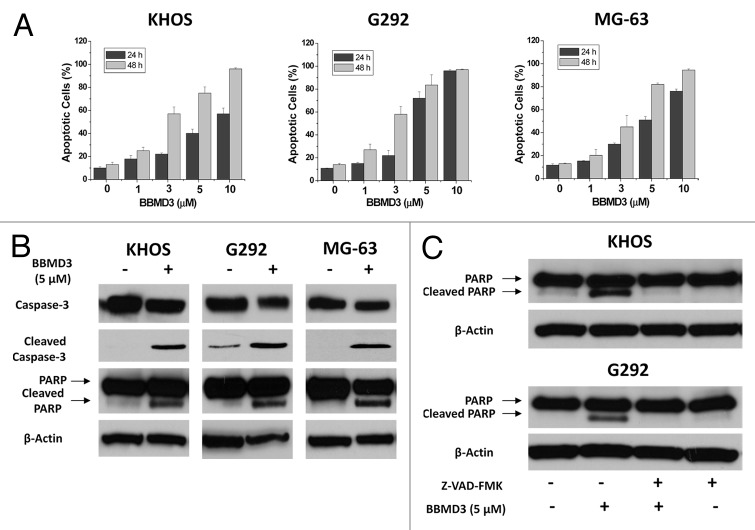
Figure 2. BBMD3 induces apoptosis of KHOS, G292 and MG-63 human osteosarcoma cells. (A) Apoptotic cells were analyzed by Annexin V-FITC and PI staining and flow cytometry. KHOS, G292, and MG-63 cells were treated with BBMD3 (0, 1, 3, 5, 10 µM) for 24 h and 48 h, respectively. Apoptotic cells represented Annexin V-FITC positive (early stage of apoptosis) or PI and Annexin V-FITC double-positive (late stage of apoptosis) cells. Each experiment was performed in triplicate or duplicate and repeated twice independently. Each bar graph represents the mean, and the error bars represent ± SD (B) Cleavages of caspase-3 and PARP were increased by 24 h BBMD3 treatment through immunoblotting analyses. (C) Caspase inhibitor, Z-VAD-FMK, blocked the effects of BBMD3 on increasing expression of cleaved caspase-3 and PARP. β-actin works as a loading control.
Apoptosis induced by BBMD3 via activation of caspase-3 in osteosarcoma cells
Activation of caspase-3, a critical executor of apoptosis,22 resulted in cleavage of poly (ADP-ribose) polymerase (PARP), which is known to help cells to maintain their viability.23 To further confirm that the cell death induced by BBMD3 is apoptosis, immunoblotting analyses were used to detect the activation of caspase-3 and cleavage of PARP in total cell lysate after 24 h BBMD3 treatment. BBMD3 increased the cleaved caspase-3 (active form of caspase-3) and cleaved PARP (inactive form of PARP) levels in G292, KHOS, and MG-63 cells (Fig. 2B). Since BBMD3 induced apoptosis of human osteosarcoma cells through activation of caspase cascade, we anticipate that the inhibitors of caspases should block the effects of BBMD3. To prove this hypothesis, we employed Z-VAD-FMK, a general inhibitor of caspases. G292 and KHOS cells were treated with 40 µM Z-VAD-FMK and 5 µM BBMD3 for 24 h. Then, cells were collected and expression of total and cleaved caspase-3 and PARP was detected by immunoblottings. Z-VAD-FMK could completely block the increased cleavage of caspase-3 and PARP induced by BBMD3 in human osteosarcoma cells (Fig. 2C).
BBMD3 induces apoptosis of osteosarcoma cells via intrinsic signaling pathway
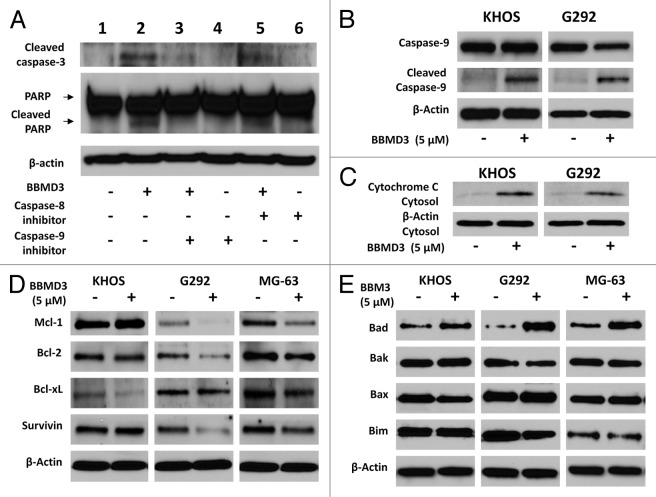
Programmed cell death or apoptosis contains two main pathways: the extrinsic or death receptor pathway and the intrinsic mitochondria pathway. Both pathways converge on same terminal target, caspase-3.24 The extrinsic signaling pathway that initiates apoptosis involves transmembrane receptor-mediated interactions and activation of caspase-8. However, the intrinsic signaling pathway is initiated by diverse “cell stress” stimuli including chemotherapeutic drugs, hypoxia, free radical, cytokines, and so on.24 All of these stimuli cause changes in the inner mitochondrial membrane that results in release of cytochrome c and activates caspase-9. Since both of caspase-8 and caspase-9 can activate caspase-3 and induce apoptosis, we employed specific inhibitors for caspase-8 and caspase-9 to determine which pathway was involved in apoptosis induced by BBMD3 in osteosarcoma cells. After KHOS cells were treated with caspase-8 inhibitor, Z-IEHD-FMK (20 µM), or caspase-9 inhibitor, Z-LEHD-FMK (20 µM), for 30 min, BBMD3 (5 µM) was added to the cells for another 24 h. Then, levels of cleaved caspase-3 and PARP were analyzed by immunoblottings. Figure 3A showed that caspase-9 inhibitor could block the increased cleavage of caspase-3 and PARP (lane 3) induced by BBMD3, while caspase-8 inhibitor could not block the cleavage of caspase-3 and PARP (lane 5) in KHOS cells. To further confirm the activation of caspase-9 by BBMD3, total caspase-9 (inactive form) and cleaved caspase-9 (active form) were detected by immunoblotings after 5 µM BBMD3 treatment for 24 h. Results exhibited that BBMD3 increased the cleavage of caspase-9 (Fig. 3B). Caspase-3 is activated by cleaved caspase-9, which activation is dependent on the release of cytochrome c from mitochondria to cytoplasm. To analyze whether BBMD3 increases the release of cytochrome c from mitochondria to cytoplasm, cytosol was isolated from KHOS and G292 cells after 24 h treatment with 5 µM BBMD3. Cytochrome c in cytosol was determined by immunoblotting assays with specific antibody (Fig. 3C). BBMD3 increased the release of cytochrome c to cytoplasm. Thus, we conclude that BBMD3 induces apoptosis of human osteosarcoma cells via intrinsic signaling pathway.
Figure 3. Caspase-9 inhibitor blocked the activation of caspase-3 induced by BBMD3 and effects of BBMD3 on regulatory proteins of apoptosis in osteosarcoma cells. (A) KHOS cells were first treated with 20 µM csapase-8 inhibitor (Z-IEHD-FMK) or caspase-9 inhibitor (Z-LEHD-FMK) for 30 min, and then 5 µM BBMD3 was added to cells for another 24 h. Expression of cleaved caspase-3 and PARP was analyzed by immunoblotting assays. (B) BBMD3 increased cleaved caspase-9 and in KHOS and G292 cells. (C) BBMD3 increased release of cytochrome c to cytosol in KHOS and G292 cells. Cells were treated with 5 µM BBMD3 for 24 h and cytosol was isolated. Cytochrome c was detected by immunoblotting assays. Effects of BBMD3 on anti-apoptotic proteins (D) and pro-apoptotic proteins (E) in KHOS, G292, and MG-63 cells were determined by immunoblotting assays after 24 h treatment.
Effects of BBMD3 on the expression of pro-apoptotic and anti-apoptotic genes in osteosarcoma cells
Bcl-2 family proteins have an important role in survival of normal and tumor cells. Members of Bcl-2 family regulate the cytochrome c/caspases/PARP pathway by controlling cytochrome c release through modulating mitochondrial outer membrane permeabilization.25 The expression of three anti-apoptotic proteins, Mcl-1, bcl-2, and Bcl-xL, and four anti-apoptotic proteins, Bad, Bak, Bax, and Bim from the Bcl-2 family, was investigated after BBMD3 treatment in osteosarcoma cells. Mcl-1 and Bcl-2 expression was inhibited by BBMD3 in G292 and MG-63 cells, while Bcl-xL expression was inhibited in KHOS and MG-63 cells (Fig. 3D). However, expression of pro-apoptotic protein Bad was increased in all three human osteosarcoma cell lines after BBMD3 treatment (Fig. 3E). BBMD3 did not affect expression of Bak, Bax, and Bim (Fig. 3E). We also studied effects of BBMD3 on survivin, an anti-apoptotic protein from the Inhibitor of Apoptosis (IAP) gene family. BBMD3 only inhibited survivin expression in G292 and MG-63 cells (Fig. 3D). Though BBMD3 showed the inhibition of anti-apoptotic proteins, increasing expression of pro-apoptotic protein Bad is a common response to BBMD3 treatment in all three cell lines.
BBMD3 suppresses the cell growth and inhibits expression of cyclin D1 and D2 in human osteosarcomas
KHOS and G292 cells (1 × 105) were seeded in 12-well plates in 1% FBS culture medium. Next day, cells were treated with BBMD3 (0, 1, 3, and 5 µM) for 24 h. Then, attached cells were collected and counted with Trypan blue staining. The numbers of live cells were showed in Figure 4A and B. BBMD3 treatment strongly decreased KHOS and G292 cell numbers in a dose-dependent manner. We further investigated the effects of BBMD3 on key cell cycle regulators, including positive regulators, cyclins D1, D2, and E, and negative regulators, p21Cip1 and p27Kip1. BBMD3 reduced protein expression of cyclin D1 and D2 in KHOS, G292, and MG-63 cells by immunoblotting analysis (Fig. 4C). Cyclin E was decreased in G292 and MG-63 cells and p21Cip1 was increased in KHOS in MG-63 cells (Fig. 4C). Therefore, inhibition of cyclin D1 and D2 expression is a common response after BBMD3 treatment in all three osteosarcoma cell lines.
Figure 4. Effects of BBMD3 on cell growth and regulatory proteins of cell cycle progression in osteosarcoma cells. (A and B) Rates of KHOS and G292 cell growth were determined by counting live cells after 24 h BBMD3 treatment with Trypan blue staining. (C) Effects of BBMD3 on cyclin D1, D2 and E, positive regulators for cell cycle, and p21Cip1 and p27Kip1, negative regulator for cell cycle in KHOS, G292, and MG-63 cells after 24 h BBMD3 treatment.
BBMD3 increases the phosphorylation of JNK1 and JNK2 in osteosarcoma cells
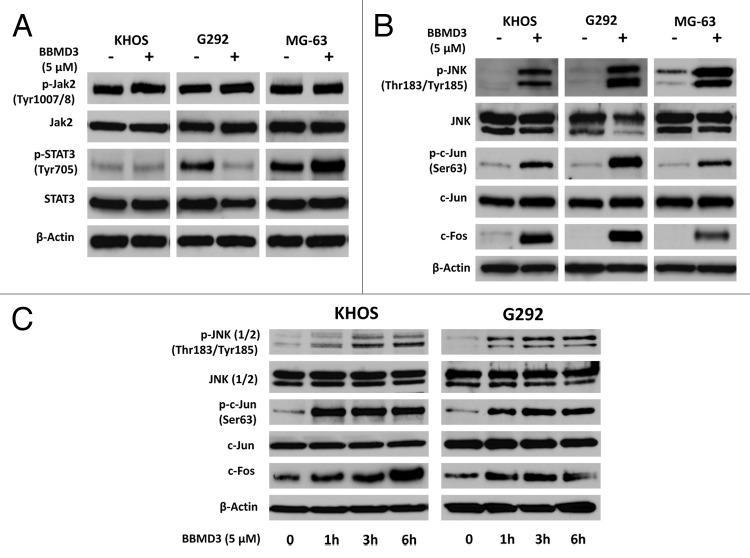
Since BBMD3 is reported to inhibit JAK2-STAT3 signaling pathway in melanoma cells.12 We investigated whether BBMD3 also inhibited this pathway in human osteosarcoma cells. After 5 µM BBMD3 24 h treatment, total proteins were isolated from KHOS, MG-63, and G292 cells. Immunoblotting assays showed that BBMD3 did not inhibit the phosphorylation of JAK2, while phosphorylated STAT3 was only inhibited in G292 cells, not KHOS and MG-63 cells (Fig. 5A). Therefore, anti-cancer effects of BBMD3 on osteosarcoma cells are not due to inhibiting JAK2-STAT3 signaling pathway. To further explore the mechanism which mediates the action of BBMD3 in KHOS, MG-63, and G292 cells, we determined the activation of stress-response signaling pathway JNK after BBMD3 treatment. After KHOS, MG-63, and G292 cells were treated with 5 µM BBMD3 for 24 h, total proteins were prepared and JNK1 (46 kD) and JNK2 (54 kD) isoforms were detected by specific antibodies. Surprisingly, BBMD3 strongly increased the phosphorylation (active form) of JNK1/2 in KHOS, MG-63, and G292 cells (Fig. 5B). The activation of JNK1/2 induced by BBMD3 occurred as early as 1 h treatment (Fig. 5C).
Figure 5. BBMD3 increased phosphorylation of JNK (1/2), c-Jun, and total protein of c-Fos in human osteosarcoma cells by immunoblotting assays. (A) Effects of BBMD3 on expression of total and phosphorylated JAK2 and STAT3 in KHOS, G292, and MG-63 cells after 24 h BBMD3 treatment. (B) Phosphorylated JNK (1/2), c-Jun, and total c-Fos were increased by 24 h BBMD3 treatment in KHOS, G292, and MG-63 cells. (C) KHOS and G292 cells were treated with 5 µM BBMD3 for 0, 1, 3, and 6 h. Then, expression of phosphorylated JNK (1/2) and c-Jun, and total JNK, c-Jun, and c-Fos were determined by immunoblotting analyses.
BBMD3 increases the phosphorylation of c-Jun, JNK downstream target, in osteosarcoma cells
c-Jun, the most extensively studied protein of the activator protein-1 (AP-1) complex, is involved in numerous cell activities, such as proliferation, apoptosis, survival, tumorigenesis, and tissue morphogenesis. c-Jun is a downstream target of JNK signaling pathway. Since BBMD3 activated JNK1 and JNK2, we investigated whether BBMD3 also activated c-Jun. As we expected, BBMD3 increased the phosphorylated c-Jun in all three osteosarcoma cell lines (Fig. 5B). BBMD3 also increased total c-Fos (Fig. 5B), another major component of transcriptional factor AP-1. Upregulation of phosphorylated JNKs and c-Jun, and c-Fos total protein by BBMD3 was detected after 1 h treatment in both KHOS and G292 cells (Fig. 5C). Thus, upregulation of JNK/AP-1 signaling pathway after BBMD3 treatment is an early response and lasts at least 24 h in these osteosarcoma cells.
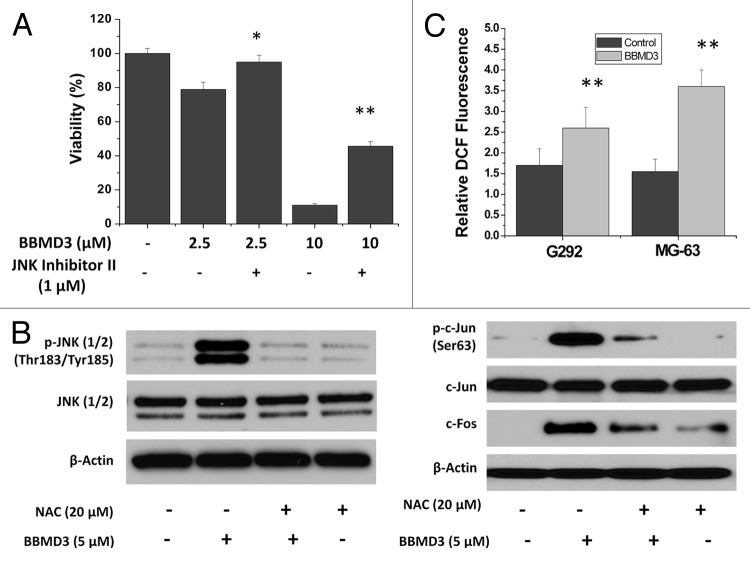
JNK inhibitor II partially blocks the effects of BBMD3 on osteosarcoma cells
To further confirm the role of JNK signaling pathway in the action of BBMD3 on human osteosarcoma cells, we employed the JNK inhibitr II to inhibit JNK signaling pathway. The G292 cells were first treated with 1 µM JNK inhibitor II for 30 min, and then 2.5 and 10 µM BBMD3 was added to the cells. After 24 h incubation, cell viability was determined. Results in Figure 6A showed that treatment with JNK inhibitor II partially blocked the effects of BBMD3 on the inhibiting viability of G292 cells.
Figure 6. JNK inhibitor and ROS scavenger could block the effects of BBMD3 on human osteosarcoma cells. (A) G292 cells were first treated with 1 µM JNK inhibitor II for 30 min. Then, 2.5 and 10 µM BBMD3 were added to cells for 24 h and viability of these cells was determined. (B) Treatment of G292 cells with NAC, a ROS scavenger, blocked the increase of phosphorylated JNK (1/2), phosphorylated c-Jun and total c-Fos induced by 24 h BBMD3 treatment. (C) Effects of BBMD3 on production of ROS after 24 h BBMD3 treatment in MG-63 and G292 cells. Values of mean fluorescence intensity in ten thousand cells were presented. *P < 0.05; **P < 0.01.
N-acetylcysteine, a ROS scavenger, blocks the effects of BBMD3 on upregulation of phosphorylated JNKs, c-Jun, and total c-Fos in osteosarcoma cells
It has been well established that reactive oxygen species (ROS) are potent inducers of JNK. Oxidative stress has been defined as an imbalance between the elevated level of ROS and/or impaired function of the antioxidant defense system. JNK signaling pathway is a key modulator in cell death mediated by ROS.26 Therefore, we investigate whether activation of JNK signaling pathway by BBMD3 in osteosarcoma cells is involved in production of ROS. We employed N-acetylcysteine (NAC), a ROS scavenger, to inhibit the effects of ROS. G292 osteosarcoma cells were first treated with 20 µM NAC for 1 h, and then 5 µM BBMD3 was added to the cells for 6 h. Total proteins were prepared and expression of phosphorylated JNK and c-Jun, and total c-Fos was determined by immunoblotting assays. The results showed that NAC treatment blocked the effects of BBMD3 on upregulation of JNK and AP-1 signaling pathways (Fig. 6B). Therefore, the activation of JNK/AP-1 signaling pathway by BBMD3 in osteosarcoma cells is mediated by ROS.
BBMD3 increases production of reactive oxygen species in osteosarcoma cells
Reactive oxygen species (ROS) regulate apoptosis and proliferation in response to a variety of stimuli. Since the ROS scavenger, NAC, could block the phosphorylation of JNK and c-Jun induced by BBMD3, we speculated that BBMD3 might increase production of ROS in osteosarcoma cells. To confirm our speculation, G292 and MG-63 cells were treated with 10 µM BBMD3 for 24 h, followed by incubation with 10 µM 2′,7′-dichlorodihydrofluorescein diacetate (DCF) for 30 min at 37 °C. Then, fluorescence density was determined by FACS analysis. Data was shown in Figure 6C and mean values of fluorescence intensity in ten thousand cells were reported. Results indicated that BBMD3 increased the production of ROS.
Discussion
Osteosarcoma is derived from primitive mesenchymal cells and is the most common pediatric bone malignancy. It is urgent to find new drugs for the osteosarcoma patients with metastatic and recurrent diseases. In this study, BBMD3, a novel synthetic derivative from natural product berbamine, showed strong effects on induction of apoptosis in conventional chemotherapy-resistant human osteosarcoma cells. Apoptosis induced by BBMD3 in these cells is associated with activation of stress-response JNK signaling pathway and JNK downstream target, AP-1 transcriptional factor.
BBMD3 was originally screened by its stronger antitumor activity compared with its parental berbamine in human melanoma and prostate cancer cells.12 Human osteosarcoma cell lines, KHOS, G292, and MG-63, are completely resistant or less sensitive to conventional chemotherapy, cisplatin and methotrexate. However, these cells are very sensitive to BBMD3 treatment. IC50 values of BBMD3 in both KHOS and G292 cells are less 5 µM after 24 h or 48 h treatment. BBMD3 inhibited cell viability and induced apoptosis of human osteosarcoma cells in a dose- and time-dependent manner. Apoptosis induced by BBMD3 in these osteosarcoma cells was confirmed by Annexin-V staining and increased cleavage of caspase-3 and PARP. Caspase-9 inhibitor Z-LEHD-FMK could block the activation of caspase-3 by BBMD3, while caspase-8 inhibitor Z-IEHD-FMK did not have such role. Therefore, BBMD3 induced apoptosis of osteosarcoma cells via activation caspase-9/caspase-3 pathway, i.e., the intrinsic signaling pathway.24 Members of Bcl-2 family play very important role in the regulation of intrinsic apoptotic pathway of via controlling mitochondrial membrane permeability. BBMD3 increased expression pro-apoptotic protein Bad in all three osteosarcoma cell lines. Bad belongs to BH3 only pro-apoptotic proteins which are cell death initiators.27
Though BBMD3 was first reported to inhibit tumor cell viability associated with inhibition of JAK2-STAT3 signaling pathway in human melanoma cells,12 BBMD3 did not inhibit JAK2 activity in these osteosarcoma cells and only reduced phosphorylated STAT3 in G292 cells, not KHOS and MG-63 cells. Therefore, anticancer activity of BBMD3 in human osteosarcoma cells may be through different signaling pathway. Our data showed that BBMD3 strongly increased the phosphorylation of JNK, the stress-response signaling, at both early time point (1 h) and late time point (24 h) in KHOS, G292, and MG-63 osteosarcoma cells. JNK signaling pathway contributes to cell proliferation and apoptosis. Suggestion of JNK to involve in apoptosis came from the observation that Jnk1−/−jnk2−/− mice were resistant to apoptosis induced by UV irradiation, anisomycin, and DNA-alkylating agent methyl methanesulfate.28 JNK is an intrinsic component of the mitochondrial-dependent death pathway during stress-induced apoptosis. Our data has showed that cell death induced by BBMD3 in human osteosarcoma cells is an apoptotic process via the intrinsic signaling pathway. Since JNK inhibitor II can partially block the effects of BBMD3 on osteosarcoma cells, the anticancer action of BBMD3 in these cells is dependent on the activation of JNK signaling pathway. After activation of JNK, consequently BBMD3 increased phosporylated c-Jun and total protein of c-Fos, the downstream targets of JNK. c-Jun and c-Fos are major components of AP-1, a transcriptional factor, which regulates proliferation, inflammation, differentiation, apoptosis, and cellular migration.18 Many evidences indicate that a role for JNK-c-Jun/AP-1 mediated expression of pro-apoptotic genes in JNK-mediated apoptosis.29 Oxidative stress is an apoptotic signal for activation of JNK-c-Jun/AP-1 signaling pathway. BBMD3 increased the production of ROS and NAC, a ROS scavenger, can block the phosphorylation of JNK or c-Jun induced by BBMD3 in osteosarcoma cells. Therefore, the activation of JNK-c-Jun/AP-1 signaling pathway by BBMD3 is through increasing ROS production. JNK and AP-1 have pro- or anti-apoptotic functions, depending on cell type, nature of the death stimulus, duration, and the activity of other signaling pathway.18,19
Though BBMD3 exhibited a strong antitumor activity in human osteosarcoma cells which are resistant or less sensitive to conventional chemotherapies, BBMD3 has a lower toxicity to normal human cells.12 Thus, BBMD3 will be a promising agent for treatment of osteosarcoma patients with metastasis or recurrence.
Materials and Methods
Reagents and antibodies
BBMD3 was synthesized by reaction of natural BBM with NH2-containing substituents. Structure and purity were analyzed using HNMR spectroscopy. BBMD3 displayed over 98% purity in NMR analysis. JNK inhibitor II was purchased from EMD Biosciences. Anti-cyclin D1 was obtained from Calbiochem. Anti-cyclin E was obtained from BD Biosciences. Anti-cyclin D2 and anti-Mcl-1 were obtained from Santa Cruz. Horseradish peroxidase-labeled anti-mouse and anti-rabbit secondary antibodies were from GE Healthcare. All other antibodies were purchased from Cell Signaling.
Cell cultures
G292, KHOS/NP, and MG-63 human osteosarcoma cell lines were from American Type Culture Collection (ATCC). G292 cells were maintained in McCoy’s 5A medium and KHOS and MG-63 cells were maintained in MEM (Eagle) with l-glutamine supplemented with 10% fetal bovine serum (FBS) and 1% Antibiotic-Antimycotic (AA). All cultured cells were grown in a humidified atmosphere of 5% CO2 at 37 °C.
Cell viability assays
Cell viability assays were performed with CellTiter 96 Aqueous One Solution Cell proliferation Assay from Promega which contains 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS). Each well of 96-well plate was seeded with 5000 cells in culture medium. After overnight culture (16 h) the cells were treated with different concentrations of drugs. After 24 h or 48 h treatment, MTS was added to the cells according to the supplier’s protocol and absorbance was measured at 490 nm using an automated ELISA plate reader.
Apoptosis assay
G292, KHOS, and MG-63 cells (2 × 105) were seeded in 60 mm culture dishes in culture medium containing 1% FBS. The following day the cells were treated with indicated concentrations of BBMD3 for 48 h. After treatment, all cells including both detached and attached cells were collected, and the apoptotic cells were detected by Annexin V-FITC Apoptosis Detection Kit (BD Biosciences). The cells were stained with Annexin V-FITC and propidium iodide (PI) according to the supplier’s instructions. Viable and apoptotic cells were detected by flow cytometry in the Analytical Cytometry Core at City of Hope National Medical Center. Apoptotic cells include both the early apoptotic portion (Annexin V-positive) and the late apoptotic portion (Annexin V- and PI-positive).
Live cells assay
G292 and KHOS cells (1 × 105) were seeded in 12-well plates. Next day, cells were treated with 0, 1, 3, and 5 µM BBMD3 for 24 h. Then, the cells were washed with cold PBS and attached cells were collected by trypsinization. Live cells were measured by using Vi-cellTM XR analyzer (Beckman Coulter).
Immunoblotting analysis
Twenty micrograms total proteins were resolved in a 4–15% gradient Tris-HCl gel from BIO-RAD. After gel electrophoresis, the proteins were transferred to Hybond-C membranes (Amersham). The membranes were blocked for 1 h at room temperature (RT) in 10% non-fat dry milk in PBST (1× PBS with 0.1% Tween-20), followed by an overnight incubation at 4 °C with primary antibodies in PBST with 2% non-fat dry milk. Horseradish peroxidase labeled anti-mouse or anti-rabbit secondary antibodies were incubated 1 h at RT. Immunoreactivity was detected with SuperSignal West Pico substrate (Pierce).
Cytosol isolation
Cytosol of KHOS and G292 cells was isolated as described.30 In brief, after 24 h treatment with 5 µM BBMD3, cells were collected and resuspended in 1 ml of lysis buffer containing 20 mM HEPES-KOH (pH 7.5), 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 10 µg/ml leupeptin, 10 µg/ml aprotinin, and 250 mM sucrose. After homogenization with a 26-gauge needle syringe 5 times, cells were centrifuged at 750 × g for 10 min at 4 °C. Then the supernatant was centrifuged at 10 000 × g for 15 min at 4 °C, and the resulting supernatant was collected (i.e., the cytosolic extract).
Measurement of intracellular ROS
Cells were incubated with 10 µM 2′,7′-dichlorodihydrofluorescein diacetate (DCF, Cayman Chemical Company) for 30 min at 37 °C. Then, cells were collected by trypsinization and cell pellets were dissolved in 1 ml of PBS, followed by FACS analysis. Values of mean fluorescence intensity in ten thousand cells were reported.
Statistics
The Student t test was used to evaluate statistical significance of differences between two groups and P < 0.05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This project was dedicated to the memory of Brad Bridenbecker. This work was generously supported in part by Brad Bridenbecker’s family, and NCI grant CA1155674 (to RJ).
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/26045
References
- 1.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–43. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21(Suppl 7):vii320–5. doi: 10.1093/annonc/mdq276. [DOI] [PubMed] [Google Scholar]
- 3.Chou AJ, Gorlick R. Chemotherapy resistance in osteosarcoma: current challenges and future directions. Expert Rev Anticancer Ther. 2006;6:1075–85. doi: 10.1586/14737140.6.7.1075. [DOI] [PubMed] [Google Scholar]
- 4.Niswander LM, Kim SY. Stratifying osteosarcoma: minimizing and maximizing therapy. Curr Oncol Rep. 2010;12:266–70. doi: 10.1007/s11912-010-0106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakamoto A, Iwamoto Y. Current status and perspectives regarding the treatment of osteo-sarcoma: chemotherapy. Rev Recent Clin Trials. 2008;3:228–31. doi: 10.2174/157488708785700267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.PosthumaDeBoer J, Witlox MA, Kaspers GJ, van Royen BJ. Molecular alterations as target for therapy in metastatic osteosarcoma: a review of literature. Clin Exp Metastasis. 2011;28:493–503. doi: 10.1007/s10585-011-9384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du HP, Shen JK, Yang M, Wang YQ, Yuan XQ, Ma QL, Jin J. 4-Chlorobenzoyl berbamine induces apoptosis and G2/M cell cycle arrest through the PI3K/Akt and NF-kappaB signal pathway in lymphoma cells. Oncol Rep. 2010;23:709–16. doi: 10.3892/or_00000688. [DOI] [PubMed] [Google Scholar]
- 8.Liang Y, Xu RZ, Zhang L, Zhao XY. Berbamine, a novel nuclear factor kappaB inhibitor, inhibits growth and induces apoptosis in human myeloma cells. Acta Pharmacol Sin. 2009;30:1659–65. doi: 10.1038/aps.2009.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, Liu Q, Zhang Y, Liu K, Yu P, Liu K, Luan J, Duan H, Lu Z, Wang F, et al. Suppression of growth, migration and invasion of highly-metastatic human breast cancer cells by berbamine and its molecular mechanisms of action. Mol Cancer. 2009;8:81. doi: 10.1186/1476-4598-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan H, Luan J, Liu Q, Yagasaki K, Zhang G. Suppression of human lung cancer cell growth and migration by berbamine. Cytotechnology. 2010;62:341–8. doi: 10.1007/s10616-009-9240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang GY, Lv QH, Dong Q, Xu RZ, Dong QH. Berbamine induces Fas-mediated apoptosis in human hepatocellular carcinoma HepG2 cells and inhibits its tumor growth in nude mice. J Asian Nat Prod Res. 2009;11:219–28. doi: 10.1080/10286020802675076. [DOI] [PubMed] [Google Scholar]
- 12.Nam S, Xie J, Perkins A, Ma Y, Yang F, Wu J, Wang Y, Xu RZ, Huang W, Horne DA, et al. Novel synthetic derivatives of the natural product berbamine inhibit Jak2/Stat3 signaling and induce apoptosis of human melanoma cells. Mol Oncol. 2012;6:484–93. doi: 10.1016/j.molonc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–49. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 14.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–44. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–35. [PubMed] [Google Scholar]
- 16.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/S0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 17.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–48. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 18.Shaulian E. AP-1--The Jun proteins: Oncogenes or tumor suppressors in disguise? Cell Signal. 2010;22:894–9. doi: 10.1016/j.cellsig.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- 20.Shieh JM, Huang TF, Hung CF, Chou KH, Tsai YJ, Wu WB. Activation of c-Jun N-terminal kinase is essential for mitochondrial membrane potential change and apoptosis induced by doxycycline in melanoma cells. Br J Pharmacol. 2010;160:1171–84. doi: 10.1111/j.1476-5381.2010.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei G, Wang M, Carr BI. Sorafenib combined vitamin K induces apoptosis in human pancreatic cancer cell lines through RAF/MEK/ERK and c-Jun NH2-terminal kinase pathways. J Cell Physiol. 2010;224:112–9. doi: 10.1002/jcp.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994;269:30761–4. [PubMed] [Google Scholar]
- 23.Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JM. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem. 1998;273:33533–9. doi: 10.1074/jbc.273.50.33533. [DOI] [PubMed] [Google Scholar]
- 24.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–9. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 26.Shen HM, Liu ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;40:928–39. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 27.Danial NN. BAD: undertaker by night, candyman by day. Oncogene. 2008;27(Suppl 1):S53–70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- 28.Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–4. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 29.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–51. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhoopathi P, Chetty C, Kunigal S, Vanamala SK, Rao JS, Lakka SS. Blockade of tumor growth due to matrix metalloproteinase-9 inhibition is mediated by sequential activation of beta1-integrin, ERK, and NF-kappaB. J Biol Chem. 2008;283:1545–52. doi: 10.1074/jbc.M707931200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]