Maternal placental syndromes (MPS) occur as a consequence of abnormal placental vessel formation, and refer to hypertensive disorders of pregnancy and related placental abnormalities, such as placental abruption and infarction.[1] In the affected pregnancies, adverse pregnancy outcomes, including preterm delivery, foetal growth retardation, and intrauterine foetal demise, are common. Hypertensive disorders of pregnancy (HPD) complicate about 5–10% of pregnancies worldwide[2] and cover a spectrum of conditions. Most notable of these is preeclampsia, a multisystem pregnancy-specific disorder clinically characterised by hypertension and proteinuria, and which remains a leading cause of foetal and maternal morbidity and mortality. Evidence suggests that preeclampsia is associated with the release of anti-angiogenic factors by an ischaemic placenta, which, in turn, may lead to maternal endothelial dysfunction.[3, 4]
In 1927, Corwin and Herrick reported an association between ‘hypertensive toxaemia of pregnancy’, as preeclampsia was once called, and chronic cardiovascular disease.[5] Since then, there have been multiple epidemiological studies that confirm these authors’ observation. HPD have been associated with chronic hypertension, ischaemic heart disease, stroke, venous thromboembolism, cardiovascular death;[6, 7] evidence suggests that other features of the metabolic syndrome are present as well.[8] Similarly, the development of a maternal placental syndrome has been shown to double the risk for the development of cardiovascular disease later in life.[1] However, studies evaluating an association between HPD and future arrhythmias and heart failure are limited.
In this issue of Heart, Ray, et al.,[9] discuss these specific cardiovascular outcomes, heart failure and arrhythmias, in the context of a previous history of maternal placental syndromes. In this unique retrospective cohort study, using a large administrative database in Ontario, Canada, the authors evaluate the frequency of these two outcomes in women with previous maternal placental syndromes, defined as placental abruption, placental infarction, preeclampsia/eclampsia or gestational hypertension. Their primary composite outcome was hospitalisation for heart failure, atrial or ventricular arrhythmias, one year or more following the index pregnancy.
The results of this study demonstrate a 61% relative increase in the risk of heart failure or dysrhythmias among women who had a history of placental syndrome, compared to those without such a condition; the risk was further increased in the co-presence of features of metabolic syndrome, preterm delivery, poor foetal growth, and severe preeclampsia. Ventricular arrhythmias were not significantly increased. As MPS confer an increased risk of hypertension and ischaemic heart disease, conditions which are the most common risk factors for heart failure and arrhythmias, this result was not unexpected. Indeed, the authors also demonstrate an increased frequency of features of the metabolic syndrome, i.e. risk factors for coronary artery disease, as well as incident coronary artery disease in the exposed group. What is unique about this study, however, is that the authors were able to take their observations a step further, adjusting the outcomes for the development of known risk factors for heart failure and arrhythmias. The effect of MPS on the outcomes was found to persist, even when adjusted for coronary artery disease, hypertension, metabolic syndrome and thyroid disease.
The high prevalence of heart failure and arrhythmias, which have sex-specific differences, underlies the importance of focusing on them as outcomes in the context of female-specific conditions. Atrial fibrillation is the most common arrhythmia, and in fact, one of the most common conditions encountered in medical practice today: at age 40, there is a lifetime risk up to 25% of developing atrial fibrillation.[10] Heart failure is the most common cause of hospitalisation of adults above the age of 65 years, and the lifetime risk of development of heart failure at the age of 40 is one in five.[11] There are important sex differences in the epidemiology of heart failure and arrhythmias. For instance, women are significantly more prone to heart failure with a preserved ejection fraction than men, [12, 13] and women have higher rates of atrial fibrillation-related ischaemic stroke and disability without prophylactic antithrombotic therapy.[14] The results of the current study suggest that development of heart failure and atrial fibrillation in women may be linked to their adverse pregnancy outcomes, thus offering the opportunity for early screening and identification of those at risk.
The HPD, and by extension, MPS, have been suggested to be a ‘failed stress test’, identifying women who have an underlying predisposition to cardiovascular disease. Preeclampsia and cardiovascular disease share common risk factors (Figure 1), such as metabolic syndrome, and it is thought that these shared risk factors mediate the increased risk for HPD and cardiovascular disease at different times in a woman’s life.[15] Another possibility is that HPD may induce long-term vascular and metabolic changes that may increase future cardiovascular risk (Figure 1). The supporting evidence comes from studies showing that, despite normalisation of blood pressure, these seemingly healthy women may demonstrate unfavourable metabolic and vascular changes,[15] such as an impaired brachial artery flow- mediated (endothelium dependent) dilatation, a measure of endothelial dysfunction, three years after the diagnosis of preeclampsia.[16] With respect to heart failure and arrhythmia outcomes, several studies have indicated that cardiac changes, which occur during hypertensive pregnancies and may contribute to these outcomes, and do not revert to normal upon delivery. During a hypertensive pregnancy, there is eccentric and concentric ventricular remodelling,[17] impaired contractility and diastolic dysfunction.[18] Left atrial size is increased, accompanied by increased atrial natriuretic peptide levels,[19, 20, 21] and there is global right ventricular systo-diastolic dysfunction. Melchiorre, et al., performed echocardiographic studies of women post-partum, demonstrating that at one year post-partum, women with preeclampsia, especially preterm preeclampsia, had an increased risk of altered left ventricular geometry (concentric remodelling, eccentric hypertrophy), impaired left ventricular relaxation with global diastolic dysfunction, and mildly impaired radial systolic function (ejection fraction 45%–55%) compared to normotensive pregnancy.[22] Zandstra, et al. demonstrated a fourfold increase in the risk of diastolic dysfunction at six months post-partum in women who had placental syndromes.[23] Conceivably, these persistent abnormalities may contribute to the risk for future heart failure and arrhythmia (Figure 1), but the mechanisms underlying the associations between these outcomes and a history of maternal placental syndrome remain to be determined in future research, as rightly so recognised by and Ray, et al [9].
Figure 1.
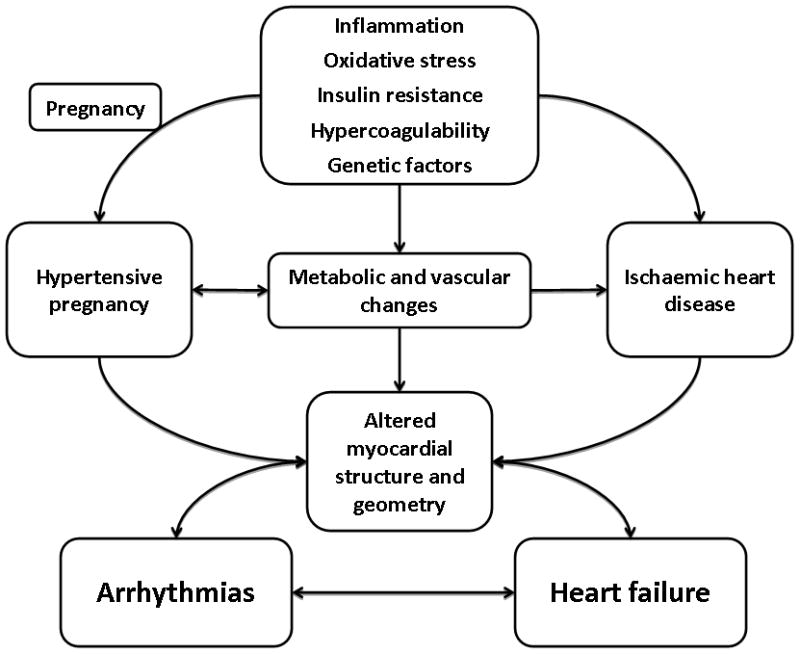
Possible mechanisms of the association of hypertensive pregnancy disorders, arrhythmias and heart failure
There are two potential clinical implications of the current study. First, guidelines for the prevention of cardiovascular disease in women recommend referral of women with a history of hypertensive pregnancy to primary care or cardiology in order to facilitate monitoring and control of risk factors.[24] Given Ray, et al.’s findings [9], a history of maternal placental syndrome may also identify women at risk for heart failure and arrhythmia, suggesting that increased post-partum vigilance, lifestyle modifications and early detection and treatment of risk factors may affect these outcomes as well. Second, these data may further challenge the current guidelines for the management of hypertensive pregnancy, which recommend lenient blood pressure control in the absence of end organ damage.[25] Some centres hold anti-hypertensive medications in women with chronic hypertension, only re-instituting them when the blood pressure becomes elevated above 150–160 mmHg systolic, 100–190 mmHg diastolic. The rationale is that there is no evidence that pharmacologic treatment improves foetal outcome,[25] with the concern that the drug therapy may potentially harm the foetus without clear gain.
With a growing body of evidence demonstrating persistent cardiovascular impairment in the immediate post-partum period, and its likely effect on the risk of heart failure and arrhythmia, we should revisit the question of that ‘gain’. One may postulate that the atrial stretch implied by elevated atrial natriuretic peptide levels and left atrial enlargement is due to atrial pressure overload caused by poor hypertension control during pregnancy. The diastolic dysfunction that occurs during pregnancy, and that persists for years afterward, is likely directly related to hypertension as well. Would improved blood pressure control during pregnancy therefore lead to decreased atrial arrhythmias and heart failure long term? Would the introduction of neuro-hormonal modulating agents decrease these risks? Would this treatment need to be a short term measure to return cardiovascular function to normal, or would it need to be continued long term? Would they need to be instituted during pregnancy for adequate effect? These are important clinical questions, the answers to which can be obtained only through carefully designed and adequately powered studies. Until then, treatment of hypertension in pregnancy should remain a matter of carefully weighing the risk/benefit ratio for each individual patient, coupled regular surveillance post-partum, in order to optimise maternal and foetal pregnancy outcomes, as well as future maternal health.
We must, however, be careful not to extrapolate too many conclusions from this observational study, as many details are wanting. We know neither the nature of the atrial arrhythmias, nor the heart failure subtypes involved. The details of the obstetric management are unknown, as well as the adequacy of risk factor management outside of pregnancy. Despite these limitations, the findings of this study are intriguing, expand our knowledge of the life-long effects of HPD, and create a solid base for future research, which may ultimately address the mechanisms underlying the associations between MPS and future heart failure and arrhythmias, leading to specific diagnostic, preventive, and therapeutic measures.
Acknowledgments
The project described was supported by Award Number K08HD051714 (Vesna D. Garovic) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
This is a commentary on article Ray JG, Schull MJ, Kingdom JC, Vermeulen MJ. Heart failure and dysrhythmias after maternal placental syndromes: HAD MPS Study. Heart. 2012;98(15):1136-41.
Footnotes
The authors have no conflict of interest or relationship with industry to disclose
References
- 1.Ray JG, Vermeulen MJ, Schull MJ, et al. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366:1797–803. doi: 10.1016/S0140-6736(05)67726-4. [DOI] [PubMed] [Google Scholar]
- 2.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011 doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Granger JP, Alexander BT, Llinas MT, et al. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension. 2001;38:718–22. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
- 4.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–69. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CORWIN J, HERRICK WW. RELATION OF HYPERTENSIVE TOXEMIA OF PREGNANCY TO CHRONIC CARDIOVASCULAR DISEASE. Journal of the American Medical Association. 1927;88:457–9. [Google Scholar]
- 6.Bellamy L, Casas JP, Hingorani AD, et al. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald SD, Malinowski A, Zhou Q, et al. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918–30. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 8.Pouta A, Hartikainen A-L, Sovio U, et al. Manifestations of Metabolic Syndrome After Hypertensive Pregnancy. Hypertension. 2004;43:825–31. doi: 10.1161/01.HYP.0000120122.39231.88. [DOI] [PubMed] [Google Scholar]
- 9.Ray JG. Heart Failure and Dysrhythmias after Maternal Placental Syndromes: HAD MPS Study. Heart. 2012 doi: 10.1136/heartjnl-2011-301548. In press. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–72. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 12.Masoudi FA, Havranek EP, Smith G, et al. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217–23. doi: 10.1016/s0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 13.Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol. 2011;26:562–8. doi: 10.1097/HCO.0b013e32834b7faf. [DOI] [PubMed] [Google Scholar]
- 14.Fang MC, Singer DE, Chang Y, et al. Gender differences in the risk of ischemic stroke and peripheral embolism in atrial fibrillation: the AnTicoagulation and Risk factors In Atrial fibrillation (ATRIA) study. Circulation. 2005;112:1687–91. doi: 10.1161/CIRCULATIONAHA.105.553438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garovic VD, Hayman SR. Hypertension in pregnancy: an emerging risk factor for cardiovascular disease. Nat Clin Pract Nephrol. 2007;3:613–22. doi: 10.1038/ncpneph0623. [DOI] [PubMed] [Google Scholar]
- 16.Chambers JC, Fusi L, Malik IS, et al. Association of maternal endothelial dysfunction with preeclampsia. Jama. 2001;285:1607–12. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- 17.Melchiorre K, Sutherland GR, Baltabaeva A, et al. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension. 2011;57:85–93. doi: 10.1161/HYPERTENSIONAHA.110.162321. [DOI] [PubMed] [Google Scholar]
- 18.Bamfo JE, Kametas NA, Chambers JB, et al. Maternal cardiac function in normotensive and pre-eclamptic intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;32:682–6. doi: 10.1002/uog.5311. [DOI] [PubMed] [Google Scholar]
- 19.Tihtonen KM, Koobi T, Vuolteenaho O, et al. Natriuretic peptides and hemodynamics in preeclampsia. Am J Obstet Gynecol. 2007;196:328, e1–7. doi: 10.1016/j.ajog.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 20.Adam B, Malatyalioğ, et al. Plasma Atrial Natriuretic Peptide Levels in Preeclampsia and Eclampsia. J Matern Fetal Investig. 1998;8:85–8. [PubMed] [Google Scholar]
- 21.Furuhashi N, Kimura H, Nagae H, et al. Brain natriuretic peptide and atrial natriuretic peptide levels in normal pregnancy and preeclampsia. Gynecol Obstet Invest. 1994;38:73–7. doi: 10.1159/000292452. [DOI] [PubMed] [Google Scholar]
- 22.Melchiorre K, Sutherland GR, Liberati M, et al. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58:709–15. doi: 10.1161/HYPERTENSIONAHA.111.176537. [DOI] [PubMed] [Google Scholar]
- 23.Zandstra M, Stekkinger E, van der Vlugt MJ, et al. Cardiac diastolic dysfunction and metabolic syndrome in young women after placental syndrome. Obstet Gynecol. 2010;115:101–8. doi: 10.1097/AOG.0b013e3181c4f1e8. [DOI] [PubMed] [Google Scholar]
- 24.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women-2011 update a guideline from the american heart association. J Am Coll Cardiol. 2011;57:1404–23. doi: 10.1016/j.jacc.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National High Blood Pressure Education Program Working Group on High Blood Pressure in P. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]


