Abstract
Objective
The variations in perilipin gene (PLIN) were previously associated with obesity and insulin sensitivity. We examined whether PLIN variability was associated with diabetes risk and whether obesity status modified such associations.
Research Methods and Procedures
We conducted a nested case-control study of 431 incident cases of type 2 diabetes and 791 healthy control women from the Nurses' Health Study. Obesity was defined by body mass index or waist circumference (central obesity).
Results
In the sample of all participants, PLIN variations were not significantly associated with the incidence of diabetes. The central obesity status (By NCEP ATP III definition of waist circumference greater than 35 inches) significantly interacted with PLIN polymorphisms in relation to diabetes risk (P for interaction=0.027, 0.009, and 0.02 for rs2289487, rs8179043, and rs894160 respectively). In non-obese (central) women, carriers of rs2289487, rs8179043 and rs894160 had significantly greater risk of type 2 diabetes, adjusting for diabetes risk factors (OR=1.52, 1.03–2.25; 1.54, 1.07–2.23, and 1.57, 1.09–2.27 respectively). Haplotypes possessing the three polymorphisms were also significantly associated with diabetes risk (global test, P=0.01). As compared with the most common haplotype 111, haplotype 222 and 211 (1 codes the common and 2 codes the minor alleles) were associated with 44% (OR=1.44, 95% CI 1.09–1.91), P=0.01) and 70% (OR=1.70, 95% CI 1.04–2.77; P=0.03) greater risk respectively. The PLIN variations were not significantly associated with the disease risk among women with central obesity.
Discussion
Our data indicate that central obesity may modify the associations between PLIN variations and diabetes risk in women.
INTRODUCTION
Adipose tissue is now recognized as an important endocrine organ that plays pivotal roles in regulating energy balance, glucose and lipid metabolisms, and insulin sensitivity (1; 2). The alteration of the metabolic and endocrine functions of adipose tissue is frequently associated with insulin resistance and type 2 diabetes (3; 4). Moreover, the variability in the genes regulating adipose metabolism has been found to predispose to both obesity and type 2 diabetes (5–9).
White adipose tissue stores most of the body’s fat reservoir in lipid droplets, which are earning recognition as active organelles regulating energy homeostasis and adipose metabolism (10). Perilipin covers the lipid droplet surface and modulates the turnover of the stored fat (11–13). Ablation of perilipin results in a lean phenotype with high level of adipocyte lipolysis, enhanced leptin production, and peripheral insulin resistance in animals (11; 13; 14). In earlier analyses, we have identified several sequence variations in perilipin gene (PLIN) that were associated with diabetes risk factors including central obesity, fasting glucose levels, and insulin sensitivity especially in women (15–18). However, little is known whether PLIN variations affect diabetes risk.
In this study, we examined the associations between common polymorphisms in PLIN gene and the risk of type 2 diabetes in a prospective, nested case-control study from the Nurses’ Health Study. Given the tight relation between PLIN and adiposity, especially central fatness, we particularly assessed the modification effects of obesity on the associations between PLIN variations and diabetes risk.
SUBJECTS and METHODS
Study population
The Nurses’ Health Study cohort was established in 1976 when 121,700 female registered nurses aged 30–55 years and residing in 11 large US states completed a mailed questionnaire on their medical history and lifestyle (19). The lifestyle factors, including smoking, menopausal status and postmenopausal hormone therapy, and body weight, have been updated by validated questionnaires every 2 years. Samples for the present study were selected from a subcohort of 32,826 women who provided a blood sample between 1989 and 1990 and were free from diabetes, cardiovascular disease, stroke, or cancer at the time of blood collection. Incident cases were defined as self-reported diabetes confirmed by a validated supplementary questionnaire and diagnosed at least 1 year after blood collection through 2000. The supplementary questionnaire obtained information on symptoms, diagnostic tests, and hypoglycemic therapy used to define type 2 diabetes cases. Medical record review confirmed the diagnosis of type 2 diabetes using this questionnaire for 98% of cases using the National Diabetes Data Group criteria (20). We used the American Diabetes Association diagnostic criteria for diagnosis of diabetes cases during the 1998 and 2000 cycles (21). The present study included 431 diabetes cases and 791 control subjects who were successfully measured on waist circumference and body mass index.
Definition of obesity
In 1986, participants were instructed to measure their waist circumference at the level of the umbilicus and their hips at the largest circumference with a tape measure while standing relaxed and to report values to the nearest quarter inch. In 1987, the validity of self reported waist and hip measures was assessed in a random sample of 140 participants living in the greater Boston, Massachusetts area (22). The average of two technician measurements spaced six months apart was compared with the self-reported current weight and waist and hip circumference values on the most recent questionnaire. After adjustment for age and within-person variability, the Pearson correlation coefficients between the self-reported measures and the average of the two technician assessments were 0.95 for waist circumference and 0.88 for hip circumference. Body mass index was calculated as weight in kilograms divided by the square of height in meters. We defined obesity as those with BMI more than 30 kg/m2. Both waist circumference and waist-to-hip ratio have been used as measures of central obesity in epidemiological studies (23). We used waist circumference as the primary measure for central fatness because of its better correlation with the technician-measurements in the validation study than waist-to-hip ratio. Central obesity was defined by waist circumference greater than 35 inches according to National Cholesterol Education Program-Adult Treatment Panel III (ATP III) definition (24).
SNP selection and genotype Determination
DNA was extracted from the buffy coat fraction of centrifuged blood using the QIAmp Blood Kit (Qiagen, Chatsworth, CA). We included four previously reported SNPs at PLIN locus, rs2289487, rs894160, rs2304795, and rs1052700 (also known as PLIN1 6209T>C, PLIN4 11482G>A, PLIN5 13041A>G and PLIN6 14995A>T respectively) (16). We also selected SNPs from HapMap database (HapMap data Release 20/phase II). Two SNPs (from five available SNPs) that are commonly distributed (CEU, minor allele frequency ≥ 5%) were included in the present study. The SNPs were genotyped using Taqman SNP allelic discrimination by means of an ABI 7900HT (Applied Biosystems, Foster City, CA). Replicate quality control samples were included and genotyped with >99% concordance.
Statistical analyses
A chi-square test was used to assess whether the genotypes were in Hardy-Weinberg equilibrium (HWE) and to compare the genotype and allele frequencies between case and control subjects. Odds ratios (ORs) were calculated using unconditional logistic regression adjusting for type 2 diabetes risk factors, including age, physical activity (<1.5, 1.5–5.9, 6.0–11.9, 12–20.9, and ≥21.0 metabolic equivalent hours/week), smoking (never, past, and current), alcohol intake (nondrinker and drinker [0.1–4.9, 5–10, or >10 g/day]), family history of diabetes, menopausal status (pre- or postmenopausal [never, past, or current hormone use]), and BMI. The interactions between obesity and PLIN genotypes were assessed using a likelihood ratio test. We created two interaction terms for the heterozygotes and the minor allele homozygotes and used a 2 degree of freedom test. The SAS statistical package was used for the analyses (SAS, Version 8.2 for UNIX). Haplotype analysis was conducted using THESIAS program that is based on the Stochastic-EM algorithm (SEM) (25). All P-values are two-sided.
RESULTS
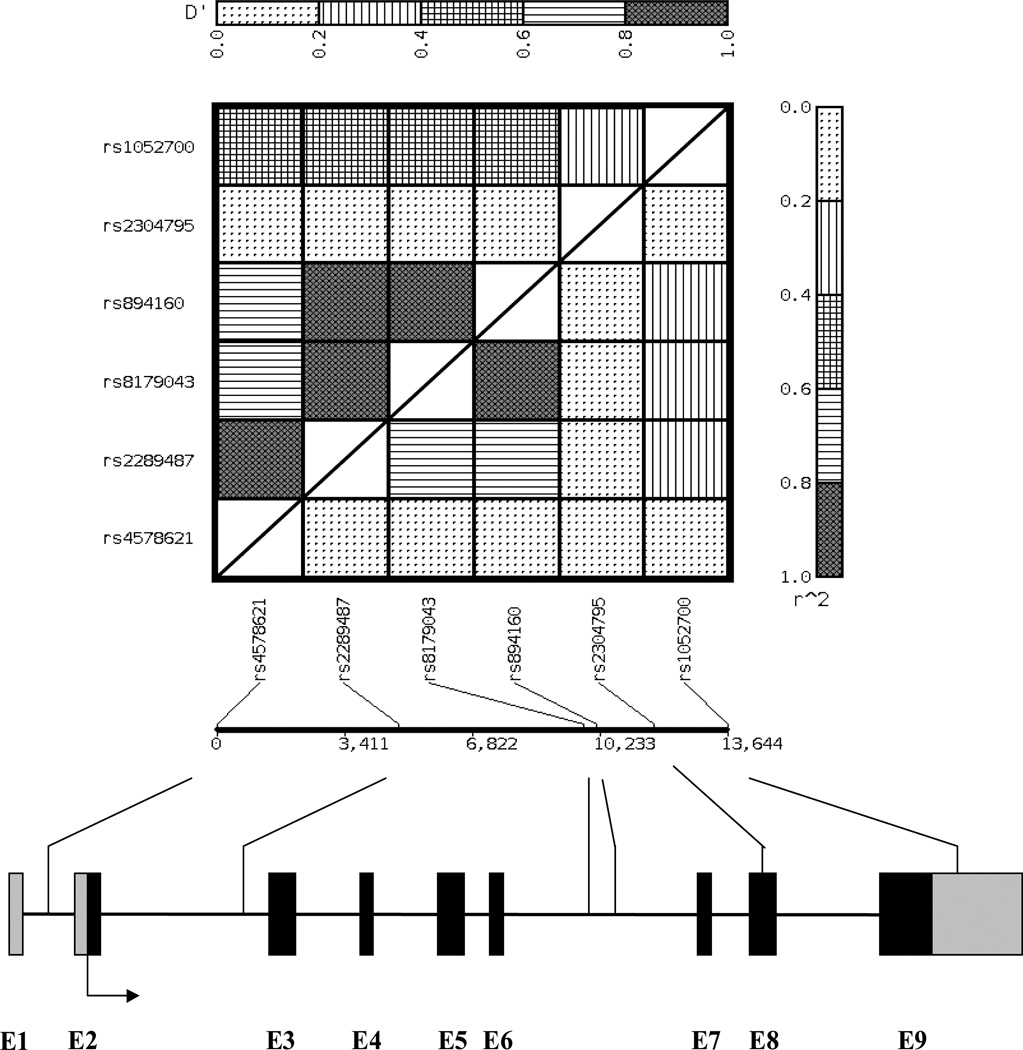
The allele frequency of the PLIN polymorphisms ranged from 0.09 to 0.38 in the healthy women and the genotype distribution did not significantly deviate from HWE (P>0.05). Polymorphisms rs2289487 (intron 2), rs8179043 (intron 6) and rs894160 (intron 6) were in strong pair-wise LD (D’>0.99; and r2 ranges from 0.64 to 0.93) (Figure 1). Table 1 presents the baseline characteristics of diabetes cases and control subjects by central obesity, which defined by waist circumference greater than 35 inches (by NCEP ATP III definition) (24). In women without central obesity, the cases had significantly higher BMI than the controls. Such a difference was not observed in women with central obesity.
FIGURE 1.
The location of PLIN polymorphisms (not drawn to scale) and pairwise linkage disequilibrium matrix (D’ is presented above the diagonal and r2 is presented below the diagonal). The exons of PLIN gene are depicted by black boxes and the promoter and 3’ UTR are denoted by smaller gray boxes. The direction of transcription is labeled with arrows. The position and identity of polymorphisms are indicated with lines.
Table 1.
Baseline characteristics of diabetes patients and controls by central obesity
| Non-obese | Obese | |||||
|---|---|---|---|---|---|---|
| Diabetes | Controls | P | Diabetes | Controls | P | |
| n of participants | 201 | 575 | 230 | 216 | ||
| Age, years | 57±7 | 57±7 | 0.51 | 57±9 | 57±9 | 0.80 |
| Body mass index, kg/m2 | 26.6±3.7 | 24.4±3.8 | <0.001 | 32.5±4.9 | 32.4±5.3 | 0.89 |
| Physical activity, MET hours/week | 13.2±14.6 | 17.0±20.0 | 0.01 | 11.4±14.6 | 11.8±15.2 | 0.76 |
| Alcohol consumption, g/day | 3.2±7.7 | 5.6±9.1 | <0.001 | 2.5±6.3 | 4.5±10.8 | 0.02 |
| Current smoker, % | 12.9 | 9.9 | 0.33 | 12.6 | 7.4 | 0.12 |
| Postmenopausal status, % | 81.1 | 81.0 | 0.98 | 85.6 | 82.4 | 0.35 |
| Family history of diabetes, % | 47.3 | 19.8 | <0.001 | 50.0 | 26.8 | <0.001 |
Central obesity was defined according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) definition, as waist circumference greater than 35 inches; the data are presented as mean ± SD (continuous) and percentage (categorical).
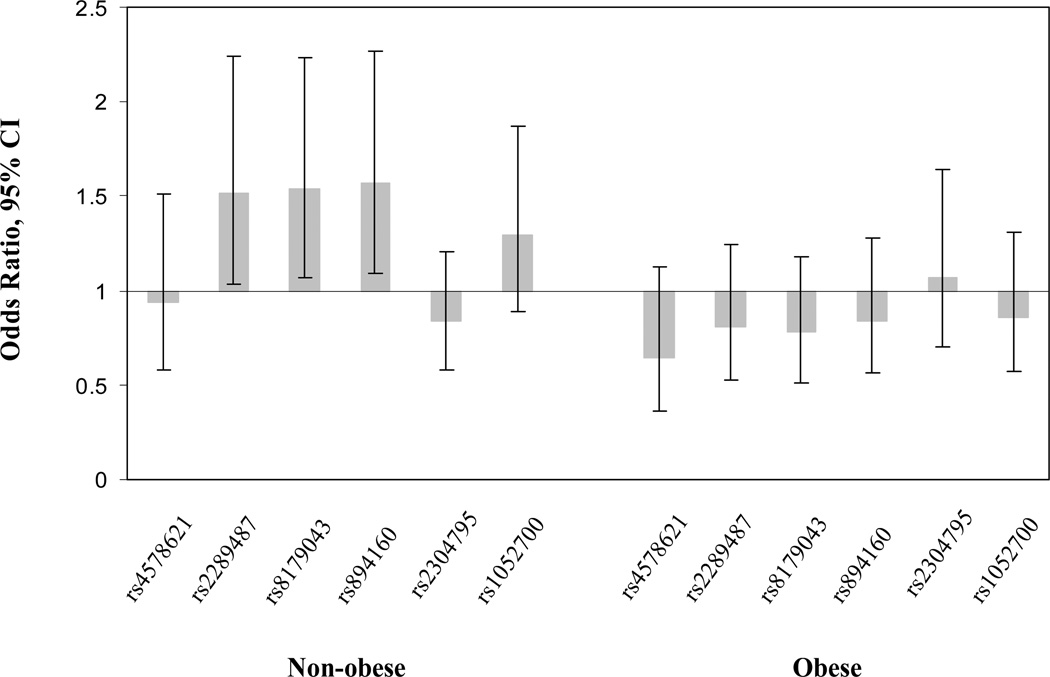
We first examined the associations between PLIN genotypes and diabetes risk in all study samples including obese and non-obese women. Overall, there was not significant difference in the distribution of PLIN genotypes between diabetes patients and healthy controls (data not shown). We then assessed the potential interactions between obesity and PLIN genotypes in relation to diabetes risk. To evaluate the effects of body fat distribution, we used waist circumference to define central obesity and use BMI to represent the overall obesity. Significant interactions were found between polymorphisms rs2289487, rs8179043, and rs894160 (P for interaction=0.027, 0.009, and 0.02 respectively) and central obesity in relation to diabetes risk. Polymorphisms rs2289487, rs8179043, and rs894160 were associated with significantly increased risk of type 2 diabetes in non-obese women, adjusting for conventional diabetes risk factors including age, BMI, smoking, alcohol consumption, physical activity, family history of diabetes, and menopausal status (Table 2). The genetic effects fit dominant inheritance models (OR=1.52, 95%CI 1.03–2.25; OR=1.54, 95%CI 1.07–2.23; and OR=1.57, 95CI 1.09–2.27; Figure 2). In women with central obesity, no polymorphism was significantly associated with diabetes risk. There was not significant interaction between obesity defined by BMI and PLIN variations in relation to diabetes risk.
Table 2.
Associations between PLIN polymorphisms and the risk of type 2 diabetes by central obesity
| No. (%) | Odds Ratio | |||
|---|---|---|---|---|
| Cases | Controls | (95% CI) | ||
| Non-obese | ||||
| rs2289487 | TT | 62 (32.6) | 210 (38.5) | 1 |
| TC | 91 (47.9) | 262 (48.0) | 1.35 (0.89–2.04) | |
| CC | 37 (19.5) | 74 (13.5) | 2.18 (1.26–3.78) | |
| TC+CC | 1.52 (1.03–2.25) | |||
| rs8179043 | CC | 82 (42.0) | 279 (50.1) | 1 |
| CT | 91 (46.7) | 225 (40.4) | 1.51 (1.03–2.23) | |
| TT | 22 (11.3) | 53 (9.5) | 1.67 (0.91–3.08) | |
| CT+TT | 1.54 (1.07–2.23) | |||
| rs894160 | GG | 86 (44.3) | 285 (51.8) | 1 |
| GA | 90 (46.4) | 218 (39.6) | 1.55 (1.05–2.28) | |
| AA | 18 (9.3) | 47 (8.6) | 1.67 (0.87–2.21) | |
| GA+AA | 1.57 (1.09–2.27) | |||
| Obese | ||||
| rs2289487 | TT | 98 (44.9) | 81 (38.4) | 1 |
| TC | 94 (43.2) | 95 (45.0) | 0.89 (0.57–1.41) | |
| CC | 26 (11.9) | 35 (16.6) | 0.60 (0.31–1.13) | |
| TC+CC | 0.81 (0.53–1.24) | |||
| rs8179043 | CC | 118 (53.7) | 97 (45.8) | 1 |
| CT | 83 (37.7) | 95 (44.8) | 0.79 (0.51–1.23) | |
| TT | 19 (8.6) | 20 (9.4) | 0.70 (0.33–1.47) | |
| CT+TT | 0.78 (0.51–1.18) | |||
| rs894160 | GG | 116 (53.7) | 100 (47.6) | 1 |
| GA | 81 (37.5) | 92 (43.8) | 0.82 (0.53–1.28) | |
| AA | 19 (8.8) | 18 (8.6) | 0.80 (0.38–1.70) | |
| GA+AA | 0.82 (0.54–1.24) | |||
Adjusted for age and BMI, cigarette smoking, alcohol consumption, physical activity, and menopausal status.
FIGURE 2.
The adjusted associations of PLIN variations (carriers v.s non-carriers) with the risk of type 2 diabetes by central obesity. The Odds ratio and 95% confidence intervals (CI) are presented. Analyses were adjusted for age, BMI, smoking, alcohol consumption, physical activity, family history of diabetes, and menopausal status.
We further analyzed the haplotype associations in women with and without central obesity. Because rs2289487, rs8179043, and rs894160 were in a LD block (Figure 1), we inferred the haplotypes from these three polymorphisms. In line with the analyses for individual polymorphisms, the three-SNP haplotypes were significantly associated with diabetes risk in women without central obesity (global test, P=0.01). Haplotypes 222 and 211 were associated with 44% and 70% increased risk of type 2 diabetes compared with the most common haplotype 111 (1 codes the common and 2 codes the minor allele, Table 3). PLIN haplotypes were not associated with diabetes risk in women with central obesity.
Table 3.
PLIN haplotypes and the risk of type 2 diabetes according to central obesity
| Polymorphisms | Frequency | Odds Ratios | P | Global, P | ||||
|---|---|---|---|---|---|---|---|---|
| rs2289487 | rs8179043 | rs894160 | Cases | Controls | (95% CI) | |||
| Non-obese | ||||||||
| H1 | 1 | 1 | 1 | 55.9 | 62.5 | 1.0 | - | 0.01 |
| H2 | 2 | 2 | 2 | 32.7 | 28.5 | 1.44, 1.09–1.91 | 0.01 | |
| H3 | 2 | 1 | 1 | 9.4 | 7.3 | 1.70, 1.04–2.77 | 0.03 | |
| Obese | ||||||||
| H1 | 1 | 1 | 1 | 66.2 | 60.9 | 1.0 | - | 0.32 |
| H2 | 2 | 2 | 2 | 27.2 | 30.0 | 0.86, 0.62–1.19 | 0.37 | |
| H3 | 2 | 1 | 1 | 5.2 | 7.1 | 0.66, 0.36–1.21 | 0.18 | |
H1-H3, haplotype 1-haplotype 3. ‘1’ codes the common and ‘2’ codes the minor alleles; analyses were adjusted for age, body mass index, alcohol consumption, physical activity, family history of diabetes, and menopausal status.
DISCUSSION
Unequivocal evidence from experimental, epidemiological and clinical studies during the past decades causally links dysfunction of adipose tissue with the development of type 2 diabetes (26; 27). Also, variations in genes regulating adipose metabolisms have been associated with diabetes risk (5–9). Perilipin is a key regulatory protein for adipose metabolism. The function of perilipins is to prevent lipolysis in basal condition, favoring the fat deposition. Animals lacking perilipin were lean, resistant to diet-induced or genetic obesity, and had peripheral insulin resistance (11; 13; 14). In human studies, the common variations in PLIN gene have been associated with several risk factors for diabetes, including obesity, weight gain, insulin resistance, and hypertension (16; 28–30).
Little is known about the relation between PLIN variations and diabetes risk. Polymorphism rs894160 (also known as 11482G>A) in intron 6 of PLIN gene was previously associated with lower perilipin contents and increased lipolysis in women (31). As such, elevated lipolysis may lead to augmentation in fatty acid release from adipose tissue. It is well established that fatty acids from adipose tissue are closely related to the metabolism of glucose and adversely affect peripheral insulin sensitivity (32–34). This may partly account for the elevated diabetes risk associated with rs894160 and other polymorphisms in LD with it. In addition, adipose tissue is increasingly recognized as a key endocrine organ synthesizing and secreting a wide range of hormonal products (namely adipokines) that are involved in the pathogenesis of diabetes (35). Adipose lipolysis may reprogram adipokine expression (36). It is possible the metabolic changes related to PLIN polymorphisms may affect the endocrine function of adipose tissue that in turn leads to the development of diabetes. Nevertheless, data linking PLIN and the endocrine function of adipocytes are sparse. Further studies are needed to test these hypotheses. The polymorphisms associated with diabetes risk in non-obese women were in strong LD. We assume that the genetic effects are likely attributed to the same causal variant.
The data from the present study indicate that the genetic effects of PLIN may be dependent on obesity status, especially the centric distribution pattern of body fat. The accumulation of abdominal fat has strong associations with many factors that are constituents of type 2 diabetes (34). Compelling evidence has shown that abdominal fat is more pathogenic for the metabolic disorders than the other fat depots (34; 37; 38). We used waist circumference as a convenient surrogate for abdominal adiposity (39). It has been documented that the transcription of perilipin may differ in central adipose tissue from adipose tissue in other deports and central obesity may substantially affect the expression of perilipin (40–42). Our findings support a potential modulation effect of abdominal fat on the genetic effects of PLIN. The genetic associations were observed in non-obese (central) women only. We assume this maybe partly because the obese individuals, who in general had high levels of many metabolic risk factors, were less sensitive to the gene-associated changes.
Our study was conducted in a prospective setting. Such a study design has an advantage of avoiding the potential influence of several sources of bias inherent in the studies enrolling prevalent patients, e.g. selection bias and survival bias. Also, having comprehensively measured lifestyle components, we were able to adjust for the potential confounding effects of the non-genetic factors. As a limitation, population stratification may bias the associations. However, the majority of the participants are white (~96%). Further adjustment for ethnicity or removing the minorities from the analyses did not appreciably change the results. In addition, our findings are restricted to women and may not be generalizable to men.
In summary, our data for the first time indicate that central obesity may significantly modify the associations between the genetic variations in PLIN and the risk of type 2 diabetes in women. Further studies are warranted to replicate our findings and to delve into the underlying mechanisms.
Acknowledgement
This study was supported by research grants (DK58845 and CA87969) from the National Institutes of Health.
REFERENCE
- 1.Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53(Suppl 1):S143–S151. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- 2.Mora S, Pessin JE. An adipocentric view of signaling and intracellular trafficking. Diabetes Metab Res Rev. 2002;18:345–356. doi: 10.1002/dmrr.321. [DOI] [PubMed] [Google Scholar]
- 3.Tataranni PA, Ortega E. A burning question: does an adipokine-induced activation of the immune system mediate the effect of overnutrition on type 2 diabetes? Diabetes. 2005;54:917–927. doi: 10.2337/diabetes.54.4.917. [DOI] [PubMed] [Google Scholar]
- 4.Frayn KN. Obesity and metabolic disease: is adipose tissue the culprit? Proc Nutr Soc. 2005;64:7–13. doi: 10.1079/pns2004403. [DOI] [PubMed] [Google Scholar]
- 5.Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl MC, Nemesh J, Lane CR, Schaffner SF, Bolk S, Brewer C, Tuomi T, Gaudet D, Hudson TJ, Daly M, Groop L, Lander ES. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet. 2000;26:76–80. doi: 10.1038/79216. [DOI] [PubMed] [Google Scholar]
- 6.Hu FB, Doria A, Li T, Meigs JB, Liu S, Memisoglu A, Hunter D, Manson JE. Genetic variation at the adiponectin locus and risk of type 2 diabetes in women. Diabetes. 2004;53:209–213. doi: 10.2337/diabetes.53.1.209. [DOI] [PubMed] [Google Scholar]
- 7.Memisoglu A, Hu FB, Hankinson SE, Manson JE, De Vivo I, Willett WC, Hunter DJ. Interaction between a peroxisome proliferator-activated receptor gamma gene polymorphism and dietary fat intake in relation to body mass. Hum Mol Genet. 2003;12:2923–2929. doi: 10.1093/hmg/ddg318. [DOI] [PubMed] [Google Scholar]
- 8.Shen H, Qi L, Tai ES, Chew SK, Tan CE, Ordovas JM. Uncoupling protein 2 promoter polymorphism-866G/A, central adiposity, and metabolic syndrome in Asians. Obesity (Silver Spring) 2006;14:656–661. doi: 10.1038/oby.2006.74. [DOI] [PubMed] [Google Scholar]
- 9.Qi L, van Dam RM, Meigs JB, Manson JE, Hunter D, Hu FB. Genetic variation in IL6 gene and type 2 diabetes: tagging-SNP haplotype analysis in large-scale case-control study and meta-analysis. Hum Mol Genet. 2006;15:1914–1920. doi: 10.1093/hmg/ddl113. [DOI] [PubMed] [Google Scholar]
- 10.Beckman M. Cell biology. Great balls of fat. Science. 2006;311:1232–1234. doi: 10.1126/science.311.5765.1232. [DOI] [PubMed] [Google Scholar]
- 11.Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, Reitman ML, Deng CX, Li C, Kimmel AR, Londos C. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci U S A. 2001;98:6494–6499. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tansey JT, Sztalryd C, Hlavin EM, Kimmel AR, Londos C. The central role of perilipin a in lipid metabolism and adipocyte lipolysis. IUBMB Life. 2004;56:379–385. doi: 10.1080/15216540400009968. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Botas J, Anderson JB, Tessier D, Lapillonne A, Chang BH, Quast MJ, Gorenstein D, Chen KH, Chan L. Absence of perilipin results in leanness and reverses obesity in LepRdb/db) mice. Nat Genet. 2000;26:474–479. doi: 10.1038/82630. [DOI] [PubMed] [Google Scholar]
- 14.Saha PK, Kojima H, Martinez-Botas J, Sunehag AL, Chan L. Metabolic adaptations in the absence of perilipin: increased beta-oxidation and decreased hepatic glucose production associated with peripheral insulin resistance but normal glucose tolerance in perilipin-null mice. J Biol Chem. 2004;279:35150–35158. doi: 10.1074/jbc.M405499200. [DOI] [PubMed] [Google Scholar]
- 15.Qi L, Tai ES, Tan CE, Shen H, Chew SK, Greenberg AS, Corella D, Ordovas JM. Intragenic linkage disequilibrium structure of the human perilipin gene (PLIN) and haplotype association with increased obesity risk in a multiethnic Asian population. J Mol Med. 2005;83:448–456. doi: 10.1007/s00109-004-0630-4. [DOI] [PubMed] [Google Scholar]
- 16.Qi L, Corella D, Sorli JV, Portoles O, Shen H, Coltell O, Godoy D, Greenberg AS, Ordovas JM. Genetic variation at the perilipin (PLIN) locus is associated with obesity-related phenotypes in White women. Clin Genet. 2004;66:299–310. doi: 10.1111/j.1399-0004.2004.00309.x. [DOI] [PubMed] [Google Scholar]
- 17.Qi L, Shen H, Larson I, Schaefer EJ, Greenberg AS, Tregouet DA, Corella D, Ordovas JM. Gender-specific association of a perilipin gene haplotype with obesity risk in a white population. Obes Res. 2004;12:1758–1765. doi: 10.1038/oby.2004.218. [DOI] [PubMed] [Google Scholar]
- 18.Corella D, Qi L, Sorli JV, Godoy D, Portoles O, Coltell O, Greenberg AS, Ordovas JM. Obese subjects carrying the 11482G>A polymorphism at the perilipin locus are resistant to weight loss after dietary energy restriction. J Clin Endocrinol Metab. 2005;90:5121–5126. doi: 10.1210/jc.2005-0576. [DOI] [PubMed] [Google Scholar]
- 19.Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 20.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 21.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 22.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Willett W. Nutritional Epidemiology. New York: Oxford University Press; 1998. [Google Scholar]
- 24.Grundy SM, Hansen B, Smith SC, Jr, Cleeman JI, Kahn RA. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation. 2004;109:551–556. doi: 10.1161/01.CIR.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- 25.Tregouet DA, Escolano S, Tiret L, Mallet A, Golmard JL. A new algorithm for haplotype-based association analysis: the Stochastic-EM algorithm. Ann Hum Genet. 2004;68:165–177. doi: 10.1046/j.1529-8817.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- 26.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 28.Kang ES, Cha BS, Kim HJ, Kim SH, Hur KY, Lee HJ, Shim WS, Ahn CW, Lee HC. The 11482G >A polymorphism in the perilipin gene is associated with weight gain with rosiglitazone treatment in type 2 diabetes. Diabetes Care. 2006;29:1320–1324. doi: 10.2337/dc05-2466. [DOI] [PubMed] [Google Scholar]
- 29.Corella D, Qi L, Tai ES, Deurenberg-Yap M, Tan CE, Chew SK, Ordovas JM. Perilipin gene variation determines higher susceptibility to insulin resistance in Asian women when consuming a high-saturated fat, low-carbohydrate diet. Diabetes Care. 2006;29:1313–1319. doi: 10.2337/dc06-0045. [DOI] [PubMed] [Google Scholar]
- 30.Yan W, Chen S, Huang J, Shen Y, Qiang B, Gu D. Polymorphisms in PLIN and hypertension combined with obesity and lipid profiles in Han Chinese. Obes Res. 2004;12:1733–1737. doi: 10.1038/oby.2004.214. [DOI] [PubMed] [Google Scholar]
- 31.Mottagui-Tabar S, Ryden M, Lofgren P, Faulds G, Hoffstedt J, Brookes AJ, Andersson I, Arner P. Evidence for an important role of perilipin in the regulation of human adipocyte lipolysis. Diabetologia. 2003;46:789–797. doi: 10.1007/s00125-003-1112-x. [DOI] [PubMed] [Google Scholar]
- 32.Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 33.Raz I, Eldor R, Cernea S, Shafrir E. Diabetes: insulin resistance and derangements in lipid metabolism. Cure through intervention in fat transport and storage. Diabetes Metab Res Rev. 2005;21:3–14. doi: 10.1002/dmrr.493. [DOI] [PubMed] [Google Scholar]
- 34.Bergman RN, Kim SP, Catalano KJ, Hsu IR, Chiu JD, Kabir M, Hucking K, Ader M. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006;14(Suppl 1):16S–19S. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
- 35.Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf) 2006;64:355–365. doi: 10.1111/j.1365-2265.2006.02474.x. [DOI] [PubMed] [Google Scholar]
- 36.Hertzel AV, Smith LA, Berg AH, Cline GW, Shulman GI, Scherer PE, Bernlohr DA. Lipid metabolism and adipokine levels in fatty acid-binding protein null and transgenic mice. Am J Physiol Endocrinol Metab. 2006;290:E814–E823. doi: 10.1152/ajpendo.00465.2005. [DOI] [PubMed] [Google Scholar]
- 37.Gastaldelli A, Miyazaki Y, Pettiti M, Matsuda M, Mahankali S, Santini E, DeFronzo RA, Ferrannini E. Metabolic effects of visceral fat accumulation in type 2 diabetes. J Clin Endocrinol Metab. 2002;87:5098–5103. doi: 10.1210/jc.2002-020696. [DOI] [PubMed] [Google Scholar]
- 38.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, Willett WC, Manson JE. Abdominal adiposity and coronary heart disease in women. Jama. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 40.Arvidsson E, Blomqvist L, Ryden M. Depot-specific differences in perilipin mRNA but not protein expression in obesity. J Intern Med. 2004;255:595–601. doi: 10.1111/j.1365-2796.2004.01314.x. [DOI] [PubMed] [Google Scholar]
- 41.Kern PA, Di Gregorio G, Lu T, Rassouli N, Ranganathan G. Perilipin expression in human adipose tissue is elevated with obesity. J Clin Endocrinol Metab. 2004;89:1352–1358. doi: 10.1210/jc.2003-031388. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Sullivan S, Trujillo M, Lee MJ, Schneider SH, Brolin RE, Kang YH, Werber Y, Greenberg AS, Fried SK. Perilipin expression in human adipose tissues: effects of severe obesity, gender, and depot. Obes Res. 2003;11:930–936. doi: 10.1038/oby.2003.128. [DOI] [PubMed] [Google Scholar]