Abstract
Background
The onset of major depressive disorder is likely precipitated by a combination of heredity and life stress. The present study tested the hypothesis that rats selectivity bred on a trait related to emotional reactivity would show differential susceptibility or resilience to the development of depression-like signs in response to chronic mild variable intermittent stress (CMS).
Methods
Male Sprague-Dawley rats that were bred based on the trait of either high or low locomotor activity in response to a novel environment were exposed to four weeks of CMS or control conditions. Changes in hedonic behavior were assessed using weekly sucrose preference tests and anxiety-like behavior was evaluated using the novelty-suppressed feeding test.
Results
During four weeks of CMS, bred low responder (bLR) rats became anhedonic at a faster rate and to a larger degree than bred high responder (bHR) rats, based on weekly sucrose preference tests. Measures of anxiety-like behavior in the novelty-suppressed feeding test were also significantly increased in the CMS-exposed bLR rats, though no differences were observed between CMS-exposed bHR rats and their unstressed controls.
Conclusions
These findings present further evidence that increased emotional reactivity is an important factor in stress susceptibility and the etiology of mood disorders, and that bHR and bLR rats provide a model of resistance or vulnerability to stress-induced depression. Furthermore, exposing bHR and bLR rats to CMS provides an excellent way to study the interaction of genetic and environmental factors in the development of depression-like behavior.
Keywords: depression, chronic mild stress, high responder, low responder, selectively bred rat, vulnerability
Introduction
Reliable animal models of depression are crucial to the success of preclinical studies of mood disorders such as major depressive disorder. Chronic mild stress (CMS), an animal model of depression first developed in the late 1980's, has gained favor in recent years for several reasons. First, it uses variable and unpredictable chronic intermittent stress, an established risk factor for depression [1, 2]. Second, it provokes changes in behavior and physiology that closely resemble the human disorder [3, 4]. Finally, CMS-induced depression-like signs can be reversed with antidepressant treatment [4-7]. Additionally, the extended timecourse of the CMS paradigm, which typically lasts 4-6 weeks, is more appropriate for studying the effects of chronic drug treatment than acute measurements of so-called “despair behavior” such as the forced swim test [3, 8-11]. The depression-like behavior produced by CMS has typically been evaluated by measuring anhedonia, a key component of DSM-IV criteria for major depressive disorder. Reduced sucrose preference, for instance, can be reversed by chronic but not acute administration of anti-depressant drugs and is not changed by drugs that are ineffective as antidepressants [12-15]. The CMS model of depression in rodents strongly mimics both the symptoms of major depressive disorder and the time course of typical responsiveness to pharmacological treatment.
However, environmental factors are not solely responsible for the onset of depression, and a key question in the study of mood disorders is that of vulnerability and resilience among individuals [16]. Researchers have begun to address this issue by examining baseline individual differences in behavior that correlate with either vulnerability or resistance to behavioral, genetic, hormonal, and molecular markers of mood disorders [17-21]. One behavioral trait that has been examined in this regard in rats is locomotor behavior in novel environments [17, 20, 22-24]. Outbred Sprague-Dawley rats can be classified based on their activity level in a novel environment. Rats classified as high-responders (HR) have high levels of exploratory activity, whereas rats with low activity in a novel environment are classified as low responders (LR). Low exploratory activity is interpreted as a sign of anxiety-like behavior due to rodents' innate aversion to novel, open spaces. Likewise, increased exploration is considered indicative of less anxiety-like behavior. Since the HR/LR designation is based on exploration of a novel environment, it is not surprising that this classification predicts emotional reactivity in other behavioral tests. Specifically, outbred rats classified as LR have higher anxiety-like behavior than HR rats in the elevated plus maze, light-dark box, and the open field test [17, 22, 25]. LR rats' tendency toward higher emotional reactivity is also apparent in the forced swim test, in which they display more depression-like behavior than HR rats [26].
In order to study the possible interaction of genetics and behavioral phenotype in HR versus LR animals, rats have been selectively bred based on that trait [17, 27]. These selectively-bred strains of each type (bred HR [bHR] and bred LR [bLR], respectively) represent the extremes of the normal distribution of the Sprague-Dawley population. The patterns of anxiety- and depression-like behavior discussed above have been preserved and enriched across generations of bHR and bLR rats [17, 27], raising the possibility that the bHR/bLR rat strains may represent a model of inherited susceptibility or resilience to mood disorders. We therefore hypothesized that studying bHR/bLR rats in the CMS model of depression would reveal an interaction of genetic predisposition and environmental stress, providing a model of vulnerability or resilience to stress-induced depression.
Methods
Animals and Housing
Rats (male, Sprague-Dawley) used in this study were selectively-bred for locomotor traits according to procedures outlined in Stead et al., 2006 [17] and Clinton et al., 2007 [27]. These rats were from the F20 generation bred at the Molecular and Behavioral Neuroscience Institute at the University of Michigan; 16 Low-Responder (bLR) rats and 15 High Responder (bHR) rats were shipped to the University of Pittsburgh when they were approximately 3 months old (400-450g). Prior to shipping, locomotor activity in a novel environment similar to the housing cage, was tested in all rats. All subsequent testing was performed at the University of Pittsburgh. Rats were housed individually in plastic cages (length × width × height: 40 × 22 × 19 cm) with 3-5 cm of bedding (course cut Aspen chips; P.J. Murphy) and wire lids. Standard rat chow (Purina) and tap water were available ad libitum. Rooms were kept at a constant temperature of 23°C under 12 h light: 12 h dark lighting conditions (lights on at 0700). CMS-exposed and Control groups (see descriptions below) were housed in separate rooms under similar conditions. The University of Pittsburgh Animal Care and Use Committee approved all animal protocols that were used.
Chronic Mild Stress Protocol
After a two-week period to collect baseline measurements, rats were either exposed to CMS for a total of 5 weeks, or were handled according to standard animal care practices (Control group). CMS and Control groups were single-housed in separate rooms. The following individual stressors were used in the CMS protocol in varying order across different weeks (Figure 1): continuous overnight lighting; overnight water deprivation (18h) followed by 1 hour of empty water bottle replacement; 40-degree cage tilt; stroboscopic lighting (2-6 h; Chauvet mini-strobe CH-730; 8-12 flashes per second, 35 watts); overnight paired housing with another CMS-exposed rat (18h); damp bedding (300-500mL lukewarm water added to cage bedding); white noise (radio static, 85dB, 1-4 h, continuous or intermittent); and predator odor exposure (30-60 minutes exposure to 20uL undiluted 2,4,5-trimethylthiazoline (TMT; Pherotech Intl.) placed on a piece of filter paper and hung in each rat's cage). These mild environmental stressors were chosen to minimize the amount of interaction between experimenter and animal and have been shown in previous studies to be effective in inducing depression-like behaviors in rats [9, 13, 28-33]. All CMS rats were exposed to the same schedule of stressors, although the schedule of these stressors was altered on a weekly basis and multiple stressors occasionally overlapped. However, overnight food- and water-deprivation was only combined prior to the sucrose preference test (below).
Figure 1. Typical chronic mild stress (CMS) schedule.

CMS-exposed rats were exposed to a variety of intermittent stressors, which were applied each week, on different days and in different combinations. Control rats were housed in a separate room and were handled according to usual animal care protocols with the addition of a weekly sucrose preference test (SPT). SPT was always administered on Friday.
Sucrose Preference Test
The CMS protocol has been shown to decrease rodents' preference for sweet solutions (see [4] for a review), which is thought to represent anhedonia, a core symptom of major depression [34]. This anhedonic behavior is commonly assessed in rats via the sucrose preference test (SPT) [12, 15, 28-33] and can be reversed by chronic antidepressant treatment [5-7]. To accustom the rats to the taste of sucrose, ad libitum water was replaced with 1% sucrose solution for one week. Tap water was returned one day before the first baseline SPT was administered. SPT was administered once per week to both CMS and Control groups. Rats were food- and water-deprived for 18 hours prior to the test. Two graduated burets were placed on the cage filled with either 1% sucrose solution or tap water. Rats were allowed to drink freely for one hour and total volume consumed was recorded. Preference for sucrose was calculated as [(mL sucrose/total mL consumed) *100].
Novelty-suppressed Feeding
The novelty-suppressed feeding test (NSF) is often used as a measure of depression-like behaviors. Like the open field test, the NSF test is based on rodents' innate fear of novel spaces. However, the NSF test introduces an additional component of motivation, as the food-deprived animal's drive to eat conflicts with its fear of novel open spaces. Chronic, but not acute, administration of antidepressants reduces these latencies, giving the NSF test excellent predictive validity for the timecourse of antidepressant efficacy [12, 35, 36].
During CMS week 5, rats were food-deprived 24 hours prior to the test, with free access to water and were moved to the dimly lit testing room one to two hours before the test. Rats were placed into one corner of an open field apparatus (17 in. × 17 in. × 12 in.) with clear acrylic walls and an opaque white acrylic floor. Light level in the open field was maintained at 16-20 Lux and the walls and floor were wiped with Novalsan (chlorhexidine diacetate) between trials. A food pellet was placed in the center of the open field and rats were placed in one corner. Latencies to approach and to begin eating were recorded with a limit of 15 minutes. As soon as the rat was observed to eat, or the 15-minute time limit was reached, the rat was removed from the open field and placed in the home cage and observed until it began to eat in the home cage. Previous studies have demonstrated that home cage consumption is the same across treatment and control groups [12, 35, 36].
Data Analysis
Statistics were performed using Microsoft Excel and SPSS 16.0. Values are expressed as mean ± SEM. For comparisons of four groups, 2-way ANOVA (strain*treatment) was used, with Tukey HSD post-hoc tests where appropriate. Repeated measures ANOVA was used to identify significant differences in SPT measures across time, also with Tukey HSD post-hoc test. To compare proportion of animals in each group with SPT scores less than 50%, a χ2-analysis was performed. Pearson correlation was used to examine the relationship between body weight and sucrose preference or fluid intake. For all tests, a two-tailed p-value of 0.05 or less was considered significant.
Results
Body Weight
Body weights were measured once per week, at the same time that food and water were removed prior to the weekly sucrose preference test. At baseline, before exposure to CMS, there were no significant differences within strains (Table 1). However, body weights were significantly higher in bHR rats than bLR rats (p<0.01), a difference that persisted throughout the experiment and that we have observed in previous populations of bHR/bLR rats (unpublished observation, Clinton, Kerman, Akil & Watson). There was also a significant difference between treatment groups (p<0.01) in the total amount of weight gained during the experiment. While both strains of control rats gained similar amounts of weight, CMS-exposed rats did not gain weight during the experiment (Table 1).
Table 1. Comparison of body weight (BW) at Baseline and at each of four weeks of CMS.
| n | Baseline | CMS Week 1 | CMS Week 2 | CMS Week 3 | CMS Week 4 | Δ BW (g) | |
|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
| bHR - Control | 7 | 553 ± 24* | 570 ± 23* | 581 ± 22* | 589 ± 22* | 576 ± 27* | 22 ± 17 |
| bHR - CMS | 8 | 574 ± 21* | 577 ± 23* | 569 ± 22* | 576 ± 22* | 573 ± 20* | -1 ± 4** |
| bLR - Control | 8 | 500 ± 10 | 505 ± 6 | 507 ± 6 | 515 ± 7 | 519 ± 7 | 19 ± 7 |
| bLR - CMS | 8 | 492 ± 19 | 495 ± 19 | 491 ± 19 | 489 ± 19 | 488 ± 19 | -4 ± 7** |
p<0.01 compared to opposite strain
p<0.01 compared to opposite treatment
Characterization of bHR/bLR rats
To confirm behavioral phenotype, bHR (n=15) and bLR (n=16) rats that were used in this study were exposed to a novel environment before being shipped to the University of Pittsburgh. As anticipated, bHR rats were markedly more active in a novel environment (Figure 2). The two groups were further assigned to CMS-exposed or control groups (n=8 per group, except bHR-Control n=7) and there were no baseline difference (p > 0.05) in locomotor scores between pre-assigned treatment groups (Figure 2).
Figure 2. Locomotor activity in response to a novel environment in bHR and bLR rats.

Prior to other experimental procedures, locomotor activity in response to a novel environment was tested. There was a significant difference between bHR (n=15) and bLR rats (n=16) in baseline locomotor activity (p<0.01) but no difference within strains in the subgroups of rats that were subsequently exposed to CMS or not.
Exposure to chronic mild stress
Rats of each rat strain were exposed to 4 weeks of intermittent CMS (Figure 1) and sucrose preference, used as a measure of anhedonia, was tested at weekly intervals. At baseline, all groups had similar robust preference for 1% sucrose versus water. In control rats of both strains, sucrose preference remained stable during the experimental period. In contrast, after two weeks of CMS, bLR-CMS rats showed significantly reduced sucrose preference (p=0.01; Figure 3) compared to both control groups and bHR-CMS rats (p=0.02), as well as their own baseline (p<0.001). bLR-CMS rats had SPT scores that were significantly less than 50% (p<0.05), which persisted throughout the CMS period. bHR-CMS rats did not have significantly reduced SPT scores until Week 4. Body weight was not correlated with either sucrose preference (r =.023, n.s.) or sucrose intake during the SPT (r = 0.37, n.s.).
Figure 3. Percent preference for sucrose across four weeks of CMS.
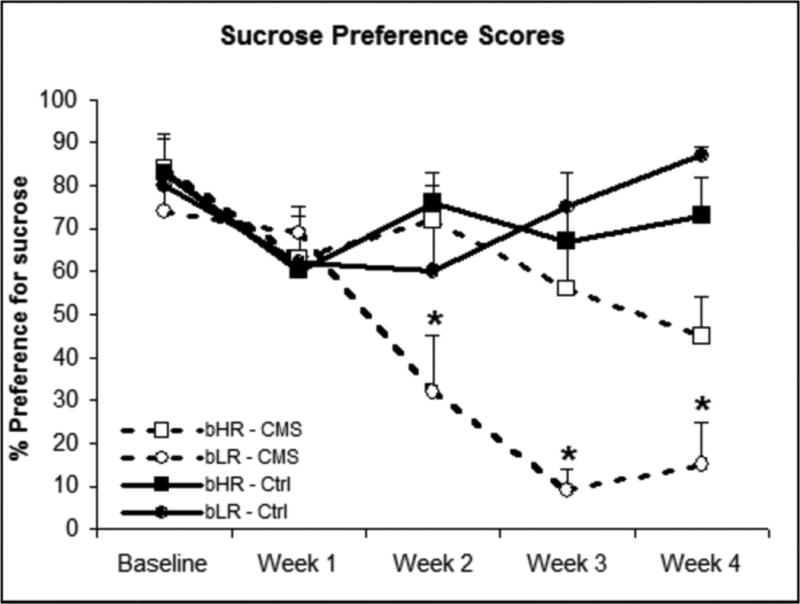
Sucrose preference was assessed at weekly intervals. At baseline and Week 1, groups had no significant difference in sucrose preference. bLR-CMS rats (n=8) had reduced sucrose preference compared to the other three groups starting in Week 2. In Weeks 3 and 4 there was a significant Treatment × Strain interaction (p=0.02). Values are means ± SEM. *:p<0.05 compared to other groups.
Performance in novelty-suppressed feeding test
The NSF test was used as an additional measurement of depressive behavior [12, 35-37]. Latency to approach the food and latency to consume the food were recorded, with longer latencies in each parameter considered indicative of anxiety-like behavior [12, 35-37]. There was a significant interaction of strain and treatment; the bLR-CMS group scored higher on both measures, taking significantly longer to approach (Figure 4A) and begin to eat the food pellet (Figure 4B). In fact, only one of the eight bLR-CMS rats ate the food pellet within the 15-minute time limit of the test. Scores were not significantly different between bHR-CMS rats and either control group (Figure 4A & 4B). All rats were observed eating chow within two minutes of being returned to the home cage, with no significant differences among groups. Finally, only bLR rats (both CMS-exposed and control) defecated during the NSF test, a factor that is often interpreted to indicate increased anxiety during behavioral tests [38-41].
Figure 4. Latency to approach and consume a food pellet in the novelty-suppressed feeding test.
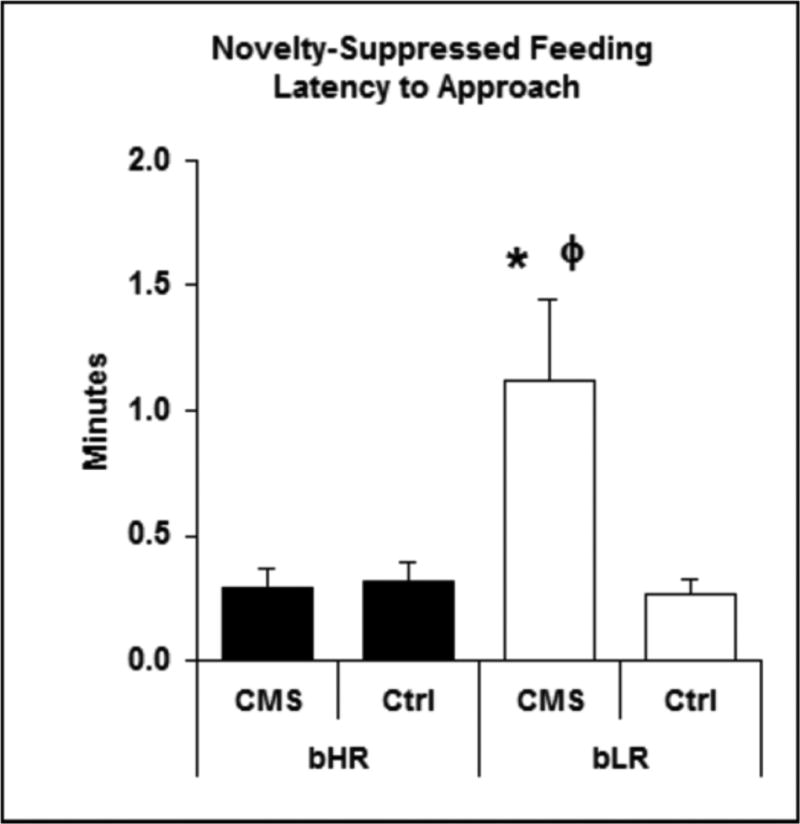

Depression-related anxiety was further assessed in the Novelty-Suppressed Feeding test. Following 24 h food deprivation, bLR-CMS rats had increased latency to approach (4A) and eat (4B) a food pellet placed in the center of an open field. Values are means ± SEM. *: Significant difference from opposite treatment. ϕ: Significant difference from opposite strain.
4A: Latency to approach food pellet. Significant effects of treatment (p = 0.03) and strain (p = 0.05) as well as an interaction of the two (p = 0.03) were found.
4B: Latency to eat food pellet. Treatment, Strain, and Treatment*Strain: p<0.001
Discussion
The key observation from these studies is that rats selectively bred for low and high locomotor responses to a novel environment show markedly different susceptibility to develop signs of depression in response to chronic mild intermittent stress. bLR rats showed signs of anhedonia sooner and to a greater degree than bHR rats, and this was confirmed with behavior in the NSF test, a test which is sensitive to antidepressant drugs [12, 37].
Supplier-bred Sprague-Dawley rats exhibit a range of behavior in novel environments [20, 42]. Based solely on this locomotor behavior, Sprague-Dawley rats can be separated into two groups: high-responders and low-responders (HR & LR), and these designations appear to predict emotional reactivity [17, 27]. The rats used in this study were selectively bred for novelty-seeking behavior in a novel environment over multiple generations. Differences in behavior and neurobiology have been well-characterized in bHR and bLR rats; in stressful or novel environments bHR rats have exaggerated locomotor activity, higher HPA activity and less anxiety [17, 27]. Additionally, bHR rats have increased drive for reward, are more inclined to self-administer drugs of abuse, and have higher dopaminergic tone in nucleus accumbens, compared to bLR rats [43, 44]. Based on these studies it has been postulated that differences in emotional reactivity between bHR and bLR rats represent a model of vulnerability to stress and mood disorders and may be a valuable tool for exploring genetic and environmental interactions of such disorders.
The current study used intermittent CMS as a model of stress-induced depression. CMS reproduces many of the complex symptoms typically observed in depressed human patients, including the core symptom of anhedonia, making it a highly useful animal model of depression [1-9, 11]. In rodents, anhedonia is most commonly measured by a reduction in sucrose preference, which can be reversed by antidepressant drugs with a timecourse consistent with antidepressant efficacy and is not changed by drugs that are ineffective as antidepressants [12-14]. These results demonstrate the validity of the CMS time course in predicting antidepressant drug actions. After four weeks of CMS exposure, bLR rats showed significantly greater anhedonia, as compared to either bHR rats exposed to CMS or control (non-CMS-exposed) groups of both strains. It is notable that this reduction in sucrose preference in bLR rats occurred at a faster rate and to a greater degree compared not only to the bHR rats in this study, but also to supplier-bred Sprague-Dawley rats in studies from other labs [28-33] including ours (Stedenfeld & Sved, unpublished observation). Furthermore, these scores were significantly less than 50%, the expected score if rats exhibited no preference for either sucrose or water. It is possible, then, that this markedly reduced preference may reflect not only a lack of interest, but an actual aversion to the previously-rewarding sucrose solution. Such extreme anhedonia is noted in severe cases of major depression in human patients, including the melancholic subtype [34, 45, 46], which may be more likely to occur following stressful life events and in patients with low sensation-seeking personalities [47-52]. The melancholic subtype also may be related to a reduction in reward processing [53, 54], paralleling similar observations in bLR rats such as a reduction in dopaminergic tone in nucleus accumbens [44] and decreased propensity to self-administer drugs of abuse [43]. Melancholic patients also display increased anxiety, increased HPA axis responses to stress [55, 56] and increased levels of stress-related neuropeptides in the paraventricular nucleus of the hypothalamus (PVN) [57].
Differences between bHR and bLR behavior were also seen in the NSF test, which is used to measure anxiety-like behavior as well as antidepressant efficacy [35, 36]. Although these may seem like two very different endpoints for a single test, there is, in fact a high rate of comorbidity between anxiety disorders and depression [58, 59], and the two disorders share some overlapping characteristics such as irritability, sleep disturbances, and difficulty concentrating [34]. However, in addition to being a central symptom of anxiety disorders, anxiety itself is often a component of the negative affect associated with depression [60, 61]. Alhough anxiety is not explicitly stated in the diagnostic criteria for major depression [34], up to 90% of depressed patients report anxiety as a symptom [62]. Indeed, anxiety without depression is much more common than depression without anxiety [60, 61]. Depressed patients with anxiety symptoms at the time of remission are more likely to experience a relapse than patients without anxiety [63]. This is especially true in the melancholic subtype of depression discussed above [49, 50].
We found that bLR-CMS rats took a substantially longer time than bHR-CMS rats to approach and then to consume the offered food pellet. In fact, only one of the eight bLR-CMS rats ate within the fifteen-minute time limit of the test, though there were no significant differences in home cage consumption among groups. Home cage food consumption is an important control for this particular test and indicates that NSF behavior in the open field is due to increased anxiety in bLR-CMS rats, rather than a difference in appetite or food-related reward.
While feelings of anxiety may be a component of both depression and anxiety disorders, the core symptom of anhedonia is unique to depression, [34]. bLR rats show heightened anhedonia (reduced sucrose preference) as well as anxiety-like behavior in the NSF compared to bHR rats, suggesting that the bLR/bHR model is a model of susceptibility or resilience to depression, rather than anxiety disorder per se. These substantial contrasts in behavior are most likely due to the interaction of inborn changes in neurobiology and the stress of the CMS model.
There are a number of rat strains available that have been selectively bred for behavioral characteristics that may correspond to changes in mood or emotionality. One such strain is the Flinders Sensitive Line (FSL), which was originally derived by breeding Sprague Dawley rats for altered sensitivity to the acetylcholinesterase inhibitor diisopropyl fluorophosphate. This strain was later described as a putative model of depression because the rats exhibit behavioral and physiological similarities to depressed patients such as abnormalities in sleep and reduced appetite and psychomotor function [64, 65]. However, although the FSL rats share several characteristics in common with depression, they lack what many consider a key characteristic: anhedonia [64-67]. Interestingly, although both FSL and FRL rats develop signs of anhedonia in response to CMS [65, 67] they do not differ from each other in this regard. Thus, the FSL model lacks evidence of a key criterion of depression and the FSL-FRL rats cannot be considered a dichotomous model of vulnerability versus resistance to depression.
Another selectively-bred rat strain was developed by breeding Wistar rats based on either high (HAB) or low anxiety-like behavior (LAB) in the elevated plus maze (EPMZ) [68]. The heightened anxiety-like behavior in HAB rats extends to other similar behavioral measures of anxiety (e.g. light-dark box, [69]) and neurobiological correlates of increased anxiety, such as increases in corticotrophin releasing hormone (CRH) in the paraventricular hypothalamus of HAB rats, as compared to LAB rats [70]). It has been suggested that HAB rats may be a model of depression because of their increased immobility in the forced swim test (FST), a model of learned helplessness frequently used to screen for antidepressant drug efficacy[71]. However, the FST has proven to be more useful as a tool for drug discovery than as a standalone model of depression-like behavior, given that acute SSRI treatment increases active behaviors and decreases immobility in unstressed rats [71]. However, measures of anhedonia (e.g., SPT) do not appear to have been reported for HAB rats at baseline and during CMS. Additionally, LAB rats show a marked increase in so-called antisocial behavior [69] and aggression [72, 73]. These findings suggest that the HAB/LAB model may be useful in the study of anxiety and social behavior, but, at present, it is difficult to compare HAB/LAB rats to bLR/bHR rats as a model of selective vulnerability to depression.
A different approach to developing animal models of neuropsychiatric disorders has been to target specific neurotransmitter systems. For example, a transgenic rat has recently been introduced that lacks the serotonin transporter (5-HTT) [74]. These rats, as well as mice lacking the 5-HTT, display evidence of increased behavioral anxiety and increased immobility in the FST [75-77]. The 5-HTT knockout rat also shows reduced 24-hour sucrose consumption [75]. These and other transgenic rodent lines with specific neurochemical alterations may be beneficial in determining the role of individual neurotransmitters in depression and have been suggested to be useful animal models for research on the mechanisms underlying depression and its treatment.
In summary, the present study demonstrates that bLR and bHR rats provide a model of vulnerability and resistance to depression-like behavior following a period of chronic intermittent stress. This model provides excellent opportunities to further study the interaction of genetic predisposition to mood disorders and environmental stress. Future studies will further elucidate the underlying neurobiology associated with these differences in behavior and will explore the relationship between depressive behavior and altered autonomic and cardiovascular regulation in bLR rats.
Research Highlights.
Exposed rats bred for high/low response to novelty to 4 weeks chronic mild stress
Low response rats became anhedonic faster & to a larger degree than high responders
Depression-like behavior was also examined via the novelty-suppressed feeding test
Results suggest a model of resistance/vulnerability to stress-induced depression
Acknowledgments
This study was supported by National Institute of Mental Health Grant 5K99MH081927-02 (IAK), NARSAD Young Investigator Award (IAK), Office of Naval Research Grant N00014-02-1-0879 (HA), and National Institute on Drug Abuse Grant PPG 5P01DA021633-02 (SJW). The authors have no conflicts of interest to declare. The authors would like to thank Myriam Stricker and Xia Li of the University of Pittsburgh for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 2.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 3.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–29. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 4.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 5.Muscat R, Papp M, Willner P. Antidepressant-like effects of dopamine agonists in an animal model of depression. Biol Psychiatry. 1992;31:937–46. doi: 10.1016/0006-3223(92)90119-k. [DOI] [PubMed] [Google Scholar]
- 6.Sampson D, Willner P, Muscat R. Reversal of antidepressant action by dopamine antagonists in an animal model of depression. Psychopharmacology (Berl) 1991;104:491–5. doi: 10.1007/BF02245655. [DOI] [PubMed] [Google Scholar]
- 7.Stein EJ, da Silveira Filho NG, Machado DC, Hipolide DC, Barlow K, Nobrega JN. Chronic mild stress induces widespread decreases in thyroid hormone alpha1 receptor mRNA levels in brain--reversal by imipramine. Psychoneuroendocrinology. 2009;34:281–6. doi: 10.1016/j.psyneuen.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Willner P, Mitchell PJ. The validity of animal models of predisposition to depression. Behav Pharmacol. 2002;13:169–88. doi: 10.1097/00008877-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Gronli J, Murison R, Bjorvatn B, Sorensen E, Portas CM, Ursin R. Chronic mild stress affects sucrose intake and sleep in rats. Behav Brain Res. 2004;150:139–47. doi: 10.1016/S0166-4328(03)00252-3. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell PJ. Antidepressant treatment and rodent aggressive behaviour. Eur J Pharmacol. 2005;526:147–62. doi: 10.1016/j.ejphar.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 11.Johnson AK, Grippo AJ. Sadness and broken hearts: neurohumoral mechanisms and co-morbidity of ischemic heart disease and psychological depression. J Physiol Pharmacol. 2006;57(Suppl 11):5–29. [PubMed] [Google Scholar]
- 12.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 13.Jayatissa MN, Bisgaard C, Tingstrom A, Papp M, Wiborg O. Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology. 2006;31:2395–404. doi: 10.1038/sj.npp.1301041. [DOI] [PubMed] [Google Scholar]
- 14.Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- 15.Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–31. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- 16.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–57. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, et al. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Ballaz SJ, Akil H, Watson SJ. Previous experience affects subsequent anxiety-like responses in rats bred for novelty seeking. Behav Neurosci. 2007;121:1113–8. doi: 10.1037/0735-7044.121.5.1113. [DOI] [PubMed] [Google Scholar]
- 20.Tonissaar M, Herm L, Eller M, Koiv K, Rinken A, Harro J. Rats with high or low sociability are differently affected by chronic variable stress. Neuroscience. 2008;152:867–76. doi: 10.1016/j.neuroscience.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Touma C, Bunck M, Glasl L, Nussbaumer M, Palme R, Stein H, et al. Mice selected for high versus low stress reactivity: a new animal model for affective disorders. Psychoneuroendocrinology. 2008;33:839–62. doi: 10.1016/j.psyneuen.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–8. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallo T, Alttoa A, Koiv K, Tonissaar M, Eller M, Harro J. Rats with persistently low or high exploratory activity: behaviour in tests of anxiety and depression, and extracellular levels of dopamine. Behav Brain Res. 2007;177:269–81. doi: 10.1016/j.bbr.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Pawlak CR, Ho YJ, Schwarting RK. Animal models of human psychopathology based on individual differences in novelty-seeking and anxiety. Neurosci Biobehav Rev. 2008;32:1544–68. doi: 10.1016/j.neubiorev.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–45. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- 26.Jama A, Cecchi M, Calvo N, Watson SJ, Akil H. Inter-individual differences in novelty-seeking behavior in rats predict differential responses to desipramine in the forced swim test. Psychopharmacology (Berl) 2008;198:333–40. doi: 10.1007/s00213-008-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinton SM, Vazquez DM, Kabbaj M, Kabbaj MH, Watson SJ, Akil H. Individual differences in novelty-seeking and emotional reactivity correlate with variation in maternal behavior. Horm Behav. 2007;51:655–64. doi: 10.1016/j.yhbeh.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1333–41. doi: 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- 29.Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav. 2003;78:703–10. doi: 10.1016/s0031-9384(03)00050-7. [DOI] [PubMed] [Google Scholar]
- 30.Grippo AJ, Santos CM, Johnson RF, Beltz TG, Martins JB, Felder RB, et al. Increased susceptibility to ventricular arrhythmias in a rodent model of experimental depression. Am J Physiol Heart Circ Physiol. 2004;286:H619–26. doi: 10.1152/ajpheart.00450.2003. [DOI] [PubMed] [Google Scholar]
- 31.Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, et al. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology (Berl) 2005;179:769–80. doi: 10.1007/s00213-004-2103-4. [DOI] [PubMed] [Google Scholar]
- 33.Grippo AJ, Beltz TG, Weiss RM, Johnson AK. The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol Psychiatry. 2006;59:309–16. doi: 10.1016/j.biopsych.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Association A.P. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washingtion, DC: American Psychiatric Association; 1994. [Google Scholar]
- 35.Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29:771–83. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- 37.Bessa JM, Mesquita AR, Oliveira M, Pego JM, Cerqueira JJ, Palha JA, et al. A trans-dimensional approach to the behavioral aspects of depression. Front Behav Neurosci. 2009;3:1. doi: 10.3389/neuro.08.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez V, Rivier J, Wang L, Tache Y. Central injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks CRF- and stress-related alterations of gastric and colonic motor function. J Pharmacol Exp Ther. 1997;280:754–60. [PubMed] [Google Scholar]
- 39.Bonaz B, Tache Y. Water-avoidance stress-induced c-fos expression in the rat brain and stimulation of fecal output: role of corticotropin-releasing factor. Brain Res. 1994;641:21–8. doi: 10.1016/0006-8993(94)91810-4. [DOI] [PubMed] [Google Scholar]
- 40.Stam R, Croiset G, Akkermans LM, Wiegant VM. Psychoneurogastroenterology: interrelations in stress-induced colonic motility and behavior. Physiol Behav. 1999;65:679–84. doi: 10.1016/s0031-9384(98)00208-x. [DOI] [PubMed] [Google Scholar]
- 41.Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 42.Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Response to novelty predicts the locomotor nucleus accumbens dopamine response to cocaine. Synapse. 1991;9:121–8. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- 43.Davis BA, Clinton SM, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacol Biochem Behav. 2008;90:331–8. doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, et al. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rush AJ, Weissenburger JE. Melancholic symptom features and DSM-IV. Am J Psychiatry. 1994;151:489–98. doi: 10.1176/ajp.151.4.489. [DOI] [PubMed] [Google Scholar]
- 46.Melartin T, Leskela U, Rytsala H, Sokero P, Lestela-Mielonen P, Isometsa E. Co-morbidity and stability of melancholic features in DSM-IV major depressive disorder. Psychol Med. 2004;34:1443–52. doi: 10.1017/s0033291704002806. [DOI] [PubMed] [Google Scholar]
- 47.Loas G. Vulnerability to depression: a model centered on anhedonia. J Affect Disord. 1996;41:39–53. doi: 10.1016/0165-0327(96)00065-1. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell PB, Parker GB, Gladstone GL, Wilhelm K, Austin MP. Severity of stressful life events in first and subsequent episodes of depression: the relevance of depressive subtype. J Affect Disord. 2003;73:245–52. doi: 10.1016/s0165-0327(01)00479-7. [DOI] [PubMed] [Google Scholar]
- 49.Harkness KL, Monroe SM. Severe melancholic depression is more vulnerable than non-melancholic depression to minor precipitating life events. J Affect Disord. 2006;91:257–63. doi: 10.1016/j.jad.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatry. 2006;60:1147–54. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horesh N, Klomek AB, Apter A. Stressful life events and major depressive disorders. Psychiatry Res. 2008;160:192–9. doi: 10.1016/j.psychres.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Muscatell KA, Slavich GM, Monroe SM, Gotlib IH. Stressful life events, chronic difficulties, and the symptoms of clinical depression. J Nerv Ment Dis. 2009;197:154–60. doi: 10.1097/NMD.0b013e318199f77b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorwood P. Neurobiological mechanisms of anhedonia. Dialogues Clin Neurosci. 2008;10:291–9. doi: 10.31887/DCNS.2008.10.3/pgorwood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin-Soelch C. Is depression associated with dysfunction of the central reward system? Biochem Soc Trans. 2009;37:313–7. doi: 10.1042/BST0370313. [DOI] [PubMed] [Google Scholar]
- 55.Gold PW, Gabry KE, Yasuda MR, Chrousos GP. Divergent endocrine abnormalities in melancholic and atypical depression: clinical and pathophysiologic implications. Endocrinol Metab Clin North Am. 2002;31:37–62. vi. doi: 10.1016/s0889-8529(01)00022-6. [DOI] [PubMed] [Google Scholar]
- 56.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–75. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 57.Meynen G, Unmehopa UA, van Heerikhuize JJ, Hofman MA, Swaab DF, Hoogendijk WJ. Increased arginine vasopressin mRNA expression in the human hypothalamus in depression: A preliminary report. Biol Psychiatry. 2006;60:892–5. doi: 10.1016/j.biopsych.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 58.Kessler RC, Gruber M, Hettema JM, Hwang I, Sampson N, Yonkers KA. Comorbid major depression and generalized anxiety disorders in the National Comorbidity Survey follow-up. Psychol Med. 2008;38:365–74. doi: 10.1017/S0033291707002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beesdo K, Pine DS, Lieb R, Wittchen HU. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch Gen Psychiatry. 2010;67:47–57. doi: 10.1001/archgenpsychiatry.2009.177. [DOI] [PubMed] [Google Scholar]
- 60.Morilak DA, Frazer A. Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int J Neuropsychopharmacol. 2004;7:193–218. doi: 10.1017/S1461145704004080. [DOI] [PubMed] [Google Scholar]
- 61.Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annu Rev Psychol. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- 62.Regier DA, Rae DS, Narrow WE, Kaelber CT, Schatzberg AF. Prevalence of anxiety disorders and their comorbidity with mood and addictive disorders. Br J Psychiatry Suppl. 1998:24–8. [PubMed] [Google Scholar]
- 63.Flint AJ, Rifat SL. Two-year outcome of elderly patients with anxious depression. Psychiatry Res. 1997;66:23–31. doi: 10.1016/s0165-1781(96)02964-2. [DOI] [PubMed] [Google Scholar]
- 64.Overstreet DH, Friedman E, Mathe AA, Yadid G. The Flinders Sensitive Line rat: a selectively bred putative animal model of depression. Neurosci Biobehav Rev. 2005;29:739–59. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 65.Overstreet DH, Pucilowski O, Djuric V. Genetic/environment interactions in chronic mild stress. Psychopharmacology (Berl) 1997;134:359–60. doi: 10.1007/s002130050468. discussion 71-7. [DOI] [PubMed] [Google Scholar]
- 66.Matthews K, Baldo BA, Markou A, Lown O, Overstreet DH, Koob GF. Rewarding electrical brain stimulation: similar thresholds for Flinders Sensitive Line Hypercholinergic and Flinders Resistant Line Hypocholinergic rats. Physiol Behav. 1996;59:1155–62. doi: 10.1016/0031-9384(95)02212-0. [DOI] [PubMed] [Google Scholar]
- 67.Ayensu WK, Pucilowski O, Mason GA, Overstreet DH, Rezvani AH, Janowsky DS. Effects of chronic mild stress on serum complement activity, saccharin preference, and corticosterone levels in Flinders lines of rats. Physiol Behav. 1995;57:165–9. doi: 10.1016/0031-9384(94)00204-i. [DOI] [PubMed] [Google Scholar]
- 68.Landgraf R, Wigger A. High vs low anxiety-related behavior rats: an animal model of extremes in trait anxiety. Behav Genet. 2002;32:301–14. doi: 10.1023/a:1020258104318. [DOI] [PubMed] [Google Scholar]
- 69.Ohl F, Toschi N, Wigger A, Henniger MS, Landgraf R. Dimensions of emotionality in a rat model of innate anxiety. Behav Neurosci. 2001;115:429–36. [PubMed] [Google Scholar]
- 70.Bosch OJ, Kromer SA, Neumann ID. Prenatal stress: opposite effects on anxiety and hypothalamic expression of vasopressin and corticotropin-releasing hormone in rats selectively bred for high and low anxiety. Eur J Neurosci. 2006;23:541–51. doi: 10.1111/j.1460-9568.2005.04576.x. [DOI] [PubMed] [Google Scholar]
- 71.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–69. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 72.Neumann ID, Wegener G, Homberg JR, Cohen H, Slattery DA, Zohar J, et al. Animal models of depression and anxiety: What do they tell us about human condition? Prog Neuropsychopharmacol Biol Psychiatry. 2010 doi: 10.1016/j.pnpbp.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 73.Veenema AH, Torner L, Blume A, Beiderbeck DI, Neumann ID. Low inborn anxiety correlates with high intermale aggression: link to ACTH response and neuronal activation of the hypothalamic paraventricular nucleus. Horm Behav. 2007;51:11–9. doi: 10.1016/j.yhbeh.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 74.Homberg JR, Olivier JD, Smits BM, Mul JD, Mudde J, Verheul M, et al. Characterization of the serotonin transporter knockout rat: a selective change in the functioning of the serotonergic system. Neuroscience. 2007;146:1662–76. doi: 10.1016/j.neuroscience.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 75.Olivier JD, Van Der Hart MG, Van Swelm RP, Dederen PJ, Homberg JR, Cremers T, et al. A study in male and female 5-HT transporter knockout rats: an animal model for anxiety and depression disorders. Neuroscience. 2008;152:573–84. doi: 10.1016/j.neuroscience.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 76.Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology. 2002;27:914–23. doi: 10.1016/S0893-133X(02)00374-3. [DOI] [PubMed] [Google Scholar]
- 77.Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, et al. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–71. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]


