Abstract
Genetic defects in matriptase are linked to two congenital ichthyosis, autosomal recessive ichthyosis with hypotrichosis (ARIH, OMIM 610765) and, ichthyosis, follicular atrophoderma, hypotrichosis, and hypohidrosis (IFAH, OMIM602400). Mouse models with matriptase deficiency indicate an involvement of matriptase in suprabasal keratinocytes in the maintenance of the epidermal barrier. In contrast to what has been reported for mouse skin, we show that in human skin, matriptase is primarily expressed in the basal and spinous keratinocytes, but not in the more differentiated keratinocytes of the granular layer. In addition, matriptase zymogen activation was predominantly detected in the basal cells. Furthermore, using skin organotypic cultures as a model system to monitor the course of human epidermal differentiation, we found elevated matriptase zymogen activation during early stages of epidermal differentiation, coupled with a loss of matriptase expression in the late stages of this process. We also show here that matriptase deficiency in HaCaT cells modestly reduces cell proliferation and temporally affects calcium-induced expression of differentiation markers. These collective data suggests that, unlike mouse matriptase, human matriptase may be involved in regulation of keratinocyte growth and early differentiation, rather than terminal differentiation, providing mechanistic insights for the pathology of the two congenital ichthyoses, ARIH and IFAH.
Introduction
The genetic skin disorders ARIH and IFAH have been recently shown to be associated with mutations in matriptase, a type 2 integral membrane serine protease (Basel-Vanagaite et al., 2007;Avrahami et al., 2008;Alef et al., 2009). Defects in skin barrier function represent the underlying pathogenic mechanisms for patients with these disorders, as observed in a majority of other congenital ichthyoses (Akiyama and Shimizu, 2008). Such defects in skin barrier function for ichthyosis pathogenesis result from impairments in at least one of the three major components of the stratum corneum barrier: 1) intercellular lipid layers, 2) cornified cell envelop and 3) keratin/profilaggrin degradation products (Akiyama and Shimizu, 2008). Disruptions in profilaggrin processing appear to be the principal contributor to the impaired skin barrier observed in patients with matriptase mutations (Alef et al., 2009). Histological and ultrastructural analyses of the skin of ARIH patients further reveals that impaired degradation of corneodesmosomes and thickened stratum spinosum (acanthosis) as well as thickened stratum corneum associated with this condition (Basel-Vanagaite et al., 2007). Degradation of both corneodesmosomes and profilaggrin requires intensive proteolysis. It is tempting to speculate that matriptase may be involved in these proteolytic processes, but its role, direct or indirect, in either corneodesmosomes or profilaggrin degradation has yet to be elucidated. The acanthosis and the thickened stratum corneum associated with matriptase mutations could result from the impact of the mutation on keratinocyte proliferation and differentiation that is propagated through multiple downstream steps rather than just the result of one specific proteolytic event. Therefore, matriptase may indirectly regulate multiple processes in skin from the formation of stratum corneum barrier to the maintenance of epidermal homeostasis through the desquamation and/or the replenishment of keratinocytes.
Animal model systems with targeted deletion of matriptase have been generated and provided insights into the role of matriptase in skin barrier formation and its dysregulation in ichthyosis. In addition to replicating the ichthyotic phenotype and impaired profilaggrin processing seen in the ARIH and IFAH patients, impaired tight junction activity has also been identified to play an important role in the loss of epidermal barrier function in the skin of matriptase knockout mice (List et al., 2009). Significantly, experiments with matriptase knockout mice further revealed that zymogen activation of prostasin, a glycosylphosphatidylinositol (GPI)-anchored serine protease was compromised in the mouse skin (Netzel-Arnett et al., 2006). Given the almost identical epidermal defects observed in both matriptase and prostasin knockout mice and the co-localization of the two proteases in the outermost layer of the viable keratinocytes, the lack of matriptase-mediated zymogen activation of prostasin is likely responsible for the epidermal defects in matriptase knockout mice. Interestingly, the lack of prostasin zymogen activation was also reported in the skin of the IFAH patients (Alef et al., 2009). The functional link between the two membrane-bound serine proteases was also demonstrated in immortalized human keratinocytes in which prostasin zymogen activation was shown to be dependent on matriptase (Chen et al., 2010b). Zymogen activation of both serine proteases is under extremely tight control of hepatocyte growth factor activator inhibitor (HAI)-1, a Kunitz type serine protease inhibitor, in a manner such that HAI-1 binds to and inhibits the serine proteases virtually at the same time that zymogen activation of the proteases occurs (Chen et al., 2010b). It is worthy to note that targeted deletion of HAI-1 in mice also causes ichthyosis-like skin with aberrant pro-filaggrin processing and acanthosis (Nagaike et al., 2008). The matriptase-prosatssin-HAI-1 cell surface protease network, therefore, appears to be an essential process for maintenance of skin barrier function.
Matriptase-knockout mice may appear to be an excellent tool to study the physiological roles of matriptase in the skin and to understand development and progression of human skin diseases, given the shared epidermal defects associated with matriptase deficiency, the propagation through the same downstream substrate, prostasin, and the control by the same antiprotease mechanism, HAI-1, between human and rodent. This is particularly true for the matriptase hypomorphic mice which phenocopy ARIH patients (List et al., 2007). However, the less severe non-life threatening clinical presentation of IFAH patients with deletion of matriptase when compared to the uniform death within 48 hours after birth due to impaired skin barrier function observed in matriptase knockout mice indicates that there are important differences in matriptase functions in humans versus mice (List et al., 2002). Therefore, there is a need to establish human-relevant models to understand the role of matriptase in the ARIH and IFAH. To this end, we investigated the distribution profiles of matriptase expression in human skin as well as in an in vitro skin organotypic culture model in which the dynamic processes of robust epidermal differentiation and tissue regeneration can be tracked. The state of matriptase zymogen activation was also probed for using a unique matriptase mAb that can distinguish between activated matriptase from matriptase zymogen. In addition, HaCaT human keratinocytes were used to investigate the effects of matriptase knockdown. Regarded as a whole, our data suggest that human matriptase is likely to be involved in the regulation of proliferation and early differentiation primarily in basal keratinocytes. This conclusion is in a stark contrast to the role played by mouse matriptase, which is expressed by suprabasal but not basal keratinocytes, with highest expression observed in the outermost viable keratinocytes, indicating involvement in late stages of keratinocyte differentiation.
Results
Distribution and zymogen activation of matriptase in human skin
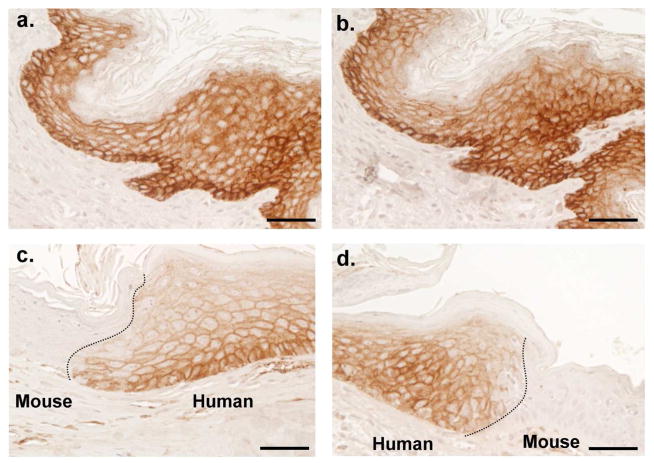
We analyzed the distribution profiles of matriptase by immunohistochemistry (IHC). The specificity of the matriptase mAbs used in this study has been established in multiple previous publications by immunoblot analysis and fluorescent immunocytochemical staining (Benaud et al., 2001;Benaud et al., 2002;Chen et al., 2010b;Hung et al., 2004;Tseng et al., 2010;Wang et al., 2009). In order to cross-validate the pattern of matriptase staining observed in this study, however, we conducted IHC staining for matriptase on sections of the same sample of human neonatal foreskin using two independent anti-matriptase mAbs, M32 and M24 (Fig. 1, a and b). We also stained skin sections from a human-mouse xenograft model that contained both human and mouse epidermis (Fig. 1, c and d). We conducted these additional studies due to the stark contrast between the matriptase expression pattern we find in human skin versus what has been reported in rodent skin (Netzel-Arnett et al., 2006). These findings are also at odds with two previous reports of the distribution of matriptase in human skin discussed below (Mildner et al., 2006;Bocheva et al., 2009)
Figure 1. The specificity of matriptase mAbs in immunohistochemical staining.
Paraffin embedded tissue sections prepared from human neonatal foreskin and human skin graft onto the nude mice. The foreskin sections (upper panels) immunostained with two monoclonal antibodies for total matriptase, M32 (a) and M24 (b). The human skin graft immunostained with M32 (c and d). Nuclei of cells were counterstained blue with hematoxylin. Scale bar: 50 μm.
Additional human skin sections in which the basal layer (SB), spinous layer (SS) and granular layer (SG) can clearly be discriminated were stained for matriptase (Fig. 2a). Matriptase was detected at cell-cell junctions in the SB and SS (Fig. 2b). However, decreasing expression of matriptase was observed along with the progression of differentiation from the SS to the SG. The expression of matriptase becomes undetectable in SG keratinocytes with dense cytoplasm that border the cornified layer (SC), which is the outermost layer and is also matriptase-negative (Fig. 2b). These data suggest that matriptase expression might be differentiation-regulated, and that the physiological function of matriptase in skin likely lies in the earlier stages of differentiation (SB and SS layers) rather than the later stage of differentiation (SG layer). In addition to the samples of neonatal foreskin, human skin xenograft, and samples of non-affected skin from patients with skin disease studied, the distribution profile of matriptase expression was also observed in the skin specimens from the scalp of a healthy adult (data not shown). These expression analyses suggest a consistent distribution profile for matriptase in human epidermis regardless of the origins of skin samples.
Figure 2. Cellular localization of matriptase and HAI-1 in normal human skin.
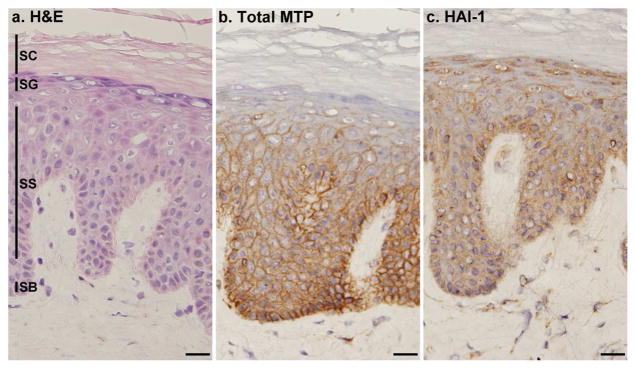
Frozen sections from normal human skin were stained with hematoxylin and eosin (a. H&E) or immunostained with mAb M32 for total matriptase (b. Total MTP) or mAb M19 for HAI-1 (c. HAI-1). Nuclei of cells in all three panels were counterstained blue with hematoxylin. The epidermal layers are indicated by SB for the stratum basale, SS for the stratum spinosum, SG for the stratum granulosum and SC for the stratum corneum. Scale bar: 25 μm.
Similar to the matriptase expression pattern, HAI-1 expression was also observed at the cell-cell junctions of the cells in the SB and SS layers (Fig. 2c). However, in contrast to the lack of matriptase expression in the SG layer, high-levels of HAI-1 expression were observed in this layer. Moreover, the HAI-1 present in the SG layer exhibited altered subcellular localization compared to the other layers, being present primarily in the cytoplasm rather than at the cell-cell junctions (Fig. 2c). The observed differential expression of matriptase and HAI-1 at the SG layer points towards a functional divergence of this cognate protease and protease inhibitor pair in terminally differentiated human keratinocytes.
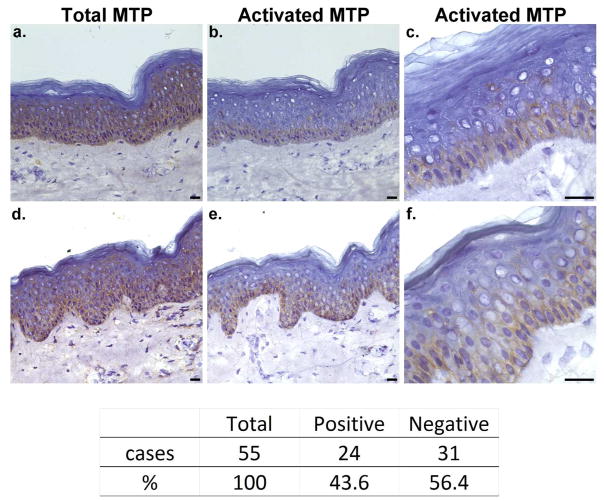
Next we sought to examine the status of matriptase zymogen activation in human skin. To this end, we compared the staining patterns of 55 frozen sections of human skin using either the total matriptase-specific mAb or the activated matriptase-specific mAb (Fig. 3). While all 55 samples were positive for total matriptase (100%), only 24 of the 55 samples were positive for activated matriptase (44%). Two representative positive sections for each mAb are shown in Fig. 3. Inspection of these sections reveals distinctly different distribution patterns of activated matriptase in comparison to total matriptase. While the level of positive staining for total matriptase in the basal cell layer was similar to the corresponding level in the spinous cell layer, positive staining for activated matriptase was observed almost exclusively in the basal layer, with positive for activated matriptase in spinous cells rarely being observed. When present, the activated matriptase-positive spinous cells were typically present as isolated individual cells or as cell clusters next to basal cells. On the odd occasion, activated matriptase-positive staining was observed in spinous cells that were a couple of layers above the basal layer. At high magnification (Fig. 3c and f), activated matriptase appeared as discontinuous bar-like bridges connecting two neighboring cells. These collective observations suggest that the basal keratinocyte layer is likely the primary location in which matriptase actively exercises its physiological function in human epidermis.
Figure 3. Matriptase activation in the basal layer of epidermis.
Frozen sections of human skin from 55 specimens were immunostained with mAb M32 for total matriptase (a and d, Total MTP) or mAb M69 for activated matriptase (b and e, Activated MTP). Higher magnification for the micrographs b and e are presented as c and f, respectively. Nuclei were counterstained blue with hematoxylin. A negative control using Mouse IgG was also performed (not shown). The table shows the numbers and percentages of skin specimens with positive and negative activated matriptase. Scale bar: 25 μm.
Elevated matriptase activation at the early stages of epidermal differentiation and loss of matriptase expression at the late stages of epidermal differentiation
The expression and zymogen activation state of matriptase in human skin revealed by our immunohistochemistry data thus far suggested that matriptase may be more important in the early stages of keratinocyte differentiation rather than the later stages. To further examine this hypothesis, we analyzed matriptase expression and zymogen activation along the course of epidermal differentiation in organotypic skin cultures using human primary keratinocytes isolated from foreskin. This in vitro skin culture system closely mimics epidermal regeneration and keratinocyte differentiation in skin (Akgul et al., 2005;Westphal et al., 2009;Chong et al., 2009).
Primary human keratinocytes maintained in low Ca2+ culture medium in a monolayer expressed the zymogen form of matriptase as can be seen in the immunoblot shown in Fig. 4a, lane M. In the organotypic cell culture system, primary keratinocytes are induced to differentiate by growing them on a fibroblast-primed collagen matrix plug in medium containing high Ca2+ for two days and then lifting the plugs such that the cells are grown at the collagen-air interface. Keratinocytes that have been induced to undergo differentiation in this manner exhibited high-levels of activated matriptase (Fig. 4a, lanes R), in contrast to the uninduced primary keratinocytes that only expressed matriptase zymogen (Fig. 4a, lane M). These results support the hypothesis that matriptase zymogen activation occurs upon keratinocyte differentiation.
Figure 4. Matriptase and HAI-1 in an organotypic skin culture.
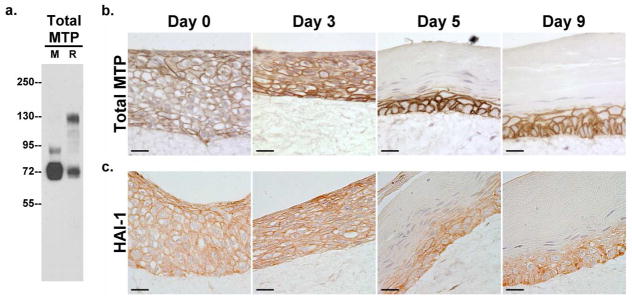
a. Expression and zymogen activation of matriptase. Primary human keratinocytes were grown in monolayer (M) or in an organotypic skin culture system (R). Cells were harvested on the day before they were lifted to the air-liquid interface and analyzed by immunoblot for total matriptase (Total MTP). The appearance of the 120-kDa matriptase complex with HAI-1 indicates the zymogen activation of matriptase. b and c. Expression of matriptase and HAI-1 over the course of epidermal differentiation. Organotypic skin cultures on Days 0, 3, 5, and 9 after lifting them to the air-liquid interface were analyzed by immunohistochemistry for total matriptase (Total MTP) using mAb M24 and HAI-1 (HAI-1) using mAb M19. Nuclei were counterstained blue with hematoxylin. Scale bar: 20 μm.
To further explore matriptase and HAI-1 expression and localization patterns during epidermal differentiation, primary keratinocytes that had been induced to undergo differentiation in the organotypic cultures were analyzed by immunohistochemistry on days 0, 3, 5 and 9 after lifting the cells to the air-liquid interface (Fig. 4b and c). Both matriptase and HAI-1 were homogenously detected on the cell surface in cells of all layers on day 0 when the keratinocytes were lifted to the air (Fig. 4b and c, Day 0). These data suggest that the keratinocytes that are undergoing early steps of differentiation induced by Ca2+ and the collagen matrix still maintained the serine protease system. However, by day 3, loss of matriptase expression begins to occur at the outermost layer of keratinocytes, whose nuclei now appear to be slightly condensed and elongated (Fig. 4b, Day 3). By day 5, when robust differentiation was observed with the appearance of the keratinocytes either with condensed nuclei or without nuclei on the upper layers, the expression of matriptase and HAI-1 was confined to a couple of keratinocyte layers near the collagen matrix (Fig. 4b and c, Day 5). By day 9, the skin raft contained basal cells with altered orientation and thick cornified layers, and expression of matriptase and HAI-1 were again limited to the same couple of layers as observed on day 5. In summary, our results reveal that expression of matriptase and HAI-1 is differentiation-dependent; they are highly expressed in proliferating (in monolayer culture in low Ca2+ medium) and early differentiating keratinocytes but become lost during late stages of differentiation.
Role of matriptase in keratinocyte proliferation and differentiation
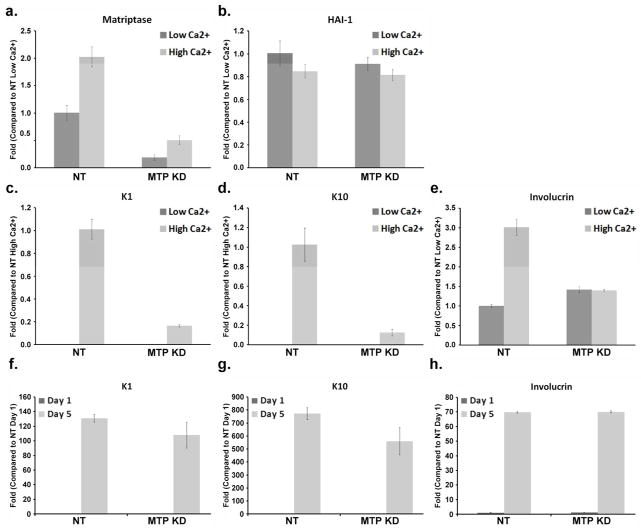
We next used the human keratinocyte cell line HaCaT in which matriptase expression was knocked down with matriptase-targeting small hairpin RNA (shRNA) to further explore the role of matriptase in keratinocyte proliferation and early stage differentiation. Fig. 5a shows that matriptase expression was significantly reduced in the pools expressing the matriptase-targeting shRNA, while the non-target shRNA expressing pools exhibited robust matriptase expression. HAI-1 expression was not affected by the cellular expression of either shRNA (Fig. 5a). Fig 5b shows the comparison of growth curves of matriptase-targeting (MTP KD) and non-target (NT) control pools using the crystal violet staining proliferation assay. Knockdown of matriptase expression in HaCaT cells resulted in a statistically significant decrease in cellular proliferative ability by 37.5% (p <0.01) in comparison with the non-target control cells (Fig. 5b). These data suggest that matriptase expression levels impact human keratinocyte proliferation.
Figure 5. Role of matriptase in keratinocyte proliferation.
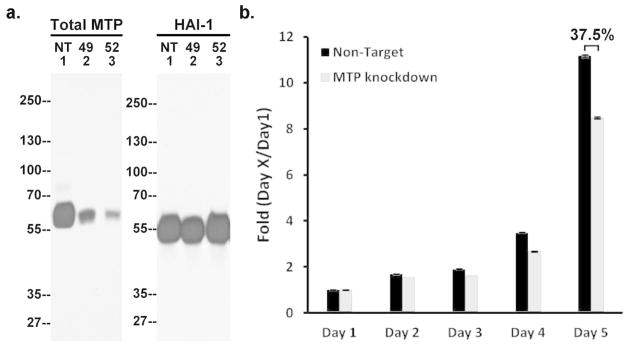
a. Matriptase and HAI-1 expression in matriptase knockdown cells. The cell lysates of the two matriptase knockdown pools (49 and 52) and the NT control (NT) were analyzed by immunoblot for matriptase (Total MTP) or HAI-1 (HAI-1). b. Decreased proliferation in matriptase knockdown cells. Growth curves of the matriptase knockdown pools, 52 (MTP knockdown) and the non-target control (Non-target) were determined by the crystal violet staining assay. Each point corresponds to the mean and standard error of experiments carried out in quadruplicate. The results presented are from one experiment representative of the four performed.
We further investigated the impact of matriptase knockdown on keratinocyte differentiation by using RT-PCR to determine the mRNA levels of three differentiation markers, keratin 1 (K1), keratin 10 (K10), and involucrin. For these studies, both MTP KD and NT HaCaT pools were adapted to the basal cell-like state by being maintained in low calcium media (Deyrieux and Wilson, 2007). We were unable to detect K1 or K10 expression in the NT pools which has been adapted in low Ca2+ media, with the expression of these two proteins being detected only when the cells were grown in high Ca2+ media that induces differentiation (Fig. 6c and d). Growth of NT HaCaT cells in the high Ca2+ media also increased the expression level of matriptase itself as well as involucrin (Fig. 6a and e), while having minimal impact, if any, on the expression level of HAI-1. These data are consistent with the notion that high Ca2+ media induce differentiation in HaCaT cells.
Figure 6. Role of matriptase in keratinocyte differentiation.
The mRNA levels of matriptase, HAI-1, K1, K10, and involucrin were analyzed by real-time PCR in matriptase knockdown pools 52 (MTP KD) and the non-target control (NT). The cells were grown either in low Ca2+ or high Ca2+ media for 24 hr (panels a–e), as indicated. In the panels f, g, and h, the cells were grown in low Ca2+ for 1 day or 5 days, as indicated. The fold changes of mRNA levels were quantified by normalization relative to the controls indicated. Mean values ± SD (n=4) are plotted. The results presented are from one experiment representative of the four performed.
After exposing the cells in high calcium media for one day, the K1 and K10 expression levels were significantly lower in the MTP KD HaCaT pools as compared to the NT control pools (Fig. 6c and d). Similarly, the 24 hour-exposure to high Ca2+ media failed to increase the expression level of involucrin in the MTP KD pools (Fig. 6e). As observed for the NT HaCaT pools, higher matriptase expression was observed in MTP KD clones grown in high Ca2+ media versus low Ca2+ media (Fig. 6a), while the expression of HAI-1 was not affected by the calcium concentration of the growth media (Fig. 6b). The impact of matriptase knockdown on the calcium-induced expression of the three differentiation markers seems to be a temporal event since the expression of the differentiation markers is highly dependent on cell density regardless of the presence of calcium. At very confluence, E-cadherin adherens junctions are formed (data not shown) and the expression of these differentiation makers increase to high levels. This phenomenon can be seen not only for the NT cells but also for MTP KD cells (Fig. 6f, g, and h). These data suggest that matriptase might affect keratinocyte differentiation by transiently reducing expression of the differentiation markers. The transient nature of this effect on the expression of K1, K10 and involucrin in our culture cell model is consistent with the observation that the expression of these markers is not altered in ARIH patients, where the delay in differentiation would be difficult to detect (Basel-Vanagaite et al., 2007).
Discussion
Genotypic studies of patients with the hereditary disorders ARIH and IFAH have revealed the importance of matriptase in human skin. The results described in this paper provided mechanistic insights into matriptase function in human keratinocytes, which has implications on how matriptase dysregulation could impact skin ichthyosis observed in these genetic disorders. We find that matriptase is highly expressed by the keratinocytes in the basal and spinous layers of the epidermis, but not in the granular layer. In vitro organotypic skin rafts recapitulate the differentiation-dependent distribution profile of matriptase expression. We also present evidence that matriptase zymogen activation is maximal in the basal layer of skin. Our collective results suggest that the mutations associated with matriptase in ARIH and IFAH would likely impact biological functions of basal keratinocytes the most, which then leads to ichthyosis.
The basal layer contains three subpopulations of keratinocytes: 1) stem cells, 2) transit amplifying cells, and 3) post-mitotic differentiating cells (Ross et al., 1995;Wheater et al., 1993). The major function of the basal keratinocytes is to maintain the stem cell population through mitosis and to replenish the cells in the epidermis through differentiation. Once differentiation is initiated, keratinocytes move towards the spinous layer and continue to differentiate from one layer to the next until they reach the final layer, stratum corneum (Eckert, 1989;Lindsay, 1996). Given that we observed homogenous matriptase expression in the entire basal layer, and that its zymogen activation, when present, was also associated with the entire basal layer, we propose that matriptase participates in a shared program among the subpopulations of basal keratinocytes. In other words, our studies are consistent with the hypothesis that matriptase is involved in the growth regulation and early differentiation of basal keratinocytes. This hypothesis is supported by our functional analysis using matriptase knockdown cells. Keratinocytes in which matriptase has been knocked down grow more slowly and transiently express lower levels of the three differentiation markers, K1, K10 and involucrin in response to the differentiation agent Ca2+.
Our results indicate that matriptase likely plays a very limited role in epidermal terminal differentiation due to its diminishing expression along the progression of differentiating keratinocytes from the SS to the SG. Given this limited role in granular keratinocytes, it implies that matriptase is less likely to participate in the formation of skin barrier through direct involvement of any the three major barrier components, the intercellular lipid layers, the cornified cell envelop, and the keratin-filaggrin degradation products. Interestingly, impaired filaggrin processing has been seen in some animal models involving altered function of enzymes such as 12R-lipoxygenase that have no obvious roles in degradation and/or modification of profilaggrin (Epp et al., 2007). Defects in epidermal differentiation processes even at early stages of differentiation could affect downstream filaggrin processing. The defects in filaggrin degradation seen in the keratinocytes isolated from IFAH patient might, therefore, be an indirect event associated with the loss of matriptase. The likely indirect role of matriptase in the formation of stratum corneum barrier may also explain the much milder clinical manifestation of matriptase deficiency in IFAH patients. This is in stark contrast to what has been observed in matriptase knockout mice. For these mice, matriptase has been clearly detected by gene trapping in suprabasal keratinocytes with highest expression of matriptase at the outmost viable layer in which the formation of stratum corneum barrier is actively at work (Netzel-Arnett et al., 2006). The much more severe defects in skin barrier in matriptase knockout mice could result from a direct involvement of mouse matriptase in the late stage of epidermal differentiation. In spite of the stark contrast in the distribution of matriptase expression in the normal mouse versus human skin, matriptase has been reported to be expressed at high levels in the basal and spinous layers of mouse skin after exposure to carcinogens (Szabo et al., 2011). This interesting phenomenon suggests that mouse matriptase might play a greater role than simply in terminal differentiation. It is tempting to speculate that mouse matriptase could also contribute to the proliferation and early differentiation, as human matriptase does.
Despite the unusually tight functional and regulatory linkage between matriptase and HAI-1 in keratinocytes, as well as in other types of epithelial cells, the observation that HAI-1, but not matriptase, is highly expressed in the granular layer (SG) of skin implies that there may be a decoupling of matriptase-HAI-1 activities in the cells of the SG layer. Unlike matriptase, HAI-1 may play an active role in the terminal differentiation of keratinocytes in SG layer, perhaps by the inhibition of serine proteases other than matriptase. The intracellular punctate staining of HAI-1 in the granular keratinocytes suggests that the Kunitz inhibitor might be incorporated into keratohyalin granules and/or the lamellar granules during the processes of terminal differentiation in the granular layer. Therefore, HAI-1 is more likely than matriptase to be directly involved in the regulation of profilaggrin processing in keratohyalin granules, and in lipid matrix formation in the lamellar granules in human skin.
The differences in the distribution profile of matriptase in the skin of human versus rodent could explain the physiological basis for the differences in clinical phenotype associated with matriptase loss in the two species. It is, however, worthwhile considering the different methodology employed for the detection of matriptase in both species. We have used five different extensively characterized matriptase- and HAI-1-specific mAbs (M32, M24, M19, M58, and M69) for the immunohistochemistry analyses in our current studies and in our previously reported work (Benaud et al., 2001;Benaud et al., 2002;Chen et al., 2010b; Hung et al., 2004;Tseng et al., 2010;Wang et al., 2009). The staining patterns observed with our antibodies clearly indicate that these proteins are localized at cell-cell junctions, as expected for integral membrane proteins such as matriptase and HAI-1. The different distribution profile between total matriptase and activated matriptase, and between total matriptase and HAI-1 would be seen to exclude the possibility of non-specific staining by our mAb of any of the abundant structural proteins found in skin. Our conclusions regarding the matriptase distribution profile in human skin was further bolstered by our data generated using human skin grafted to the back of nude mice and the in vitro skin equivalents. The former further demonstrates the specificity of our matriptase mAb M32 which does not interact with mouse matriptase. The latter clearly shows the dynamics of matriptase expression over the course of differentiation with the loss of matriptase expression at the late stage of differentiation, consistent with what has been observed in human skin. Furthermore, matriptase mRNA was clearly detected in both the basal and spinous layers of human skin by in situ hybridization in our previous study (Oberst et al., 2003), consistent with the detection of matriptase protein in the both layers in the current study (Fig. 2). Regarding the assessment of whether matriptase is expressed in the granular layer of human skin, a tissue section with clear granular layer is required. In our previous study (Oberst et al., 2003), the granular layer was not as easy to discern as it is in samples used in our current study. As a result, the expression of matriptase in the granular layer could not be clearly determined and was, we believe, misinterpreted as being positive due to the proximity of the strong staining in the spinous layers. Our current study is also at odds with two previous studies in which human matriptase protein was detected by immunohistochemistry in the suprabasal layers but not in the basal layer of human skin (Bocheva et al., 2009) and skin raft cultures (Mildner et al., 2006). In both of these studies, however, matriptase was mainly detected intracellularly, which is inconsistent with the known integral membrane protein nature of matriptase.
In ARIH patients, two different matriptase mutations were identified. One (G827R) is in the serine protease domain (Basel-Vanagaite et al., 2007), likely resulting in defects in zymogen activation (Desilets et al., 2008), whereas the other (M1I) replaces the translation initiator, resulting in the loss of the intracellular, transmembrane, and a small portion of extracellular domain (Avrahami et al., 2008). If translated, the matriptase M1I mutant might become intracellular protein. In IFAH patients, a splice-site mutation and a frame-shift deletion were identified, both resulting in the loss of matriptase expression. Given the differential distribution and likely different biology of matriptase in human versus mouse, development of human relevant models would be desired for understanding the disease progression for ARIH and IFAH. The organotypic skin culture system using human primary keratinocytes provides a relevant model which faithfully presents the distribution profile of matriptase expression in normal human skin. Our high efficiency matriptase knockdown system can significantly reduce matriptase expression in HaCaT human keratinocytes, which has allowed us to probe the role of matriptase in keratinocyte proliferation and early differentiation. The HaCaT cell model is consistent with the distribution profile of matriptase expression observed in the immunohistochemistry of human skin samples and rafts. Furthermore, the adaptation of HaCaT cells to a basal cell-like state through Ca2+-induced keratinocyte differentiation allows us to study the potential role of matriptase over the course of keratinocyte differentiation. In addition to revealing the role of human matriptase in the proliferation and early differentiation, both models and their combination would provide a tool to further understand the etiology of ARIH and IFAH.
In summary, we have provided several lines of evidence that lend support to the hypothesis that the primary physiological function of matriptase in human skin lies in proliferation and early differentiation of keratinocytes, rather than terminal differentiation. Dysregulated matriptase as observed in ARIH and IFAH could impact the functions of basal and spinous keratinocytes. The divergence between human and mouse in matriptase distribution and physiological role in the skin underscores the necessity to develop more human relevant models for the study in role of matriptase in skin diseases.
Material and Methods
Cell lines and Cell cultures
Human primary keratinocytes were obtained from neonatal foreskin and maintained in Keratinocyte-SFM (Invitrogen) supplemented with bovine pituitary extract (20–30 μg/ml), recombinant epidermal growth factor (rEGF, 0.1–0.2 ng/ml), 100 units/ml penicillin, and 100 μg/ml streptomycin. Human keratinocyte HaCaT cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM, Mediatech Inc., Manassas, VA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gemini, West Sacramento, CA), 100 units/ml penicillin, and 100 μg/ml streptomycin. HaCaT clones of matriptase knockdown and the non-target control, generated with matriptase small hairpin RNAs (shRNA) as previously described (Chen et al., 2010b), were maintained in DMEM supplemented with 10% FBS, 2 μg/ml puromycin, 100 units/ml penicillin, and 100 μg/ml streptomycin. The stably basal-like HaCaT cells were generated by culturing HaCaT cells in the completed Keratinocyte-SFM, which contains only 0.03 mM calcium, for at least one month as previously described (Deyrieux and Wilson, 2007;Micallef et al., 2009). These basal-like HaCaT cells were switched from low calcium completed Keratinocyte-SFM to high calcium completed DMEM in order to induce differentiation (Deyrieux and Wilson, 2007). All cells were incubated in a humidified incubator at 37° with 5% CO2.
Antibodies
The total matriptase monoclonal antibodies M24 and M32 and the activated matriptase mAb M69 were generated using matriptase-HAI-1 complex as antigen as described previously (Lin et al., 1999). The total matriptase mAbs recognize both latent and activated forms of matriptase. M69 mAb recognizes an epitope only present on activated matriptase and therefore is able to distinguish activated matriptase from latent matriptase (Chen et al., 2010b;Tseng et al., 2010). Human HAI-1 protein was detected using the HAI-1 mAb M19.
Immunoblot analysis
Proteins for immunoblot analyses were prepared by dissolving cells in DPBS containing 1% Triton X-100 and 1 mM (5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB). Insoluble debris was removed by centrifugation, and the protein concentration determined using the Bradford Assay (Sigma-Aldrich, St. Louis, MD) according to the manufacturer’s instructions. Samples containing the same amount of total protein were diluted with 5X sample buffer in the absence of reducing agent and resolved by 7.5% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) followed by transfer to nitrocellulose membrane (Pall Corp., Pensacola, FL). The membranes were probed with the desired monoclonal antibodies and an HRP-conjugated secondary antibody (Kirkegaard & Perry Laboratories, Gaithersburg, MD), followed by signal detection with Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Sciences, Waltham, MA).
Crystal violet cell proliferation assay
Matriptase knockdown HaCaT cells and the non-target control cells were seeded in 6-well plates at 104 cells per well. Cells were fixed for 10 min in a solution of 3.7% buffered formalin on the desired days. The cells were washed with DPBS and subsequently stained with 0.5% crystal violet dissolved in 20% methanol in DPBS for 10 min. After removing the excess stain and washing the cells with water, the crystal violet stained cells were dissolved in 2 ml of a 1% SDS solution by agitating the plates on an orbital shaker until color was uniformly distributed in the solution. The optical density of the extracted dye was read with a spectrophotometer (Bechman, DTX 880) at 595 nm. Optical density is proportionate to the number of cells in the well and values were normalized to the reading on Day 1.
Real-time polymerase chain reaction (PCR)
Total RNA was extracted from cells using Trizol reagent (Invitrogen) following the manufacturer’s instructions. Briefly, chloroform was added to homogenized samples followed by centrifugation to isolate the aqueous layer. After the RNA was precipitated, it was washed in 70% ethanol three times, air-dried and dissolved in water. One microgram of total RNA was treated with DNase and used for cDNA synthesis. The cDNA synthesis step included a control reaction without reverse transcriptase. Real-time PCR reactions were conducted using SYBR green and the following gene-specific primers: matriptase forward: 5′-ATCGCCTACTACTGGTCTGA G-3′ and matriptase reverse: 5′-GTTTTGGAGTCCGTGGGGAAA-3′; HAI-1 forward: 5′-GTC GGGGTGTGCAAGGTGGG-3′ and HAI-1 reverse: 5′-GCGGAACTGGGTGGGCTGAC-3′; K1 forward: 5′-ATTTCTGAGCTGAATCGTGTGATC-3′ and K1 reverse: 5′-CTTGGCATCCT TGAGGGCATT-3′; K10 forward: 5′-TGATGTGAATGTGGAAATGAATGC-3′ and K10 reverse: 5′-GTAGTCAGTTCCTTGCTCTTTTCA-3′; involucrin forward: 5′-GGGTGGTTATT TATGTTTGGGTGG-3′ and involucrin reverse: 5′-GCCAGGTCCAAGACATTCAAC-3′; GAPDH forward 5′-TGCACCACCAACTGCTTAGC-3′ and GAPDH reverse 5′-GGCATGGA CTGTGGTCATGAG-3′. All reactions were normalized to GAPDH as an internal control. The RT-PCR reaction conditions were initiated by 2 min at 50 °C and then 10 min at 95 °C, followed by 45 cycles of switching between 95°C for 15 sec and 60 °C for 1 min.
Immunohistochemistry
Immunohistochemical staining was performed as previously described (Chen et al., 2010a;Chen et al., 2011). Tissue sections of paraffin-embedded human skin, skin equivalents, and human skin xenograft, frozen sections of human skin, and paraffin-embedded human skin xenograft were stained using the total matriptase mAb M24 or M32, activated matriptase mAb M69, and HAI-1 mAb M19. Frozen human skin tissue sections were obtained from Tri-Service General Hospital, National Defense Medical Center under the IRB 099-05-019, approved by TSGHIRB. The human skin xenograft was obtained from a human-mouse xenograft model, which is generated by engrafting normal human skin harvested from elective abdominoplasty resections onto athymic nude (nu/nu) mice (Harlan Laboratories). The specimens stained here were harvested 140 days after engraftment. This study was approved by the Georgetown University Medical Center Animal Care and Use Committee (GUACUC# 2011-050). The use of unidentified tissue is not considered human subjects research and therefore this research did not require prospective review of approval by the Georgetown University Medical Center Institutional Review Board (IRB). DAB (3,3′-Diaminobenzidine) and the secondary antibody (EnVision+ Dual Link System Peroxidase) (Dako, Glostrup, Denmark) were used for the detection of positive staining. Cell nuclei were counterstained with hematoxylin. Images were captured using an Olympus AH2 Vanox Microscope System (Olympus, Melville, NY).
Organotypic culture
Organotypic rafts were generated based on the protocol described previously with minor revision (Chen et al., 2010b;Stark et al., 1999;Stark et al., 2004). Briefly, Type I collagen (BD Biosciences, Bedford, MA) plugs with 7.5×104 human primary fibroblasts per plug were prepared in 6-well cell culture inserts (BD Biosciences) and incubated for 2–5 days. A total of 106 primary keratinocytes were applied to the center of each collagen plug and incubated for 1–4 days. Differentiation of the rafts was initiated by lifting them to the air-liquid interface.
Acknowledgments
This study was supported by National Cancer Institute (NCI) Grants RO1 CA 123223 (M.D. J and C.-Y. L), and Maryland Cigarette Reconstitute Fund (C.-Y. L) and Taiwan Department of Defense Grant MAB101-47 (to J.-K. W). The human-mouse xenograft studies were funded by award numbers KL2RR031974 and UL1TR000101 (previously UL1RR031975) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through the Clinical and Translational Science Awards Program (CTSA). We are grateful for the assistance of following Shared Resources at the Lombardi Comprehensive Cancer Center which are supported in part by NIH/NCI P30-CA051008: Tissue Culture Shared Resource, Microscopy and Imaging Shared Resource, Histology and Tissue Shared Resource.
Abbreviation
- ARIH
autosomal recessive ichthyosis with hypotrichosis
- HAI-1
hepatocyte growth factor activator inhibitor
- IFAH
ichthyosis, follicular atrophoderma, hypotrichosis, and hypohidrosis
- MTP KD
matriptase knockdown
- SB
stratum basale
- SC
stratum corneum
- SG
stratum granulosum
- shRNA
small hairpin RNA
- SS
stratum spinosum
Footnotes
Conflict of Interest Statement
The C.-Y.L is an inventor on US patents #6,077,938 and #6,677,377 and M.D.J and C.-Y.L are inventors on US patent #7,355,015.
References
- Akgul B, Garcia-Escudero R, Ghali L, et al. The E7 protein of cutaneous human papillomavirus type 8 causes invasion of human keratinocytes into the dermis in organotypic cultures of skin. Cancer Res. 2005;65:2216–2223. doi: 10.1158/0008-5472.CAN-04-1952. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Shimizu H. An update on molecular aspects of the non-syndromic ichthyoses. Exp Dermatol. 2008;17:373–382. doi: 10.1111/j.1600-0625.2007.00691.x. [DOI] [PubMed] [Google Scholar]
- Alef T, Torres S, Hausser I, et al. Ichthyosis, follicular atrophoderma, and hypotrichosis caused by mutations in ST14 is associated with impaired profilaggrin processing. J Invest Dermatol. 2009;129:862–869. doi: 10.1038/jid.2008.311. [DOI] [PubMed] [Google Scholar]
- Avrahami L, Maas S, Pasmanik-Chor M, et al. Autosomal recessive ichthyosis with hypotrichosis syndrome: further delineation of the phenotype. Clin Genet. 2008;74:47–53. doi: 10.1111/j.1399-0004.2008.01006.x. [DOI] [PubMed] [Google Scholar]
- Basel-Vanagaite L, Attia R, Ishida-Yamamoto A, et al. Autosomal recessive ichthyosis with hypotrichosis caused by a mutation in ST14, encoding type II transmembrane serine protease matriptase. Am J Hum Genet. 2007;80:467–477. doi: 10.1086/512487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaud C, Dickson RB, Lin CY. Regulation of the activity of matriptase on epithelial cell surfaces by a blood-derived factor. Eur J Biochem. 2001;268:1439–1447. doi: 10.1046/j.1432-1327.2001.02016.x. [DOI] [PubMed] [Google Scholar]
- Benaud C, Oberst M, Hobson JP, et al. Sphingosine 1-phosphate, present in serum-derived lipoproteins, activates matriptase. J Biol Chem. 2002;277:10539–10546. doi: 10.1074/jbc.M109064200. [DOI] [PubMed] [Google Scholar]
- Bocheva G, Rattenholl A, Kempkes C, et al. Role of Matriptase and Proteinase-Activated Receptor-2 in Nonmelanoma Skin Cancer. J Invest Dermatol. 2009;129:1816–1823. doi: 10.1038/jid.2008.449. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Wu BY, Tsao PI, et al. Increased matriptase zymogen activation in inflammatory skin disorders. Am J Physiol Cell Physiol. 2011;300:C406–415. doi: 10.1152/ajpcell.00403.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Lee MS, Lucht A, et al. TMPRSS2, a Serine Protease Expressed in the Prostate on the Apical Surface of Luminal Epithelial Cells and Released into Semen in Prostasomes, Is Misregulated in Prostate Cancer Cells. Am J Pathol. 2010a;176:2986–2996. doi: 10.2353/ajpath.2010.090665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Wang JK, Chou FP, et al. Regulation of the matriptase-prostasin cell surface proteolytic cascade by hepatocyte growth factor activator inhibitor-1 (HAI-1) during epidermal differentiation. J Biol Chem. 2010b;285:31755–31762. doi: 10.1074/jbc.M110.150367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong HC, Tan MJ, Philippe V, et al. Regulation of epithelial-mesenchymal IL-1 signaling by PPARbeta/delta is essential for skin homeostasis and wound healing. J Cell Biol. 2009;184:817–831. doi: 10.1083/jcb.200809028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desilets A, Beliveau F, Vandal G, et al. Mutation G827R in matriptase causing autosomal recessive ichthyosis with hypotrichosis yields an inactive protease. J Biol Chem. 2008;283:10535–10542. doi: 10.1074/jbc.M707012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyrieux AF, Wilson VG. In vitro culture conditions to study keratinocyte differentiation using the HaCaT cell line. Cytotechnology. 2007;54:77–83. doi: 10.1007/s10616-007-9076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert RL. Structure, function, and differentiation of the keratinocyte. Physiol Rev. 1989;69:1316–1346. doi: 10.1152/physrev.1989.69.4.1316. [DOI] [PubMed] [Google Scholar]
- Epp N, Furstenberger G, Muller K, et al. 12R-lipoxygenase deficiency disrupts epidermal barrier function. J Cell Biol. 2007;177:173–182. doi: 10.1083/jcb.200612116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung RJ, Hsu I, Dreiling JL, et al. Assembly of adherens junctions is required for sphingosine 1-phosphate-induced matriptase accumulation and activation at mammary epithelial cell-cell contacts. Am J Physiol Cell Physiol. 2004;286:C1159–C1169. doi: 10.1152/ajpcell.00400.2003. [DOI] [PubMed] [Google Scholar]
- Lin CY, Anders J, Johnson M, et al. Purification and characterization of a complex containing matriptase and a Kunitz-type serine protease inhibitor from human milk. J Biol Chem. 1999;274:18237–18242. doi: 10.1074/jbc.274.26.18237. [DOI] [PubMed] [Google Scholar]
- Lindsay DT. The integument. In: Simth JM, editor. Functional human anatomy. Mosby Year Book; St. Louis: 1996. pp. 345–375. [Google Scholar]
- List K, Currie B, Scharschmidt TC, et al. Autosomal ichthyosis with hypotrichosis syndrome displays low matriptase proteolytic activity and is phenocopied in ST14 hypomorphic mice. J Biol Chem. 2007;282:36714–36723. doi: 10.1074/jbc.M705521200. [DOI] [PubMed] [Google Scholar]
- List K, Haudenschild CC, Szabo R, et al. Matriptase/MT-SP1 is required for postnatal survival, epidermal barrier function, hair follicle development, and thymic homeostasis. Oncogene. 2002;21:3765–3779. doi: 10.1038/sj.onc.1205502. [DOI] [PubMed] [Google Scholar]
- List K, Kosa P, Szabo R, et al. Epithelial Integrity Is Maintained by a Matriptase-Dependent Proteolytic Pathway. Am J Pathol. 2009;175:1453–1463. doi: 10.2353/ajpath.2009.090240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef L, Belaubre F, Pinon A, et al. Effects of extracellular calcium on the growth-differentiation switch in immortalized keratinocyte HaCaT cells compared with normal human keratinocytes. Exp Dermatol. 2009;18:143–151. doi: 10.1111/j.1600-0625.2008.00775.x. [DOI] [PubMed] [Google Scholar]
- Mildner M, Ballaun C, Stichenwirth M, et al. Gene silencing in a human organotypic skin model. Biochem Biophys Res Commun. 2006;348:76–82. doi: 10.1016/j.bbrc.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Nagaike K, Kawaguchi M, Takeda N, et al. Defect of Hepatocyte Growth Factor Activator Inhibitor Type 1/Serine Protease Inhibitor, Kunitz Type 1 (Hai-1/Spint1) Leads to Ichthyosis-Like Condition and Abnormal Hair Development in Mice. Am J Pathol. 2008;173:1464–1475. doi: 10.2353/ajpath.2008.071142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzel-Arnett S, Currie BM, Szabo R, et al. Evidence for a matriptase-prostasin proteolytic cascade regulating terminal epidermal differentiation. J Biol Chem. 2006;281:32941–32945. doi: 10.1074/jbc.C600208200. [DOI] [PubMed] [Google Scholar]
- Oberst MD, Singh B, Ossandon M, et al. Characterization of matriptase expression in normal human tissues. J Histochem Cytochem. 2003;51:1017–1025. doi: 10.1177/002215540305100805. [DOI] [PubMed] [Google Scholar]
- Ross MH, Romrell LJ, Kaye GI. Integumentary system. In: Coryell PA, editor. Histology, a text and atlas. Williams & Wilkins; Baltimore: 1995. pp. 370–403. [Google Scholar]
- Stark HJ, Baur M, Breitkreutz D, et al. Organotypic keratinocyte cocultures in defined medium with regular epidermal morphogenesis and differentiation. J Invest Dermatol. 1999;112:681–691. doi: 10.1046/j.1523-1747.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- Stark HJ, Szabowski A, Fusenig NE, et al. Organotypic cocultures as skin equivalents: A complex and sophisticated in vitro system. Biol Proced Online. 2004;6:55–60. doi: 10.1251/bpo72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo R, Rasmussen AL, Moyer AB, et al. c-Met-induced epithelial carcinogenesis is initiated by the serine protease matriptase. Oncogene. 2011;30:2003–2016. doi: 10.1038/onc.2010.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng IC, Xu H, Chou FP, et al. Matriptase activation, an early cellular response to acidosis. J Biol Chem. 2010;285:3261–3270. doi: 10.1074/jbc.M109.055640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JK, Lee MS, Tseng IC, et al. Polarized epithelial cells secrete matriptase as a consequence of zymogen activation and HAI-1-mediated inhibition. Am J Physiol Cell Physiol. 2009;297:C459–C470. doi: 10.1152/ajpcell.00201.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal K, Akgul B, Storey A, et al. Cutaneous human papillomavirus E7 type-specific effects on differentiation and proliferation of organotypic skin cultures. Cell Oncol. 2009;31:213–226. doi: 10.3233/CLO-2009-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheater PR, Burkitt HG, Daniels VG. Skin. In: Burkitt HG, Young B, Heath JW, editors. Wheater’s functional histology, a text and colour atlas. Churchill Livingston; New York: 1993. pp. 153–169. [Google Scholar]