Abstract
Accumulating data have shown that bile acids are important cell signaling molecules, which may activate several signaling pathways to regulate biological processes. Bile acids are endogenous ligands for the farnesoid X receptor (FXR) and TGR5, a G-protein coupled receptor. Gain- and loss-of-function studies have demonstrated that both FXR and TGR5 play important roles in regulating lipid and carbohydrate metabolism and inflammatory responses. Importantly, activation of FXR or TGR5 lowers hepatic triglyceride levels and inhibits inflammation. Such properties of FXR or TGR5 have indicated that these two bile acid receptors are ideal targets for treatment of non-alcoholic fatty liver disease, one of the major health concerns worldwide. In this article, we will focus on recent advances on the role of both FXR and TGR5 in regulating hepatic triglyceride metabolism and inflammatory responses under normal and disease conditions.
Keywords: FXR, TGR5, triglyceride, cholesterol, inflammation
1. Introduction
1.1 Nonalcoholic fatty liver disease (NAFLD)
Nonalcoholic fatty liver disease (NAFLD) is a very common disorder that affects up to 25% of the US population. It refers to a spectrum of liver disease ranging from simple steatosis to non-alcoholic hepatosteatosis (NASH) and liver cirrhosis. NAFLD begins with simple steatosis, which can progress to NASH. NASH is featured by the presence of hepatocyte ballooning and apoptosis, inflammation and pericellular fibrosis. NASH may further progress to liver cirrhosis, eventually leading to hepatocellular carcinoma and liver failure. When NAFLD progresses to this late stage, liver transplantation is the only option for treatment [1, 2]
Although NAFLD is considered hepatic manifestations of insulin resistance, the pathogenesis of NAFLD has been poorly understood to date. The simple liver steatosis is a benign condition, about 20% of which may progress to NASH [2, 3]. Day and James proposed a “two-hits” hypothesis for the pathogenesis of NASH, in which hepatic triglyceride (TG) accumulation as the first hit and oxidative stress followed by subsequent inflammation as the second hit [4]. The abnormal hepatic TG accumulation in NAFLD results from an imbalance between TG synthesis, hydrolysis and secretion [5]. Since TG results from esterification of free fatty acids (FFAs) and glycerol, the imbalance between fatty acid input (which may come from dietary fats, adipose tissue and de novo lipogenesis) and FA output (hepatic fatty acid oxidation; FAO) may result in hepatic TG accumulation. Hepatic TG lipolysis is mediated by lipases, which releases FFA for oxidation in mitochondria or peroxisomes. After synthesis, hepatic TG may be stored as lipid droplets or packaged with ApoB into very low-density lipoprotein (VLDL) and then secreted into circulation.
Inflammation and fibrosis in NASH can be triggered by multiple factors [3]. Increased hepatic FFA influx associated with obesity and insulin resistance produces excessive reactive oxygen species (ROS), leading to activation of c-Jun N-terminal kinase pathway and accumulation of harmful lipid peroxidation products [6]. A variety of cytokines also contribute to the inflammation and fibrosis in NASH, such as TNF-α, interleukin (IL)-6, IL-1 and TGF-β. These cytokines promote fibrosis through activating hepatic stellate cells, which generate and secrete collagen in the liver upon stimulation [3].
By far, no efficient treatments have been established for treatment of NAFLD. Weight loss and life style change are the only options, indicating an urgent need to develop novel therapeutics for treatment of this common disease. An ideal therapeutic approach for NAFLD should not only improve steatosis but also ameliorate inflammation and fibrosis. The bile acid receptors, farnesoid X receptor (FXR) and TGR5, have been shown to improve lipid and glucose homeostasis and inhibit inflammatory response. Therefore, these two receptors may represent a class of promising candidates for treatment of NAFLD.
1.2. Bile acid receptors
1.2.1. Bile acid (BA) metabolism
Bile acids are important amphipathic biomolecules which can help absorption of dietary lipids and fat-soluble vitamins in the intestine. The synthesis of BAs from cholesterol occurs exclusively in the liver and involves two distinct pathways—the classic pathway and alternative pathway. Chenodeoxycholic acid (CDCA) and cholic acid (CA) represent two major primary BA products generated by these two pathways which involve multiple steps of modification of the sterol ring, oxidation and shortening of the side chain. Cholesterol 7α-hydroylase (CYP7A1) is the rate-limiting enzyme of the classic pathway whereas CYP27A1 is the first enzyme of alternative pathway. Sterol 12α-hydroxylase (CYP8B1) is the key enzyme in CA biosynthesis and determines the relative abundance of CA versus CDCA. Once synthesized, the free BAs are conjugated to amino acids (taurine or glycine) and then secreted to the bile canaliculi and stored in gallbladder. Upon ingestion of a meal, cholecystokinin (CCK) simulates gallbladder to secrete bile into intestinal lumen, where BAs facilitate the absorption of dietary fats through forming micelles. In the intestine, the gut flora further metabolize primary BAs to secondary bile acids by dehydroxylation and deconjugation of primary BAs to form lithocholic acid (LCA) and deoxycholic acid (DCA). About 95% of BAs secreted to the intestine are reabsorbed in the lateral ileum and transported back to the liver via enterohepatic circulation. Only 5% of BAs are excreted into the feces. The loss of BAs in the feces represents the principal means of eliminating cholesterol from the body [7–9].
In addition to help absorption of lipids and fat-soluble vitamins in the intestine, accumulating data have also shown that BAs may function as signaling molecules to regulate biological processes. For instance, BAs may regulate metabolism through binding to the BA receptors FXR and TGR5.
1.3.1 FXR
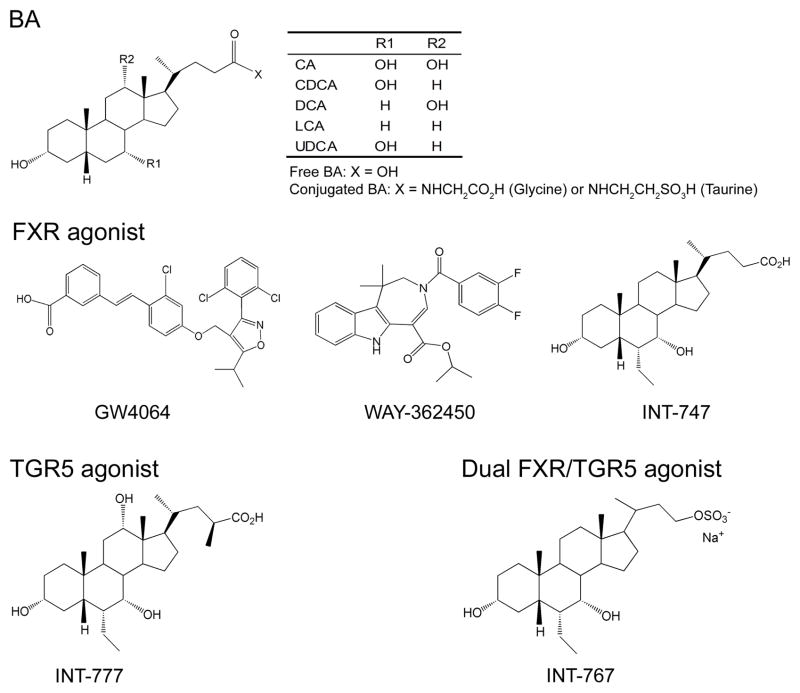
In 1999, BAs were identified as endogenous FXR ligands with high affinity. Both conjugated and unconjugated BAs can activate FXR. The order of potency of BAs is CDCA>LCA=DCA>CA. UDCA cannot activate FXR. The EC50 for the most potent BA CDCA is less than 10 μM, a dose which is comparable to the postprandial BA concentration in the plasma. These studies have suggested for the first time that BAs may serve as endocrine hormones to regulate metabolism via FXR [7, 8, 10]. Since bile acids may also activate other pathways independent of FXR, the use of potent and selective FXR agonists, such as GW4064, INT-767 and WAY-362450 (Figure 1) has provided important insights into to the role of FXR in metabolic control.
Figure 1.
Chemical structures of BAs, FXR agonists, TGR5 agonists and FXR/TGR5 dual agonists.
FXR is highly expressed in the liver, intestine, kidney and adrenal gland [7–10]. Like other nuclear receptor members, FXR has an N-terminal activation domain (AF1) for interaction with cofactors, a conserved DNA binding domain (DBD), a unique ligand binding domain (LBD) allowing receptor dimerization, and a C-terminal activation domain (AF2) for co-regulator interactions [8]. FXR binds to an FXR response element (FXRE) as a heterodimer with RXR or as monomer to regulate gene expression [8].
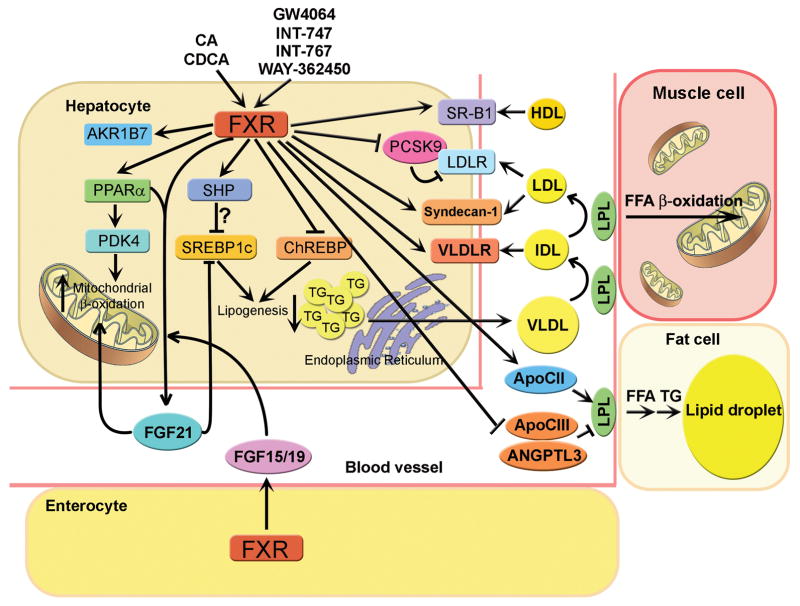
Over the past decade, gain- and loss-of-function data have demonstrated that FXR is a multipurpose nuclear receptor that plays an essential role in maintaining BA, lipid and glucose homeostasis [8–10]. FXR is also shown to play a role in liver regeneration and in preventing inflammation and liver or intestine tumorigenesis [8–10]. Among all these FXR-regulated pathways, the role of FXR in BA metabolism has been well established. FXR is now known to control every single step of BA metabolism. Since numerous review articles on FXR have been published, we will focus on the role of FXR in lipid metabolism (Figure 2) and inflammation, particularly in NAFLD.
Figure 2. Role of FXR in lipid metabolism.
Bile acids (CA and CDCA), synthetic FXR agonists (GW4064, INT-747 and WAY-362450) and a dual FXR/TGR5 agonist (INT-767) activate hepatic FXR, resulting in modulation of hepatic and plasma lipid homeostasis. FXR lowers hepatic triglyceride levels likely through multiple mechanisms. The FXR-SHP-SREBP-1c pathway does not appear to play a role in this process [25, 29–32]. Instead, other genes/pathways, including FGF21 [43], ChREBP [33], PPARα [36, 41], and AKR1B7 [34] may play a role in FXR-mediated reduction in hepatic triglyceride levels. Activation of FXR regulates plasma cholesterol and triglyceride levels through modulating several genes, including SR-BI [19], LDLR [16], Syndecan-1[39], VLDLR [38], ApoCII, ApoCIII [37] and ANGPTL3 [25].
1.4.1. TGR5
TGR5 is a member of the rhodopsin-like superfamily of G protein-coupled receptors. Like FXR, it is originally regarded as an orphan receptor without known ligands [7, 11]. Recent findings demonstrate a variety of bile acids can activate TGR5 with LCA being the most potent ligand, followed by DCA, CDCA and CA [7, 11]. In addition, TGR5 is also activated by semi-synthetic BA derivatives such as INT-777 and INT-767 [12, 13] (Figure 1 and 3). TGR5 is expressed in many tissues, with highest expression in gallbladder, followed by intestine, primarily in ileum and colon. It is also expressed with lower abundance in brown adipose tissue, liver, muscle and central nervous system. In the liver, TGR5 is expressed in sinusoidal endothelial cells and kupffer cells — the resident macrophages of the liver, but not in hepatocytes [11].
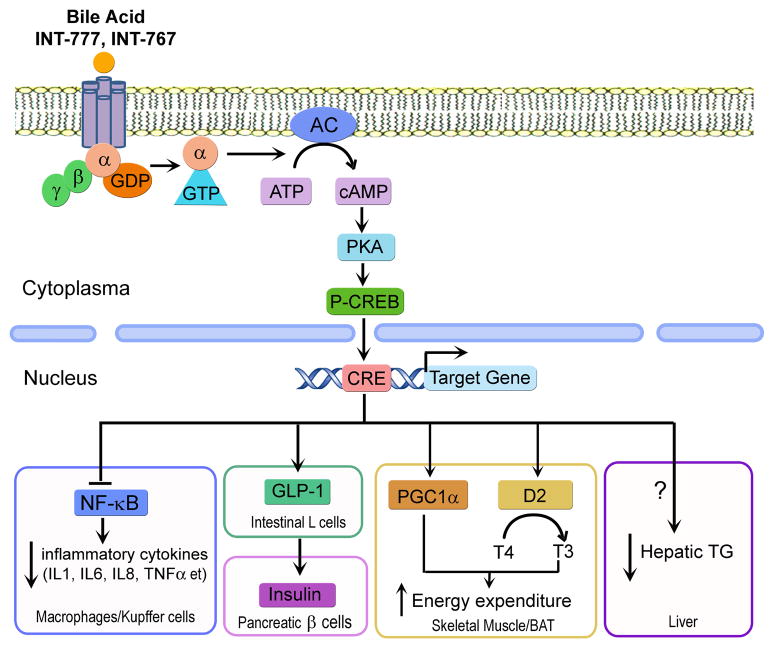
Figure 3. Role of TGR5 in lipid metabolism, inflammation and energy homeostasis.
In the absence of a ligand, TGR5 is tightly bound to a G protein complex consisting of α, β and γ subunits. Upon binding to a ligand (bile acids, synthetic TGR5 agonist (INT-777), synthetic dual FXR/TGR5 agonist (INT-767)), the G protein complex dissociates to form α and βγ protein subunits. The α protein subunit then activates adenylate cyclase which in turn converts ATP to cAMP. The resultant cAMP accumulation activates protein kinase A, which exerts downstream effects [11]. In macrophages and hepatic Kupffer cells, TGR5 attenuates inflammatory cytokine production through antagonizing the activity of NF-κB [69]. In intestinal L cells, TGR5 induces GLP1 secretion, which subsequently stimulates insulin secretion from pancreatic β cells to regulate glucose homeostasis [62]. In skeletal muscle and brown adipose tissue (BAT), TGR5 increases energy expenditure through inducing mitochondrial thermogenesis via PGC1α and T3 [61]. In the liver, TGR5 attenuated triglyceride accumulation through a yet-to-be-determined mechanism [62].
TGR5 is a typical G-protein coupled receptor (GPCR). In its inactive form, the receptor is tightly bound to a G protein complex consisting of α, β and γ subunits. Upon BA binding, the G protein complex dissociates to form α and βγ protein subunits. The α protein subunit then activates adenylate cyclase which converts ATP to cAMP [11]. The resultant cAMP accumulation activates protein kinase A, which in turn exerts downstream effects (Figure 3). TGR affects several aspects of bile acid metabolism. In gallbladder, TGR5 was shown to play a role in regulating bile composition and gallstone formation [11] as well as gallbladder refilling [14]. Tgr5−/− mice have been shown to have a smaller BA pool size by 21–25% [11]. These data suggest that TGR5 plays an important role in maintaining BA homeostasis.
2. Role of FXR in lipid and glucose metabolism and inflammation
Numerous gain- and loss-of-function studies have demonstrated that FXR is an important regulator of lipid and glucose metabolism as well as inflammatory response. Activated FXR improves lipid and glucose homeostasis and inhibits inflammation. Such properties of FXR suggest that FXR is an ideal target for treatment of NAFLD.
2.1. FXR and cholesterol metabolism
Since FXR plays an important role in BA metabolism, it is not surprising to find that FXR also regulates cholesterol metabolism. It has long been known the BA sequestrants decrease plasma LDL-cholesterol [15]. Now it is known that FXR controls several genes involved in cholesterol and lipoprotein metabolism, such as low-density lipoprotein receptor (LDLR), proprotein convertase subtilisin kexin type 9 (PCSK9) and scavenger receptor group B type I (SR-B1). CDCA treatment increases LDLR expression in human hepatocyte cell lines [16]. FXR activation suppresses the activity of PCSK9 [17], a repressor of LDLR, thus increasing the activity of LDLR. Fxr−/− mice display elevated levels of plasma total cholesterol level and HDL-cholesterol [18]. In contrast, FXR ligand treatment reduces plasma HDL-cholesterol by increasing the expression of hepatic SR-B1 [19]. Importantly, activation of FXR increases reverse cholesterol transport [20], a process by which extra-hepatic cholesterol is transported back to the liver for secretion to the bile and feces. Consistent with the latter data, activation of FXR significantly inhibits the development of atherosclerosis in Ldlr−/− or ApoE−/− mice [21, 22].
2.2. FXR and triglyceride metabolism
The relationship between BA and TG metabolism dates back to early 1970s, when CDCA, which was originally used for the treatment of individuals suffered from cholesterol gallstone disease, was found to reduce plasma TG levels [23]. Accordingly, LDL-lowering agents, BA sequestrants, such as cholestyramine, were found to increase plasma TG [24]. Such an effect of BAs on TG metabolism was found to be mediated at least partially through FXR. Consistent with such a finding, synthetic and specific FXR agonists, such as GW4064, INT-747 and WAY-362450, reverse the lipid disorder in various NAFLD animal models via an FXR-dependent pathway [19, 25–27]. In contrast, Fxr−/− mice displayed significantly elevated plasma TG levels, VLDL production and hepatic steatosis [28].
Although activation of FXR lowers hepatic TG levels, the underlying mechanism remains elusive. SHP was thought to be an important mediator in this process through repressing the transcription of SREBP1c, a master transcription factor that controls the expression of genes involved in fatty acid biosynthesis. Activation of FXR is known to induce SHP expression [10]. In Shp−/− mice, the repression on SREBP1c and its target genes by CA or GW4064 was not observed [25]. Meanwhile, the hepatic TG-lowering effect of CA or GW4064 was lost in Shp−/− mice [25]. These data suggest that FXR lowers hepatic TG levels through an FXR-SHP-SREBP-1c pathway [25]. However, recent findings challenge this observation. Firstly, transgenic mice over-expressing SHP in the liver exhibited higher expression of SREBP-1c and steatosis in the liver [29]. Secondly, Shp−/− mice have reduced hepatic TG level when fed a high fat diet [30]. Thirdly, knockout of Shp in ob/ob mice prevents hepatic TG accumulation [31]. Lastly, although activation of FXR represses SREBP-1c expression, activation of FXR does not suppress SREBP-1c target genes, such as fatty acid synthase (FAS) [32]. These latter observations suggest that activation of FXR lowers hepatic TG levels independent of the FXR-SHP-SREBP1 pathway (Figure 2). Thus, other yet-to-be-determined mechanism(s) should be involved in FXR-mediated reduction in hepatic TG levels.
Recently, FXR was found in human hepatocytes to inhibit the transcriptional activity of carbohydrate response element-binding protein (ChREBP) [33], another master regulator controlling hepatic glucose and lipid metabolism. FXR inhibits glucose-induced gene expression through a trans-repressive mechanism involving recruiting co-repressor to and releasing ChREBP from carbohydrate response element (ChORE). The induction of FAS and ApoC-III by high glucose was blunted by GW4064 treatment in hepatocytes. Further in vivo studies are needed to verify whether FXR modulates hepatic lipid metabolism through regulating ChREBP transcriptional activity. Aldo-keto reductase 1B7 (AKR1B7), represents another novel FXR target which may contribute to FXR-mediated amelioration of hepatic steatosis. Originally suggested to be involved in lipid peroxidation, AKR1B7 was found to be a direct target of FXR in both the liver and intestine. Adenovirus-mediated overexpression of AKR1B7 in db/db mice ameliorated hepatic steatosis [34].
Activation of FXR lowers plasma TG levels mainly though increasing plasma lipoprotein clearance. CA feeding increased hepatic expression of ApoC-II, a lipoprotein lipase (LPL) activator, specifically through FXR as this effect was not observed in Fxr−/− mice [35]. Fxr−/− mice in ob/ob background displayed elevated plasma TG and concomitant decrease in both hepatic Apo-CII and ApoA-V compared to control mice [36]. The expression of ApoC-III and angiopoietin-like 3 (ANGPTL3), both of which are LPL inhibitors, were suppressed by FXR activation [25, 37]. In addition, FXR activation increases the expression of VLDL receptor [38] and syndecan-1 [39], which are responsible for increased clearance of TG-rich lipoprotein and remnant particles, respectively.
Other mechanisms may also be involved in FXR-mediated lipid lowering effects. Human PPARα, a key regulator governing hepatic FAO, was induced after CDCA and GW4064 treatment in HepG2 cells and primary hepatocytes [40]. Fxr−/− mice in ob/ob background exhibited significantly reduced hepatic expression of PPARα and its target gene, carnitine palmitoyltransferase 1α (CPT1α), a key enzyme involved in FAO [36]. GW4064 treatment increased the expression of PDK4, a PPARα target gene involved in substrate switch, in both hepatocytes and in vivo [41]. Recently, FGF21, an important cytokine modulating systematic carbohydrate and lipid metabolism, was found to be a direct target of FXR. FGF21 increases the rates of FAO and ketogenesis through increasing lipolysis in adipose tissue [42]. FXR activation by BA or GW4064 increased the expression and secretion of FGF21 [43]. Furthermore, FXR also up-regulated the expression of FGF21 through FGF19, a cytokine secreted from intestine after FXR activation [43]. In Fxr−/− mice, the induction of FGF21 by a ketogenic diet was significantly attenuated, suggesting that FXR may play an important role in ketogenesis during fasting [43]. Increased hepatic expression of FGF21 has been shown to reduce hepatic TG levels [11, 44, 45]. In addition, FGF21 was also reported to inhibit lipogenesis through suppressing the transcriptional activity of SREBP-1c [46]. Thus, the FXR-FGF21 pathway may play a role in FXR-mediated decrease in hepatic TG levels. Overall, FXR regulates hepatic and plasma TG metabolism likely through multiple mechanisms (Figure 2).
2.3. FXR and inflammation
Inflammation plays an important role in the pathogenesis of NAFLD. Fxr−/− mice display elevated levels of inflammation such as elevated interferon-γ, tumor necrosis factor-α and interleukin-1β (IL-1β), and develop spontaneous HCC at age of 9–12 months [47]. Diabetes/insulin resistance facilitates the progression HCC when FXR is deficient [48]. The increased levels of inflammation observed in Fxr−/− mice may partly be explained by elevated BA levels in these mice. In contrast, FXR activation is shown to antagonize nuclear factor kappa B (NF-κB) pathway, including inhibition of iNOS and COX-2 in hepatocytes [49]. It remains to be determined how activation of FXR antagonizes NF-κB function.
2.4. FXR and NAFLD
The findings that FXR plays an important role in regulating both lipid homeostasis and inflammation suggest that FXR may modulate the progression of NAFLD. Indeed, Fxr−/− mice have increased hepatic TG accumulation. FXR deficiency causes pathologic manifestations of NASH in Ldlr−/− mice after a high fat feeding; the liver of these mice displayed massive steatosis, inflammatory infiltrate and fibrosis [50]. In one study on NAFLD patients, the hepatic expression of FXR is significantly decreased, whereas the expression of hepatic LXR, SREBP-1 and FAS are induced [51]. The reduced FXR expression may thus play a role in the pathogenesis of human NAFLD. Another very recent study on 113 NAFLD patients reveals a close association between BA synthesis and plasma BA concentration and the severity of NAFLD [52]. In these patients, both CYP7A1 and the bile acid transporter Na+/taurocholate cotransporter (NTCP) expression is increased, likely as a result of increased FFA levels [52].
Administration of the FXR agonist WAY-362450 attenuated hepatic inflammation and fibrosis, and lowered serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in C57BL/6 mice fed a methionine and choline-deficient [53] diet, a well-established nutritional model of NASH [27]. Inflammatory factors, such as keratinocyte-derived chemokine (mKC) and monocyte chemotactic protein-1 (MCP-1) were also reduced. WAY-362450 treatment also down-regulated hepatic fibrosis genes, such as collagen, TGF-β1, matrix metalloproteinase 2 (MMP-2) and tissue inhibitor of metalloproteinase 1 (TIMP-1). These effects are FXR dependent since no improvement was observed in MCD-fed Fxr−/− mice [27].
INT-747, also known as Obeticholic acid [52], is a 6α-ethyl derivative of CDCA. It is reported to ameliorate hepatic steatosis and inflammation on a variety of animal models. OCA treatment improved insulin resistance and hepatic steatosis in Zucker fa/fa rats, which is associated with decreased expression of genes involved in hepatic lipogenesis and gluconeogenesis [54]. OCA also inhibits hepatic inflammatory responses induced by NF-κB while maintaining or enhancing the cell survival response. This drug is under clinical trial at present. The phase II clinical trial in patients with type 2 diabetes and NAFLD demonstrates that OCA treatment increased systematic insulin sensitivity as evidenced by increased glucose infusion rate in hyperinsulinemic-euglycemic clamp study [55, 56]. There was also a drop in plasma γ-glutamyltransferase (GGT) and alanine aminotransferase (ALT) [55, 56], suggesting reduced liver injury. Liver fibrosis, which occurred in 81% of the patients, was also improved significantly by OCA treatment, as evidenced by reduced hyaluronic acid, procollagen III amino terminal peptide, and tissue inhibitor of metalloproteinase 1 [55, 56]. The Phase IIb clinical study with NASH patients is underway.
2.5. FXR and glucose metabolism and energy expenditure
Administration of C57BL/6J mice with CA decreased hepatic transcription of PEPCK and G6Pase as well as fasting glucose levels [57]. This effect was absent in either Fxr−/− or Shp−/− mice, suggesting that the hypoglycemic effect of FXR is through inhibiting hepatic gluconeogenesis via the FXR-SHP pathway [58]. In line with this report, adenovirus-mediated constitutive activation of FXR in liver or GW4064 administration to db/db or KK-A(y) mice reversed hyperglycemia and decreased hepatic expression of PEPCK and G6Pase [19]. FXR activation was also reported to modulate glycogen storage [19].
Although activation of FXR results in multiple favorable effects on both glucose and lipid metabolism, one potential adverse effect of FXR activation may warrant some attention. The FXR agonist GW4064 attenuated energy expenditure in high fat diet (HFD)-induced obese mice as a result of reduced BA pool size [59]. In contrast, Fxr−/− mice showed resistance to diet-induced obesity [60] and Fxr deficiency in ob/ob mice protects against body weight gain and obesity [48]. This negative effect of FXR on energy expenditure may pose a concern on the long term use of FXR agonist(s).
3. Role of TGR5 in lipid and energy metabolism and inflammation
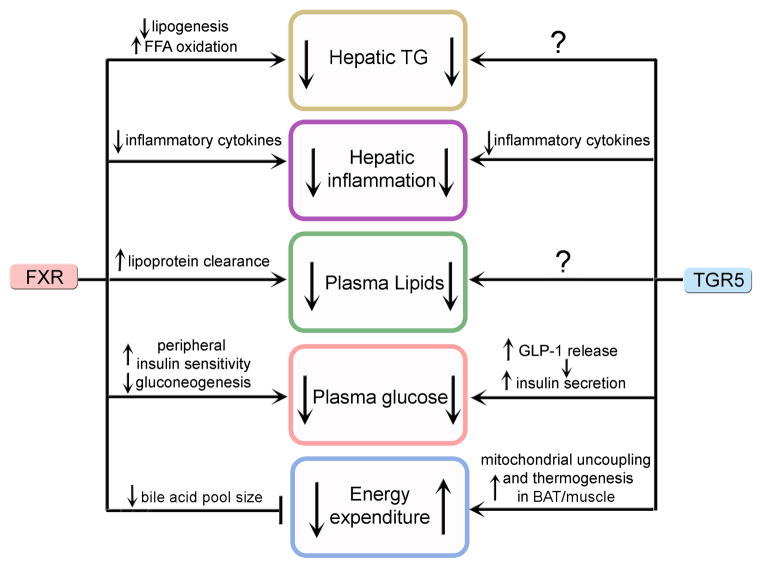
TGR5 has been well recognized not only for its role in BA homeostasis but also for its role in glucose and lipid homeostasis as well as energy expenditure (Figures 3 and 4). It regulates the expression of genes involved in inflammation, modulates plasma glucose and lipid levels, and increases energy expenditure in skeletal muscle and brown adipose tissue [11, 61–63].
Figure 4. Major pathways regulated by FXR and TGR5.
Activation of either FXR or TGR5 lowers hepatic TG levels and inhibits inflammation, thus protecting against the development of NAFLD. Activation of either receptor also lowers plasma lipids and improves glucose homeostasis. Activation of FXR reduces energy expenditure. In contrast, activation of TGR5 increases energy expenditure. The mechanism by which TGR5 lowers hepatic TG levels or plasma lipids remains to be determined.
3.1. TGR5 and glucose and energy homeostasis
In the enteroendocrine STC-1 cell line, activation of TGR5 leads to increased secretion of glucagon like peptide-1 (GLP-1) [64]. This TGR5-induced GLP-1 secretion has been shown to improve pancreatic and liver function and also to improve glucose tolerance in obese mice [62]. While the mechanism behind this is unclear, there may be an involvement of oxidative phosphorylation. The resultant increase in the ATP/ADP ratio can induce membrane depolarization and calcium mobilization in a similar fashion as that occurs before insulin secretion in pancreatic β cells [62]. In contrast, Tgr5−/− mice have worse glucose clearance as compared to their wild-type littermates.
Activation of TGR5 by BAs also leads to increased expression and activity of 2-iodothyronine deiodinase (D2), which is able to increase mitochondrial oxidative phosphorylation and energy expenditure in brown adipose tissue and muscle, by converting inactive thyroxine (T4) into its active form 3,5,3-tri-iodothyronine (T3) which then binds and activates the thyroid hormone receptor, thus inducing energy expenditure [61]. CA attenuates body weight gain in HFD-fed wild-type mice [61]. However, it remains to be determined whether this latter effect is through TGR5. In addition, the synthetic TGR5 agonist INT-777 is found to increase energy expenditure and prevent HFD-induced obesity [62].
3.2. TGR5 and hepatic triglyceride metabolism
In HFD-fed mice, INT-777 treatment not only prevented body weight gain but also reduced liver steatosis and plasma FFAs, LDH, AST and ALT [62]. In another study, it was reported that HFD-fed male but not female knockouts of TGR5 showed significantly higher levels of liver steatosis [65]. The above findings are counterintuitive considering that TGR5 is not expressed in hepatocytes. One possibility is that the liver takes up less FFAs from adipose tissue because of reduced obesity that results from increased energy expenditure in brown fat/skeletal muscle. Additional studies are needed to explore other underlying mechanisms.
3.3. TGR5 and inflammation and atherosclerosis
It has been shown that TGR5 is expressed in several immune cells, such as monocytes, alveolar macrophages, and Kupffer cells and that BAs modulate the inflammatory response in these cells [66, 67]. cAMP is reported to inhibit LPS-induced cytokine secretion [68]. Treatment with BAs activates TGR5 and increases cAMP production in THP-1 cell [67], resulting in attenuation of macrophage’s effector functions including reduction of phagocytic activity as well as generation of LPS-stimulated cytokines (TNF-α, IL-1α, IL-1β, IL-6, and IL-8) [66] (Figure 3). Agonist-induced TGR5 activation inhibits the expression of inflammatory mediators in response to Toll-like receptor 4 (TLR4) activation by LPS in WT but not in Tgr5−/− mouse liver, thus identifying TGR5 as a negative regulator of liver inflammation [69] (Figures 3 and 4)
TGR5 also participates in regulation of intestinal inflammation. It is reported that the expression of TGR5 increases in response to inflammation in rodent models of colitis and in inflamed tissues obtained from Crohn’ disease patients. Ciprofloxacin and oleanolic acid, two TGR5 ligands, attenuate colon inflammation in rodent models of colitis [70].
The inhibition of inflammation through TGR5 activation can be also beneficial for atherosclerosis. The development and progression of atherosclerosis is accelerated by dyslipidemia and chronic inflammation, and macrophages play a key role in this process [71]. Macrophages scavenge modified forms of LDL, leading to the formation of foam cells and local production of cytokines and chemokines that initiate chronic inflammation of the vessel wall, one of the initial steps in the pathogenesis of atherosclerosis [63]. It was recently shown that TGR5 activation inhibits the inflammatory response in the macrophage and prevents the development of atherosclerosis. The activation of TGR5 by INT-777 attenuated atherosclerosis in Ldlr−/− Tgr5+/+ mice but not in Ldlr−/− Tgr5−/− mice. The inhibition of lesion formation was associated with decreased intraplaque inflammation and less plaque macrophage content [63].
3.4. TGR5 and the progression of NAFLD
TGR5 is highly expressed in Kupffer cells and sinusoidal endothelial cells [66, 72]. These Kupffer cells are capable of secreting proinflammatory cytokines which can in turn contribute to the progression of NAFLD [73]. When Kupffer cells were treated with INT-777, there was a reduction in the lipopolysaccharide (LPS)-induced production of inflammatory cytokines through the TGR5-cAMP-dependent pathway. INT-777 treatment blunts the expression of inflammatory mediators by antagonizing NF-κB activity in wild-type mice but not in Tgr5−/− mice [69]. The anti-inflammatory and anti-steatotic properties of TGR5 suggest that TGR5 may protect against the development and progression of NAFLD. Future studies are needed to test whether TGR5 prevents the progression of NAFLD.
4. FXR and TGR5 dual agonists
Activation of either FXR or TGR5 has beneficial effects on lipid and glucose homeostasis. Although BAs can activate both FXR and TGR5, BAs may also activate other pathways independent of these two receptors [10]. Therefore, the development of specific/selective agonist(s) that can activate both FXR and TGR5 may provide another tool for fighting lipid disorder and diabetes.
INT-767 (6-ethyl-23(S)-methyl-3,7,12-trihydroxy-5β-cholan-24-oic acid), a bile acid derive, is a novel and potent agonist for both FXR and TGR5 [60]. INT-767 treatment decreased plasma total cholesterol, HDL cholesterol and TG levels in streptozotocin-treated mice that were fed a Western diet [60]. INT-767 treatment was also shown to significantly reduce serum liver enzyme levels and improve hepatic inflammation and biliary fibrosis in Mdr2−/− mice [13], a murine model of chronic cholangiopathy, and to significantly improve hepatic steatosis and inflammation in db/db mice [74]. INT-767 decreases the levels of proinflammatory cytokines and increases IL-10 production, leading to down-regulation of Ly6C both in vitro and in vivo. Furthermore, treatment with INT-767 increased the proportion of intrahepatic Ly6Clow monocytes which have anti-inflammatory property [74]. Despite these exciting data, more studies are needed to characterize the role of the dual FXR/TGR5 agonist in lipid and carbohydrate metabolism and the benefits of its use over FXR or TGR5 agonists.
5. Should we target FXR or TGR5, or both?
Activation of either FXR or TGR5 or both FXR and TGR5 has been shown to display beneficial effects on hepatic triglyceride and glucose homeostasis and inflammation (Figure 4). One intriguing question is whether we should target FXR, TGR5 or both in treatment of NAFLD or metabolic syndrome. Activated FXR or TGR5 lowers hepatic TG levels and plasma lipids, inhibits inflammation, and improves glucose homeostasis (Figure 4). In addition, activation of FXR reduces energy homeostasis whereas activation of TGR5 increases energy homeostasis (Figure 4). Since FXR and TGR5 may complement each other functionally, simultaneous activation of FXR and TGR5 may receive the maximum beneficial effect on lipid and glucose homeostasis. However, additional studies are needed to elucidate whether this is the case and whether activation of TGR5 has any detrimental effects.
Although both FXR and TGR5 are expressed in the liver, FXR is expressed exclusively in hepatocytes whereas TGR5 is expressed in Kupffer cells but not hepatocytes. In the case of NAFLD, FXR activation has a pronounced effect on lowering hepatic TG levels but less effect on inhibition of inflammation. In contrast, TGR5 activation has a striking inhibitory effect on inflammation but less effect on hepatic TG levels. Therefore, the use of a dual FXR/TGR5 agonist may be a better approach for treatment of NAFLD.
6. Conclusions
Accumulating data have clearly demonstrated that both FXR and TGR5 regulate BA, lipid and glucose homeostasis. Importantly, activation of FXR or TGR5 has beneficial effects on lipid and glucose homeostasis and inflammatory response. Treatment with agonists for FXR or TGR5 significantly protects against NAFLD. However, the mechanism(s) underlying the protection against liver steatosis by FXR or TGR5 remain to be determined. Since FXR and TGR5 may function synergistically and complement each other, the utilization of a dual FXR/TGR5 agonist may represent a new approach for treatment of NAFLD and other metabolic disease.
Acknowledgments
This work was supported by NIH grants R15DK088733, R01HL103227, and R01DK095895 to Y.Z. We thank Jiesi Xu and Yang Xu for their contribution to this article. We apologize for not being able to cite many of the relevant references as the journal only allows us to cite a maximum of 75 references.
References
- 1.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. The Journal of clinical investigation. 2008;118:829–38. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–23. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujii H, Kawada N. Inflammation and fibrogenesis in steatohepatitis. Journal of gastroenterology. 2012;47:215–25. doi: 10.1007/s00535-012-0527-x. [DOI] [PubMed] [Google Scholar]
- 4.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–5. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 332:1519–23. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson N, Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev. 2008;60:311–57. doi: 10.1124/pr.108.00001. [DOI] [PubMed] [Google Scholar]
- 7.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–93. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Edwards PA. FXR signaling in metabolic disease. FEBS Lett. 2008;582:10–8. doi: 10.1016/j.febslet.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–91. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 10.Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. FXR, a multipurpose nuclear receptor. Trends Biochem Sci. 2006;31:572–80. doi: 10.1016/j.tibs.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol. 54:1263–72. doi: 10.1016/j.jhep.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellicciari R, Gioiello A, Macchiarulo A, Thomas C, Rosatelli E, Natalini B, et al. Discovery of 6alpha-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J Med Chem. 2009;52:7958–61. doi: 10.1021/jm901390p. [DOI] [PubMed] [Google Scholar]
- 13.Rakhshandehroo M, Knoch B, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 2010 doi: 10.1155/2010/612089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furihata T, Hosokawa M, Nakata F, Satoh T, Chiba K. Purification, molecular cloning, and functional expression of inducible liver acylcarnitine hydrolase in C57BL/6 mouse, belonging to the carboxylesterase multigene family. Arch Biochem Biophys. 2003;416:101–9. doi: 10.1016/s0003-9861(03)00286-8. [DOI] [PubMed] [Google Scholar]
- 15.Insull W., Jr Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: a scientific review. South Med J. 2006;99:257–73. doi: 10.1097/01.smj.0000208120.73327.db. [DOI] [PubMed] [Google Scholar]
- 16.Nakahara M, Fujii H, Maloney PR, Shimizu M, Sato R. Bile acids enhance low density lipoprotein receptor gene expression via a MAPK cascade-mediated stabilization of mRNA. J Biol Chem. 2002;277:37229–34. doi: 10.1074/jbc.M206749200. [DOI] [PubMed] [Google Scholar]
- 17.Langhi C, Le May C, Kourimate S, Caron S, Staels B, Krempf M, et al. Activation of the farnesoid X receptor represses PCSK9 expression in human hepatocytes. FEBS Lett. 2008;582:949–55. doi: 10.1016/j.febslet.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 18.Lambert G, Amar MJ, Guo G, Brewer HB, Jr, Gonzalez FJ, Sinal CJ. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 2003;278:2563–70. doi: 10.1074/jbc.M209525200. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1006–11. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Yin L, Anderson J, Ma H, Gonzalez FJ, Willson TM, et al. Identification of novel pathways that control farnesoid X receptor-mediated hypocholesterolemia. J Biol Chem. 2010;285:3035–43. doi: 10.1074/jbc.M109.083899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartman HB, Gardell SJ, Petucci CJ, Wang S, Krueger JA, Evans MJ. Activation of farnesoid X receptor prevents atherosclerotic lesion formation in LDLR−/− and apoE−/− mice. Journal of lipid research. 2009;50:1090–100. doi: 10.1194/jlr.M800619-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flatt B, Martin R, Wang TL, Mahaney P, Murphy B, Gu XH, et al. Discovery of XL335 (WAY–362450), a highly potent, selective, and orally active agonist of the farnesoid X receptor (FXR) J Med Chem. 2009;52:904–7. doi: 10.1021/jm8014124. [DOI] [PubMed] [Google Scholar]
- 23.Bateson MC, Maclean D, Evans JR, Bouchier IA. Chenodeoxycholic acid therapy for hypertriglyceridaemia in men. Br J Clin Pharmacol. 1978;5:249–54. doi: 10.1111/j.1365-2125.1978.tb01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crouse JR., 3rd Hypertriglyceridemia: a contraindication to the use of bile acid binding resins. Am J Med. 1987;83:243–8. doi: 10.1016/0002-9343(87)90692-9. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. The Journal of clinical investigation. 2004;113:1408–18. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. Journal of lipid research. 2009 doi: 10.1194/jlr.M001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang S, Wang J, Liu Q, Harnish DC. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J Hepatol. 2009;51:380–8. doi: 10.1016/j.jhep.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–44. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 29.Boulias K, Katrakili N, Bamberg K, Underhill P, Greenfield A, Talianidis I. Regulation of hepatic metabolic pathways by the orphan nuclear receptor SHP. EMBO J. 2005;24:2624–33. doi: 10.1038/sj.emboj.7600728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Liu J, Saha P, Huang J, Chan L, Spiegelman B, et al. The orphan nuclear receptor SHP regulates PGC-1alpha expression and energy production in brown adipocytes. Cell Metab. 2005;2:227–38. doi: 10.1016/j.cmet.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Huang J, Iqbal J, Saha PK, Liu J, Chan L, Hussain MM, et al. Molecular characterization of the role of orphan receptor small heterodimer partner in development of fatty liver. Hepatology. 2007;46:147–57. doi: 10.1002/hep.21632. [DOI] [PubMed] [Google Scholar]
- 32.Matsukuma KE, Bennett MK, Huang J, Wang L, Gil G, Osborne TF. Coordinated control of bile acids and lipogenesis through FXR-dependent regulation of fatty acid synthase. Journal of lipid research. 2006;47:2754–61. doi: 10.1194/jlr.M600342-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Ip E, Farrell GC, Robertson G, Hall P, Kirsch R, Leclercq I. Central role of PPARalpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology. 2003;38:123–32. doi: 10.1053/jhep.2003.50307. [DOI] [PubMed] [Google Scholar]
- 34.Ge X, Yin L, Ma H, Li T, Chiang JY, Zhang Y. Aldo-keto reductase 1B7 is a target gene of FXR and regulates lipid and glucose homeostasis. J Lipid Res. 52:1561–8. doi: 10.1194/jlr.M015859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kast HR, Nguyen CM, Sinal CJ, Jones SA, Laffitte BA, Reue K, et al. Farnesoid X-activated receptor induces apolipoprotein C-II transcription: a molecular mechanism linking plasma triglyceride levels to bile acids. Mol Endocrinol. 2001;15:1720–8. doi: 10.1210/mend.15.10.0712. [DOI] [PubMed] [Google Scholar]
- 36.Prawitt J, Abdelkarim M, Stroeve JH, Popescu I, Duez H, Velagapudi VR, et al. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes. 60:1861–71. doi: 10.2337/db11-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Claudel T, Inoue Y, Barbier O, Duran-Sandoval D, Kosykh V, Fruchart J, et al. Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology. 2003;125:544–55. doi: 10.1016/s0016-5085(03)00896-5. [DOI] [PubMed] [Google Scholar]
- 38.Sirvent A, Claudel T, Martin G, Brozek J, Kosykh V, Darteil R, et al. The farnesoid X receptor induces very low density lipoprotein receptor gene expression. FEBS Lett. 2004;566:173–7. doi: 10.1016/j.febslet.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 39.Anisfeld AM, Kast-Woelbern HR, Meyer ME, Jones SA, Zhang Y, Williams KJ, et al. Syndecan-1 Expression Is Regulated in an Isoform-specific Manner by the Farnesoid-X Receptor. J Biol Chem. 2003;278:20420–8. doi: 10.1074/jbc.M302505200. [DOI] [PubMed] [Google Scholar]
- 40.Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17:259–72. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- 41.Savkur RS, Bramlett KS, Michael LF, Burris TP. Regulation of pyruvate dehydrogenase kinase expression by the farnesoid X receptor. Biochem Biophys Res Commun. 2005;329:391–6. doi: 10.1016/j.bbrc.2005.01.141. [DOI] [PubMed] [Google Scholar]
- 42.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–25. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Cyphert HA, Ge X, Kohan AB, Salati LM, Zhang Y, Hillgartner FB. Activation of the farnesoid X receptor induces hepatic expression and secretion of fibroblast growth factor 21. J Biol Chem. 287:25123–38. doi: 10.1074/jbc.M112.375907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furihata T, Hosokawa M, Satoh T, Chiba K. Synergistic role of specificity proteins and upstream stimulatory factor 1 in transactivation of the mouse carboxylesterase 2/microsomal acylcarnitine hydrolase gene promoter. The Biochemical journal. 2004;384:101–10. doi: 10.1042/BJ20040765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konig B, Koch A, Spielmann J, Hilgenfeld C, Hirche F, Stangl GI, et al. Activation of PPARalpha and PPARgamma reduces triacylglycerol synthesis in rat hepatoma cells by reduction of nuclear SREBP-1. Eur J Pharmacol. 2009;605:23–30. doi: 10.1016/j.ejphar.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Lei T, Huang JF, Wang SB, Zhou LL, Yang ZQ, et al. The link between fibroblast growth factor 21 and sterol regulatory element binding protein 1c during lipogenesis in hepatocytes. Mol Cell Endocrinol. 342:41–7. doi: 10.1016/j.mce.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940–6. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Ge X, Heemstra LA, Chen WD, Xu J, Smith JL, et al. Loss of FXR protects against diet-induced obesity and accelerates liver carcinogenesis in ob/ob mice. Mol Endocrinol. 2012;26:272–80. doi: 10.1210/me.2011-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang YD, Chen WD, Wang MH, Yu DN, Forman BM, Huang WD. Farnesoid X Receptor Antagonizes Nuclear Factor kappa B in Hepatic Inflammatory Response. Hepatology. 2008;48:1632–43. doi: 10.1002/hep.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kong B, Luyendyk JP, Tawfik O, Guo GL. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther. 2009;328:116–22. doi: 10.1124/jpet.108.144600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang ZX, Shen W, Sun H. Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatology international. 2010;4:741–8. doi: 10.1007/s12072-010-9202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bechmann LP, Kocabayoglu P, Sowa JP, Sydor S, Best J, Schlattjan M, et al. Free fatty acids repress small heterodimer partner (SHP) activation and adiponectin counteracts bile acid-induced liver injury in superobese patients with nonalcoholic steatohepatitis. Hepatology. 2013;57:1394–406. doi: 10.1002/hep.26225. [DOI] [PubMed] [Google Scholar]
- 53.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–25. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. Journal of lipid research. 2010;51:771–84. doi: 10.1194/jlr.M001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adorini L, Pruzanski M, Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug discovery today. 2012;17:988–97. doi: 10.1016/j.drudis.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 56.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, et al. Efficacy and Safety of the Farnesoid X Receptor Agonist Obeticholic Acid in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 57.Yamagata K, Daitoku H, Shimamoto Y, Matsuzaki H, Hirota K, Ishida J, et al. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J Biol Chem. 2004;279:23158–65. doi: 10.1074/jbc.M314322200. [DOI] [PubMed] [Google Scholar]
- 58.Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. The Journal of clinical investigation. 2006;116:1102–9. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watanabe M, Horai Y, Houten SM, Morimoto K, Sugizaki T, Arita E, et al. Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. J Biol Chem. 286:26913–20. doi: 10.1074/jbc.M111.248203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rizzo G, Passeri D, De Franco F, Ciaccioli G, Donadio L, Rizzo G, et al. Functional Characterization of the Semisynthetic Bile Acid Derivative INT-767, a Dual Farnesoid X Receptor and TGR5 Agonist. Molecular Pharmacology. 2010;78:617–30. doi: 10.1124/mol.110.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–9. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 62.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–77. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pols TW, Nomura M, Harach T, Lo Sasso G, Oosterveer MH, Thomas C, et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14:747–57. doi: 10.1016/j.cmet.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–90. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 65.Talayero BG, Sacks FM. The role of triglycerides in atherosclerosis. Current cardiology reports. 2011;13:544–52. doi: 10.1007/s11886-011-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keitel V, Donner M, Winandy S, Kubitz R, Haussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78–84. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- 67.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–40. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 68.Yoshimura T, Kurita C, Nagao T, Usami E, Nakao T, Watanabe S, et al. Inhibition of tumor necrosis factor-alpha and interleukin-1-beta production by beta-adrenoceptor agonists from lipopolysaccharide-stimulated human peripheral blood mononuclear cells. Pharmacology. 1997;54:144–52. doi: 10.1159/000139481. [DOI] [PubMed] [Google Scholar]
- 69.Wang YD, Chen WD, Yu D, Forman BM, Huang W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-kappaB) in mice. Hepatology. 2011;54:1421–32. doi: 10.1002/hep.24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cipriani S, Mencarelli A, Chini MG, Distrutti E, Renga B, Bifulco G, et al. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS One. 2011;6:e25637. doi: 10.1371/journal.pone.0025637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 72.Keitel V, Reinehr R, Gatsios P, Rupprecht C, Gorg B, Selbach O, et al. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45:695–704. doi: 10.1002/hep.21458. [DOI] [PubMed] [Google Scholar]
- 73.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212–23. doi: 10.1016/j.jhep.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McMahan RH, Wang XX, Cheng LL, Krisko T, Smith M, El Kasmi K, et al. Bile Acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic Fatty liver disease. J Biol Chem. 2013;288:11761–70. doi: 10.1074/jbc.M112.446575. [DOI] [PMC free article] [PubMed] [Google Scholar]