Abstract
We present the case of a 63-year-old white male with bilateral chronic leg ulcers due to polycythemia vera and hydroxyurea therapy who demonstrated dramatic healing of his wounds in response to ruxolitinib (Jakafi®, Novartis), a novel Janus kinase-1 and -2 inhibitor. This patient’s wound had previously been refractory to multiple surgical interventions and immunosuppression. After the initiation of ruxolitinib, the patient underwent successful split-thickness skin grafting, with resultant healing of his wounds. He was stable without prednisone and other immunosuppressant therapy and had healed at 6 months. Ruxolitinib therapy could represent a novel option for patients who develop persistent inflammatory wounds in the setting of polycythemia vera and hydroxyurea therapy.
Keywords: chronic wound, hydroxyurea, leg ulcer, polycythemia vera, pyoderma gangrenosum, split-thickness skin graft
Leg ulceration is a known complication of hydroxyurea use in the setting of polycythemia vera (PV) and sickle cell disease that can be very challenging to treat (1–3). Although the ulcers often respond to withdrawal of hydroxyurea, this is not always feasible owing to the underlying hematologic condition (4). Ruxolitinib is an oral selective inhibitor of Janus kinase (JAK)-1 and -2 recently approved by the U.S. Food and Drug Administration for the treatment of intermediate-and high-risk myelofibrosis and PV (5–7). Patients with leg ulcers were excluded from the original studies of ruxolitinib in myelodys-plastic disease because of concerns about infection. However, we report the use of ruxolitinib in a patient with resistant PV-associated leg ulceration. The impressive response of this patient was very promising, suggesting a possible role for JAK inhibition in patients with chronic leg ulceration associated with myelofibrotic disorders.
Case Report
A 63-year-old white male with bilateral foot and ankle ulceration associated with PV and chronic hydroxyurea therapy was followed up at our institution from January 2006 to April 2013. At presentation, the patient had had refractory leg ulcers for 1 year. Wound debridement and grafts with biologic alternative tissues had previously been unsuccessful. Because of a presumptive diagnosis of pyoderma gangrenosum, he was started on high-dose prednisone at a dose of 80 mg/day and mycophenolate mofetil (CellCept®, Genentech, South San Francisco, CA) 1.5 g twice daily.
Approximately 6 months before the presentation, the patient had developed a left leg deep venous thrombosis and began anticoagulation with warfarin. Concurrently with this, he also reported development of Raynaud’s phenomenon of the digits and erythematous papules on the dorsum of the bilateral digits, consistent with Gottron’s papules. He denied muscle weakness, malar and discoid rashes, sicca symptoms, oral ulcers, cough, chest pain, and shortness of breath. The patient had a remote history of colon cancer treated 15 years before presentation with hemi-colectomy and chemotherapy. The gastrointestinal workup was negative for colon cancer recurrence or evidence of inflammatory bowel disease.
He had no family history of autoimmune disease, diabetes, or leg ulceration. The patient worked full time as a defense contractor. He did not smoke, use illicit substances, or drink alcohol.
On the initial examination, the patient was afebrile. He had a blood pressure of 120/56 mm Hg and pulse of 104 beats/min regular rhythm. The head and neck evaluation was remarkable only for bilateral hearing aids; he had no malar or discoid or heliotrope rashes. He had no oral ulcers and had a normal oral aperture and normal salivary pooling. His neck was supple, with no lymphadenopathy or thyromegaly. The cardiovascular, respiratory, and abdominal examination findings were unremarkable. On examination of the hands, he had scaly erythematous lesions over the metacarpophalangeal joints and proximal interphalangeal joints. He had no rash on the torso or chest. His muscle strength was 5 of 5 in all muscle groups. The examination of the legs revealed bilateral deep necrotic ulcers (Fig. 1).
Fig. 1.
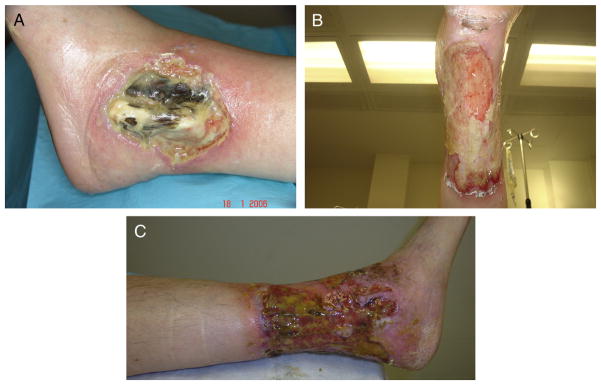
(A) Lateral malleolar ulcer at presentation demonstrating central necrosis of the tendons with extensive inflammatory slough and deep overhanging gunmetal gray borders consistent with pyoderma gangrenosum. (B) and (C) Progression of ulceration to become circumferential by 3 years after presentation.
Pathologic examination of the wound biopsy specimen demonstrated necrosis with dense neutrophilic and mixed inflammatory infiltrate. Superficial capillaries demonstrated thick periodic acid-Schiff stain-positive walls, and the dermal capillaries demonstrated thick walls and almost occluded lumens (Fig. 2).
Fig. 2.
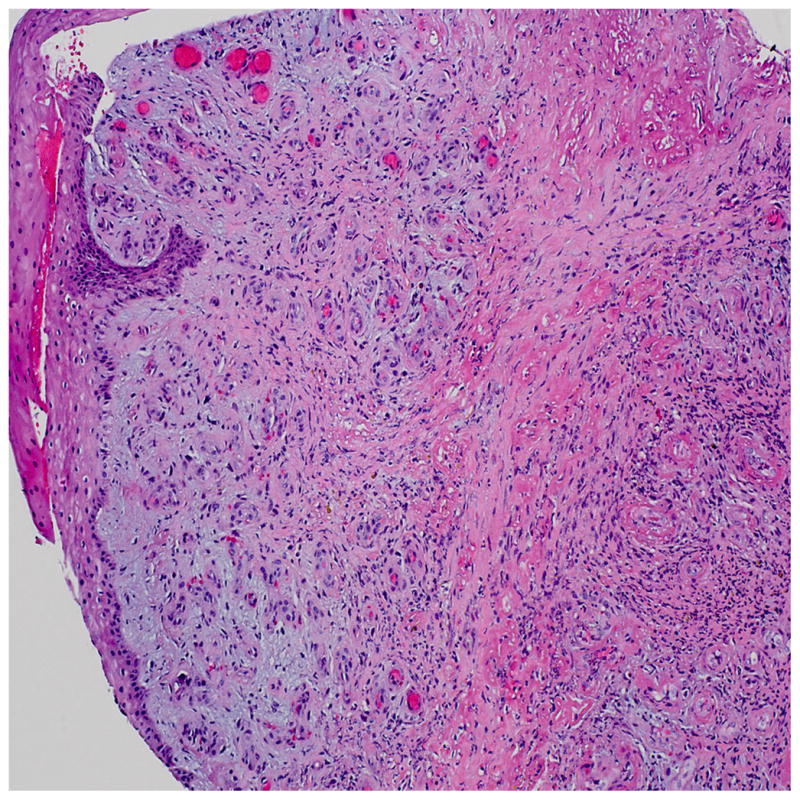
Histopathologic findings from wound biopsy specimen demonstrating ulcer border showing mixed inflammatory infiltrate, including prominent neutrophils, granulation tissue formation, and fibrinous occlusive vasculopathy (hematoxylin and eosin stain, original magnification ×10).
Laboratory Data
At presentation with the leg ulcerations, the laboratory workup revealed mild leukocytosis and hemoglobin of 14.3 g/dL, consistent with his known PV. He was homozygous for the JAK-2 mutation. The autoimmune profile was negative (Table 1). The results of the arterial studies were within normal limits. The venous studies showed an incompetent deep venous system, with reflux involving the common femoral, proximal femoral, mid-femoral, distal femoral, and popliteal veins.
Table 1.
Hematologic parameters and autoimmune profile at presentation
| Variable | Patient’s Findings | Normal Range |
|---|---|---|
| Janus kinase-2 mutation | Homozygous | |
| White blood cell count (k/μL) | 14.3 | 4.0–10.8 |
| Differential (%) | ||
| Neutrophils | 93.9 | |
| Lymphocytes | 4.0 | |
| Hemoglobin (g/dL) | 14.3 | 12.5–16.5 |
| Hematocrit (%) | 37.4 | 41–53 |
| Red blood cell distribution width (%) | 16.6 | 11–15 |
| Platelet count (k/μL) | 398 | 145–400 |
| Sodium (mmol/L) | 136 | 137–145 |
| Potassium | 4.7 | 3.5–5.1 |
| Blood urea nitrogen (mg/dL) | 20 | 9–20 |
| Creatinine (mg/dL) | 0.9 | 0.66–1.50 |
| Alkaline phosphatase (U/L) | 93 | 38–126 |
| Aspartate aminotransferase (U/L) | 23 | 3–34 |
| Alanine aminotransferase (U/L) | 29 | 15–41 |
| ANA immunofluorescence | Negative | Negative |
| dsDNA antibodies | Negative | Negative |
| Antineutrophil cytoplasmic antibodies | Negative | Negative |
| Sjögren’s antibodies (SSA and SSB) | Negative | Negative |
| Sm antibody | Negative | Negative |
| RNP antibody | Negative | Negative |
| Thyroid-stimulating hormone (IU/mL) | 1.58 | 0.40–4.0 |
| Uric acid (mg/dL) | 2.4 | 3.5–7.2 |
| Hemoglobin A1c (%) | 4.7 | 4.2–5.6 |
| β2-Glycoprotein I antibodies IgG (U/mL) | <4.0 | <10.0, negative |
| β2-Glycoprotein I antibodies IgA (U/mL) | <4.0 | <10.0, negative |
| β2-Glycoprotein I antibodies IgM (U/mL) | <4.0 | <10.0, negative |
| Anticardiolipin antibodies IgG (U) | <23 | <23, negative |
| Anticardiolipin antibodies IgA (U) | <11 | <11, negative |
| Anticardiolipin antibodies IgM | <11 units | <11, negative |
| Lupus anticoagulant ratio | Negative | 1.2–1.5 (weakly positive) |
| 1.5–2.0 (moderately positive) | ||
| >2.0 (strongly positive) | ||
| Sedimentation rate (mm/hr) | 10 | 0–16 |
| C-reactive protein (mg/L) | 1.2 | 0.00–3.00 |
| Human immunodeficiency virus 1 and 2 | Negative | Negative |
| Hepatitis B surface antibody | Negative | Negative |
| Hepatitis C antibody | Negative | Negative |
| Plasminogen activator inhibitor mutation | 4G/5G, heterozygous | 5G/5G |
| Factor V Leiden (R506Q) mutation | Negative | Negative |
| Methyl-tetrahydrofolate reductase C677T mutation | C677T, homozygous | Negative |
| Prothrombin gene (G20210A) mutation | Negative | Negative |
Abbreviations: ANA, antinuclear antibody; dsDNA, double-stranded DNA; RNP, ribonucleoprotein.
The patient had had several hospitalizations during the course of several years. He was initially treated with prednisone, mycophenolate mofetil, intravenous immunoglobulin, pentoxifylline, and sulfasalazine. He underwent a venous ablation procedure to address the venous insufficiency, with no improvement in the ulcers. Because of the lack of a response to immunosuppression and because of the skin lesions, which were consistent with the amyopathic vasculitic rashes known to be associated with hydroxyurea therapy, an attempt was made to wean the patient off the hydroxyurea. However, his ulcers worsened, and the hydroxyurea had to be increased back to 1500 mg/day.
In conjunction with the intravenous immunoglobulin therapy, the patient developed an additional deep venous thrombosis, and the warfarin therapy was changed to low-molecular-weight heparin (enoxaparin) at a dose of 1 mg/kg twice daily. Despite all these measures, the ulcers remained refractory to therapy. Immunosuppression was changed to methotrexate and tumor necrosis factor-α inhibition. He underwent repeated debridement, repeated attempts at bioengineered alternative tissue grafting, split-thickness skin grafting, hyperbaric oxygen therapy, local wound care, and low-frequency ultrasound therapy (MIST®, Celleration, Eden Prairie, MN), but the ulcers continued to worsen. Amputation was discussed, but the patient declined.
Approximately 5 years after the initial presentation, because of the persisting leg ulceration, another attempt was made to stop the hydroxyurea. The patient developed progressive leukocytosis, weight loss, splenomegaly, and headaches. Magnetic resonance imaging of the brain revealed a 6 × 5-mm hypothalamic mass, which was thought to be consistent with extramedullary hematopoiesis secondary to postpolycythemia myeloid metaplasia. The tumor necrosis factor-α inhibitor was discontinued, and hydroxyurea was reinstituted. A bone marrow biopsy was not performed, but peripheral blood cytogenetic testing was negative for acute myeloid leukemia.
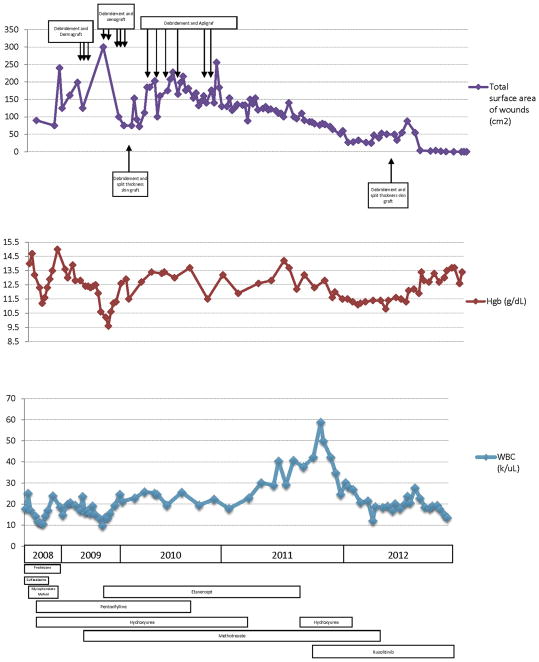
After a discussion of the risks, ruxolitinib was added at a dose of 10 mg twice daily, and the hydroxyurea dosage was decreased. Approximately 5 weeks after beginning this therapy, the white blood cell count began to decline; as a result, the hydroxyurea was discontinued. The wounds began to heal, and the patient underwent split-thickness skin grafting with a more than 80% take. The methotrexate dose was tapered and then withdrawn. The residual wound healed by secondary intention. Fig. 3 shows the timeline of the patient’s total wound surface area as it covaried with the hemoglobin and total white blood cell count, with the dates of the surgical interventions and medication exposures superimposed. At 6 months of ruxolitinib therapy, the wounds had entirely healed (Fig. 4). The other skin manifestations attributed to hydroxyurea use, including the Gottron papules on the digits persisted, but the Raynaud phenomenon, which was likely related to hyperviscosity, had totally resolved. The headaches had also resolved, and repeat imaging demonstrated stability of the hypothalamic mass. At the 12-month visit, the patient was mobilizing independently and regaining full function and range of motion at the ankle through physical therapy. However, 15 months after the final surgery and 18 months after initiation of ruxolitinib, the patient died of a cerebral hemorrhage.
Fig. 3.
Graphic timeline of (top) total surface area of wounds, (middle) hemoglobin, and (bottom) total white blood cell count, along with a timeline of medication exposures (bars) and surgical interventions (arrows).
Fig. 4.
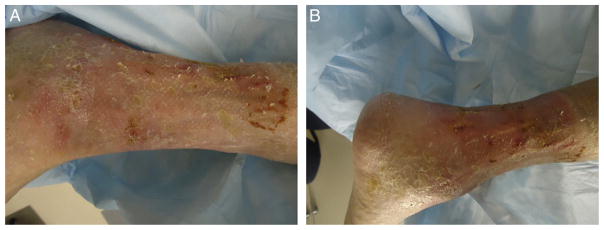
Photograph of leg demonstrating complete healing of the wounds after split-thickness skin grafting and initiation of ruxolitinib therapy.
Discussion
PV is a chronic myeloproliferative disorder characterized by an increased red blood cell mass with a low erythropoietin level and the presence of the JAK-2 mutation (V617F) in 92% of cases (8–10).
Hydroxyurea is an antimetabolite used in the treatment of PV that impairs DNA repair by inhibiting ribonucleotide reductase. It has been recommended for PV therapy in conjunction with phlebotomy for patients who are older than 60 years and those with previous thrombosis. Hydroxyurea is usually fairly well tolerated but is known to be associated with a variety of dermatologic side effects, including alopecia, diffuse hyperpigmentation, erythema, skin atrophy, an amyopathic dermatomyositis presentation (11), nail changes, poikilodermatous dermatitis (11,12), and resistant leg ulcers (1–3,13–15). Leg ulceration occurs in approximately 9% of patients receiving hydroxyurea in the setting of myeloproliferative syndromes and in approximately 29% of patients taking hydroxyurea for management of sickle cell anemia (1). This complication seems to be dose dependent, with studies reporting an association with a mean cumulative exposure to hydroxyurea of 3.2 (range 1.4 to 5.5) kg and mean duration of hydroxyurea treatment of 6.1 (range 2 to 15) years. Biopsy specimens have shown nonspecific changes, with 1 series reporting epidermal atrophy, dermal fibrosis, and occasional fibrin occlusive vasculopathy similar to that seen in livedoid vasculitis (2). Although it has been postulated that the pathogenesis of leg ulcers relates in part to the underlying prothrombotic state (4,16–19), to date, the only effective therapy for these ulcers has been withdrawal of hydroxyurea (2,13,14,20); however, the underlying hematologic state often precludes this.
The JAK signal transducer and activator of transcription pathway is a final common pathway of signal transduction for various cytokines and growth factors, including erythropoietin, thrombopoietin, granulocyte macrophage colony-stimulating factor, granulocyte colony-stimulating factor, and interleukin-3, -5, and -6 (10). Under normal conditions, JAK-2 becomes activated in response to growth factor or cytokine binding; however, in myelodysplastic syndromes such as PV, the acquired gain of function mutations in JAK-2 result in constitutive activation in the absence of any ligand (8,21).
Ruxolitinib is an oral selective inhibitor of JAK-1 and -2 recently approved by the U.S. Food and Drug Administration for the treatment of intermediate- and high-risk myelofibrosis (5–7). Patients with leg ulcers were excluded from the original studies of ruxolitinib to treat myelodysplastic disease because of concerns about infection. However, we report the use of ruxolitinib in a patient with resistant PV-associated leg ulceration. The impressive wound healing seen in the present case merits additional investigation and suggests a possible role for JAK inhibition in patients with chronic leg ulceration associated with myelofibrotic disorders.
Acknowledgments
Dr. Shanmugam and Mr. McNish are currently supported by awards KL2RR031974 and UL1TR000101 (previously UL1RR031975) from the National Center for Advancing Translational Sciences, National Institutes of Health, through the Clinical and Translational Science Awards Program, and by award R01NR013888 from the National Institute of Nursing Research. Dr. Shara is currently supported by award UL1TR000101 from the National Center for Advancing Translational Sciences. Dr. Attinger is supported by award R01NR013888 from the National Institute of Nursing Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Financial Disclosure: None reported.
Conflict of Interest: None reported.
References
- 1.Chaine B, Neonato M, Girot R, Aractingi S. Cutaneous adverse reactions to hydroxyurea in patients with sickle cell disease. Arch Dermatol. 2001;137:467–470. [PubMed] [Google Scholar]
- 2.Sirieix M, Debure C, Baudot N, Dubertret L, Roux M, Morel P, Frances C, Loubeyres S, Beylot C, Lambert D, Humbert P, Gauthier O, Dandurand M, Guillot B, Vaillant L, Lorette G, Bonnetblanc JM, Lok C, Denoeux JP. Leg ulcers and hydroxyurea: forty-one cases. Arch Dermatol. 1999;135:818–820. doi: 10.1001/archderm.135.7.818. [DOI] [PubMed] [Google Scholar]
- 3.Kato N, Kimura K, Yasukawa K, Yoshida K. Hydroxyurea-related leg ulcers in a patient with chronic myelogenous leukemia: a case report and review of the literature. J Dermatol. 1999;26:56–62. doi: 10.1111/j.1346-8138.1999.tb03510.x. [DOI] [PubMed] [Google Scholar]
- 4.Kikuchi K, Arita K, Tateishi Y, Onozawa M, Akiyama M, Shimizu H. Recurrence of hydroxyurea-induced leg ulcers after discontinuation of treatment. Acta Derm Venereol. 2010;91:373–374. doi: 10.2340/00015555-1048. [DOI] [PubMed] [Google Scholar]
- 5.Harrison C, Kiladjian J-J, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, McQuitty M, Hunter DS, Levy R, Knoops L, Cervantes F, Vannucchi AM, Barbui T, Barosi GJAK. Inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 6.Tefferi A. Polycythemia vera and essential thrombocythemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol. 2012;87:284–293. doi: 10.1002/ajh.23135. [DOI] [PubMed] [Google Scholar]
- 7.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, Catalano JV, Deininger M, Miller C, Silver RT, Talpaz M, Winton EF, Harvey JH, Jr, Arcasoy MO, Hexner E, Lyon RM, Paquette R, Raza A, Vaddi K, Erickson-Viitanen S, Koumenis IL, Sun W, Sandor V, Kantarjian HM. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355:2452–2466. doi: 10.1056/NEJMra063728. [DOI] [PubMed] [Google Scholar]
- 9.Spivak JL. Polycythemia vera, the hematocrit, and blood-volume physiology. N Engl J Med. 2013;368:76–78. doi: 10.1056/NEJMe1213283. [DOI] [PubMed] [Google Scholar]
- 10.Spivak JL. Narrative review: thrombocytosis, polycythemia vera, and JAK2 mutations: the phenotypic mimicry of chronic myeloproliferation. Ann Intern Med. 2010;152:300–306. doi: 10.7326/0003-4819-152-5-201003020-00008. [DOI] [PubMed] [Google Scholar]
- 11.Suehiro M, Kishimoto S, Wakabayashi T, Ikeuchi A, Miyake H, Takenaka H, Okano A, Hirai H, Shimazaki C, Yasuno H. Hydroxyurea dermopathy with a dermatomyositis-like eruption and a large leg ulcer. Br J Dermatol. 1998;139:748–749. doi: 10.1046/j.1365-2133.1998.02486.x. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy B, Smith L, Goltz R. Skin changes secondary to hydroxyurea therapy. Arch Dermatol. 1975;111:183–187. [PubMed] [Google Scholar]
- 13.Best PJ, Daoud MS, Pittelkow MR, Petitt RM. Hydroxyurea-induced leg ulceration in 14 patients. Ann Intern Med. 1998;128:29–32. doi: 10.7326/0003-4819-128-1-199801010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Daoud MS, Gibson LE, Pittelkow MR. Hydroxyurea dermopathy: a unique lichenoid eruption complicating long-term therapy with hydroxyurea. J Am Acad Dermatol. 1997;36:178–182. doi: 10.1016/s0190-9622(97)70276-7. [DOI] [PubMed] [Google Scholar]
- 15.Saravu K, Velappan P, Lakshmi N, Shastry BA, Thomas J. Hydroxyurea induced perimalleolar ulcers. J Korean Med Sci. 2006;21:177–179. doi: 10.3346/jkms.2006.21.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stahl RL, Silber R. Vasculitic leg ulcers in chronic myelogenous leukemia. Am J Med. 1985;78:869–872. doi: 10.1016/0002-9343(85)90297-9. [DOI] [PubMed] [Google Scholar]
- 17.Weinlich G, Fritsch P. Leg ulcers in patients treated with hydroxyurea for myeloproliferative disorders: what is the trigger? Br J Dermatol. 1999;141:171–172. doi: 10.1046/j.1365-2133.1999.02956.x. [DOI] [PubMed] [Google Scholar]
- 18.Wirth K, Schoepf E, Mertelsmann R, Lindemann A. Leg ulceration with associated thrombocytosis: healing of ulceration associated with treatment of the raised platelet count. Br J Dermatol. 1998;138:533–535. doi: 10.1046/j.1365-2133.1998.02141.x. [DOI] [PubMed] [Google Scholar]
- 19.Bader U, Banyai M, Boni R, Burg G, Hafner J. Leg ulcers in patients with myeloproliferative disorders: disease- or treatment-related. Dermatology. 2000;200:45–48. doi: 10.1159/000018315. [DOI] [PubMed] [Google Scholar]
- 20.Weinlich G, Schuler G, Greil R, Kofler H, Fritsch P. Leg ulcers associated with long-term hydroxyurea therapy. J Am Acad Dermatol. 1998;39:372–374. doi: 10.1016/s0190-9622(98)70394-9. [DOI] [PubMed] [Google Scholar]
- 21.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR. Cancer Genome Project. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]