Abstract
Staphylococcus aureus community-acquired pneumonia is often associated with influenza or an influenza-like syndrome. Morbidity and mortality due to methicillin-resistant S. aureus (MRSA) or influenza and pneumonia, which includes bacterial co-infection, are among the top causes of death by infectious diseases in the United States. We developed a non-lethal influenza A virus (IAV) (H3N2)/S. aureus co-infection model in cynomolgus macaques (Macaca fascicularis) to test the hypothesis that seasonal IAV infection predisposes non-human primates to severe S. aureus pneumonia. Infection and disease progression were monitored by clinical assessment of animal health; analysis of blood chemistry, nasal swabs, and X-rays; and gross pathology and histopathology of lungs from infected animals. Seasonal IAV infection in healthy cynomolgus macaques caused mild pneumonia, but unexpectedly, did not predispose these animals to subsequent severe infection with the community-associated MRSA clone USA300. We conclude that in our co-infection model, seasonal IAV infection alone is not sufficient to promote severe S. aureus pneumonia in otherwise healthy non-human primates. The implication of these findings is that comorbidity factors in addition to IAV infection are required to predispose individuals to secondary S. aureus pneumonia.
Keywords: Staphylococcus aureus, influenza a virus, coinfection, USA300, MRSA, pneumonia
Introduction
Influenza and pneumonia, which includes bacterial co-infection or secondary infection, was ranked as the eighth leading cause of death in the United States in 2009.1 Mortality caused by influenza alone is surprisingly low—2808 deaths attributed to influenza alone in 2009 vs. 50 774 deaths attributed to pneumonia.1 Consistent with these data, it is now well accepted that bacterial pneumonia is a primary cause of death during influenza A virus (IAV) epidemics.2-7 For example, Staphylococcus aureus pneumonia was associated with increased mortality during IAV epidemics that occurred in 1918, 1940–1941, 1957, and 1968–1969.3-6 In one IAV outbreak at Camp Jackson, SC, in September 1918, S. aureus was the most abundant bacterial species cultured from lungs postmortem, and was present in 49 percent (153) of fatal cases.6 More recently, there has been an increase in the incidence of S. aureus community-acquired pneumonia in the United States, and in many cases influenza or an influenza-like illness is associated with this syndrome.8-10 The majority of S. aureus isolates from these co-infections have been identified as pulsed-field type USA300, a community-associated methicillin-resistant S. aureus (CA-MRSA) strain that has been epidemic in the United States.11-13 Although progress has been made, the molecular determinants of S. aureus community-acquired pneumonia remain incompletely determined. This is in part due to lack of an appropriate animal infection model that closely approximates human disease, especially for co-infection studies.
Previous studies have demonstrated that the pathology and pathogenesis of influenza viruses are appropriately modeled using non-human primates.14 In particular, the study of 1918 IAV infection has been invaluable in identifying the underlying viral and immunological basis for the severe disease caused by the 1918 virus in primates.15 Since emerging influenza viruses with pandemic potential are of high public health concern, evaluation of viruses posing an immediate threat to humans and enhanced understanding of the pathogenic potential and mechanisms leading to serious illness in humans, which includes S. aureus co-infection, are of high priority. Furthermore, the high prevalence of CA-MRSA and IAV infections make co-infection a serious potential public health concern in the United States. Understanding pathogenesis of influenza/S. aureus co-infection in the macaque model is an important step toward understanding this disease process in humans. To that end, we developed a non-lethal model of seasonal H3N2 IAV/USA300 co-infection in cynomolgus macaques to test the hypothesis that IAV infection promotes severe S. aureus respiratory tract infection.
Results
Nonhuman primate infection model of seasonal IAV and S. aureus pneumonia
To test the hypothesis that antecedent IAV infection is a predisposing factor to severe disease caused by USA300, we developed a non-lethal co-infection model of pneumonia in cynomolgus macaques (Macaca fascicularis). The parameters used for this co-infection study are based in part on a recent non-human primate (NHP) infection model that we established to evaluate USA300 pathogenesis and determine the role of Panton–Valentine leukocidin (PVL) in lower respiratory tract infection without antecedent IAV infection.16 In that study, 12 adult cynomolgus macaques were administered 106 USA300 wild-type or isogenic lukS/F-PV deletion (Δpvl) strains via bronschoscopic instillation into the right middle lobe of the lung and pathogenesis assessed 2, 4, and 8 d following infection. Notably, all 12 monkeys developed mild S. aureus pneumonia, and pathology was irrespective of PVL.16 In the present study, 12 adult animals—all negative for IAV-specific antibodies—were randomly assigned to three groups of four animals and each animal was infected with a total dose of 7 × 106 PFUs of seasonal IAV via inoculation by four routes: intratracheal, nasal, oral, and ocular (Table 1). On day 5 post-IAV infection, two NHP groups received 106 CFUs of USA300 wild-type or isogenic lukS/F-PV deletion (Δpvl) strains via intrabronchiole instillation, and the remaining group received DPBS alone (Fig. 1). The relatively low inoculum of S. aureus was chosen to approximate CFUs present in aspirated saliva.17 Although our previous study failed to determine a role for PVL in S. aureus lower respiratory tract infection in NHPs, contribution of the toxin to disease was not tested in the context of co-infection. The times for co-infection with MRSA and necropsy were selected for specific purposes. Previous studies have shown there is replication of H3N2 IAV in cynomolgus macaques 3–5 d postinfection.18 We chose 4 and 8 d post-MRSA infection (9 and 13 d post-IAV) for necropsy because we recently established these time-points as appropriate for establishing MRSA pneumonia in cynomolgus macaques, and these animals serve as important historic controls for the present study.16 In addition, the timing of necropsy chosen for our model coincided with the two highest mortality periods for S. aureus pneumonia (days 6–10 and 11–15 after initial symptoms of IAV infection) reported for an IAV outbreak in 1918 at Camp Jackson, SC.6 At necropsy, the right lung of each NHP was fixed to preserve the integrity of fine structure for histopathology analysis. The left lung (contralateral to bacterial inoculation) was sectioned and used to detect virus and bacteria.
Table 1. Cynomolgus macaques used in the study.
| Animal ID | Age (y) | Weight (kg) | Gender | Necropsy | Nasal col. | Infection group |
|---|---|---|---|---|---|---|
| 82–150 | 8.5 | 5.12 | F | Day +9 | Y (CC30) | H3N2 + PBS |
| L971 | 18 | 4.00 | F | Day +9 | Y (Unk) | H3N2 + PBS |
| RML639 | 8 | 4.28 | F | Day +13 | Y (CC30) | H3N2 + PBS |
| RML615 | 13.5 | 4.70 | F | Day +13 | Y (CC30) | H3N2 + PBS |
| 121–236 | 9 | 4.28 | F | Day +9 | Y (Unk) | H3N2 + USA300 |
| RML622 | 12.5 | 4.34 | F | Day +9 | Y (CC30) | H3N2 + USA300 |
| RML636 | 8.5 | 4.20 | F | Day +13 | Y (CC30) | H3N2 + USA300 |
| RML117 | 13 | 7.42 | M | Day +13 | Y (Unk) | H3N2 + USA300 |
| RML683 | 9.5 | 4.58 | F | Day +9 | Y (CC30) | H3N2 + USA300Δpvl |
| RML600 | 16 | 4.96 | F | Day +9 | Y (CC30) | H3N2 + USA300Δpvl |
| 62–249 | 9 | 8.48 | M | Day +13 | Y (CC30) | H3N2 + USA300Δpvl |
| RML602 | 16 | 7.04 | F | Day +13 | Y (CC30) | H3N2 + USA300Δpvl |
Shading indicates the three infection groups. Y, yes; CC, clonal complex; Unk, unknown.
Figure 1. Experimental design and procedures performed. Twelve cynomolgus macaques were divided into 3 groups of 4 animals as shown (see Table 1 for additional details on animals used). All animals were inoculated with ~7 × 106 PFUs of H3N2 IAV by intratracheal, intranasal, oral, and ocular routes on day (D) 0 as described in Methods. Five days after infection with IAV (D +5), groups of 4 animals each were inoculated with 106 CFUs of the indicated USA300 strains or PBS by intrabrochiole instillation. Two animals from each group were euthanized 9 and 13 d after infection with influenza virus (4 and 8 d after bacterial co-infection) as described in Methods. Procedures used to monitor animal health and disease, which included collection of blood and nasal swabs, and taking chest X-rays (blood, X-ray, nasal swab), were performed on the indicated days. The circles represent individual monkeys. The shaded left half of the circle indicates that the monkeys received H3N2 IAV. The right half indicates that the animals received PBS (unshaded), USA300 (red), or USA300Δpvl (pink).
Pathogenesis of experimental IAV and S. aureus co-infection in NHPs
All NHPs were observed in person several times daily by trained veterinary personnel who were blinded to the experimental treatment groups to monitor clinical signs indicative of disease progression according to an established protocol.19 Consumption of feed and water, and condition of feces was noted daily. In addition, each animal received a complete clinical examination under general anesthesia prior to inoculation of IAV and on days 2, 5, 9, 11, and 13 following IAV infection. The physical examination included measurement of body temperature, weight, blood pressure, heart rate, and blood oxygen saturation. Blood was obtained to determine both cellular composition and chemistry and the respiratory system was monitored by auscultation of lung sounds and X-ray analysis (Tables S1 and S2).
Analysis of the clinical data collected during the postinoculation observation period revealed evidence of a mild lower respiratory tract infection in the majority of NHPs. Clinical symptoms included slight elevations in body temperature and respiration rate, infrequent coughing and sneezing, abnormal feces, decreased food and water intake, and crackling lung sounds by auscultation. Consistent with these findings, analysis of the chest radiographs showed either indiscernible or subtle changes in lung fine structure that were suggestive of mild or subclinical disease (Fig. S1). The majority of NHPs showed mild increases in neutrophil counts following infection with both IAV and S. aureus (Table S1), and blood analytes tested remained relatively unchanged (Table S2). Inasmuch as the clinical features of IAV and S. aureus infection were mild, no clinically apparent differences were observed between NHP treatment groups. These findings are consistent with a recent report by Chertow and Memoli in which the authors conclude that the clinical presentation of human cases of bacterial co-infection with influenza A (H1N1) is similar to influenza A occurring alone.20
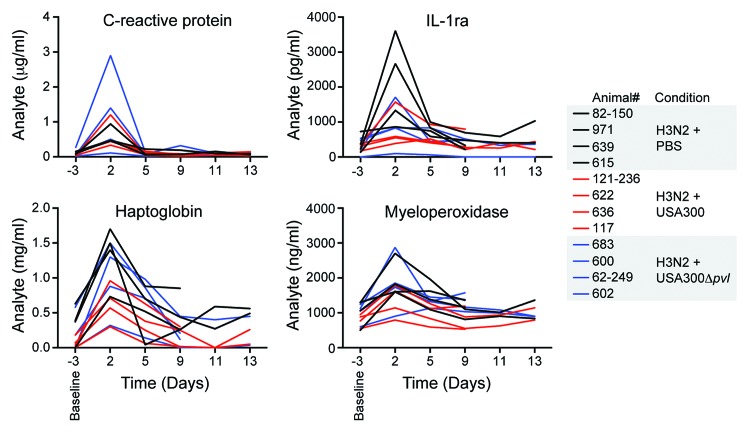
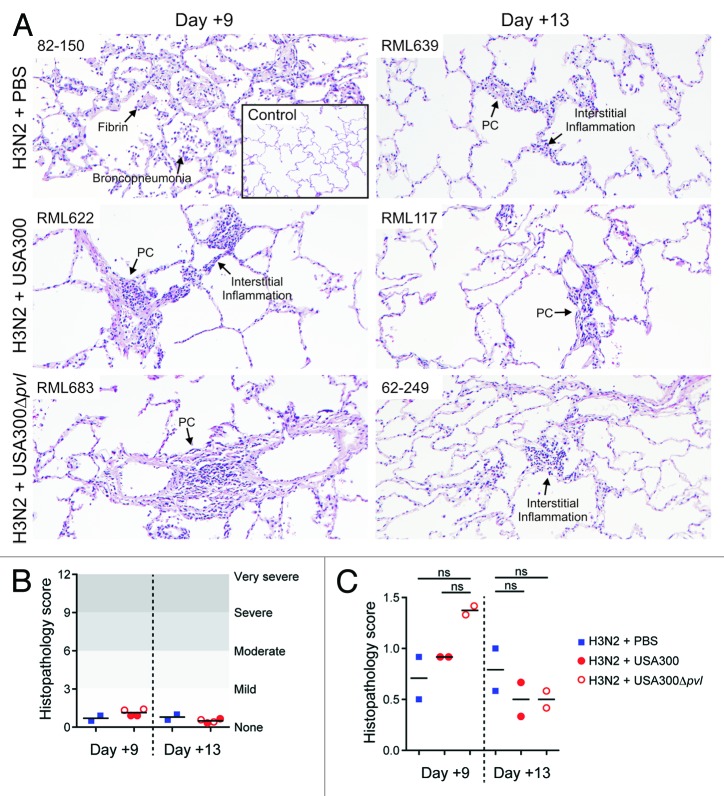
To further assess the effects of MRSA on IAV-infected NHPs, we conducted visual inspection of the lungs excised at necropsy and noted gross morphological characteristics of the thoracic cavity (Fig. S2). In general, the lungs appeared relatively healthy by manual examination with the exception of small areas of mild discoloration without consolidation. All NHPs showed evidence of mild to moderately enlarged hilar lymph nodes. Microbiological analysis of the left lung at the time of necropsy demonstrated that IAV RNA was detectable in all NHPs and verified that the animals were infected successfully (Table 2). Consistent with these findings, the majority of NHPs had increased serum levels of C-reactive protein, haptoglobin, interleukin-1 receptor antagonist (IL-1ra), and myeloperoxidase following IAV infection (Fig. 2). By contrast, we were unable to recover S. aureus from the contralateral lung following necropsy, indicating that bacteria were either cleared, contained in discrete foci, or failed to disseminate widely following inoculation. To further characterize the pathogenesis of seasonal IAV and CA-MRSA coinfection in NHPs, we next performed detailed microscopic examination of lung tissue obtained at necropsy (Fig. 3). Consistent with the clinical findings of a mild lower respiratory tract infection, analysis of histopathological sections from all monkeys showed features of mild multifocal, multilobar, interstitial pneumonia. In most cases there were foci of a minimal to mild increase in cellularity in the alveolar walls and less frequent evidence of alveolar wall thickening. Virtually all NHPs showed evidence of mild acute pneumonia predominantly characterized by slight increases in neutrophils and necrotic debris in the air space with little evidence of consolidation. Furthermore, examination of lung tissue showed evidence of perivascular lymphocyte cuffing in all NHPs. Overall, the mean histopathology scores for all NHPs were consistent with mild disease (Fig. 3B). In addition, we observed no statistically significant differences between NHP treatment groups (Fig. 3C).
Table 2. Detection of influenza A virus RNA in lung and upper respiratory track tissues by PCR.
| Animal ID | Upper lung | Middle lung | Lower lung | Bronch LN | Trachea | Necropsy | Infection group |
|---|---|---|---|---|---|---|---|
| 82–150 | −/− | +/− | +/+ | +/+ | −/− | Day +9 | H3N2 + PBS |
| L971 | −/− | −/− | +/+ | +/+ | −/− | Day +9 | H3N2 + PBS |
| RML639 | −/− | −/− | −/+ | +/− | −/− | Day +13 | H3N2 + PBS |
| RML615 | −/− | +/− | −/− | +/+ | −/− | Day +13 | H3N2 + PBS |
| 121–236 | −/− | +/+ | +/+ | +/+ | −/− | Day +9 | H3N2 + USA300 |
| RML622 | −/− | −/− | +/+ | +/+ | −/− | Day +9 | H3N2 + USA300 |
| RML636 | −/− | −/− | +/− | +/+ | −/− | Day +13 | H3N2 + USA300 |
| RML117 | −/− | −/− | −/− | −/+ | −/− | Day +13 | H3N2 + USA300 |
| RML683 | −/− | +/− | +/+ | +/+ | −/− | Day +9 | H3N2 + USA300Δpvl |
| RML600 | −/− | −/− | +/+ | +/+ | −/− | Day +9 | H3N2 + USA300Δpvl |
| 62–249 | −/− | −/− | −/− | +/+ | −/− | Day +13 | H3N2 + USA300Δpvl |
| RML602 | +/+ | −/− | +/+ | −/− | −/+ | Day +13 | H3N2 + USA300Δpvl |
Influenza A virus RNA was detected by PCR as described in Methods. Data from two separate PCR runs were scored as positive (+) or negative (−) (each run is separated by a forward slash). Bronch LN, bronchial lymph node.
Figure 2. Inflammatory markers and acute phase reactants in sera of infected cynomolgus macaques. Serum was isolated from NHP blood prior to infection (Day −3, baseline) and 2, 5, 9, 11, and 13 d following IAV inoculation. NHPs receiving S. aureus (USA300 and USA300Δpvl) were inoculated on day 5. Sera from individual animals were analyzed for C-reactive protein, IL-1ra, haptoglobin, and myeloperoxidase as described in the Methods.
Figure 3. Histopathology analysis of lungs from infected animals. Infected animals were euthanized 9 or 13 d after infection with seasonal H3N2 IAV as indicated. The right lung of each animal was insufflated with formalin and fixed for at least 7 d prior to processing for histopathology as described in Methods. (A) Images are from a representative H and E stained section of lung tissue in one animal from each group as indicated. Selected features are indicated. PC, perivascular cuffing. (B and C) Sections of the right lung of each animal were scored by histopathology analysis as described in Methods. Lines indicated the mean score of the two cynomolgus macaques for each condition and time point. ns, not statistically significant (P > 0.05, one-way ANOVA with the Tukey post-test). See Figure 1 for details of the experimental set-up.
Discussion
The incidence of S. aureus colonization among healthy (non-hospitalized) individuals (~28%),21 coupled with the overall burden of CA-MRSA skin and soft tissue infections and seasonal IAV infections, presents an opportunity for increased morbidity and mortality as a result of co-infection. Indeed, studies from the late 1950s first linked antecedent S. aureus skin infections to secondary S. aureus pneumonia following influenza.22 Despite the seemingly favorable conditions for co-infection in recent times, and although there has been an increase in the proportion of S. aureus co-infection in children,10 MRSA community-acquired pneumonia remains relatively uncommon in the United States (~2% prevalence).23-25 These observations seem at variance with the high prevalence of S. aureus pneumonia that occurred during historic IAV epidemics,3-6 but are consistent with our finding that IAV/USA300 co-infection in cynomolgus macaques caused very mild disease. That said, we were surprised by our findings in NHPs, because numerous studies using rodent models have demonstrated that antecedent IAV infection causes a significant increase in morbidity and mortality following secondary S. aureus respiratory tract infection.26-29 There are several possible explanations for the noted differences.
First, we used a human-adapted seasonal H3N2 IAV that alone produced limited disease and pathology in NHPs, whereas previous S. aureus co-infection studies in mice have typically used H1N1 strains—sometimes mouse-adapted—and high inocula (e.g., 5 × 104 PFUs/mouse) to produce significant pathology or fatal disease.26,30 The inoculum used in our current study (7 × 106 PFUs/animal or ~1.32 × 103 PFUs/g based on an average weight of 5.3 kg for the 12 animals) is similar to published high doses used in mice (e.g., ~1.67 × 103 PFUs/g in the work by Lee et al.26 based on an estimated mouse weight of 30 g). Thus, although the inocula are comparable, differences in IAV strains used could account, in part, for the differences in outcome between NHP and rodent co-infection models. It is noteworthy that F. Macfarlane Burnet reported the ability of high titer IAV to cause fatal primary infection of cynomolgus monkeys, and he further suggested that the strains used had varied capacity to cause disease.31
Previous work has also demonstrated that the timing of antecedent IAV infection is critical for the development of severe or fatal bacterial pneumonia in animal infection models.28,29,32,33 For example, Iverson et al. found that the greatest mortality in S. aureus co-infected mice occurred 3 and 7 d after IAV infection.28 This timing corresponds well with our model (co-infection with USA300 5 d after infection with IAV). In addition, Rimmelzwaan et al. reported replication of H3N2 IAV in cynomolgus macaques 3–6 d after intratracheal inoculation,18 which is primarily the reason we chose day 5 post-IAV inoculation for S. aureus co-infection in our study. Chickering et al. found that the greatest number of human fatalities resulting from IAV/S. aureus co-infection during an IAV outbreak in 1918 occurred 6–15 d after the onset of disease.6 The endpoints in our NHP model (8 and 13 d after IAV infection) correspond well with these historic data. Taken together, it seems unlikely that the timing of co-infection in our model explains the observed mild disease and/or differences in severity between rodent and monkey infection models.
Although our cynomolgus macaque model of co-infection differs from those using rodents in many ways, it has many features that mimic humans and/or human infections. For example, all of the NHPs used in our studies were colonized in the anterior nares with S. aureus, and 50–60% of humans are either permanently or intermittently colonized with S. aureus.21,34,35 Importantly, S. aureus is a natural and prominent cause of secondary respiratory infections in monkeys.36 The inoculum of S. aureus used in our model (106 CFUs) is less than that typically used for similar studies in rodents,26,28 but is more similar to a challenge dose that might be expected during multiple saliva aspirations.17 Using this inoculum, Olsen et al. found that lower respiratory tract infection of cynomolgus monkeys with USA300 causes a mild pneumonia that has clinical features of early mild pneumonia in humans.16
Panton–Valentine leukocidin (PVL, encoded by lukSF-PV) is a S. aureus secreted toxin often present in isolates recovered from patients with severe community-acquired necrotizing pneumonia.37,38 In our previous studies of S. aureus lower respiratory tract infection in cynomolgus monkeys (Olsen et al.), we found that disease severity was similar in monkeys infected with USA300 wild-type or isogenic lukSF-PV-negative strains.16 Notably, patients with severe S. aureus community-acquired necrotizing pneumonia frequently have antecedent influenza or an influenza-like illness.38 Therefore, it is possible that antecedent IAV infection facilitates involvement of PVL in respiratory tract infection caused by S. aureus, a notion tested in our studies. However, we found no difference in disease severity or progression in monkeys infected with USA300 wild-type or isogenic lukSF-PV-negative strains (Fig. 3; Figs. S1 and S2), a finding consistent with previous co-infection studies in a mouse model.26 Another possible confounding factor in these studies is the susceptibility of host cells to PVL. Compared with human granulocytes—a known PVL target cell—those isolated from mice and monkeys are relatively insensitive to cytolysis by PVL in vitro,39 and such resistance might explain the negative results from murine and monkey co-infection models. On the other hand, cytolysis in vitro may have limited bearing on the contribution of PVL during S. aureus infection in vivo. This notion is supported by recent studies indicating PVL functions as an immune modulator and priming agent in vitro and in vivo.40-42 For example, at sublytic concentrations, PVL triggers release of proinflammatory molecules from mouse and human leukocytes, and human lung epithelial cells,42 and primes for enhanced killing of S. aureus by human neutrophils.41 Mice inoculated with isogenic lukSF-PV-negative S. aureus strains in the lungs have decreased survival compared with those infected with wild-type strains, a phenomenon that can be explained by PVL-mediated immune priming.40,42 In addition, Malachowa et al. recently reported that highly purified PVL elicits an inflammatory response in monkey skin, an observation indicating cells of cynomolgus monkeys respond to PVL.43 Whether species-specificity is an issue for the animal infection model here remains unknown, but the idea that antecedent IAV accentuates or promotes involvement of PVL in severe S. aureus necrotizing pneumonia merits further investigation.
Collectively, data obtained from our infection model indicate that seasonal H3N2 IAV infection alone is insufficient to promote severe secondary S. aureus pneumonia in otherwise healthy NHPs. One caveat of NHP studies in general is that only a limited number of animals can be used for such studies (e.g., we used 4 animals infected with IAV alone and 8 animals infected with IAV + S. aureus). Although the number of animals used in our studies is typical of that used for NHP infection models, it is possible our sample size was not large enough to reveal an ability of IAV to predispose animals to S. aureus pneumonia. Nevertheless, a potential broad scope implication of these findings is that underlying risk factors—other than simply the combination of IAV and S. aureus—are necessary to promote S. aureus pneumonia. Co-morbidities such as immunosuppression were epidemiologically linked to morbidity and mortality of children and adults co-infected with S. aureus during the recent H1N1 epidemic.44,45 Inasmuch as the strain of virus might also be important in this context, it will be important to test IAV strains that cause more than mild disease in NHPs.
Materials and Methods
Bacterial strains and culture
USA300 wild-type and isogenic lukS/F-PV deletion mutant strains (LAC and LACΔpvl; labeled USA300 and USA300Δpvl herein) were described previously.46 Bacteria were cultured overnight from frozen stock in trypticase soy broth (TSB; Becton Dickinson) at 37 °C with shaking (250 rpm), diluted 1:200 in fresh TSB the following morning, and then cultured to mid exponential phase of growth (optical density [OD]600 = 0.75). To prepare inocula, bacteria were washed in Dulbecco’s phosphate-buffered saline (DPBS) and suspended in DPBS at 1 × 106 colony-forming units (CFUs)/mL. CFUs were confirmed by quantitative culture. S. aureus strains were isolated from nasopharyngeal swabs of NHPs prior to initiation of experiments and were analyzed by spa typing.47 Quantitative bacterial culture from infected tissue was performed as described previously.16
Influenza A virus (IAV) culture
A/NewYork/470/2004 (H3N2; NY470) IAV was passaged in MDCK cells (ATCC CCL-34) in Dulbecco’s modified Eagle’s medium (DMEM; Quality Biological) containing 1 μg/mL TPCK-Trypsin (Sigma-Aldrich), as described.48 Viral titers were determined by plaque assay and were expressed as plaque-forming units per milliliter (PFU/mL) using standard protocols.49 Back titration of the inoculum was also performed using 50% tissue culture infectious dose (TCID50) as described.50
Non-human primate (NHP) co-infection studies
All experimental animal studies conformed to the guidelines set forth by the National Institutes of Health and were reviewed, approved, and supervised by the institutional animal care and use committee at Rocky Mountain Laboratories (RML), National Institute of Allergy and Infectious Diseases (protocol 2009–34). RML is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The animals were housed according to regulations and recommendations detailed in the Animal Welfare Act, Public Health Service and National Institutes of Health Animal Care and Use Policies, DHHS Guide for the Care and Use of Laboratory Animals. The animals were individually housed in standard nonhuman primate caging (Primate Products) and fed a commercial nonhuman primate diet (Purina 4047 high protein jumbo biscuits, PMI Nutrition International) twice daily. The diet was supplemented with fruit at each feeding. The room was maintained on a 12-h light/dark cycle with lights on at 6:00 AM. Environmental enrichment for the macaques was achieved by use of treats, fresh fruits and vegetables, and toys. All NHP experimental procedures were performed under ketamine anesthesia (10 mg/kg intramuscular), and all efforts were made to minimize suffering. All animals were euthanized by exsanguination via intracardiac needle following induction of deep anesthesia (10 mg/kg ketamine intramuscular, followed by 25 mg/kg ketamine intravenous).
Two male and 10 female cynomolgus macaques (Macaca fasicularis; Rocky Mountain Laboratories colony) age 8 to 16 y and weight 4 to 8 kg, were assigned to three groups of four animals (Table 1). All NHP used in this study tested negative for the presence of influenza virus-specific antibodies by ELISA prior to experimental infection (Influenza A Virus NP Antibody Inhibition Test, Virusys Corporation). All three groups of animals were experimentally infected with 7 × 106 plaque forming units (PFU) of H3N2 IAV by intratracheal, intranasal, oral, and ocular routes as described previously.50 All 12 animals were anesthetized on day 5 postinfluenza infection and two groups received 1 × 106 colony-forming units of USA300 wild-type or Δpvl strains by intrabronchiole instillation to the right lung,16 and the control group received 2 ml DPBS. Infection and disease progression were monitored daily by visual assessment of animal health as described previously.50 Animals were examined under anesthesia on days −3, 0, 2, 5, 9, 11, and 13 post IAV-infection, at which time vital signs (blood pressure, pulse rate, respiration rate, and temperature) and X-rays were taken, and a blood sample was acquired from each animal (Fig. 1). Two animals in each group were euthanized on days 4 and 8 post-MRSA co-infection.
Radiography
A mobile digital radiography unit with a flat panel digital detector (Sound Technologies tru/Dr®) was utilized for obtaining thoracic radiographs. The unit is equipped with a portable X-ray generator (Poskom® model PXP-HF). The system operates on a veterinary specific software system (Vetpacs®). The animals were positioned for ventrodorsal radiography by using a Lexan® v-shaped thoracic positioner. Thoracic radiographs were acquired under ketamine anesthesia and the digital images were interpreted as described previously.19
Gross and microscopic pathology analyses
Tissues were collected and lungs were assessed for discoloration and evidence of inflammation and were weighed. Lung pathology was scored at necropsy in a semiquantitative fashion as previously described.16 After weighing, the right lung was insufflated with 10% phosphate-buffered formalin and fixed for at least 7 d prior to standard histological processing. For the upper and middle lung lobes, the pleura was removed by sharp dissection and the entirety of the parenchyma was processed as a single block. For the lower lung lobe, the pleura was removed by sharp dissection and the lung was bisected (blade cut from superior/cephalad to inferior/caudal) to create medial and lateral portions. Although some minor trimming of corners and margins was required to fit each tissue into one cassette, each section represents the entirety of the lung lobe. Five micron thick, paraffin-embedded tissue sections were stained with hematoxylin and eosin on an automated tissue stainer. Stained slides were examined on an Olympus BX51 microscope. Lung histopathology was assessed by a veterinary pathologist who was blinded to the strain treatment groups and sacrifice time. A mean lung microscopic pathology score was calculated for the right lobe based on three criteria, each semiquantitatively scored on a scale of 0–4 per slide, including: (1) severity of bronchopneumonia; (2) severity of interstitial pneumonia; and (3) proportion of the arteries with perivascular lymphocyte cuffing.
Virus detection
Tissue samples were placed in RNAlater (Ambion) for subsequent RNA extraction by RNAeasy kit (QIAGEN). Real-time RT-PCR was performed as previously described50 with the following primer/probe sequences: NPforward: GCCATAAGGA CCAGAAGTGG; NPreverse: TCTGCATTGT CTCCGAAGAA ATA; NPprobe: 6FAM-TTTCGTCCGA GAGCTCGAAG ACTCC-BBQ. RT-PCR was used to pre-screen swabs, blood, and organ samples for the presence of IAV RNA.51
Quantitative bacterial culture and multiplexed immunoassays, hematology, and blood chemistry
For quantitative bacterial culture of the left lung (contralateral to the side of inoculation), five representative sections of lung tissue (one from the upper lobe and two each from the middle and lower lobes), each measuring approximately 0.5 cm were homogenized (Omni, USA Scientific) in 3 ml sterile PBS, weighed, diluted serially, and plated on trypticase soy agar plates supplemented with 5% sheep blood. The plates were incubated for 24 h at 37 °C. Quantitative multiplexed immunoassays were performed on NHP serum using the HumanMAP v1.6 immunoassay (Rules Based Medicine). Hematology was performed on EDTA-treated whole blood samples using a Hemavet 950FS (Drew Scientific). Plasma biochemistry was analyzed from heparinized blood using the iSTAT1 blood chemistry analyzer (Abbott Point of Care) with the EC8+ and Crea cartridges.
Statistical analysis
All statistical analyses were performed using GraphPad Prism (GraphPad Software). Semiquantitative histopathology data were assessed using a one-way analysis of variance (AVOVA) and Tukey posttest for correction of multiple comparisons.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Anita Mora (NIAID) for performing photography and preparation of images for publication, and the staff of the Rocky Mountain Veterinary Branch (NIAID) for assistance with basic animal care and procedures. This article was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/26572
References
- 1.Kochanek KD, Kirmeyer SE, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2009. Pediatrics. 2012;129:338–48. doi: 10.1542/peds.2011-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–70. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarzmann SW, Adler JL, Sullivan RJ, Jr., Marine WM. Bacterial pneumonia during the Hong Kong influenza epidemic of 1968-1969. Arch Intern Med. 1971;127:1037–41. doi: 10.1001/archinte.127.6.1037. [DOI] [PubMed] [Google Scholar]
- 4.Robertson L, Caley JP, Moore J. Importance of Staphylococcus aureus in pneumonia in the 1957 epidemic of influenza A. Lancet. 1958;2:233–6. doi: 10.1016/S0140-6736(58)90060-6. [DOI] [PubMed] [Google Scholar]
- 5.Finland M, Peterson OL, Strauss E. Staphylococcic pneumonia occurring during an epidemic of influenza. Arch Intern Med. 1942;70:183–205. doi: 10.1001/archinte.1942.00200200003001. [DOI] [Google Scholar]
- 6.Chickering HT, Park JH. Staphylococcus aureus pneumonia. J Am Med Assoc. 1919;72:617–26. doi: 10.1001/jama.1919.02610090001001. [DOI] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1) - United States, May-August 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1071–4. [PubMed] [Google Scholar]
- 8.Hageman JC, Uyeki TM, Francis JS, Jernigan DB, Wheeler JG, Bridges CB, Barenkamp SJ, Sievert DM, Srinivasan A, Doherty MC, et al. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003-04 influenza season. Emerg Infect Dis. 2006;12:894–9. doi: 10.3201/eid1206.051141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kallen AJ, Brunkard J, Moore Z, Budge P, Arnold KE, Fosheim G, Finelli L, Beekmann SE, Polgreen PM, Gorwitz R, et al. Staphylococcus aureus community-acquired pneumonia during the 2006 to 2007 influenza season. Ann Emerg Med. 2009;53:358–65. doi: 10.1016/j.annemergmed.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 10.Finelli L, Fiore A, Dhara R, Brammer L, Shay DK, Kamimoto L, Fry A, Hageman J, Gorwitz R, Bresee J, et al. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics. 2008;122:805–11. doi: 10.1542/peds.2008-1336. [DOI] [PubMed] [Google Scholar]
- 11.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–9. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 12.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA, EMERGEncy ID Net Study Group Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy AD, Otto M, Braughton KR, Whitney AR, Chen L, Mathema B, Mediavilla JR, Byrne KA, Parkins LD, Tenover FC, et al. Epidemic community-associated methicillin-resistant Staphylococcus aureus: recent clonal expansion and diversification. Proc Natl Acad Sci U S A. 2008;105:1327–32. doi: 10.1073/pnas.0710217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verlinde JD, Makstenieks O. Experimental respiratory infection in monkeys produced by influenza A virus and Staphylococcus aureus. Arch Gesamte Virusforsch. 1954;5:345–60. doi: 10.1007/BF01243004. [DOI] [PubMed] [Google Scholar]
- 15.Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–23. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 16.Olsen RJ, Kobayashi SD, Ayeras AA, Ashraf M, Graves SF, Ragasa W, Humbird T, Greaver JL, Cantu C, Swain JL, et al. Lack of a major role of Staphylococcus aureus Panton-Valentine leukocidin in lower respiratory tract infection in nonhuman primates. Am J Pathol. 2010;176:1346–54. doi: 10.2353/ajpath.2010.090960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohara-Nemoto Y, Haraga H, Kimura S, Nemoto TK. Occurrence of staphylococci in the oral cavities of healthy adults and nasal oral trafficking of the bacteria. J Med Microbiol. 2008;57:95–9. doi: 10.1099/jmm.0.47561-0. [DOI] [PubMed] [Google Scholar]
- 18.Rimmelzwaan GF, Baars M, van Beek R, van Amerongen G, Lövgren-Bengtsson K, Claas EC, Osterhaus AD. Induction of protective immunity against influenza virus in a macaque model: comparison of conventional and iscom vaccines. J Gen Virol. 1997;78:757–65. doi: 10.1099/0022-1317-78-4-757. [DOI] [PubMed] [Google Scholar]
- 19.Brining DL, Mattoon JS, Kercher L, LaCasse RA, Safronetz D, Feldmann H, Parnell MJ. Thoracic radiography as a refinement methodology for the study of H1N1 influenza in cynomologus macaques (Macaca fascicularis) Comp Med. 2010;60:389–95. [PMC free article] [PubMed] [Google Scholar]
- 20.Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. JAMA. 2013;309:275–82. doi: 10.1001/jama.2012.194139. [DOI] [PubMed] [Google Scholar]
- 21.Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, Fosheim GE, Jensen BJ, Killgore G, Tenover FC, Kuehnert MJ. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001-2004. J Infect Dis. 2008;197:1226–34. doi: 10.1086/533494. [DOI] [PubMed] [Google Scholar]
- 22.Goslings WR, Mulder J, Djajadiningrat J, Masurel N. Staphylococcal pneumonia in influenza in relation to antecedent staphylococcal skin infection. Lancet. 1959;2:428–30. doi: 10.1016/S0140-6736(59)90417-9. [DOI] [PubMed] [Google Scholar]
- 23.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, Albrecht V, Limbago B, Talan DA, EMERGEncy ID NET Study Group Prevalence of methicillin-resistant staphylococcus aureus as an etiology of community-acquired pneumonia. Clin Infect Dis. 2012;54:1126–33. doi: 10.1093/cid/cis022. [DOI] [PubMed] [Google Scholar]
- 24.Murray RJ, Robinson JO, White JN, Hughes F, Coombs GW, Pearson JC, Tan HL, Chidlow G, Williams S, Christiansen KJ, et al. Community-acquired pneumonia due to pandemic A(H1N1)2009 influenzavirus and methicillin resistant Staphylococcus aureus co-infection. PLoS One. 2010;5:e8705. doi: 10.1371/journal.pone.0008705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, Harriman K, Harrison LH, Lynfield R, Farley MM, Active Bacterial Core Surveillance Program of the Emerging Infections Program Network Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–44. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 26.Lee MH, Arrecubieta C, Martin FJ, Prince A, Borczuk AC, Lowy FD. A postinfluenza model of Staphylococcus aureus pneumonia. J Infect Dis. 2010;201:508–15. doi: 10.1086/650204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weeks-Gorospe JN, Hurtig HR, Iverson AR, Schuneman MJ, Webby RJ, McCullers JA, Huber VC. Naturally occurring swine influenza A virus PB1-F2 phenotypes that contribute to superinfection with Gram-positive respiratory pathogens. J Virol. 2012;86:9035–43. doi: 10.1128/JVI.00369-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iverson AR, Boyd KL, McAuley JL, Plano LR, Hart ME, McCullers JA. Influenza virus primes mice for pneumonia from Staphylococcus aureus. J Infect Dis. 2011;203:880–8. doi: 10.1093/infdis/jiq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sellers TF, Jr., Schulman J, Bouvier C, McCUNE R, Kilbourne ED. The influence of influenza virus infection on exogenous staphylococcal and endogenous murine bacterial infection of the bronchopulmonary tissues of mice. J Exp Med. 1961;114:237–56. doi: 10.1084/jem.114.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakab GJ, Warr GA, Knight ME. Pulmonary and systemic defenses against challenge with Staphylococcus aureus in mice with pneumonia due to influenza A virus. J Infect Dis. 1979;140:105–8. doi: 10.1093/infdis/140.1.105. [DOI] [PubMed] [Google Scholar]
- 31.Burnet FM. Influenza virus “A” infections of cynomolgus monkeys. Aust J Med Sci Med Biol. 1941;19:281–90. doi: 10.1038/icb.1941.43. [DOI] [Google Scholar]
- 32.McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis. 2002;186:341–50. doi: 10.1086/341462. [DOI] [PubMed] [Google Scholar]
- 33.Janssen RJ, Chappell WA, Gerone PJ. Synergistic activity between Pr8 Influenza virus and Staphylococcus aureus in the guinea pig. Am J Hyg. 1963;78:275–84. [PubMed] [Google Scholar]
- 34.Eriksen NH, Espersen F, Rosdahl VT, Jensen K. Carriage of Staphylococcus aureus among 104 healthy persons during a 19-month period. Epidemiol Infect. 1995;115:51–60. doi: 10.1017/S0950268800058118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams RE. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol Rev. 1963;27:56–71. doi: 10.1128/br.27.1.56-71.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Good RC, May BD. Respiratory pathogens in monkeys. Infect Immun. 1971;3:87–93. doi: 10.1128/iai.3.1.87-93.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lina G, Piémont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, Vandenesch F, Etienne J. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–32. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 38.Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, Vandenesch F, Piémont Y, Brousse N, Floret D, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–9. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 39.Löffler B, Hussain M, Grundmeier M, Brück M, Holzinger D, Varga G, Roth J, Kahl BC, Proctor RA, Peters G. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010;6:e1000715. doi: 10.1371/journal.ppat.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoong P, Pier GB. Antibody-mediated enhancement of community-acquired methicillin-resistant Staphylococcus aureus infection. Proc Natl Acad Sci U S A. 2010;107:2241–6. doi: 10.1073/pnas.0910344107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graves SF, Kobayashi SD, Braughton KR, Whitney AR, Sturdevant DE, Rasmussen DL, Kirpotina LN, Quinn MT, DeLeo FR. Sublytic concentrations of Staphylococcus aureus Panton-Valentine leukocidin alter human PMN gene expression and enhance bactericidal capacity. J Leukoc Biol. 2012;92:361–74. doi: 10.1189/jlb.1111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoong P, Pier GB. Immune-activating properties of Panton-Valentine leukocidin improve the outcome in a model of methicillin-resistant Staphylococcus aureus pneumonia. Infect Immun. 2012;80:2894–904. doi: 10.1128/IAI.06360-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malachowa N, Kobayashi SD, Braughton KR, Whitney AR, Parnell MJ, Gardner DJ, Deleo FR. Staphylococcus aureus leukotoxin GH promotes inflammation. J Infect Dis. 2012;206:1185–93. doi: 10.1093/infdis/jis495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rice TW, Rubinson L, Uyeki TM, Vaughn FL, John BB, Miller RR, 3rd, Higgs E, Randolph AG, Smoot BE, Thompson BT, NHLBI ARDS Network Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit Care Med. 2012;40:1487–98. doi: 10.1097/CCM.0b013e3182416f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Randolph AG, Vaughn F, Sullivan R, Rubinson L, Thompson BT, Yoon G, Smoot E, Rice TW, Loftis LL, Helfaer M, et al. Pediatric Acute Lung Injury and Sepsis Investigator’s Network and the National Heart, Lung, and Blood Institute ARDS Clinical Trials Network Critically ill children during the 2009-2010 influenza pandemic in the United States. Pediatrics. 2011;128:e1450–8. doi: 10.1542/peds.2011-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voyich JM, Otto M, Mathema B, Braughton KR, Whitney AR, Welty D, Long RD, Dorward DW, Gardner DJ, Lina G, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–70. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 47.Shopsin B, Gomez M, Montgomery SO, Smith DH, Waddington M, Dodge DE, Bost DA, Riehman M, Naidich S, Kreiswirth BN. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol. 1999;37:3556–63. doi: 10.1128/jcm.37.11.3556-3563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Memoli MJ, Jagger BW, Dugan VG, Qi L, Jackson JP, Taubenberger JK. Recent human influenza A/H3N2 virus evolution driven by novel selection factors in addition to antigenic drift. J Infect Dis. 2009;200:1232–41. doi: 10.1086/605893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cottey R, Rowe CA, Bender BS. Influenza virus. Curr Protoc Immunol 2001; Chapter 19:Unit 19.11. [DOI] [PubMed] [Google Scholar]
- 50.Safronetz D, Rockx B, Feldmann F, Belisle SE, Palermo RE, Brining D, Gardner D, Proll SC, Marzi A, Tsuda Y, et al. Pandemic swine-origin H1N1 influenza A virus isolates show heterogeneous virulence in macaques. J Virol. 2011;85:1214–23. doi: 10.1128/JVI.01848-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richt JA, Rockx B, Ma W, Feldmann F, Safronetz D, Marzi A, Kobasa D, Strong JE, Kercher L, Long D, et al. Recently emerged swine influenza A virus (H2N3) causes severe pneumonia in Cynomolgus macaques. PLoS One. 2012;7:e39990. doi: 10.1371/journal.pone.0039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.