Abstract
Staphylococcus aureus plays an important role in numerous human cases of food poisoning, soft tissue, and bone infections, as well as potentially lethal toxic shock. This common bacterium synthesizes various virulence factors that include staphylococcal enterotoxins (SEs). These protein toxins bind directly to major histocompatibility complex class II on antigen-presenting cells and specific Vβ regions of T-cell receptors, resulting in potentially life-threatening stimulation of the immune system. Picomolar concentrations of SEs ultimately elicit proinflammatory cytokines that can induce fever, hypotension, multi-organ failure, and lethal shock. Various in vitro and in vivo models have provided important tools for studying the biological effects of, as well as potential vaccines/therapeutics against, the SEs. This review succinctly presents known physical and biological properties of the SEs, including various intervention strategies. In particular, SEB will often be portrayed as per biodefense concerns dating back to the 1960s.
Keywords: SEB, S. aureus, vaccine, therapeutic, superantigen, animal model, cytokine
Introduction
Staphylococcus aureus is a formidable pathogen linked to many human diseases.1-3 Planktonic and sessile (biofilm-based) versions of S. aureus can occur in an infected host. This facultative, β-hemolytic, gram-positive, halo-tolerant bacterium readily colonizes skin, various mucosal surfaces, soft tissues, and bone, as well as indwelling medical devices. Approximately 30% of humans are asymptomatic carriers of S. aureus strains, harboring genes for antibiotic-resistance, staphylococcal enterotoxins (SEs), and other virulence factors.4 Within the non-institutionalized population of the US, Caucasian males less than 65 years old and possessing minimal education are those most likely colonized by S. aureus. Another interesting finding by Graham et al. reveals that SED is strongly correlated with methicillin-resistant strains of S. aureus (MRSA).4
In addition to the SEs that stimulate specific subsets of T cells,2,5 S. aureus also possesses many other virulence factors that include adhesins, collagenases, protein A, coagulases, hemolysins, and leukocidins.2,3,6 Clearly, the bacterium is very adept at surviving in/on a host via a hefty, diverse arsenal. Often mentioned in popular and scientific literature is an ever-increasing resistance of S. aureus toward antibiotics like methicillin and now vancomycin, which represents a serious societal concern for both humans and animals.7,8 In hospitals and nursing homes, antibiotic-resistant strains are a particularly deadly bane. Strict adherence to infection control plans is necessary to check inadvertent spread of S. aureus among staff and patients. Indeed, S. aureus is an important health and economic concern throughout the world.9 From a biodefense perspective spanning decades of research, SEB is considered a Category B select agent by the Centers for Disease Control and Prevention that is harmful following inhalation.10,11
When naturally derived by ingestion, the SEs (A–U, and counting) are associated with one of the most prevalent forms of food poisoning found throughout the world.2,12 It is evident that various populations are naturally exposed to these toxins, as demonstrated by SEB seroconversion rates in humans.13 Whether toxin-specific antibodies are developed after ingesting contaminated food, and/or colonization of humans by a toxin-producing strain of S. aureus, is to date unknown. Furthermore, whether pre-existing antibody titers in some individuals among the normal population protect against a biological attack using SEB remains an unanswered question.
SE poisoning naturally occurs after ingesting processed meats or dairy products previously contaminated by improper handling and storage. Such conditions are conducive to S. aureus growth, and pending strain, release of one (or more) SEs into the tainted food. Only microgram quantities of consumed toxin are needed to cause emesis and diarrhea within approximately 4 h, and one may still experience a general malaise 24 to 72 h later.14 As food poisoning by SEs is non-fatal and of short duration, supportive care is indicated and includes over-the-counter medication for symptomatic relief of gastrointestinal discomfort. Little effort is devoted toward developing countermeasures of foodborne illness induced by SEs. Poisoning by the SEs via many different food types is rarely fatal for healthy individuals, and occurs around the world; however, the very young and old represent higher risk groups.15 Furthermore, recent murine studies suggest that low, chronic levels of SEB can also experimentally induce autoimmunity.16 This brings up an interesting, yet largely unexplored, aspect of health effects upon humans following chronic colonization by toxin-producing S. aureus. We foresee future work in this area of toxin-induced autoimmunity becoming interestingly fruitful.
Exactly how the SEs cause enteric illness is still remarkably unresolved, but prostaglandins and leukotrienes may mediate the effects.17,18 In addition to causing food poisoning, the SEs (historically SEB) have a nefarious potential for biological warfare and bioterrorism.10,11 After inhalation, SEB can induce within 2 h several symptoms that include: head and muscle aches, tachycardia, coughing, nausea, vomiting, diarrhea, and conjunctiva irritation.10 These forms of incapacitation occur at nanogram levels, while microgram quantities of SEB can be fatal. As the toxin alters immunity, it is plausible that other agents (viral and bacterial) may act in a synergistic/opportunistic manner with co-administered SEB. Again, from a biodefense or civilian medical perspective, enhancement of opportunistic infections via co-exposure to bacterial toxin(s) is an unexplored (and admittedly complex) area of research.
Pre-existing antibodies do play an important role in susceptibility to staphylococcal toxic shock elicited by toxic shock syndrome toxin-1 (TSST-1).19 As described below, similar circumstances exist for the SEs in various animal models. Individuals not seroconverted toward TSST-1 due to toxin-induced hyporesponsive T cells,20 and/or T-cell-dependent B cell apoptosis,21 are more likely to relapse. Perhaps these findings emphasize a need for vaccines that may break tolerance toward TSST-1, and other staphylococcal superantigens, especially among high-risk populations.22-27
The therapeutic use of immunoglobulins can help prevent staphylococcal-induced shock. Intravenous immunoglobulin (IVIg), pooled from human donors, is particularly beneficial in the clinic but problems exist with batch to batch variation that logically include neutralizing titers and targeted antigens.28,29 Use of humanized monoclonal antibodies, in a “cocktail” targeting unique epitopes on the SEs and TSST-1, represents a logical step forward.30 This is akin to that described for Clostridium botulinum neurotoxin A, another bacterial protein that is of high concern within the biodefense community.31
“Superantigen”, a term used often in this review, commonly describes the SEs, TSST-1 and structurally related streptococcal pyrogenic exotoxins (SPEs) of Streptococcus pyogenes. This designation originated in the late 1980s to define microbial proteins that activate a large population of specific T cells at picogram levels.32,33 Superantigens are in contrast with “conventional” antigens that typically stimulate far fewer T cells at higher concentrations. Superantigen interactions with host cells further differ from conventional antigens by: (1) direct interactions on the outside of the peptide-binding groove of major histocompatibility complex class II (MHC II), (2) binding to various MHC II types, and (3) exerting biological effects upon the host without internalization and antigen processing.32-34 Additionally, recognition of a superantigen:MHC II complex by the T-cell receptor (TCR) depends upon the variable region within a TCR variable β chain (Vβ), and not a Vα-Vβ chain combination commonly used by conventional peptide antigens.34 Microbial superantigens are also produced by other bacteria and even viruses, thus suggesting a conserved and successful strategy employed throughout nature.
Toxin Structure and Receptor Binding
The SEs and TSST-1 are 22- to 30-kD, single-chain proteins secreted by S. aureus that form distinct homology groups based upon amino acid sequence.2,5 There are more than 20 SE variants described in the literature. Furthermore, there are approximately ten SE-like (SEL) proteins produced by S. aureus that lack emetic properties or have not been tested to date.35 Among the different SE “serotypes” originally described decades ago, SEA, SED, and SEE share the highest amino acid sequence homology ranging from 53% to 81%. SEB is 50–66% homologous with SECs (1, 2, and 3 subtypes).2,5
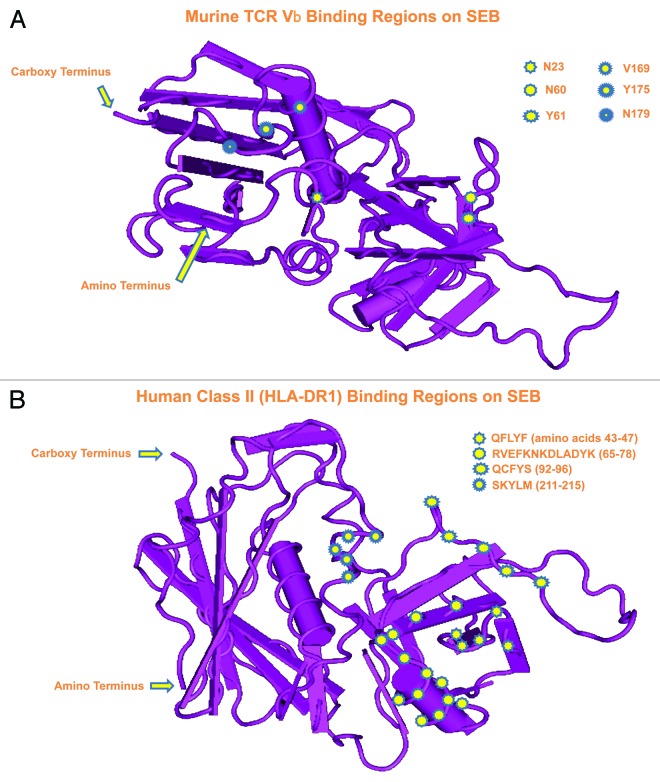
Despite varying sequences, structural studies, and X-ray crystallography of SEA, SEB, SEC2, and TSST-1 reveal quite conserved conformations with two tightly-packed domains containing β-sheet plus α-helix structures separated by a shallow groove.36,37 Structure-function studies with site-directed mutagenesis and overlapping peptides of these toxins, along with crystallographic analysis of toxin–MHC II complexes, provide further clues regarding specific residues critical for binding to MHC II and TCR.26,38,39 The SEs and TSST-1 additionally share similar structures (i.e., epitopes) as evidenced by cross-reactivity and neutralization with antibodies.22-27,40-42 Figure 1 shows two orientations of SEB and regions involved in binding to murine TCR (Vβ 8.1) as well as human MHC II (HLA-DR1).37-39,43
Figure 1. Crystal structure of SEB at 1.5 Å from Papageorgiou et al.37 using the Molecular Modeling Database (MMDB) of the National Center for Biotechnology Information (NCBI).155 Two different orientations reveal SEB residues important for binding to (A) murine TCR (Vβ8.1) and (B) human MHC II (HLA-DR1).38,39,43
The staphylococcal superantigens bind to conserved elements of MHC II with high micro- to low nanomolar affinity.2,5,44,45 However, each toxin preferentially binds to distinct alleles which suggests different contact sites on MHC II. Upon comparing the binding attributes of staphylococcal superantigens, SEA has the highest affinity for HLA-DR mediated by two binding sites.
Co-crystals of SEB or TSST-1, complexed with HLA-DR1 and associated peptide antigen, also clearly reveal distinct binding differences between these toxins.43,46 For example, SEB interacts exclusively with the α chain of HLA-DR1 and is unaffected by the associated peptide. Overall, it is clear that diverse methods exist for SEs and TSST-1 binding to both MHC II and TCR, which can partly explain differential activation of T cells.47,48
The groove formed between conserved domains of staphylococcal superantigens represents an important interaction site for the TCR Vβ chain (Fig. 1).38,39,48 Each toxin binds to a distinct repertoire of Vβ-bearing T cells, thus displaying a unique biological “fingerprint” that might be helpful in the clinic for diagnosing superantigen exposure.47 Mutations within the MHC II binding domains of SEA differentially affect binding to TCR Vβ,48 as evidenced by a small increase in superantigen affinity for MHC II, thus overcoming a large decrease in affinity for TCR Vβ. Furthermore, disulfide-linked homodimers of CD28 represent an additional binding site for SEB important for T-cell stimulation and subsequent biological effects.49 This same study interestingly reveals direct binding of SEB to CD28, without MHC II or TCR.
Signal Transduction and Cellular Responses
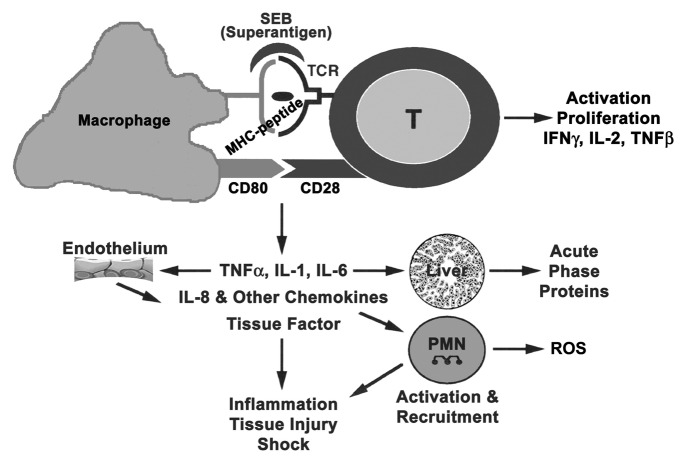
Recognition of the superantigen/MHC II complex by TCR results in cell signaling, proliferation, and subsequent release of cytokines/chemokines.32,33,44,45,50 Immune cell activation by superantigens and subsequent cellular changes are similar to those of conventional antigens and requires three important signals. Figure 2 shows the cells and mediators involved in eliciting the biological effects of superantigens.50 Signal 1 comes from superantigen interaction with TCR and activation of protein tyrosine kinases (PTKs), which in turn phosphorylate tyrosine-based motifs of the TCR intracellular components and other cellular substrates plus adaptors.34 Activation of phospholipase C gamma (PLCγ) through phosphorylation by TCR-induced kinases generates second messengers. The latter subsequently activate protein kinase C (PKC), the proto-oncogene Ras, and also increase intracellular calcium levels. Engagement of costimulatory molecules on antigen-presenting cells (APCs) and T cells, upon superantigen binding, results in a second signal that optimizes T-cell activation. Expression of intercellular adhesion molecule (ICAM) on an APC promotes stable cell conjugates and an immunological synapse. The interactions between adhesion (LFA-1 with ICAM-1) and costimulatory (CD28 with CD80) molecules have been implicated in superantigen-mediated T-cell activation.51 Other cell-surface molecules such as CD2, CD11a/ICAM-1, and ELAM facilitate optimal activation of endothelial cells and T cells by SEB.52 PKC and PTK activation lead to other downstream signaling pathways including mitogen-activated protein kinase (MAPK), extracellular signal regulated kinase (ERK), and c-Jun N-terminal kinase (JNK) pathways, ultimately activating transcriptional factors NFκB, NF-AT, and AP-1.34,50 Many proinflammatory cytokine genes contain NFκB binding sites within the promoter/enhancer region and are induced by NFκB. Interleukin (IL)-1, tumor necrosis factor α (TNFα), interferon gamma (IFNγ), IL-2, IL-6, and chemokines, specifically monocyte chemoattractant protein-1 (MCP-1), are induced directly by superantigens and represent the third signal for T-cell activation. IL-1 and TNFα can also activate fibroblasts plus epithelial and endothelial cells to produce other mediators, thus providing an inflammatory environment for T-cell activation. The binding of TCR, costimulatory receptors, T-cell cytokines (IL-2 and IFNγ) plus chemokines to their respective receptors activate the lipid kinase, phosphoinositide 3 kinase (PI3K), which in turn activates the mammalian target of rapamycin (mTOR). The PI3K/Akt/mTOR pathway regulates many physiological and pathological processes as it controls cell survival, growth and migration. Myeloid differentiation protein 88 (MyD88) is also an adaptor protein involved in SEB-induced cytokine signaling.53 Clearly, SEB-based activation of cells involves a multi-factorial event encompassing multiple host molecules. Biological effects induced by superantigens are triggered by low, non-saturating occupancy rates indicative of “low affinity” binding to MHC II.
Figure 2. Cells and mediators participating in superantigen-induced toxic shock.
Human whole blood and purified peripheral blood mononuclear cells (PBMCs) are commonly used in vitro to study cell activation by staphylococcal superantigens, as well as potential therapeutic agents against these toxins.54-60 PBMCs release cytokines and chemokines following SE or TSST-1 exposure, such as: IL-1, IL-2, IL-6, TNFα, IFNγ, macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and MCP-1. Although monocytes alone can produce many chemokines as well as proinflammatory cytokines, like IL-1, IL-6, and TNFα, T cells enhance mediator levels.59,60 There are contradictory reports regarding APCs and T-cell responses to these bacterial toxins without the other cell type, as evidenced by cytokine/chemokine production by human monocytic lines or freshly isolated cells.59 MHC II-linked stimulation of T cells by SEs is a general requirement, but those with select TCR Vβs can independently respond with less efficiency.
Additional cell types that respond to superantigens include B, nasal, intestinal and vaginal epithelial, as well as intestinal fibroblasts and synovial myofibroblasts.18,52,61,62 The cross-linking of TCR with MHC II by superantigen triggers B-cell proliferation and differentiation into immunoglobulin-producers in a dose-dependent manner, but high concentrations of superantigen inhibit immunoglobulin synthesis.62 Suppression of antibody synthesis by TSST-1 reportedly occurs via apoptosis,20,21 which can clearly hamper protective immunity against this toxin.19 Such an effect upon B cells is likely linked to recurring bouts of toxic shock following persistent, vaginal colonization by TSST-1 producing S. aureus. Upon activation by SEB, nasal epithelial cells produce granulocyte colony-stimulating factor and various chemokines including MCP-1.61 Transcytosis of SEB across intestinal epithelial cells has been observed in vitro,63,64 and in vivo the toxin penetrates the gut lining that then leads to local and systemic immune responses.65
In Vivo Effects: Animal Models and More
Monkeys: the laboratory standard (but expensive!)
The SEs readily induce emesis in primates when ingested, but not after i.v. injection, in low microgram quantities. Pending the dose plus route, there may be more severe consequences that progress from rapid hypotension and decreased cardiac output, into lethal toxic shock not reversed by epinephrine.14,66,67 Humans are more sensitive to ingested SEB than monkeys. For many years, classic primate studies for SEs have been performed by various groups and are the “gold standard”. However, this animal model has become prohibitively expensive in many ways that obviously includes money, but also institutional relations with the lay public. In contrast to the SEs, structurally related TSST-1 and some tested SEL proteins do not cause vomiting in monkeys after ingestion.2,14,35
Unlike many other bacterial enterotoxins, specific cells and receptors in the intestinal tract have not been clearly associated with SE intoxication. The latter seemingly requires a complex interplay between immunological and non-immunological mechanisms involving multiple cell types. SEB stimulation of mast cells causes release of cysteinyl leukotrienes that subsequently elicit emesis and skin reactions in primates.18 In total, the immunologically-based results within the intestine are likely connected to toxin-specific stimulation of unique Vβ-bearing T cells. Table 1 reveals select animal models used to study not only the enteric, but also lung and skin effects of SEs.
Table 1. Enteric, lung, and skin models for staphylococcal superantigens.
| Animal | Inducing agent(s) | Route | Mediators, symptoms, pathology |
|---|---|---|---|
| Mouse Balb/c | SEB | i.g. | IFNγ and IL-2 increase in mucosal lymphoid tissue at 4 h65 |
| Mouse HLA-DR3 IFNγ knockout Mouse C57BL/6 |
SEB SEB |
i.p. i.p. |
IFNγ linked to intestinal pathology, increased gut permeability, toxic shock68 Acute lung inflammation, leukocyte infiltration, capillary leakage, and endothelial cell injury by six hours69 |
| Mouse (different strains) with inflammatory bowel disease | SEA or SEB | i.r. | Exacerbation of inflammatory bowel disease70 |
| Monkey cynomolgus | SEB | i.d. | Immediate-type skin reaction, emesis, cutaneous mast cell degranulation, and cysteinyl leukotriene generation18 |
| Monkey cynomolgus | SEA, SEB, or SEC1 | i.g. or i.v. | Emesis at three hours, followed by diarrhea14 |
i.d., intradermal; i.g., intragastric; i.p., intraperitoneal; i.r., intrarectal; i.v., intravenous.
Studies with human Caco-2 monolayers reveal transcytosis of SEA, SEB, and TSST-1, with ingested SEB entering the bloodstream of mice more readily than SEA.63 These data suggest that SEs cross the gastric mucosa and circulate throughout the body. In vitro, the SEs are not cytotoxins that directly disrupt human (Henle 407) intestinal cells.71 However, SEB affects the gut mucosa as evidenced by increased ion flow through a monolayer of human T84 colonic cells incubated with SEB-stimulated PBMCs.72 The interactions of most superantigens with epithelial cells are indirect via release of IL-1, TNFα, and IFNγ from superantigen-activated APCs and T cells.73,74 Furthermore, there is an age-related increase (up to ten years among humans) in CD4+ T cells that produce IFNγ following SEB exposure, thus increased susceptibility to SEs over time is linked to IFNγ levels.75
Although debatable, it appears that superantigenicity may not play a role in SE enterotoxicity as recombinant variants of SEA and SEB lacking MHC II binding and T-cell mitogenicity remain emetic. Carboxymethylation or tyrosine replacement of select histidine (H) molecules on SEA76 and SEB77 yields proteins that differ from wild type in superantigenic, enterotoxic, and lethal effects. In particular, H61 on SEA is important for emesis, but not superantigenicity; however, changes in H44, H50, H114, or H187 do not alter wild-type properties.76
As stated before, affinity for MHC II and specific TCR Vβ enables superantigens to induce high levels of proinflammatory cytokines.54-60 The SEs and TSST-1 are pyrogenic in primates as well as rabbits, a likely result of elevated levels of proinflammatory cytokines that include IFNγ, IL-1, and TNFα.2,14,33,50,78 IFNγ enhances immunological responses by elevating expression of MHC II on APCs and epithelial/endothelial cells, as well as augments the proinflammatory actions of IL-1 and TNFα. SEB causes acute lung injury and potential shock characterized by increased: (1) expression of adhesion molecules like ICAM-1 and vascular cell adhesion molecule (VCAM), (2) neutrophil and mononuclear cell infiltration, (3) endothelial cell injury, (4) serum levels of proinflammatory cytokines, and (5) vascular permeability.10,11,50,52,69
Mice: experimentally not perfect, but often preferred
Mice have historically been used by various groups as an alternative to monkeys for studying superantigen-mediated effects in vivo.79-88 From a cost perspective, mice are very feasible for basic toxin studies and discovery of therapeutics/vaccines for combating staphylococcal superantigen-induced shock. However, mice lack an emetic response and are thus questionably appropriate for studying the food poisoning aspects of SEs. Nasal application of SEB in mice elicits IL-1, IL-4, IL-6, IFNγ and lung injury still evident four days after steroid treatment.79 Interestingly, IL-17 plays a critical role in allergic rhinitis as evidenced by experiments with knockout mice not responding to SEB.85 Nasal and systemic effects toward an SEB vaccine applied to the nares, or perhaps even mucosa-dwelling S. aureus, are linked to nasopharynx-associated lymphoid tissue.86 When given intrarectally to mice, SEA or SEB elicit an inflammatory intestinal response and exacerbate a preexisting, microbial-based syndrome (inflammatory bowel disease or IBD) that further suggests an immunological-based component provided by the host.70 Additionally, mice are naturally less susceptible (vs. monkeys) to SEs and TSST-1 because of decreased affinity for MHC II.81,84 Therefore, potentiating agents such as d-galactosamine, actinomycin D, lipopolysaccharide (LPS), viruses, or even protozoa are used by various groups with different mouse strains.80-83,87,88 These agents amplify SE or TSST-1 effects in mice so that practical, lower amounts of these protein toxins elicit a quantifiable form of toxic shock (i.e., lethality or temperature change) useful for therapeutic and vaccine discovery. Of course, adding a potentiating agent with toxin provides yet another variable in deciphering derived data.
In addition to toxin-specific resistance elicited by a single oral dose of SE, chronic intravenous exposure to SEA can delete Vβ-reactive T cells in mice.89 This may be partly explained by increased frequency of FoxP3+ CD4+ regulatory T cells observed in TCR transgenic mice repeatedly exposed to SEB.90 Another study shows that mice given SEA (1 μg every week for three weeks), but not a recombinant SEA variant lacking superantigenicity, become resistant to a subsequent lethal challenge of SEA but not TSST-1.91 This form of tolerance is not linked to toxin-specific antibody or deletion/anergy of SEA-reactive T cells. However, a significant increase in serum IL-10 levels among these animals correlates with in vitro and in vivo protection against SE-induced effects.55,91 IL-10, but not IL-4, provides protection against abnormal ion flow through a human colonic monolayer following SEB stimulation of PBMCs.72
Many of our mouse studies with SEs and TSST-1 have been done via LPS-potentiation with a lethal endpoint, as it has been well established by many laboratories that a natural synergy exists between these protein toxins and LPS.80,81,84,92-94 As SEs and TSST-1 synergize with LPS many log-fold, minute quantities of each can cause severe effects in mammals. Among healthy humans there are numerous Gram-negative bacteria constituting normal intestinal, or vaginal, flora. Along with a recognized increase in these microbes among toxic shock patients, the odds of this superantigen-LPS synergy naturally occurring are plausibly high.95 There is a strong correlation between elevated serum levels of various proinflammatory cytokines (IL-1, IL-2, TNFα, and/or IFNγ) with SE- or TSST-1-induced shock.2,18,22,23,33,50,53-57,78,81,84
The interdependent effects of SEB used alone, and together with LPS, on serum cytokines/chemokines has been described in further detail using a Balb/c mouse model.78 SEB alone induces moderate serum levels of IL-2 and MCP-1, with all mice surviving a high dose (100 µg/animal). Additionally there are only low levels of TNFα, IL-1, IFNγ, and MIP-2; however, with LPS there is increased expression of these cytokines to include IL-6 and MCP-1. Thus, the synergistic action of SEB and LPS promotes early TNFα release and prolongs IL-6, IFNγ, IL-2, MIP-2, and MCP-1 release in non-survivors. Overall, the elevated and sustained levels of these key cytokines lead to lethal toxic shock within 48 h after co-administration of LPS plus SEB. Mice given antibody against IL-10 have increased serum levels of IL-2, IFNγ, plus TNFα after SEB stimulation, and become more susceptible to lethal shock.96 Additionally, these efforts correlate nicely with others employing SEA and knockout mice lacking IFNγ, ΙL-2, or TNF receptor type 1.92 Further work has been described with SE or TSST-1 intoxication among knockouts deficient in IL-10, TNF receptor types I or II, or CD43.92,97
Besides knockouts, another method for studying superantigenic shock in mice includes transgenics with inserted genes, often of human origins. As one example, mice expressing human HLA-DQ6 and CD4 succumb to normally sublethal amounts of SEB (with d-galactosamine potentiation), and the serum levels of TNFα correlate with lethal shock.98 In particular, two high doses of SEB (30 and 100 μg/mouse) are necessary to induce toxic shock in this model. Regarding mode of action, d-galactosamine is converted by hepatocytes into uridine diphosphate-galactosamine, which in turn prevents uridine triphosphate formation. Ultimately this affects RNA, and subsequent protein, synthesis that becomes lethal for the host.99
Transgenic mice with human HLA-DR3 lethally respond to SEs without a potentiating agent, thus providing a “simpler” model for future in vivo toxin studies.100 Like the HLA-DR3 model, transgenic (HLA-DQ8) mice also respond to SEB without a potentiating agent as per elevated serum levels of various cytokines.101 Following SEB exposure (aerosol) in these latter transgenics, the lung lesions, temperature fluctuations, and lethality after 96 h are also similar to those experienced by monkeys after a lethal aerosol.
Besides mice displaying human receptor on cells, transgenics that overexpress murine TCR Vβ3 also have increased mortality linked to elevated TNF and IFNγ levels following infection by SEA-producing S. aureus.102 Overall, transgenic animals can provide interesting clues to superantigenic shock in humans at a fraction of the cost vs. monkeys.
In addition to lethality as an endpoint, temperature has been used for studying SE and TSST-1-induced shock in mice. These studies were accomplished by implanting a subcutaneous transponder92 or intraperitoneal telemetry device,103 in which the latter also measured movement. Telemetry technology affords a seamless collection of data without human handling, thus negating some potential confounding factors during an experiment. There is a rapid (within 10 h) temperature decrease readily evident post-toxin injection of mice, thus providing a rapid non-lethal model. Temperature, but not movement, significantly correlates with SEB intoxication.103 None of these studies detected a temperature increase, evident in monkeys,104 thus suggesting a very rapid onset of shock in these murine models.92,103
Intranasal administration of SEB has also been used in various murine models.105,106 One consists of a two-hit (or dual-dosing) model in C3H/HeJ mice requiring SEB given two hours apart.105 The first dose is delivered i.n. and the next administered either i.n. or i.p. Increased serum levels of IL-2, IL-6, and MCP-1, accompanied by elevated lung levels of MCP-1, are evident in this dual-dosing model. MCP-1, a potent activator and chemotactic factor for T cells plus monocytes probably contributes to early leukocyte recruitment into the lung. Pathological lesions, temperature fluctuations, and time course of lethality following SEB exposure also resemble those in transgenic mice and monkeys.66,101,106,108,109 In summary, a few mouse (and other animal species) models for the staphylococcal superantigens are shown in Table 2.
Table 2. Toxic shock models for staphylococcal superantigens.
| Animal | Inducing agent(s) | Route | Mediators, symptoms, pathology |
|---|---|---|---|
| Mouse Balb/c | TSST-1 + LPS | i.v. | TNFα peaks at 1 to 2 h, lethal shock84 |
| SEB + LPS | i.p. | TNFα peaks at 1 h, IFNγ, IL-1, IL-6 increase at 2 h, lethal shock, and hypothermia81,92 | |
| Mouse Balb/c | d-galactosamine + SEB | i.p. | High levels of TNFα and IL-2 leading to lethal shock87 |
| Mouse Balb/c | Actinomycin D + SEB | i.p. | Blood congestion in lungs and intestine by 4 h, PBMCs in lungs, spleen, and liver, alveolar septa thickening at 8 h, lethal shock at 2 to 4 d82 |
| Mouse C3H/HEJ | SEB + SEB | i.n. i.n. + i.p. |
Bronchiolar epithelial degeneration, lung neutrophilic infiltration, IL-2, IL-6, and MCP-1 in serum and lung, lethal shock at 96 h105 |
| Mouse transgenic HLA-DR3 | SEB | i.n. | Neutrophilic infiltration, TNFα, IFNγ, IL-6, IL-12, and MCP-1 increase at 3 h106 |
| Rat Sprague–Dawley | Catheterized, SEB + LPS | i.v. | TNFα increase at 90 min, IFNγ at 4 h, hepatic injury and dysfunction93 |
| Rabbit Dutch Belted | TSST-1 + LPS | i.v. | TNFα peaks at 4 h leading to lethal shock84 |
| SEC + LPS | i.v. | Fever at 4 h, hypothermia, labored breathing, diarrhea, vascular collapse, lethality by 24 h94 | |
| Rabbit New Zealand white | SEA | i.v. | TNFα, IFNγ, and IL-2 increase at 1 to 2 h, peak at 3 to 5 h, febrile reaction evident at 1 h107 |
| Monkey Rhesus | SEB | aerosol | Leukocyte infiltration, intra-alveolar edema, parenchymal cell degeneration, lymphocyte necrosis, temperature fluctuation, and lethal shock10,104,108,109 |
i.n., intranasal; i.p., intraperitoneal; i.v., intravenous
The rapid, SEB-induced hyperactivation and proliferation of select Vβ T cells in mice eliminates most of these T cells within 48 h by activation-induced cell death via faulty activating protein-1 (AP-1) transcription factor.110 After SEB injection of mice, splenic Vβ8 T cells are physically deleted or non-responsive (anergic) to homologous toxin and produce less IL-2 and IFNγ. Others report that these anergic cells can secrete more IFNγ that mediates toxic shock after a subsequent dose of SEB.111 An evident paradox is that an anti-inflammatory cytokine like IL-10, which protects against SE-induced shock,55,96 is also produced by SEB-primed T cells.111 This perhaps is the host’s attempt to counter the proinflammatory effects of IFNγ? It is possible that SEB-induced anergy differentially affects CD4+ and CD8+ T cells, with the former becoming more susceptible.111
Rabbits: a common model for TSST-1
In addition to mice, rabbits have also afforded a reliable in vivo model for SE, and particularly TSST-1, induced shock as determined by temperature and lethal endpoints (Table 2).84,94,108,112-115 Some of these models employ an implanted infusion pump that delivers toxin over time, thus mimicking more naturally a S. aureus infection that leads to toxic shock.112 As evidenced in mice with the various staphylococcal superantigens, different rabbit strains also possess varying susceptibility toward TSST-1 as New Zealand whites are more susceptible vs. Dutch belted.113 As witnessed in humans with toxic shock, rabbits given TSST-1 or SEB experience elevated levels of circulating LPS eliminated by polymyxin B.113,114 From a biodefense perspective, intrabronchial instillation of SEB (SEC or TSST-1 too) into rabbits can be useful for testing potential therapies and vaccines.115 The testing of live S. aureus is also possible in this model for exploring interventions against bacterial pneumonia.
Goats, ferrets, and shrews: unusual alternatives
In addition to monkeys, mice, and rabbits, other less employed models for SE intoxication have been described in the literature. For example, goats have been used for studying in vivo effects of TSST-1 and SEB (0.02–20 μg/kg) after i.v. administration.116 Following SEB exposure, goats experience tachycardia and diarrhea, as well as elevated blood urea plus temperature. In contrast, TSST-1 does not elicit diarrhea in goats, which mimics results from monkeys given this same toxin orally.2,14,35
There is also a ferret model for oral SEB and SEC2 intoxication which elicits emesis, increased defecation, altered feces appearance, and rapid fever;117 however, this model employs milligram quantities of toxin (upwards to 10 mg/animal) that are much higher than that typically used in various mouse, monkey, or goat models. Possible explanations for requiring this large dose of SEB include receptor differences and/or more efficient degradation of SEB in the gut of ferrets vs. humans or monkeys.
Finally, another emetic model more recently described and highly developed for SE studies employs an unusual laboratory animal: the house musk shrew.118,119 When given orally, both SEA and SEC cause emesis (100 or 500 μg/animal) within 30 to 120 min.119 SEA is more effective than SEC, while TSST-1 has no effect. This model has been useful for vaccine efforts against SEA, using a recombinantly-attenuated SEA devoid of superantigen and emetic activity.118 Furthermore, sera from these vaccinated animals (vs. alum only controls) inhibit SEA (wild type)-induced proliferation of naïve shrew splenocytes (in vitro) as well as emesis. Interestingly, there are no diarrheic effects in shrews when toxin is given orally or injected directly into intestinal loops. Upon comparing models a much lower amount of SE is required in shrews, vs. ferrets, via an i.p. or oral route. However, an unpleasant readout with any emetic (or diarrheic) model is volume quantity and/or event numbers.
In summary, basic aspects of SE and/or TSST-1 intoxication have been investigated in each animal model listed above. For any investigator, there are many options pending study intent (i.e., toxic shock and/or enteric effects) plus available resources. With varying amounts of additional work, and recognition of inherent caveats, each model can ultimately be used for future vaccine and therapeutic discovery. Every model has pluses, and minuses, that ultimately do not fully capture human intoxication. Basic biology (i.e., physiology) is simply different, in different ways, between animal species. To be fair though, how staphylococcal superantigens affect humans is not totally understood to date which relates to the very complex interplay of toxin with host (i.e., immune system, intestinal tract, nervous system, etc.). It is certain that future studies involving the SEs and TSST-1 are not lacking for available animal models that can answer, with limitations, some very important questions linked to human intoxication.
Neutralization Strategies
Neither small-molecular weight therapeutics nor vaccines against SEs or TSST-1 have been approved for human use by the United States Food and Drug Administration (FDA). Given that the medical community has fewer effective tools to thwart evolving strains of S. aureus, as well as other antibiotic-resistant pathogens, progress toward discovering and subsequently developing therapeutics plus vaccines seem logical. The biodefense concern of defending against a toxin, such as SEB, is a relatively simpler “static” scenario (i.e., toxin does not reproduce) vs. overcoming a bacterial pathogen employing various virulence factors. Mitigation of SE or TSST-1 toxicity will afford some relief for an infected patient suffering from an antibiotic-resistant strain of S. aureus. Upon current understanding of SE and TSST-1 intoxication at a molecular level, potential therapies/vaccines should target at least one of three important steps: (1) TCR–toxin–MHC II interactions, (2) accessory, co-stimulatory, or adhesion molecules that include intracellular signaling molecule and adaptor (i.e., CD28, MyD88, etc.) activation of T cells, and (3) cytokine release by activated T cells and APCs. There are clearly multiple targets awaiting further investigation. The question becomes one of funding a focused endeavor(s) that leads to novel findings and a product of clinical value.
Non-immunoglobulin based protection
Attempts at in vitro and in vivo inhibition of the above toxin-exploited targets have been many and diverse, emanating from groups throughout the world. A conserved region (residues 150–161) from SEB prevents shock induced by SEB, as well as SEA or TSST-1, in mice when given 30 min after toxin.120 This peptide evidently stops transcytosis of various SEs and TSST-1 across a human colonic cell (T84) monolayer, and may block co-stimulatory signaling necessary for T-cell activation.49,52,60,63 However, subsequent studies indicate that such peptides are ineffective inhibitors of SEB-induced effects both in vitro and in vivo.121 Another study with a different SEB peptide (residues 72–86) reveals inhibition of SEA-, SEB-, and SEC-mediated responses in vivo.122 Recently, short synthetic peptides corresponding to the binding region of CD28 were shown to block SEB-induced TNFα, IFNγ, and IL-2 expression.49 Clearly this is an exciting area of research, but varied results from various groups are troublesome and suggest more work to ascertain any potential usefulness in the clinic. Furthermore, one potential problem for medicinal use of any peptides or proteins involves proteolysis by the host, and thus inactivation.
A different approach for blocking receptor interactions of SEB uses a chimera of the DRα1 domain from MHC II and TCR Vβ connected by a flexible linker.123 This construct prevents cellular activation and subsequent IL-2 release in SEB-stimulated PBMCs (human) in vitro. A potential drawback is that individual chimeras must be constructed for each SE, as TCR Vβ preferences differ among superantigens. Related to receptor blocking, another group reports that a soluble TCR Vβ mutant can neutralize SEB, and related SPEA, with picomolar affinity.124
Aptamers, consisting of peptide or single-stranded nucleic acid, are a relatively new method to detect or neutralize targets that include protein toxins. Such DNA-based molecules, fished from recombinant libraries, can directly bind SEB and prevent receptor interaction.125 In particular, aptamer technology could be useful in the food safety industry and detection of various SEs in tainted foods.
Once a staphylococcal superantigen engages surface receptor, blockade of signal pathways within a targeted cell represents the next medicinal option. The complexity of any medical intervention at this stage increases dramatically, as (1) entry of any therapeutic into a cell and (2) subsequent short-circuiting of toxin-induced toxicity represents a very daunting task. Naturally, most cells (APCs being an exception) are rather discriminating toward bringing compounds in from the external milieu. This is a very different mode of intervention, vs. those described above for disrupting toxin-receptor interactions, as signal transduction events are post-exposure and will likely work for various SEs and TSST-1.
Nuclear factor κB (NFκB) is an attractive intracellular target for therapy, as its activation is linked to transcription of many mediators involved in inflammation and carcinoma survival.126 NFκB precursor is proteolytically processed into a mature form that subsequently governs apoptosis and cell proliferation/migration. Activation of NFκB is influenced by various stimuli, such as bacteria and viruses, involving ubiquitination plus proteolysis of sequestration proteins (known as IκB or inhibitors of kappa beta) in the cytosol. In vitro and in vivo studies have shown that many of the inflammation-associated genes implicated in superantigen-induced lethal shock contain NFκB binding sites in the promoter/enhancer regions.127 A cyclic, cell-penetrating peptide (29 amino acids designated as cSN50) targeting NFκB nuclear transport attenuates SEB-induced T-cell responses and diminishes inflammatory cytokine levels in mice. There is also reduced liver apoptosis, hemorrhagic necrosis, lung damage and mortality following pre- or post-toxin use of cSN50.128 When cSN50 (i.p.) is given 30 min before SEB (i.n.), there are reduced levels of proinflammatory cytokines and chemokines in the bronchoalveolar space of mice. This compound also attenuates neutrophil/monocyte infiltration into the lung and vascular injury.128 Bortezomib, a dipeptidyl boronic acid that inhibits proteasome and NFκB activation, also decreases SEB-induced serum cytokine/chemokine levels in mice; however, there is unfortunately no beneficial effect upon mortality and liver necrosis.129
Another potent NFκB inhibitor is dexamethasone, a corticosteroid used clinically to treat various inflammatory diseases. In vitro, dexamethasone potently inhibits SE-induced proliferation of T cells, cytokine release, and activation markers in human PBMCs.50,55,130 Prevention (or at least diminishment) of circulating levels of proinflammatory cytokines is a useful strategy against any superantigen, as these cytokines activate NFκB (IL-1 and TNFα) or PI3K (IL-2, IFNγ, and chemokines) signal pathways that lead to immune cell activation. In vivo, dexamethasone also significantly reduces serum levels of cytokines and protects mice from SEB-induced shock in the two-hit SEB-only as well as SEB + LPS models.79,130 Dexamethasone at a 1.25–5 mg/kg dose attenuates the hypothermic response to SEB in both toxic shock models, markedly improving survival of mice when administered two to three hours after SEB. Resveratrol, a plant-derived phytoallexin, also acts as an anti-inflammatory that affects cyclooxygenases and NFκB pathways after SEB-induced lung injury in mice.131
Studies with human PBMCs in vitro and a mouse model show that either pentoxifylline or pirfenidone lower proinflammatory cytokine expression, thus abrogating the ill effects of SEB or TSST-1.56,57 Pentoxifylline is an FDA-approved xanthine derivative commonly used to improve peripheral blood flow that acts as a phosphodiesterase inhibitor targeting TNF. Pirfenidone is an anti-fibrotic pyridone approved for use in various countries, but not (as of this writing) in the US. Pirfenidone inhibits signaling networks linked to transforming growth factor-β1 (TGFβ1) and translocation of Smads (2 and 3) into the nucleus.132 Ultimately, pirfenidone affects the cytoskeleton, synthesis of extracellular matrix, and cell migration.
Another group has shown that IFNγ production by SEB-stimulated lymphocytes from Peyer patches significantly decreases after oral administration of tryptanthrin.133 The latter is an indole quinazoline alkaloid possessing anti-inflammatory properties derived from a medicinal mustard-family plant (Isatis tinctoria, commonly known as woad). Tryptanthrin evidently inhibits prostaglandin and leukotriene synthesis via 5-lipoxygenase.134 Discovery of other natural products may prove useful for not only neutralizing SEB, but other biodefense agents. Along these lines, a Chinese herbal medicine (Yin Zhi Huang) commonly used to treat liver disease also effectively inhibits SEB-induced proliferation of T cells.135 Ten compounds were isolated from Yin Zhi Huang with varying activities against T-cell stimulation induced by SEB, but much more work remains to ascertain potential synergy between these ingredients.
More highly purified, plant-derived compounds such as epigallocatechin gallate (EGCG) and baicalin also effectively downregulate SEB-induced cytokines in vitro by blocking NFκB activation.136,137 In addition, EGCG reduces IFNγ-induced permeability of epithelium and suppresses T-cell activation.136 A common concern linked to discovery and development of any natural product involves isolation and/or synthesis in sufficient quantities for further study.
Other strategies include a commercially-available extracellular domain of the cytotoxic T lymphocyte antigen-4 (CTLA4), fused to the Fc region of IgG1, which in turn prevents TSST-1-induced proliferation of murine T cells in vitro and lethal shock in vivo.138 CTLA4 essentially prevents TNFα and IFNγ, but not IL-2, release into the blood stream following TSST-1 co-injection in a mouse model of toxic shock. This antagonist, commercially known as Abatacept or Orencia, is normally indicated for treating rheumatoid arthritis. For biodefense purposes, use of drugs previously approved by the FDA for other indications makes sense. In theory, there should be relatively rapid discovery/approval vs. attempting to discover and subsequently develop/approve novel molecules as unique, effective therapeutics. Various avenues of current biodefense research in the United States explore off-label use of existing FDA-approved drugs.
Another FDA-approved immunosuppressant (rapamycin) protects against SEB-induced shock in mice, even when administered 24 h after SEB.139 Ironically, this drug was originally tested as an antifungal agent that ultimately failed for this purpose. Rapamycin (a macrocyclic lactone produced by Streptomyces hygroscopicus) prevents proinflammatory cytokine release and T-cell proliferation, by stopping G1 to S cycling of cells not restricted to T lymphocytes.140 Mechanistically, rapamycin binds to an immunophilin (FK-506 binding protein 12 [FKBP12]) acting as a peptidyl-prolyl isomerase important in protein folding and trafficking. The rapamycin-FKBP12 complex blocks mTOR complex 1 activity and inhibits cell cycle progression. Rapamycin represents an effective treatment post-SEB exposure since both TCR and costimulatory molecules, as well as T-cell cytokines, activate the PI3K/Akt/mTOR pathway.
A fruitful target for mitigating proinflammatory cytokine release after LPS or SE/TSST-1 exposure, in vitro and in vivo, is MyD88.53,141 Therapeutic inhibition of MyD88-based signaling occurs via binding of a synthetic BB-loop mimetic of the Toll/IL-1 receptor domain found on MyD88. Application of such a therapeutic for both endo- or exo-toxic shock is rather appealing from a clinical perspective, apparently possessing universal application. A summary of effective small molecule therapeutics in various murine models of SEB-induced shock is shown in Table 3.
Table 3. Effective small molecule therapeutics for murine models of SEB-induced shock.
| Pharmacologic agent | Target | Biological effects against SEB |
|---|---|---|
| Mimetic peptides of CD28 dimer interface | Costimulatory molecule CD28 | Attenuated SEB-induced TNFα, IL-2, IFNγ in human PBMC. Protected mice from lethal challenge with SEB by 70%.49 |
| Mimetic peptides of BB loop of MyD88 | Toll/IL-1 receptor domain of MyD88 | Reduced SEB-induced IL-1β, Il-1, TNFα and IFNγ in human PBMC. Afforded 83% protection in mouse model of SEB plus LPS-induced shock.53,141 |
| Rapamycin (FDA-approved for prevention of renal graft rejection) | Immunophilin FK506BP12 | Blocked SEB-induced MCP-1 and IL-6 in vitro and in vivo. Protected mice 100% from lethality even when administered 24 h after SEB.139 |
| Dexamethasone (FDA-approved for treating inflammatory diseases) | NFκB | Inhibited SEB-induced proinflammatory cytokines and chemokines in PBMC.55 Reduced serum levels of cytokines, attenuated hypothermia due to SEB, and prevented both SEB-induced and SEB+LPS-induced lethal shock in mice.79,130 |
| Pentoxifylline (FDA-approved for treating peripheral arterial disease) | Phosphodiesterase | Attenuated SEB-induced proinflammatory cytokines and chemokines in PBMC.54,56 Blocked cytokine release in vivo and prevented lethality in SEB+LPS-induced shock model.56 |
| Pirfenidone | TGFβ1 | Inhibited SEB-stimulated cytokines in vitro and in vivo. Improved survival of mice against SEB+LPS.57 |
A natural feedback inhibitor of various signal transducers and activators of T cells (STATs) used by IFNγ and IL-2 signaling is the suppressor of cytokine signaling 3 (SOCS3). In this regard, a cell-penetrating form of SOCS3 protects animals from lethal effects of SEB and LPS by reducing inflammatory cytokine production, as well as attenuating liver apoptosis and hemorrhagic necrosis.142
Finally, another potential therapeutic target recently identified in SEB-linked lung injury involving acute respiratory distress syndrome, is CD44.143 CD44 is involved in multiple cell functions, acting as an intracellular signaling protein and receptor for many different ligands. CD44 is increasingly expressed among PBMCs in the lung after SEB exposure. Antibody against CD44, or use of CD44-knockouts, mitigates the lung-injuring effects of SEB (i.n.) in mice that include: (1) enhanced vascular permeability, (2) increased cell infiltration, and (3) elevated cytokine levels. Perhaps, like MyD88, targeting of a protein(s) such as CD44 involved in so many inflammatory processes is logical for not only biodefense, but also civilian, medical concerns involving staphylococcal superantigens.
Immunoglobulin-based protection
Perhaps one of the more promising therapeutics against S. aureus toxins involves immunoglobulins, a successful neutralization strategy for over a hundred years against various bacterial toxins. Sometimes the old methods (or modern variants of!) prove to be best, even in light of rapidly evolving technologies evident throughout the biological sciences. It is known that recurring bouts of TSST-1 can be linked to low antibody titers, thus emphasizing the importance of host immune responses in controlling this life-threatening disease.19,144 In fact, low antibody titers to various S. aureus exotoxins (i.e., α and δ hemolysins, Panton–Valentin leukocidin, and SEC1) can also portend susceptibility to S. aureus sepsis in hospital patients.145 Furthermore, IVIg can be effective against staphylococcal-induced shock. These antibody preparations are derived from pooled human sera of those naturally hyperimmune to various S. aureus antigens.146,147 As S. aureus readily colonizes humans and grows (when given opportunity) in various consumed foods, seroconversion opportunities against various virulence factors (i.e., SEs and TSST-1) are many. With IVIg, there will naturally be many variables between lots (i.e., antibody recognition of different antigens, relative avidities, neutralizing capabilities, amounts of each antibody, etc). One way to minimize batch-to-batch variability of polyclonal antibodies is to develop human monoclonal antibodies characterized by various assays. Recent studies by different groups target select SEs, including SEB, with different types of antibodies (i.e., recombinant human or mouse–human chimeras) for various purposes (Table 4).30,42,148
Table 4. SEB-targeting monoclonal antibodies (mAbs) in recent literature (2010–12).
| Antibody | Species | Specificity | Binding affinity and use |
|---|---|---|---|
| Ch63 and Ch82M full-length mAbs | Human–mouse chimeras | Distinct, undefined epitopes on SEB. No cross-reactivity with SEA or TSST-1. | Ch63 = 437 pM (KD) for SEB by surface plasmon resonance (SPR). Ch82M = 602 pM (KD) for SEB.30 In vitro neutralization of SEB-induced proliferation in murine splenocytes from BALB/C or HLA-DR3 transgenics. Neutralization of SEB in human PBMC assays. Synergistic protection afforded by mAbs. |
| Ten fAbs with two converted to full-length mAbs | Human | No reactivity with SEA, TSST-1, or SPEA. Varying reactivities with SEB, SEC1, SEC2, and SPEC. | KD range of 1.1 μM–1.3 nM for SEB by SPR.42 In vitro neutralization of SEB, SEC1, and SPEC in human PBMC assays. Neutralization of SEB in lethal shock model (murine). |
| fAbs and full-length mAbs | Human | Varying reactivities with SEA, SEB, SEC1, and SED | KD range of three best clones (1.2 nM–320 pM) for SEB by SPR.148 In vitro neutralization of SEB in human PBMC assays. Neutralization of SEB in lethal shock model (murine). Antibodies are stable toward heat and cold over 15 d. |
| Single domain antibody (sdAb) A3 consisting of only heavy chain | Lama | Specific for SEB, no cross-reactivity with SEA, SED, or Shiga toxin | KD range of 75 - 600 pM for SEB by SPR.149 Used as capture and detector antibody in Luminex-based assays for SEB (64 pg/ml detection limits). sdAb A3 is heat stable up to 80°C. |
| fAb and single-chain variable (scFv) fragments from commercial mAb (ab53981) |
Mouse | SEB only tested with fAb and scFv fragments. Intact ab53981 recognizes N-terminal epitope 8PDELHKS,14 as well as SEC2 and SED. | fAb fragment = 4.1 pM (KD) for SEB by SPR.150 scFv = 0.8 pM (KD) Antibody fragments used in establishing detection limits for SEB by ELISA (0.5 ng) and western blot (25 ng) |
A further twist for deriving characterized immunoreagents for therapy or diagnostic purposes includes recombinant single-domain, heavy-chain antibodies of lama origins that target SEB.149 Lama antibodies consist of only heavy chains and are reportedly much more thermal stable than “traditional” antibodies from other mammals. This unique attribute of camelids, found also in sharks, could be quite useful in situations where a “cold chain” is not available for preserving reagents. fAb fragments (single-chain mouse) against SEB derived from phage display can also be useful, namely for diagnostic purposes, as host reactivity against heterologous species can be an issue.150 Overall, these recombinant antibody-based approaches seem sage, as it is possible that one (or more) antibodies can be used to target one (or more) SEs as fully characterized reagents vs. relatively uncharacterized polyclonal preparations. It would be interesting to ascertain the ability of these, and other, monoclonal antibodies to neutralize natural variants of SEB and other SEs.151 Due to evolution and natural genetic drift, molecular variants of protein toxins (and other virulence factors) should always be considered for efficacy testing with any potential vaccines and therapeutics.
In addition to therapeutics, various groups have also developed different experimental vaccines for the staphylococcal superantigens. This approach for protection is logical, as the use of IVIg has also proven useful in humans following the onset of toxic shock.28,29,146,147 Experimentally, passive transfer of SEB-specific antibodies to naïve monkeys up to 4 h after an SEB aerosol also prevents lethal shock.152 Recombinantly attenuated mutants of SEA, SEB, and TSST-1 that do not bind MHC II and/or Vβ TCR molecules represent successful, experimental vaccines for preventing toxic shock in different animal models.22-27,109,153,154 When given either parenterally or mucosally, these vaccines do not cause ill-effects and are efficacious against a toxin challenge or S. aureus infection (Table 5).
Table 5. Vaccine studies for staphylococcal superantigens.
| Animal | Immunogen/adjuvant | Route | Results |
|---|---|---|---|
| Mouse Balb/c | SEB (N23K or F44S mutants)/aluminum hydroxide | i.p. | 80% protection against 30 LD50 SEB challenge (i.p.) among vaccinated animals, vs. 7% protection for adjuvant-only controls. Sera from vaccinated mice protected naïve animals against lethal SEB challenge.153 |
| Mouse Balb/c | SEB (L45R,Y89A,Y94A triple mutant)/aluminum hydroxide (i.p. route) or cholera toxin (i.n. and oral routes) | i.p. i.n. oral | Among i.p./i.n. vaccinated mice, there was 100% protection against either an 8 LD50 (aerosol) or 30 LD50 (i.p.) SEB challenge. Oral vaccination yielded 38% and 75% protection rates toward an ip or aerosol challenge, respectively. Only 0–10% of adjuvant-only controls were protected against either SEB challenge.23,25 |
| Mouse Balb/c | TSST-1 (H135A mutant)/aluminum hydroxide | s.c. | Lethal S. aureus (i.v.) challenge resulted in 0% survival among adjuvant-only controls, vs. 60% protection for H135A-vaccinated animals27 |
| Mouse Balb/c | TSST-1 (H135A mutant)/RIBI | i.p. | Among the H135A-vaccinated animals, 67% were protected against a 15 LD50 challenge (i.p.) of TSST-1 vs. 8% for adjuvant-only controls22 |
| Mouse NMRI | SEA (L48R,Y92A,D70R triple mutant)/Freund’s | s.c. | Vaccinated mice challenged with S. aureus (i.v.) had a delayed time to death and decreased weight loss, vs. BSA- vaccinated controls. Hyperimmune serum protected naïve animals24 |
| Mouse transgenic for human HLA-DR3 and CD4 | SEB (L45R, Y89A, Y94A triple mutant)/RIBI | i.p. | 100% protection against a 10 μg SEB challenge (i.p.) and markedly decreased IFN/IL-6 levels in vaccinated, vs. adjuvant-control, animals100 |
| Monkey Rhesus | SEB (L45R, Y89A, Y94A triple mutant)/aluminum hydroxide | i.m. | A 20 μg dose given three times protected against SEB-induced hyperthermia, unlike adjuvant-only controls104 |
| Crossbred piglets | SEB (L45R, Y89A, Y94A)/ Cholera toxin | oral | No ill effects with vaccine. Toxin-specific serum IgG and fecal IgA detected but cholera toxin did not enhance antibody response. No efficacy challenge results154 |
i.m., intramuscular; i.n., intranasal; i.p., intraperitoneal; i.t., intratracheal; i.v.. intravenous; s.c., subcutaneous
The Summary
S. aureus produces various superantigens representing important virulence factors that interact with MHC II, TCR, and accessory molecules on host cells. The host’s abnormally elevated immune response toward SEs or TSST-1 via various proinflammatory cytokines can trigger severe illness and lethal shock. Similar sequence homologies, structural conformations, and biological activities among this family of protein exotoxins suggest a common pathway through divergent and/or convergent evolution. More of these fascinating proteins, not only from S. aureus but also other microbes, will no doubt be discovered and novel biological properties likely elucidated by future investigators. Different assays, and more refinement of those that exist to date, will help lead the way to further discovery of much needed therapeutics and vaccines. There is an inherent urgency for more work in this field that extends well beyond biodefense. Bacterial pathogens like S. aureus, with wide dissemination among various mammalian species, multiple virulence factors, and increasing antibiotic resistance will be an omnipresent problem for many years into the future. We simply have now few effective tools for efficiently countering this bacterium and its various toxins like the SEs and TSST-1. Society needs, and increasingly demands (rightly so!), more effective controls of toxigenic pathogens like S. aureus. The future is now.
Acknowledgments
We thank the Defense Threat Reduction Agency for generous support. The views expressed in this publication are those of the author and do not reflect the official policy or position of the Department of the Army, the Department of Defense, or the US government.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/23905
References
- 1.Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME. Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence. 2011;2:445–59. doi: 10.4161/viru.2.5.17724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uchiyama T, Imanishi K, Miyoshi-Akiyama T, Kata H. Staphylococcal superantigens and the diseases they cause. In: Alouf JE, Popoff MR, eds. The Comprehensive Sourcebook of Bacterial Protein Toxins, 3rd Edition. London, Academic Press, 2006:830-43. [Google Scholar]
- 3.Thurlow LR, Joshi GS, Richardson AR. Virulence strategies of the dominant USA300 lineage of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) FEMS Immunol Med Microbiol. 2012;65:5–22. doi: 10.1111/j.1574-695X.2012.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham PL, 3rd, Lin SX, Larson EL. A U.S. A U.S. population-based survey of Staphylococcus aureus colonization. Ann Intern Med. 2006;144:318–25. doi: 10.7326/0003-4819-144-5-200603070-00006. [DOI] [PubMed] [Google Scholar]
- 5.Fraser JD, Proft T. The bacterial superantigen and superantigen-like proteins. Immunol Rev. 2008;225:226–43. doi: 10.1111/j.1600-065X.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- 6.Tang YW, Stratton CW. Staphylococcus aureus: An old pathogen with new weapons. Clin Lab Med. 2010;30:179–208. doi: 10.1016/j.cll.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Gould IM, David MZ, Esposito S, Garau J, Lina G, Mazzei T, et al. New insights into meticillin-resistant Staphylococcus aureus (MRSA) pathogenesis, treatment and resistance. Int J Antimicrob Agents. 2012;39:96–104. doi: 10.1016/j.ijantimicag.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 8.van Hal SJ, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis. 2012;54:755–71. doi: 10.1093/cid/cir935. [DOI] [PubMed] [Google Scholar]
- 9.David MZ, Medvedev S, Hohmann SF, Ewigman B, Daum RS. Increasing burden of methicillin-resistant Staphylococcus aureus hospitalizations at US academic medical centers, 2003-2008. Infect Control Hosp Epidemiol. 2012;33:782–9. doi: 10.1086/666640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulrich RG, Wilhelmsen CL, Krakauer TD. Staphylococcal enterotoxin B and related toxins. In: Zygmund Dembek, ed. Textbook of Military Medicine: Medical Aspects of Biological Warfare, US Department of Army. Washington DC, Borden Institute, 2007: 311-22. [Google Scholar]
- 11.Madsen JM. Toxins as weapons of mass destruction. A comparison and contrast with biological-warfare and chemical-warfare agents. Clin Lab Med. 2001;21:593–605. [PubMed] [Google Scholar]
- 12.Le Loir Y, Baron F, Gautier M. Staphylococcus aureus and food poisoning. Genet Mol Res. 2003;2:63–76. [PubMed] [Google Scholar]
- 13.McGann VG, Rollins JB, Mason DW. Evaluation of resistance to staphylococcal enterotoxin B: naturally acquired antibodies of man and monkey. J Infect Dis. 1971;124:206–13. doi: 10.1093/infdis/124.2.206. [DOI] [PubMed] [Google Scholar]
- 14.Bergdoll MS. Monkey feeding test for staphylococcal enterotoxin. Methods Enzymol. 1988;165:324–33. doi: 10.1016/S0076-6879(88)65048-8. [DOI] [PubMed] [Google Scholar]
- 15.Schelin J, Wallin-Carlquist N, Cohn MT, Lindqvist R, Barker GC, Rådström P. The formation of Staphylococcus aureus enterotoxin in food environments and advances in risk assessment. Virulence. 2011;2:580–92. doi: 10.4161/viru.2.6.18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdhary VR, Tilahun AY, Clark CR, Grande JP, Rajagopalan G. Chronic exposure to staphylococcal superantigen elicits a systemic inflammatory disease mimicking lupus. J Immunol. 2012;189:2054–62. doi: 10.4049/jimmunol.1201097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jett M, Brinkley W, Neill R, Gemski P, Hunt R. Staphylococcus aureus enterotoxin B challenge of monkeys: correlation of plasma levels of arachidonic acid cascade products with occurrence of illness. Infect Immun. 1990;58:3494–9. doi: 10.1128/iai.58.11.3494-3499.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheuber PH, Denzlinger C, Wilker D, Beck G, Keppler D, Hammer DK. Staphylococcal enterotoxin B as a nonimmunological mast cell stimulus in primates: the role of endogenous cysteinyl leukotrienes. Int Arch Allergy Appl Immunol. 1987;82:289–91. doi: 10.1159/000234209. [DOI] [PubMed] [Google Scholar]
- 19.Parsonnet J, Hansmann MA, Seymour JL, Delaney ML, Dubois AM, Modern PA, et al. Persistence survey of toxic shock syndrome toxin-1 producing Staphylococcus aureus and serum antibodies to this superantigen in five groups of menstruating women. BMC Infect Dis. 2010;10:249. doi: 10.1186/1471-2334-10-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahlknecht U, Herter M, Hoffmann MK, Niethammer D, Dannecker GE. The toxic shock syndrome toxin-1 induces anergy in human T cells in vivo. Hum Immunol. 1996;45:42–5. doi: 10.1016/0198-8859(95)00145-X. [DOI] [PubMed] [Google Scholar]
- 21.Hofer MF, Newell K, Duke RC, Schlievert PM, Freed JH, Leung DY. Differential effects of staphylococcal toxic shock syndrome toxin-1 on B cell apoptosis. Proc Natl Acad Sci U S A. 1996;93:5425–30. doi: 10.1073/pnas.93.11.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stiles BG, Krakauer T, Bonventre PF. Biological activity of toxic shock syndrome toxin 1 and a site-directed mutant, H135A, in a lipopolysaccharide-potentiated mouse lethality model. Infect Immun. 1995;63:1229–34. doi: 10.1128/iai.63.4.1229-1234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stiles BG, Garza AR, Ulrich RG, Boles JW. Mucosal vaccination with recombinantly attenuated staphylococcal enterotoxin B and protection in a murine model. Infect Immun. 2001;69:2031–6. doi: 10.1128/IAI.69.4.2031-2036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson IM, Verdrengh M, Ulrich RG, Bavari S, Tarkowski A. Protection against Staphylococcus aureus sepsis by vaccination with recombinant staphylococcal enterotoxin A devoid of superantigenicity. J Infect Dis. 1999;180:1370–3. doi: 10.1086/315023. [DOI] [PubMed] [Google Scholar]
- 25.Bavari S, Dyas B, Ulrich RG. Superantigen vaccines: a comparative study of genetically attenuated receptor-binding mutants of staphylococcal enterotoxin A. J Infect Dis. 1996;174:338–45. doi: 10.1093/infdis/174.2.338. [DOI] [PubMed] [Google Scholar]
- 26.Ulrich RG, Olson MA, Bavari S. Development of engineered vaccines effective against structurally related bacterial superantigens. Vaccine. 1998;16:1857–64. doi: 10.1016/S0264-410X(98)00176-5. [DOI] [PubMed] [Google Scholar]
- 27.Hu DL, Omoe K, Sasaki S, Sashinami H, Sakuraba H, Yokomizo Y, et al. Vaccination with nontoxic mutant toxic shock syndrome toxin 1 protects against Staphylococcus aureus infection. J Infect Dis. 2003;188:743–52. doi: 10.1086/377308. [DOI] [PubMed] [Google Scholar]
- 28.Schlievert PM. Use of intravenous immunoglobulin in the treatment of staphylococcal and streptococcal toxic shock syndromes and related illnesses. J Allergy Clin Immunol. 2001;108(Suppl):S107–10. doi: 10.1067/mai.2001.117820. [DOI] [PubMed] [Google Scholar]
- 29.Toussaint S, Gerlach H. Immunoglobulins in adult sepsis and septic shock. Curr Infect Dis Rep. 2012;14:522–9. doi: 10.1007/s11908-012-0287-z. [DOI] [PubMed] [Google Scholar]
- 30.Tilahun ME, Rajagopolan G, Shah-Mahoney N, Lawlor RG, Tilahun AY, Xie C, et al. Potent neutralization of staphylococcal enterotoxin B by synergistic action of chimeric antibodies. Infect Immun. 2010;78:2801–11. doi: 10.1128/IAI.01121-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nowakowski A, Wang C, Powers DB, Amersdorfer P, Smith TJ, Montgomery VA, et al. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc Natl Acad Sci U S A. 2002;99:11346–50. doi: 10.1073/pnas.172229899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi YW, Kotzin B, Herron L, Callahan J, Marrack P, Kappler J. Interaction of Staphylococcus aureus toxin “superantigens” with human T cells. Proc Natl Acad Sci U S A. 1989;86:8941–5. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alouf JE, Muller-Alouf H. What are superantigens? In: Alouf JE, Popoff MR, eds. The Comprehensive Sourcebook of Bacterial Protein Toxins, 3rd Edition. London, Academic Press, 2006:821-9. [Google Scholar]
- 34.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lina G, Bohach GA, Nair SP, Hiramatsu K, Jouvin-Marche E, Mariuzza R, International Nomenclature Committee for Staphylococcal Superantigens Standard nomenclature for the superantigens expressed by Staphylococcus. J Infect Dis. 2004;189:2334–6. doi: 10.1086/420852. [DOI] [PubMed] [Google Scholar]
- 36.Singh BR, Fu FN, Ledoux DN. Crystal and solution structures of superantigenic staphylococcal enterotoxins compared. Nat Struct Biol. 1994;1:358–60. doi: 10.1038/nsb0694-358. [DOI] [PubMed] [Google Scholar]
- 37.Papageorgiou AC, Tranter HS, Acharya KR. Crystal structure of microbial superantigen staphylococcal enterotoxin B at 1.5 A resolution: implications for superantigen recognition by MHC class II molecules and T-cell receptors. J Mol Biol. 1998;277:61–79. doi: 10.1006/jmbi.1997.1577. [DOI] [PubMed] [Google Scholar]
- 38.Garcia C, Briggs C, Zhang L, Guan L, Gabriel JL, Rogers TJ. Molecular characterization of the putative T-cell receptor cavity of the superantigen staphylococcal enterotoxin B. Immunology. 1998;94:160–6. doi: 10.1046/j.1365-2567.1998.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kappler JW, Herman A, Clements J, Marrack P. Mutations defining functional regions of the superantigen staphylococcal enterotoxin B. J Exp Med. 1992;175:387–96. doi: 10.1084/jem.175.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kum WW, Chow AW. Inhibition of staphylococcal enterotoxin A-induced superantigenic and lethal activities by a monoclonal antibody to toxic shock syndrome toxin-1. J Infect Dis. 2001;183:1739–48. doi: 10.1086/320732. [DOI] [PubMed] [Google Scholar]
- 41.Bavari S, Ulrich RG, LeClaire RD. Cross-reactive antibodies prevent the lethal effects of Staphylococcus aureus superantigens. J Infect Dis. 1999;180:1365–9. doi: 10.1086/314977. [DOI] [PubMed] [Google Scholar]
- 42.Larkin EA, Stiles BG, Ulrich RG. Inhibition of toxic shock by human monoclonal antibodies against staphylococcal enterotoxin B. PLoS One. 2010;5:e13253. doi: 10.1371/journal.pone.0013253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jardetzky TS, Brown JH, Gorga JC, Stern LJ, Urban RG, Chi YI, et al. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature. 1994;368:711–8. doi: 10.1038/368711a0. [DOI] [PubMed] [Google Scholar]
- 44.Redpath S, Alam SM, Lin CM, O’Rourke AM, Gascoigne NR. Cutting edge: trimolecular interaction of TCR with MHC class II and bacterial superantigen shows a similar affinity to MHC:peptide ligands. J Immunol. 1999;163:6–10. [PubMed] [Google Scholar]
- 45.Yagi JJ, Rath S, Janeway CA., Jr. Control of T cell responses to staphylococcal enterotoxins by stimulator cell MHC class II polymorphism. J Immunol. 1991;147:1398–405. [PubMed] [Google Scholar]
- 46.Kim J, Urban RG, Strominger JL, Wiley DC. Toxic shock syndrome toxin-1 complexed with a class II major histocompatibility molecule HLA-DR1. Science. 1994;266:1870–4. doi: 10.1126/science.7997880. [DOI] [PubMed] [Google Scholar]
- 47.Seo KS, Park JY, Terman DS, Bohach GA. A quantitative real time PCR method to analyze T cell receptor Vbeta subgroup expansion by staphylococcal superantigens. J Transl Med. 2010;8:2. doi: 10.1186/1479-5876-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newton DW, Dohlsten M, Olsson C, Segrén S, Lundin KE, Lando PA, et al. Mutations in the MHC class II binding domains of staphylococcal enterotoxin A differentially affect T cell receptor Vbeta specificity. J Immunol. 1996;157:3988–94. [PubMed] [Google Scholar]
- 49.Arad G, Levy R, Nasie I, Hillman D, Rotfogel Z, Barash U, et al. Binding of superantigen toxins into the CD28 homodimer interface is essential for induction of cytokine genes that mediate lethal shock. PLoS Biol. 2011;9:e1001149. doi: 10.1371/journal.pbio.1001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krakauer T. Small nonpeptide inhibitors of staphylococcal superantigen-induced cytokine production and toxic shock. In: Kotb M, Fraser JD, eds. Superantigens, Molecular Basis for Their Role in Human Diseases. ASM Press, 2007.229-44. [Google Scholar]
- 51.Cemerski S, Shaw A. Immune synapses in T-cell activation. Curr Opin Immunol. 2006;18:298–304. doi: 10.1016/j.coi.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 52.Krakauer T. Costimulatory receptors for the superantigen staphylococcal enterotoxin B on human vascular endothelial cells and T cells. J Leukoc Biol. 1994;56:458–63. doi: 10.1002/jlb.56.4.458. [DOI] [PubMed] [Google Scholar]
- 53.Kissner TL, Ruthel G, Alam S, Mann E, Ajami D, Rebek M, et al. Therapeutic inhibition of pro-inflammatory signaling and toxicity to staphylococcal enterotoxin B by a synthetic dimeric BB-loop mimetic of MyD88. PLoS One. 2012;7:e40773. doi: 10.1371/journal.pone.0040773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krakauer T. Induction of CC chemokines in human peripheral blood mononuclear cells by staphylococcal exotoxins and its prevention by pentoxifylline. J Leukoc Biol. 1999;66:158–64. doi: 10.1002/jlb.66.1.158. [DOI] [PubMed] [Google Scholar]
- 55.Krakauer T. Differential inhibitory effects of interleukin-10, interleukin-4, and dexamethasone on staphylococcal enterotoxin-induced cytokine production and T cell activation. J Leukoc Biol. 1995;57:450–4. doi: 10.1002/jlb.57.3.450. [DOI] [PubMed] [Google Scholar]
- 56.Krakauer T, Stiles BG. Pentoxifylline inhibits superantigen-induced toxic shock and cytokine release. Clin Diagn Lab Immunol. 1999;6:594–8. doi: 10.1128/cdli.6.4.594-598.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hale ML, Margolin SB, Krakauer T, Roy CJ, Stiles BG. Pirfenidone blocks the in vitro and in vivo effects of staphylococcal enterotoxin B. Infect Immun. 2002;70:2989–94. doi: 10.1128/IAI.70.6.2989-2994.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hermann C, von Aulock S, Graf K, Hartung T. A model of human whole blood lymphokine release for in vitro and ex vivo use. J Immunol Methods. 2003;275:69–79. doi: 10.1016/S0022-1759(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 59.Carlsson R, Fischer H, Sjögren HO. Binding of staphylococcal enterotoxin A to accessory cells is a requirement for its ability to activate human T cells. J Immunol. 1988;140:2484–8. [PubMed] [Google Scholar]
- 60.Lagoo AS, Lagoo-Deenadayalan S, Lorenz HM, Byrne J, Barber WH, Hardy KJ. IL-2, IL-4, and IFN-gamma gene expression versus secretion in superantigen-activated T cells. Distinct requirement for costimulatory signals through adhesion molecules. J Immunol. 1994;152:1641–52. [PubMed] [Google Scholar]
- 61.Huvenne W, Callebaut I, Reekmans K, Hens G, Bobic S, Jorissen M, et al. Staphylococcus aureus enterotoxin B augments granulocyte migration and survival via airway epithelial cell activation. Allergy. 2010;65:1013–20. doi: 10.1111/j.1398-9995.2009.02313.x. [DOI] [PubMed] [Google Scholar]
- 62.Stohl W, Elliott JE, Linsley PS. Human T cell-dependent B cell differentiation induced by staphylococcal superantigens. J Immunol. 1994;153:117–27. [PubMed] [Google Scholar]
- 63.Hamad AR, Marrack P, Kappler JW. Transcytosis of staphylococcal superantigen toxins. J Exp Med. 1997;185:1447–54. doi: 10.1084/jem.185.8.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shupp JW, Jett M, Pontzer CH. Identification of a transcytosis epitope on staphylococcal enterotoxins. Infect Immun. 2002;70:2178–86. doi: 10.1128/IAI.70.4.2178-2186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spiekermann GM, Nagler-Anderson C. Oral administration of the bacterial superantigen staphylococcal enterotoxin B induces activation and cytokine production by T cells in murine gut-associated lymphoid tissue. J Immunol. 1998;161:5825–31. [PubMed] [Google Scholar]
- 66.Hodoval LF, Morris EL, Crawley GJ, Beisel WR. Pathogenesis of lethal shock after intravenous staphylococcal enterotoxin B in monkeys. Appl Microbiol. 1968;16:187–92. doi: 10.21236/ad0666852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raj HD, Bergdoll MS. Effect of enterotoxin B on human volunteers. J Bacteriol. 1969;98:833–4. doi: 10.1128/jb.98.2.833-834.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tilahun AY, Holz M, Wu T-T, David CS, Rajagopalan G. Interferon gamma-dependent intestinal pathology contributes to the lethality in bacterial superantigen-induced toxic shock syndrome. PLoS One. 2011;6:e16764. doi: 10.1371/journal.pone.0016764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neumann B, Engelhardt B, Wagner H, Holzmann B. Induction of acute inflammatory lung injury by staphylococcal enterotoxin B. J Immunol. 1997;158:1862–71. [PubMed] [Google Scholar]
- 70.Lu J, Wang A, Ansari S, Hershberg RM, McKay DM. Colonic bacterial superantigens evoke an inflammatory response and exaggerate disease in mice recovering from colitis. Gastroenterology. 2003;125:1785–95. doi: 10.1053/j.gastro.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 71.Buxser S, Bonventre PF. Staphylococcal enterotoxins fail to disrupt membrane integrity or synthetic functions of Henle 407 intestinal cells. Infect Immun. 1981;31:929–34. doi: 10.1128/iai.31.3.929-934.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu J, Philpott DJ, Saunders PR, Perdue MH, Yang PC, McKay DM. Epithelial ion transport and barrier abnormalities evoked by superantigen-activated immune cells are inhibited by interleukin-10 but not interleukin-4. J Pharmacol Exp Ther. 1998;287:128–36. [PubMed] [Google Scholar]
- 73.McKay DM. Bacterial superantigens: provocateurs of gut dysfunction and inflammation? Trends Immunol. 2001;22:497–501. doi: 10.1016/S1471-4906(01)02000-2. [DOI] [PubMed] [Google Scholar]
- 74.Krakauer T. Stimulant-dependent modulation of cytokines and chemokines by airway epithelial cells: cross talk between pulmonary epithelial and peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2002;9:126–31. doi: 10.1128/CDLI.9.1.126-131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hanna-Wakim R, Yasukawa LL, Sung P, Fang M, Sullivan B, Rinki M, et al. Age-related increase in the frequency of CD4(+) T cells that produce interferon-gamma in response to staphylococcal enterotoxin B during childhood. J Infect Dis. 2009;200:1921–7. doi: 10.1086/648375. [DOI] [PubMed] [Google Scholar]
- 76.Hoffman M, Tremaine M, Mansfield J, Betley M. Biochemical and mutational analysis of the histidine residues of staphylococcal enterotoxin A. Infect Immun. 1996;64:885–90. doi: 10.1128/iai.64.3.885-890.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alber G, Hammer DK, Fleischer B. Relationship between enterotoxic- and T lymphocyte-stimulating activity of staphylococcal enterotoxin B. J Immunol. 1990;144:4501–6. [PubMed] [Google Scholar]
- 78.Krakauer T, Buckley MJ, Fisher D. Proinflammatory mediators of toxic shock and their correlation to lethality. Mediators Inflamm. 2010;2010:517594. doi: 10.1155/2010/517594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krakauer T, Buckley MJ, Huzella LM, Alves DA. Critical timing, location and duration of glucocorticoid administration rescue mice from superantigen-induced shock and attenuate lung injury. Int Immunopharmacol. 2009;9:1168–74. doi: 10.1016/j.intimp.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 80.Krakauer T, Buckley M, Fisher D. Murine models of staphylococcal enterotoxin B-induced toxic shock. Mil Med. 2010;175:917–22. doi: 10.7205/milmed-d-10-00148. [DOI] [PubMed] [Google Scholar]
- 81.Stiles BG, Bavari S, Krakauer T, Ulrich RG. Toxicity of staphylococcal enterotoxins potentiated by lipopolysaccharide: major histocompatibility complex class II molecule dependency and cytokine release. Infect Immun. 1993;61:5333–8. doi: 10.1128/iai.61.12.5333-5338.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen JY, Qiao Y, Komisar JL, Baze WB, Hsu IC, Tseng J. Increased susceptibility to staphylococcal enterotoxin B intoxication in mice primed with actinomycin D. Infect Immun. 1994;62:4626–31. doi: 10.1128/iai.62.10.4626-4631.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sarawar SR, Blackman MA, Doherty PC. Superantigen shock in mice with an inapparent viral infection. J Infect Dis. 1994;170:1189–94. doi: 10.1093/infdis/170.5.1189. [DOI] [PubMed] [Google Scholar]
- 84.Dinges MM, Schlievert PM. Comparative analysis of lipopolysaccharide-induced tumor necrosis factor alpha activity in serum and lethality in mice and rabbits pretreated with the staphylococcal superantigen toxic shock syndrome toxin 1. Infect Immun. 2001;69:7169–72. doi: 10.1128/IAI.69.11.7169-7172.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moon IJ, Hong SL, Kim DY, Lee CH, Rhee CS, Min YG. Blocking interleukin-17 attenuates enhanced inflammation by staphylococcal enterotoxin B in murine allergic rhinitis model. Acta Otolaryngol. 2012;132(Suppl 1):S6–12. doi: 10.3109/00016489.2012.661074. [DOI] [PubMed] [Google Scholar]
- 86.Fernandez S, Cisney ED, Hall SI, Ulrich RG. Nasal immunity to staphylococcal toxic shock is controlled by the nasopharynx-associated lymphoid tissue. Clin Vaccine Immunol. 2011;18:667–75. doi: 10.1128/CVI.00477-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miethke T, Wahl C, Heeg K, Echtenacher B, Krammer PH, Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J Exp Med. 1992;175:91–8. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paiva CN, Pyrrho AS, Lannes-Vieira J, Vacchio M, Soares MB, Gattass CR. Trypanosoma cruzi sensitizes mice to fulminant SEB-induced shock: overrelease of inflammatory cytokines and independence of Chagas’ disease or TCR Vbeta-usage. Shock. 2003;19:163–8. doi: 10.1097/00024382-200302000-00013. [DOI] [PubMed] [Google Scholar]
- 89.Schartner JM, Singh AM, Dahlberg PE, Nettenstrom L, Seroogy CM. Recurrent superantigen exposure in vivo leads to highly suppressive CD4+CD25+ and CD4+CD25- T cells with anergic and suppressive genetic signatures. Clin Exp Immunol. 2009;155:348–56. doi: 10.1111/j.1365-2249.2008.03827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eroukhmanoff L, Oderup C, Ivars F. T-cell tolerance induced by repeated antigen stimulation: selective loss of Foxp3- conventional CD4 T cells and induction of CD4 T-cell anergy. Eur J Immunol. 2009;39:1078–87. doi: 10.1002/eji.200838653. [DOI] [PubMed] [Google Scholar]
- 91.Collins LV, Eriksson K, Ulrich RG, Tarkowski A. Mucosal tolerance to a bacterial superantigen indicates a novel pathway to prevent toxic shock. Infect Immun. 2002;70:2282–7. doi: 10.1128/IAI.70.5.2282-2287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stiles BG, Campbell YG, Castle RM, Grove SA. Correlation of temperature and toxicity in murine studies of staphylococcal enterotoxins and toxic shock syndrome toxin 1. Infect Immun. 1999;67:1521–5. doi: 10.1128/iai.67.3.1521-1525.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beno DW, Uhing MR, Jiyamapa-Serna VA, Goto M, Chen Y, Vasan A, et al. Differential induction of hepatic dysfunction after intraportal and intravenous challenge with endotoxin and Staphylococcal enterotoxin B. Shock. 2003;19:352–7. doi: 10.1097/00024382-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 94.Schlievert PM. Enhancement of host susceptibility to lethal endotoxin shock by staphylococcal pyrogenic exotoxin type C. Infect Immun. 1982;36:123–8. doi: 10.1128/iai.36.1.123-128.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chow AW, Bartlett KH, Percival-Smith R, Morrison BJ. Vaginal colonization with Staphylococcus aureus, positive for toxic-shock marker protein, and Escherichia coli in healthy women. J Infect Dis. 1984;150:80–4. doi: 10.1093/infdis/150.1.80. [DOI] [PubMed] [Google Scholar]
- 96.Florquin S, Amraoui Z, Abramowicz D, Goldman M. Systemic release and protective role of IL-10 in staphylococcal enterotoxin B-induced shock in mice. J Immunol. 1994;153:2618–23. [PubMed] [Google Scholar]
- 97.Manjunath N, Correa M, Ardman M, Ardman B. Negative regulation of T-cell adhesion and activation by CD43. Nature. 1995;377:535–8. doi: 10.1038/377535a0. [DOI] [PubMed] [Google Scholar]
- 98.Yeung RS, Penninger JM, Kündig T, Khoo W, Ohashi PS, Kroemer G, et al. Human CD4 and human major histocompatibility complex class II (DQ6) transgenic mice: supersensitivity to superantigen-induced septic shock. Eur J Immunol. 1996;26:1074–82. doi: 10.1002/eji.1830260518. [DOI] [PubMed] [Google Scholar]
- 99.Silverstein R. D-galactosamine lethality model: scope and limitations. J Endotoxin Res. 2004;10:147–62. doi: 10.1179/096805104225004879. [DOI] [PubMed] [Google Scholar]
- 100.DaSilva L, Welcher BC, Ulrich RG, Aman MJ, David CS, Bavari S. Humanlike immune response of human leukocyte antigen-DR3 transgenic mice to staphylococcal enterotoxins: a novel model for superantigen vaccines. J Infect Dis. 2002;185:1754–60. doi: 10.1086/340828. [DOI] [PubMed] [Google Scholar]
- 101.Roy CJ, Warfield KL, Welcher BC, Gonzales RF, Larsen T, Hanson J, et al. Human leukocyte antigen-DQ8 transgenic mice: a model to examine the toxicity of aerosolized staphylococcal enterotoxin B. Infect Immun. 2005;73:2452–60. doi: 10.1128/IAI.73.4.2452-2460.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao Y-X, Abdelnour A, Kalland T, Tarkowski A. Overexpression of the T-cell receptor V beta 3 in transgenic mice increases mortality during infection by enterotoxin A-producing Staphylococcus aureus. Infect Immun. 1995;63:4463–9. doi: 10.1128/iai.63.11.4463-4469.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vlach KD, Boles JW, Stiles BG. Telemetric evaluation of body temperature and physical activity as predictors of mortality in a murine model of staphylococcal enterotoxic shock. Comp Med. 2000;50:160–6. [PubMed] [Google Scholar]
- 104.Boles JW, Pitt ML, LeClaire RD, Gibbs PH, Ulrich RG, Bavari S. Correlation of body temperature with protection against staphylococcal enterotoxin B exposure and use in determining vaccine dose-schedule. Vaccine. 2003;21:2791–6. doi: 10.1016/S0264-410X(03)00222-6. [DOI] [PubMed] [Google Scholar]
- 105.Huzella LM, Buckley MJ, Alves DA, Stiles BG, Krakauer T. Central roles for IL-2 and MCP-1 following intranasal exposure to SEB: a new mouse model. Res Vet Sci. 2009;86:241–7. doi: 10.1016/j.rvsc.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 106.Rajagopalan G, Sen MM, Singh M, Murali NS, Nath KA, Iijima K, et al. Intranasal exposure to staphylococcal enterotoxin B elicits an acute systemic inflammatory response. Shock. 2006;25:647–56. doi: 10.1097/01.shk.0000209565.92445.7d. [DOI] [PubMed] [Google Scholar]
- 107.Huang WT, Lin MT, Won SJ. Staphylococcal enterotoxin A-induced fever is associated with increased circulating levels of cytokines in rabbits. Infect Immun. 1997;65:2656–62. doi: 10.1128/iai.65.7.2656-2662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mattix ME, Hunt RE, Wilhelmsen CL, Johnson AJ, Baze WB. Aerosolized staphylococcal enterotoxin B-induced pulmonary lesions in rhesus monkeys (Macaca mulatta) Toxicol Pathol. 1995;23:262–8. doi: 10.1177/019262339502300304. [DOI] [PubMed] [Google Scholar]
- 109.Boles JW, Pitt ML, LeClaire RD, Gibbs PH, Torres E, Dyas B, et al. Generation of protective immunity by inactivated recombinant staphylococcal enterotoxin B vaccine in nonhuman primates and identification of correlates of immunity. Clin Immunol. 2003;108:51–9. doi: 10.1016/S1521-6616(03)00066-4. [DOI] [PubMed] [Google Scholar]
- 110.Sundstedt A, Dohlsten M. In vivo anergized CD4+ T cells have defective expression and function of the activating protein-1 transcription factor. J Immunol. 1998;161:5930–6. [PubMed] [Google Scholar]
- 111.Florquin S, Amraoui Z, Goldman M. T cells made deficient in interleukin-2 production by exposure to staphylococcal enterotoxin B in vivo are primed for interferon-gamma and interleukin-10 secretion. Eur J Immunol. 1995;25:1148–53. doi: 10.1002/eji.1830250503. [DOI] [PubMed] [Google Scholar]
- 112.Parsonnet J, Gillis ZA, Richter AG, Pier GB. A rabbit model of toxic shock syndrome that uses a constant, subcutaneous infusion of toxic shock syndrome toxin 1. Infect Immun. 1987;55:1070–6. doi: 10.1128/iai.55.5.1070-1076.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Azavedo JCS, Arbuthnott JP. Toxicity of staphylococcal toxic shock syndrome toxin 1 in rabbits. Infect Immun. 1984;46:314–7. doi: 10.1128/iai.46.2.314-317.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pettit GW, Elwell MR, Jahrling PB. Possible endotoxemia in rabbits after intravenous injection of Staphylococcus aureus enterotoxin B. J Infect Dis. 1977;135:646–8. doi: 10.1093/infdis/135.4.646. [DOI] [PubMed] [Google Scholar]
- 115.Strandberg KL, Rotschafer JH, Vetter SM, Buonpane RA, Kranz DM, Schlievert PM. Staphylococcal superantigens cause lethal pulmonary disease in rabbits. J Infect Dis. 2010;202:1690–7. doi: 10.1086/657156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Van Miert AS, Van Duin CT, Schotman AJ. Comparative observations of fever and associated clinical hematological and blood biochemical changes after intravenous administration of staphylococcal enterotoxins B and F (toxic shock syndrome toxin-1) in goats. Infect Immun. 1984;46:354–60. doi: 10.1128/iai.46.2.354-360.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hui J, Cao Y, Xiao F, Zhang J, Li H, Hu F. Staphylococcus aureus enterotoxin C2 mutants: biological activity assay in vitro. J Ind Microbiol Biotechnol. 2008;35:975–80. doi: 10.1007/s10295-008-0372-3. [DOI] [PubMed] [Google Scholar]
- 118.Hu D-L, Omoe K, Sashinami H, Shinagawa K, Nakane A. Immunization with a nontoxic mutant of staphylococcal enterotoxin A, SEAD227A, protects against enterotoxin-induced emesis in house musk shrews. J Infect Dis. 2009;199:302–10. doi: 10.1086/596065. [DOI] [PubMed] [Google Scholar]
- 119.Maina EK, Hu D-L, Tsuji T, Omoe K, Nakane A. Staphylococcal enterotoxin A has potent superantigenic and emetic activities but not diarrheagenic activity. Int J Med Microbiol. 2012;302:88–95. doi: 10.1016/j.ijmm.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 120.Arad G, Levy R, Hillman D, Kaempfer R. Superantigen antagonist protects against lethal shock and defines a new domain for T-cell activation. Nat Med. 2000;6:414–21. doi: 10.1038/74672. [DOI] [PubMed] [Google Scholar]
- 121.Visvanathan K, Charles A, Bannan J, Pugach P, Kashfi K, Zabriskie JB. Inhibition of bacterial superantigens by peptides and antibodies. Infect Immun. 2001;69:875–84. doi: 10.1128/IAI.69.2.875-884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang S, Li Y, Xiong H, Cao J. A broad-spectrum inhibitory peptide against staphylococcal enterotoxin superantigen SEA, SEB and SEC. Immunol Lett. 2008;121:167–72. doi: 10.1016/j.imlet.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 123.Hong-Geller E, Möllhoff M, Shiflett PR, Gupta G. Design of chimeric receptor mimics with different TcRVbeta isoforms. Type-specific inhibition of superantigen pathogenesis. J Biol Chem. 2004;279:5676–84. doi: 10.1074/jbc.M309388200. [DOI] [PubMed] [Google Scholar]
- 124.Wang N, Mattis DM, Sundberg EJ, Schlievert PM, Kranz DM. A single, engineered protein therapeutic agent neutralizes exotoxins from both Staphylococcus aureus and Streptococcus pyogenes. Clin Vaccine Immunol. 2010;17:1781–9. doi: 10.1128/CVI.00277-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.DeGrasse JA. A single-stranded DNA aptamer that selectively binds to Staphylococcus aureus enterotoxin B. PLoS One. 2012;7:e33410. doi: 10.1371/journal.pone.0033410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.DiDonato JA, Mercurio F, Karin MNF. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 127.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 128.Liu D, Zienkiewicz J, DiGiandomenico A, Hawiger J. Suppression of acute lung inflammation by intracellular peptide delivery of a nuclear import inhibitor. Mol Ther. 2009;17:796–802. doi: 10.1038/mt.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tilahun AY, Theuer JE, Patel R, David CS, Rajagopalan G. Detrimental effect of the proteasome inhibitor, bortezomib in bacterial superantigen- and lipopolysaccharide-induced systemic inflammation. Mol Ther. 2010;18:1143–54. doi: 10.1038/mt.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Krakauer T, Buckley M. Dexamethasone attenuates staphylococcal enterotoxin B-induced hypothermic response and protects mice from superantigen-induced toxic shock. Antimicrob Agents Chemother. 2006;50:391–5. doi: 10.1128/AAC.50.1.391-395.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rieder SA, Nagarkatti P, Nagarkatti M. Multiple anti-inflammatory pathways triggered by resveratrol lead to amelioration of staphylococcal enterotoxin B-induced lung injury. Br J Pharmacol. 2012;167:1244–58. doi: 10.1111/j.1476-5381.2012.02063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Choi K, Lee K, Ryu SW, Im M, Kook KH, Choi C. Pirfenidone inhibits transforming growth factor-β1-induced fibrogenesis by blocking nuclear translocation of Smads in human retinal pigment epithelial cell line ARPE-19. Mol Vis. 2012;18:1010–20. [PMC free article] [PubMed] [Google Scholar]
- 133.Takei Y, Kunikata T, Aga M, Inoue S, Ushio S, Iwaki K, et al. Tryptanthrin inhibits interferon-γ production by Peyer’s patch lymphocytes derived from mice that had been orally administered staphylococcal enterotoxin. Biol Pharm Bull. 2003;26:365–7. doi: 10.1248/bpb.26.365. [DOI] [PubMed] [Google Scholar]
- 134.Pergola C, Jazzar B, Rossi A, Northoff H, Hamburger M, Sautebin L, et al. On the inhibition of 5-lipoxygenase product formation by tryptanthrin: mechanistic studies and efficacy in vivo. Br J Pharmacol. 2012;165:765–76. doi: 10.1111/j.1476-5381.2011.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen X, Krakauer T, Oppenheim JJ, Howard OM. Yin zi huang, an injectable multicomponent chinese herbal medicine, is a potent inhibitor of T-cell activation. J Altern Complement Med. 2004;10:519–26. doi: 10.1089/1075553041323687. [DOI] [PubMed] [Google Scholar]
- 136.Watson JL, Vicario M, Wang A, Moreto M, McKay DM. Immune cell activation and subsequent epithelial dysfunction by Staphylococcus enterotoxin B is attenuated by the green tea polyphenol (-)-epigallocatechin gallate. Cell Immunol. 2005;237:7–16. doi: 10.1016/j.cellimm.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 137.Krakauer T, Li BQ, Young H. The flavonoid baicalin inhibits staphylococcal superantigen-induced inflammatory cytokines and chemokines. FEBS Lett. 2001;500:52–5. doi: 10.1016/S0014-5793(01)02584-4. [DOI] [PubMed] [Google Scholar]
- 138.Saha B, Jaklic B, Harlan DM, Gray GS, June CH, Abe R. Toxic shock syndrome toxin-1-induced death is prevented by CTLA4Ig. J Immunol. 1996;157:3869–75. [PubMed] [Google Scholar]
- 139.Krakauer T, Buckley M, Issaq HJ, Fox SD. Rapamycin protects mice from staphylococcal enterotoxin B-induced toxic shock and blocks cytokine release in vitro and in vivo. Antimicrob Agents Chemother. 2010;54:1125–31. doi: 10.1128/AAC.01015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–37. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kissner TL, Moisan L, Mann E, Alam S, Ruthel G, Ulrich RG, et al. A small molecule that mimics the BB-loop in the Toll interleukin-1 (IL-1) receptor domain of MyD88 attenuates staphylococcal enterotoxin B-induced pro-inflammatory cytokine production and toxicity in mice. J Biol Chem. 2011;286:31385–96. doi: 10.1074/jbc.M110.204982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jo D, Liu D, Yao S, Collins RD, Hawiger J. Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat Med. 2005;11:892–8. doi: 10.1038/nm1269. [DOI] [PubMed] [Google Scholar]
- 143.Sun J, Law GP, Bridges CC, McKallip RJ. CD44 as a novel target for treatment of staphylococcal enterotoxin B-induced acute inflammatory lung injury. Clin Immunol. 2012;144:41–52. doi: 10.1016/j.clim.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 144.Kansal R, Davis C, Hansmann M, Seymour J, Parsonnet J, Modern P, et al. Structural and functional properties of antibodies to the superantigen TSST-1 and their relationship to menstrual toxic shock syndrome. J Clin Immunol. 2007;27:327–38. doi: 10.1007/s10875-007-9072-4. [DOI] [PubMed] [Google Scholar]
- 145.Adhikari RP, Ajao AO, Aman MJ, Karauzum H, Sarwar J, Lydecker AD, et al. Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis. 2012;206:915–23. doi: 10.1093/infdis/jis462. [DOI] [PubMed] [Google Scholar]
- 146.Darenberg J, Söderquist B, Normark BH, Norrby-Teglund A. Differences in potency of intravenous polyspecific immunoglobulin G against streptococcal and staphylococcal superantigens: implications for therapy of toxic shock syndrome. Clin Infect Dis. 2004;38:836–42. doi: 10.1086/381979. [DOI] [PubMed] [Google Scholar]
- 147.Ohlsen K, Lorenz U. Immunotherapeutic strategies to combat staphylococcal infections. Int J Med Microbiol. 2010;300:402–10. doi: 10.1016/j.ijmm.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 148.Karauzum H, Chen G, Abaandou L, Mahmoudieh M, Boroun AR, Shulenin S, et al. Synthetic human monoclonal antibodies toward staphylococcal enterotoxin B (SEB) protective against toxic shock syndrome. J Biol Chem. 2012;287:25203–15. doi: 10.1074/jbc.M112.364075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Graef RR, Anderson GP, Doyle KA, Zabetakis D, Sutton FN, Liu JL, et al. Isolation of a highly thermal stable lama single domain antibody specific for Staphylococcus aureus enterotoxin B. BMC Biotechnol. 2011;11:86. doi: 10.1186/1472-6750-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Urushibata Y, Itoh K, Ohshima M, Seto Y. Generation of Fab fragment-like molecular recognition proteins against staphylococcal enterotoxin B by phage display technology. Clin Vaccine Immunol. 2010;17:1708–17. doi: 10.1128/CVI.00229-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kohler PL, Greenwood SD, Nookala S, Kotb M, Kranz DM, Schlievert PM. Staphylococcus aureus isolates encode variant staphylococcal enterotoxin B proteins that are diverse in superantigenicity and lethality. PLoS One. 2012;7:e41157. doi: 10.1371/journal.pone.0041157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.LeClaire RD, Hunt RE, Bavari S. Protection against bacterial superantigen staphylococcal enterotoxin B by passive vaccination. Infect Immun. 2002;70:2278–81. doi: 10.1128/IAI.70.5.2278-2281.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Woody MA, Krakauer T, Ulrich RG, Stiles BG. Differential immune responses to staphylococcal enterotoxin B mutations in a hydrophobic loop dominating the interface with major histocompatibility complex class II receptors. J Infect Dis. 1998;177:1013–22. doi: 10.1086/515250. [DOI] [PubMed] [Google Scholar]
- 154.Inskeep TK, Stahl C, Odle J, Oakes J, Hudson L, Bost KL, et al. Oral vaccine formulations stimulate mucosal and systemic antibody responses against staphylococcal enterotoxin B in a piglet model. Clin Vaccine Immunol. 2010;17:1163–9. doi: 10.1128/CVI.00078-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Madej T, Addess KJ, Fong JH, Geer LY, Geer RC, Lanczycki CJ, et al. MMDB: 3D structures and macromolecular interactions. Nucleic Acids Res. 2012;40(Database issue):D461–4. doi: 10.1093/nar/gkr1162. [DOI] [PMC free article] [PubMed] [Google Scholar]