Abstract
Francisella tularensis is a gram-negative bacterium that causes the zoonotic disease tularemia. Francisella is highly infectious via the respiratory route (~10 CFUs) and pulmonary infections due to type A strains of F. tularensis are highly lethal in untreated patients (>30%). In addition, no vaccines are licensed to prevent tularemia in humans. Due to the high infectivity and mortality of pulmonary tularemia, F. tularensis has been weaponized, including via the introduction of antibiotic resistance, by several countries. Because of the lack of efficacious vaccines, and concerns about F. tularensis acquiring resistance to antibiotics via natural or illicit means, augmentation of host immunity, and humoral immunotherapy have been investigated as countermeasures against tularemia. This manuscript will review advances made and challenges in the field of immunotherapy against tularemia.
Keywords: Francisella, tularemia, biodefense, immunotherapy, immunity, pathogen
Tularemia
Francisella tularensis is a highly infectious, gram-negative facultative intracellular bacterium that causes the zoonotic disease tularemia. In 1911, tularemia was first described as a plague-like disease of rodents and soon after the potential of tularemia as a severe and fatal human illness was recognized.1F. tularensis infections can occur via insect or tick bites, cutaneous contact with infected animal carcasses, ingestion of contaminated food and water, or inhalation of viable organisms.2F. tularensis has long been known as a potential hazard to laboratory workers,3 and has been one of the most commonly reported laboratory-acquired infections in the United States.4 In nature, F. tularensis mainly exists in regions of the United States, Canada, Mexico, Japan, Europe, and the former Soviet Union. Several animals, including rabbits, muskrats, and beavers can serve as reservoirs of infection. Tularemia is also carried by ticks, deerflies, and mosquitoes. The type and severity of tularemia depends on the strain, dose, and route of infection.5F. tularensis subspecies tularensis (type A) and holarctica (type B) cause the majority of human cases, with subspecies tularensis being more virulent.5 Type A F. tularensis is found predominately in North America, while type B strains are found in Europe and Asia. All forms of tularemia generally present with sudden onset of fever, headaches, chills, sore throat, coryza, and generalized body aches 3–5 d after exposure.6 With appropriate antibiotic therapy, the overall mortality rate of reported tularemia cases in the United States is less than 2%.7,8
Cutaneous or ulceroglandular tularemia is the most common form of human disease (75–85% of patients), but is rarely fatal.6,9 A cutaneous papule appears at the site of infection around the time of generalized symptoms in ulceroglandular tularemia. The papule becomes a painful pustule and ulcerates within a few days of its first appearance. Regional lymph nodes also may become enlarged and tender within days of papule appearance. Even with appropriate antibiotic therapy, affected lymph nodes may rupture and become fluctuant and the ulcer and lymphadenopathy may persist for months.6,9
Pulmonary tularemia is the most severe form of disease and untreated pulmonary infections with type A F. tularensis infection have mortality rates >30%.10 Inhalation of F. tularensis results in respiratory or pneumonic tularemia and is most common in people in endemic areas who perform tasks that predispose them to infectious aerosols.5 Pulmonary tularemia can present from a mild pneumonia to an acute infection with high fever, malaise, chills, cough, delirium, and pulse-temperature dissociation.5,9 Hilar lymphadenopathy, pleural effusions, and bronchopneumonia are common radiographic findings; however, early radiologic evidence of pneumonia was found in only 25–50% of human volunteers who had developed systemic symptoms of acute illness following aerosol exposure to type A F. tularensis.9,11,12
F. tularensis as a Biological Weapon
The World Health Organization (WHO) estimated in 1970 that an aerosol dispersal of 50 kg of virulent F. tularensis over a metropolitan area of 5 million residents would result in 250 000 incapacitating casualties including 19 000 deaths.13 Disease was expected to persist for several weeks and relapses of illness would occur during the following weeks and months. In 1997, the CDC estimated that the total societal base costs of a F. tularensis aerosol attack would be $5.4 billion for every 100 000 exposed persons.14 While a live vaccine strain (LVS) derived from F. tularensis subspecies holarctica was created over 50 years ago, questions remain regarding its efficacy and possible reversion to virulence, and it is not licensed for human use.5 Due to the high infectivity (~10 microorganisms) and lethality of untreated pulmonary tularemia and the lack of available vaccines, F. tularensis has long been studied as a biological weapon by several nations. F. tularensis was tested on human subjects in occupied Manchuria as part of a Japanese germ warfare program from 1932 to 1945.15 Tularemia outbreaks that affected tens of thousands of German and Soviet soldiers in Eastern Europe in World War II were also suggested to be the result of an intentional Soviet release of F. tularensis;16 however, this hypothesis has been disputed.17 Following World War II, the United States military studied the pathophysiology of tularemia and researched vaccine development and antibiotic prophylaxis, including work investigating antibiotic therapy in human subjects exposed to virulent F. tularensis in aerosol chambers.11,12 The United States also developed weapons that would disseminate F. tularensis aerosols and F. tularensis was one of several biological weapons stockpiled by the United States in the 1960s until the inventories were destroyed in the early 1970s.18 Of particular concern would be the release of F. tularensis that had been weaponized via the introduction of antibiotic resistance. Indeed the United States developed streptomycin-resistant strains of F. tularensis before shuttering its offensive biological weapons program,19 and the Soviet Union was purported to develop antibiotic-resistant strains of Francisella perhaps until the 1990s.16 Due to these concerns, F. tularensis has been determined to be a Category A biological warfare agent by CDC, and the two major spikes in tularemia research publications have come shortly after World War II and the 2001 anthrax bioterrorist attacks in the United States (Fig. 1).
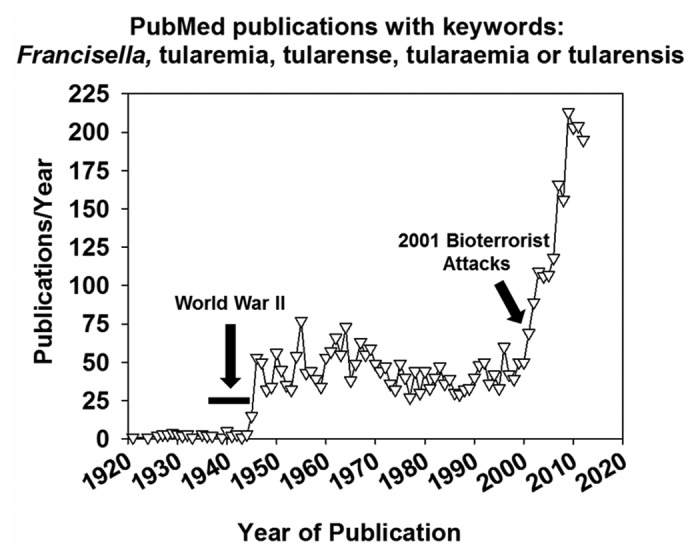
Figure 1. The number of articles published per year found in PubMed using the search phrase “Francisella or tularemia or tularense or tularaemia or tularensis”.
Precautions When Investigating Immunotherapy of Tularemia
The route of Francisella challenge affects induced immune responses,20 and rodents are generally much more susceptible to intraperitoneal (i.p.) or pulmonary challenge then to a subcutaneous (s.c.) or intradermal challenge (i.d.).21 The host species used for tularemia immunity and immunotherapy studies is also of importance due to varying susceptibilities. While mice are extremely susceptible to tularemia, rats, rabbits, and non-human primates are relatively more resistant to infection.21 Perhaps most importantly, emerging evidence indicates that immunity against virulent strains of F. tularensis that cause disease in humans differs from protective immune responses elicited by model strains of Francisella.22-25 Virulent strains of F. tularensis also employ mechanisms of immune suppression not found in model strains.26-28 In addition, vaccination and immunotherapeutic strategies that confer robust protection against attenuated strains of F. tularensis have been shown to have little to no efficacy against strains of Francisella that cause disease in humans.29-31 Therefore, the challenge strain of F. tularensis must be considered when considering the protective efficacy of immunotherapeutics. For reference, the relative virulence of common challenge strains of Francisella used in immunotherapy studies covered here is shown in Table 1 from information in reviews by Cowley and Elkins, and Wu and Lyons.21,32 In some early studies reviewed here, the challenge strain was called “virulent” but no strain designation was given. In these instances we will refer to these strains as “virulent F. tularensis”. Our review of the literature on Francisella immunotherapeutics generally follows a chronologic order under each heading or within a paragraph. However, Tables 2A, 2B, 3A, and 3B have been subdivided to clarify which immunotherapeutics are effective against virulent strains of Francisella such as SchuS4, vs. those that have only shown efficacy against model strains such as LVS or F. novicida. Also, immunotherapy strategies that confer survival of treated animals are listed before immunotherapeutics that extend the mean time to death or reduce bacterial burdens within the subdivisions of the tables. Immunity to Francisella has been recently reviewed by others,32,33 therefore in this review we will only discuss immunity in the context of protective immunotherapy. In addition, we will have a particular emphasis on immune agonists that induce protection against tularemia.
Table 1. Virulence in mammals of Francisella challenge strains in this reviewa.
| Strain | Challenge strains in this review | Human infection | Mouse infection | Rat infection |
|---|---|---|---|---|
| Subspecies tularensis type A | Schu, SchuS4, SchuS5, Vavenby | Highly infectious and can be fatal | LD50 < 10, all routes tested | LD50 = 5 × 102 IT |
| Subspecies holarctica type B | FSC 171 | Induces illness but rarely fatal | LD50 < 10, all routes tested | Unknown |
| Subspecies holarctica LVS | LVS | Attenuated Type B strain, skin or pulmonary infection induces mild side symptoms, induces protective immunity | LD50 = 106 ID, 103 IN, < 10 IP or IV | Unknown |
| F. novicida | U112 | Infections rare, sublethal, and associated with immunodeficiencies | LD50 = 103 ID, < 10 IN, IP, or IV | LD50 = 5 × 106 IT |
a Table adapted from Cowley and Elkins32
Table 2A. Immune agonists that confer protection against virulent Francisella infection.
| Agonist | Cellular receptor | Challenge strain, route, animal | Protective effect | Requirements for protection |
|---|---|---|---|---|
| Acai PS | TLR4? Carbohydrate Receptors? | Mice infected via aerosol with SchuS4 | i.n. pre- or post-treatment increased survival rates | IFN-γ |
| CLDC+MPF | TLR9+DAI | Mice infected i.n. with SchuS4 | i.v. pre-treatment increased survival rate | RNS/ROS |
| MPL | TLR4 | Mice infected i.n. with LVS or SchuS4 | i.p. pre-treatment increased survival rates in animals challenged with either strain. | ND |
| Yeast glucans | Carbohydrate receptors? | Rats infected i.p. with SchuS4 | i.v. pre-treatment increased survival rate | ND |
| Poly I:C | TLR3 | Mice infected i.n. with LVS or SchuS4 | i.n. pre- or post-treatment extended mean time to death in LVS- and SchuS4- challenged animals | ND |
| CLDC | TLR9+DAI | Mice infected i.n. with LVS or SchuS4 | i.n. pre-treatment increased survival rates in LVS-challenged mice and extended mean time to death in SchuS4-challenged mice | IFN-γ, NK cells (LVS-challenged mice) |
See text for references. ?, cellular receptor unknown or unclear; ND, not determined; astrain used for challenge unclear.
Table 2B. Immune agonists that confer protection against model strains of Francisella, but have unknown, or no protective effects against virulent F. tularensis .
| Agonist | Cellular receptor | Challenge strain, route, animal | Protective effect | Requirements for protection |
|---|---|---|---|---|
| Endotoxin | TLR4 | Mice infected i.p. with F. tularensisa | i.p. pre-treatment increased survival rate | ND |
| AGP | TLR4 | Mice infected i.n. with F. novicida | i.n. pre- and post-treatment increased survival rate | IFN-γ |
| LVS LPS | ? | Mice infected i.p. with LVS or i.n. with SchuS4 | i.p. pre-treatment increased survival rates in LVS challenged animals No protective effect observed in mice challenged with SchuS4 |
TLR2, IRAK4, B cells, antibody |
| CpG | TLR9 | Mice infected i.p. with LVS or i.n. with SchuS4 | i.p. pre-treatment increased survival rate in LVS challenged animals i.n. or i.p. pre-treatment had no protective effect in mice challenged i.n. with SchuS4 |
B cells, IFN-γ TLR9 |
| Poly I:C | TLR3 | Mice infected i.p. with F. tularensisa | i.p. pre-treatment extended mean time to death | ND |
| Tilorone | ? | Mice infected i.p. with F. tularensisa | i.p. pre-treatment extended mean time to death | ND |
| L1S | TLR4? | Mice infected i.p. with LVS | i.p. pretreatment lowered tissue burdens | ND |
See text for references. ?, cellular receptor unknown or unclear; ND, not determined; astrain used for challenge unclear.
Table 3A. Effective serum immunotherapy against virulent F. tularensis infection.
| Immunogen | Serum donor | Challenge animal, route, strain | Protective effect | Requirements for protection |
|---|---|---|---|---|
| Formalin-killed F. tularensis Schu | Horse | Rats infected s.c. with Schu | i.p. pre-treatment increased survival rate | ND |
| Live F. tularensis Schu | Goat | Rats infected s.c. with Schu | i.p. pre-treatment increased survival rate | ND |
| Formalin-killed virulent F. tularensis | Rabbit | Rats infected i.p. with virulent F. tularensis. | Mixture of serum with inoculum before i.p. challenge increased survival rate, i.p. post-treatment delayed mean time to death | ND |
| Live F. tularensis | Recovered or Convalescent Human Patient | Rats infected i.p. with virulent F. tularensis | Mixture of serum with inoculum before i.p. challenge increased survival rate | ND |
| Formalin-killed F. tularensis | Human | Rats infected i.p. with virulent F. tularensis | Mixture of serum with inoculum before i.p. challenge increased survival rate | ND |
| Formalin-killed F. tularensis | Goat | Rats infected i.p. with virulent F. tularensis | Mixture of serum with inoculum before i.p. challenge increased survival rate | ND |
| Live F. tularensis SchuS4 | Micea | Mice infected i.n. with SchuS4 | i.p. pre- or post- treatment increased survival rates | ND |
| Live F. tularensis LVS | Rat | Rats infected i.t. with SchuS4 | i.p. pre-treatment increased survival rate | IgG, CD8+ T cells |
| F. tularensis LVS MPF | Mice | Mice infected i.n. with SchuS4 | i.p. post-treatment increased survival rate when given in conjunction with suboptimal gentamicin | ND |
| ? | Horse | Mice infected i.p. with virulent F. tularensis | Mixture of hyperimmune serum with inoculum or i.p. treatment with serum immediately after challenge extended mean time to death | ND |
| Live virulent F. tularensis | Sheep | Mice infected i.p. with virulent F. tularensis | Mixture of serum with inoculum before i.p. challenge extended mean time to death | ND |
| Formalin-killed virulent F. tularensis | Rabbit | Mice infected i.p. or s.c. with virulent F. tularensis | Mixture of serum with inoculum before i.p. challenge extended mean time to death i.p. pre-treatment before s.c. challenge extended mean time to death |
ND |
| ? | Horse | Guinea pigs infected s.c. with Vavenby | Intracardial pre-treatment with hyperimmune horse serum extended mean time to death | ND |
| Acetone-killed F. tularensis SchuS5 | Mice | Mice infected i.p. or s.c. with SchuS5 | i.p. pre-treatment extended mean time to death | ND |
| ? | Mice, guinea pig, or rabbit | Mice infected i.p. with virulent F. tularensis 9K 161t | i.p. pre- and post-treatment extended mean time to death | ND |
| Live F. tularensis LVS | Mice | Mice infected i.d. with FSC 71 | i.p. pre-treatment reduced bacterial burdens and morbidity | ND |
| Live and sonicated F. tularensis LVS | Mice | Mice infected i.d. with LVS or SchuS4 | i.p. pre- or post-treatment with mAb reactive with LPS increased survival rate of LVS-challenged animals i.p. pre-treatment extended time to death of SchuS4-challenged animals |
ND |
See text for references. ?, immunogen not stated in manuscript; ND, not determined; amice were treated with levofloxacin to survive infection; broute of antibody transfer not stated.
Table 3B. Effective serum immunotherapy strategies against model strains of Francisella that have unknown, or no protective effects against virulent F. tularensis.
| Immunogen | Serum donor | Challenge animal, route, strain | Protective effect | Requirements for protection |
|---|---|---|---|---|
| Live F. tularensis LVS | Human | Mice infected i.p. with LVS | i.p. pre-treatment increased survival rate | ND |
| Live F. tularensis LVS | Mice | Mice infected i.p. with LVS | i.p. pre-treatment increased survival rate | IgG, T cells, IFN-γ |
| Live F. tularensis LVS | Mice | Wild-type or B cell-deficient mice infected i.d. with LVS | i.p. pre-treatment increased survival rates in B cell-deficient mice, and decreased bacterial burdens in both B cell-deficient and wild-type mice | ND |
| LPS from F. tularensis LVS | Mice | Mice infected i.p. with LVS | i.p. pre-treatment increased survival rate | ND |
| Live F. novicida ∆iglC | Mice | B cell-deficient mice infected i.n. with F. novicida | i.p. pre-treatment increased survival rate | ND |
| Live F. tularensis LVS | Mice | Mice infected i.n. with LVS | i.p. pre- or post-treatment increased survival rates | FCγR, IFN-γ, neutrophils, macrophages |
| Live F. tularensis LVS | Mice | Mice infected i.n. with LVS | i.p. or i.n. post-treatment with a mAb reactive with LVS LPS increased survival rates | ND |
| Heat-killed F. tularensis LVS | Mice | Mice infected i.p. with LVS | i.p. pre-treatment increased survival rate | ND |
| Live and sonicated F. tularensis LVS | Mice | Mice infected i.d. with LVS or SchuS4 | Pre- or post-treatment with FopA -reactive mAb increased survival rates in LVS-challenged animals, no effect in SchuS4-challenged animalsb | ND |
| Live and sonicated F. tularensis LVS | Mice | Mice infected i.d. with LVS | Post-treatment with LpnA-reactive mAb increased survival rateb | ND |
| F. tularensis FopA | Mice | Mice infected i.d. with LVS | Pre- and post-treatment increased survival rateb | ND |
| Live or heat-killed F. tularensis LVS | Mice | Irradiated mice infected i.d. with LVS | i.p. pre-treatment increased survival rate | ND |
See text for references. ?, immunogen not stated in manuscript; ND, not determined; amice were treated with levofloxacin to survive infection; broute of antibody transfer not stated.
Effector Mechanisms of Immunotherapeutics against Francisella
The success of Francisella as a pathogen is correlated with its ability to replicate in phagocytic cells. Therefore, activation of phagocytic cells, such as macrophages, has been investigated as immunotherapy for tularemia. IFN-γ and TNF-α can activate macrophages in vitro to restrict the replication of F. tularensis, while deficiencies in these cytokines render mice more susceptible to LVS and/or SchuS4 infection.34-37 TNF-α and IFN-γ induce nitric oxide production, which in turn can restrict the intramacrophagic growth of LVS.38 The effect of TNF-α on SchuS4 replication in macrophages is unknown; however it has been shown that IFN-γ activation of macrophages for clearance of SchuS4 is independent of reactive oxygen and nitrogen species (ROS/RNS).34 IL-12 and Toll-Like receptor (TLR) signaling are also involved in IFN-γ and TNF-α production and are required for protection against Francisella in some models which has made them targets for immunotherapy.35,39-41 The role of type I IFNs in experimental models of Francisella infection is strain-specific. Endogenous type I IFNs are protective against LVS,29 deleterious against F. novicida,42 but do not appear to play a role in protective immunity against SchuS4.42 Addition of rIFN-β to human dendritic cells does not restrict intracellular replication of SchuS4;27 however addition of rIFN-β to murine macrophages or human dendritic cells infected with LVS is protective.27,43 While the role of type I IFNs in Francisella varies depending on the model, type I IFNs and agonists that induce them have shown efficacy against experimental tularemia.
The protective mechanisms of serum against Francisella have not been completely characterized;33 however antibody treatment of experimental tularemia has been shown to have efficacy. While Francisella is generally thought of as an intracellular pathogen, recent studies have shown that Francisella has a significant extracellular phase where it would likely be recognized by antibodies.44 Several actions of immune serum may contribute to its protective capability against tularemia. Serum from LVS-vaccinees enhances phagocytosis and killing of LVS by human neutrophils and facilitates the development of a respiratory burst.45-47 Stenmark et al. showed that transfer of specific antibodies increased the expression of the cytokines TNF-α and IL-12, to the site of a cutaneous F. tularensis LVS infection.48 Neutrophil recruitment was also enhanced; however while neutrophils play a protective role when animals are challenged i.p with LVS,49 they appear to play a negligible role in immunity to pulmonary infection with LVS or type A F. tularensis.50,51 Control of LVS infections in IFN-γ treated alveolar macrophages was enhanced when LVS was opsonized with antibody prior to uptake, and the FCγ receptor was required for protection, indicating a role for opsonophagocytosis.52 Opsonization of virulent type A F. tularensis SchuS4 with antibody also increases pro-inflammatory cytokine production by macrophages. Interestingly, SchuS4, but not LVS binds plasmin, a host serine protease that degrades opsonizing antibodies, and may explain why LVS infections are more conducive to antibody immunotherapy than SchuS4 infections.26
Generation of Protective Immunity against Model Strains of Francisella by Heterologous Infection or Cytokine Therapy
Some of the earliest studies of active immunotherapy against tularemia investigated the protective effects of heterologous infection. Mice that were previously infected i.p. with Rickettsia typhi were shown to be protected against i.p. challenge with attenuated strains of F. tularensis; however no effect was obtained when the virulent Schu strain was employed.53 Infection with the Mycobacterium bovis BCG vaccine conferred potent protection against F. novicida when mice were challenged i.d. Three weeks after intravenous (i.v.) BCG infection, however, no effect was achieved against SchuS4.54 Later studies showed that i.p. pre-infection with BCG could confer 100% survival against a lethal i.p. LVS challenge in mice.55 Macrophages from BCG-infected animals were shown to have enhanced clearance of LVS in vitro, and BCG-mediated protection against LVS both in vitro and in vivo was dependent on IFN-γ, TNF-α, and reactive nitrogen oxides. Macrophage activation by nitric oxide was also presumed to be the mechanism by which co-infection with F. novicida reduced the organ burdens of LVS in rats challenged i.p. with both LVS and F. novicida.56
Cytokine administration has also shown efficacy against Francisella challenge in animal models. While the challenge strain was unclear (presumably LVS based on other studies by the authors), murine L-cell interferon (IFN-β) extended the time to death of mice infected i.p with Francisella when administered i.p. prior to challenge.57 Intranasal (i.n.) rIL-12 reduced tissue burdens and extended the time to death of mice infected i.n. with F. novicida when given 24 and 4 h prior to the time of challenge. rIL-12 conferred up to 100% survival when given i.n. in conjunction with gentamicin 8 and 24 h after F. novicida U112 challenge; however, this effect waned when treatment was delayed. rIFN-γ also enhanced the survival rates of U112-challenged mice treated with gentamicin when given 8 and 24 h post-challenge.58 Duckett et al.59 obtained similar results when testing rIL-12 as immunotherapy against F. tularensis LVS. Intranasal rIL-12 given 24 h before i.n. LVS infection reduced bacterial loads in the tissues and enhanced survival rates. This effect was dependent on IFN-γ and CD8+ T cells, but was not affected by the beige mutation in NK cells. Collectively, generation of protective immunity against Francisella by heterologous infection or cytokine administration may have potential, but efficacy against virulent challenge strains has yet to be demonstrated.
Induction of Rapid Protective Immunity against LVS with Francisella-derived LPS
In contrast to traditional lipopolysaccharide (LPS) from bacteria such as Salmonella or E. coli, Francisella LPS is not known to induce potent immune responses via TLR signaling.60 However, several groups have shown that Francisella LPS can generate rapid protective immunity against challenge with LVS. When given i.p., LVS LPS was shown to confer 100% protection against a lethal i.p. LVS challenge 2–3 d later while LPS from E. coli or Salmonella had no effect. While LPS was protective, it did not enhance proliferation or immunoglobulin secretion by murine B cells nor enhance the production of IL-4, IL-6, or IL-12 by murine splenocytes. Protection was shown to require B cells and IFN-γ, but not TLR4.61 Subsequent studies showed that i.p. LVS LPS or U112 LPS both could confer protection against i.p. LVS challenge, but no protection was generated against a F. novicida U112 challenge.62 Further mechanistic studies on LVS LPS showed that i.p. LVS LPS treatment given 2 or 7 d before i.p. LVS challenge could reduce tissue burdens and inflammatory gene expression in the liver despite the absence of detectable antibody against F. tularensis.63 However, later studies showed that LVS LPS-mediated protection against LVS required B cells and antibody produced by antigen-specific B-1a cells, but was independent of TLR4. It was also demonstrated that LVS LPS, but not LVS lipid A, was protective indicating a critical role for LPS carbohydrate in the induction of protection.64 TLR2 was required for LVS LPS-mediated protection against LVS challenge, although protection could be rescued in TLR2−/− mice when a TLR agonist such as synthetic E. coli MPL (TLR4) or flagellin (TLR5) was co-administered with LVS LPS.31 However, while LVS LPS is quite potent at protecting against LVS, no protective efficacy was obtained against an i.n. SchuS4 challenge.31
Targeting Cellular Receptors to Induce Immunity against Francisella
Perhaps the largest focus of active immunotherapy studies against Francisella is the targeting of cellular receptors such as TLRs to induce protective innate immune responses. Giron et al.57 investigated the effect of several inducers of innate immunity against Francisella. The strain used for challenge is unclear (presumably i.p. LVS); however, endotoxin pre-treatment (TLR4) decreased the mortality rate of tularemia while pre-treatment with Poly (I:C) (TLR3) or Tilorone (an activator of NK cells)65 extended the mean time to death of mice. Poly I:C was further investigated as immunotherapy by Pyles et al.66 Poly (I:C) enhanced early pulmonary cytokine expression and neutrophil recruitment while decreasing bacterial loads in the lung when administered i.n. 1 h prior to or after i.n. LVS challenge. LVS-challenged mice also displayed increased survival time when treated i.n. with Poly (I:C) 1 h prior to infection. In addition, Poly (I:C) conferred partial protection against SchuS4. Intranasal Poly (I:C) administered 1 h before or simultaneously with i.n. SchuS4 challenge extended the mean time to death by 2 and 15 d respectively. Treating mice i.n. with Poly (I:C) 1 h after SchuS4 challenge also extended the treatment window for animals to be rescued by antibiotic therapy with levofloxacin.
Bacterial DNA containing CpG motifs that signal through TLR9 were shown to be effective at inducing protective innate immunity against F. tularensis LVS when given i.p. 1–14 d before infection by Elkins et al.67 CpG-treated mice displayed lower bacterial loads and protection was dependent on B cells and IFN-γ. Subsequent studies showed that lymphocytes from the spleens of mice treated with CpG could control the intramacrophagic growth of LVS in bone marrow-derived macrophages, an effect dependent on IFN-γ TNF-α, and IL-12, but not IL-4 or perforin.38 In the same study, it was also shown that TLR9 was required for CpG-mediated protection in vivo. Klinman et al.68 showed that repeated CpG administration could prolong this enhanced immunity to F. tularensis LVS. However, later studies showed that CpG was not protective against aerosol infection with SchuS4.30 Cationic liposome-DNA complexes (CLDC) which signal through TLR969 and other receptors such as cytosolic DAI (DNA-dependent activator of IRFs)70 also provide protection against tularemia. CLDC reduced organ burdens and conferred 100% survival when administered i.n. 1 d prior to i.n. LVS challenge. Protection against LVS required NK cells and IFN-γ, but not type I IFNs. Intranasal administration of CLDC at times prior to, or after 24 h pre-infection, or administration of CLDC via s.c., i.v., or i.p. routes diminished efficacy. However, while CLDC conferred 100% survival against LVS, only a moderate increase in time to death was obtained when testing CLDC immunotherapy against a pulmonary SchuS4 challenge.29 Ireland et al. improved upon the efficacy of CLDC by combining it with a crude F. tularensis membrane protein fraction (MPF).71 When given i.v. 3 d prior to an i.n. infection with SchuS4, 60% of the animals that received CLDC + MPF survived a lethal challenge. CLDC + MPF was found to induce reactive nitrogen (RNS) and oxygen (ROS) species genes in vitro, and in vivo protection was shown to be dependent on RNS/ROS as nos2/gp91−/− mice were not protected against SchuS4 by CLDC + MPF.
Lembo et al.72 showed that i.n. administration of the synthetic TLR4 agonist AGP 48 h prior to and 24 h after aerosol F. novicida infection augmented cytokine production in the lung and reduced bacterial burdens in the tissues. This treatment regimen also enhanced the survival rate of mice by up to 50%, an effect dependent on IFN-γ; however, treatment with AGP post-infection did not affect survival. Another synthetic TLR4 agonist, MPL, conferred 43% and 20% survival when given i.p. 2 d before, or at the time of i.n. SchuS4 challenge respectively.31 However co-administration of LVS LPS, which confers potent protection against LVS infection, did not augment MPL-mediated protection against SchuS4. The L1S fragment of p60, a secreted protein from Listeria monocytogenes that also may signal through TLR4, also displayed efficacy against LVS.73 L1S enhanced activation of naïve murine NK cells in vivo and decreased tissue burdens while increasing IFN-γ levels in LVS-infected mice when L1S was administered i.p. 24 h prior to i.p. challenge.
Innate immune agonists that signal through receptors other than TLRs have also shown protection against virulent F. tularensis infection. Yeast glucans, which can induce innate immunity through various carbohydrate receptors,74 were shown to be efficacious against tularemia in rats by Reynolds et al.75 Intravenous pre-treatment of glucans for several days prior to i.p. infection with SchuS4 increased the survival rate of rats by 68%. Glucans also enhanced the survival of rats when given i.v. before aerosol challenge. In our own recent work, we have investigated the immunodulatory activity of polysaccharides derived from the pulp of the Acai berry (“Acai PS”).76 When given i.n. 1 d prior to aerosol infection with SchuS4, Acai PS conferred 80% survival against an otherwise lethal challenge.77 Acai PS was also effective when given after aerosol SchuS4 infection. Intranasal treatment of mice with Acai PS immediately after, 24 h after, or 48 h after aerosol challenge with SchuS4 resulted in respective survival rates of 73%, 60%, and 33%, making Acai PS the most potent active immunotherapeutic in the literature to treat pulmonary type A F. tularensis infection. We found that Acai PS enhanced IFN-γ expression by human NK cells in vitro, and murine NK cells in vivo, during F. tularensis infection, while neutralization of IFN-γ abrogated the protective effect of Acai PS. Further investigation of the immune responses induced by, and the cellular receptors that recognize Acai PS is ongoing, but Acai PS appears to require both TLR4/TRIF along with carbohydrate receptors (Holderness et al., manuscript in preparation) to mediate its effects. As both TLR4 and carbohydrate receptor agonists have shown efficacy against tularemia,31,75 it is possible that activation of both of these immune receptor types by Acai PS may lead to synergetic potentiation of protective immunity. Therefore, future studies of the receptors required for Acai PS-mediated signaling and immune responses induced by Acai PS could reveal receptors and pathways of immunity to be targeted for immunotherapy against F. tularensis.
Early Studies of Immune Sera as Therapy for Tularemia
Interest in antibody therapy for tularemia was piqued by human trials in the 1940s in which Foshay purported that human tularemia patients treated with immune serum from goats or horses immunized with formaldehyde-killed Francisella before the end of the second week of disease resulted in significant reductions in mortality and all aspects of morbidity.78,79 However, studies in the 1940s also showed the difficulty of demonstrating protective efficacy against lethality induced by virulent F. tularensis challenge by serum therapy in rodent models. Francis and Felton80 found that treatment of mice with hyper-immune serum from sheep (infected s.c. with virulent F. tularensis), horses (immunogen unclear), or rabbits (injected i.v. with formalin-killed virulent F. tularensis) extended the mean time to death, but did not confer survival when serum was mixed with inoculum prior to i.p. challenge of mice with virulent F. tularensis. Similar results were found in guinea pigs by Bell and Kahn81 who showed that hyperimmune horse serum extended the mean time to death of guinea pigs when given intracardially prior to s.c. challenge with the virulent Vavenby strain of F. tularensis. Studies using immune sera raised in mice against acetone-killed F. tularensis SchuS5 (a streptomycin resistant virulent type A strain) only slightly increased the mean time to death when transferred to mice 3 d prior to i.p. or s.c. SchuS5 challenge.82 Comparable findings were obtained by Thorpe and Marcus83 who showed that sera from immunized mice, guinea pigs, or rabbits (immunogen unclear) could slightly extend the mean survival time of mice when transferred concomitantly and 24 h after i.p. challenge with the virulent F. tularensis strain, 9K 161t.
As early studies showed that transfer of immune sera into mice or guinea pigs was not able to confer survival against virulent F. tularensis challenge, Foshay et al. used the more resistant rat as the recipient of immune sera. Intraperitoneal transfer of hyperimmune serum from goats (immunized with F. tularensis Schu) or horses (immunized with formalin-killed F. tularensis Schu) at the time of s.c. challenge with the Schu strain of F. tularensis could confer survival rates as high as 87.5% as compared with 3.3–6.6% survival of rats treated with normal serum.84 Larson also showed that transfer of immune sera into rats could confer survival against virulent F. tularensis infection.85 Immune serum from goats or rabbits immunized with formalin-killed virulent Francisella conferred up to 70% survival when mixed with inoculum prior to i.p. challenge of rats with virulent F. tularensis; however, studies with rabbit serum showed that the protective effect waned when serum therapy was delayed until 24 h after challenge. Perhaps most importantly, the protective efficacy of human immune serum was also demonstrated in this study. When serum from human patients that had recovered from tularemia was mixed with virulent F. tularensis prior to i.p. infection of rats, up to 100% of rats survived an otherwise completely lethal challenge. Serum from a convalescent human tularemia patient or a human that had been immunized with formalin-killed F. tularensis also slightly increased the survival rate (up to 30%) and extended the mean time to death of rats infected i.p. with virulent F. tularensis. Convalescent and vaccine-induced serum had a significantly lower agglutination titer than serum from patients that had recovered from disease, which may explain the reduced protective efficacy of convalescent and vaccine-induced serum.
Immunotherapy Using Serum from Animals Infected with Live Francisella
After a lull in research on antibody-mediated protection against Francisella, studies in the 1990s again began to investigate humoral immunotherapy of tularemia by transferring serum from animals previously infected with Francisella. Drabick et al.86 demonstrated the protective efficacy of sera from human recipients of LVS vaccination. The authors found that i.p. transfer of pooled immune sera 2 h before i.p. infection with LVS fully protected mice against a 10 000 LD50 challenge. The human sera were found to react mostly with LVS lipopolysaccharide. This sera was also found to be cross-reactive with the polysaccharide from the virulent SchuS4 strain of F. tularensis; however the efficacy of challenge against virulent F. tularensis was not investigated.
Using mice as donors of serum, i.d. vaccination with LVS was shown to produce protective antibodies by several groups. Rhinehart-Jones et al.87 showed that transfer of immune serum from mice vaccinated i.d. with LVS at the time of i.p. LVS challenge could confer 100% survival against infection. This effect was found to be predominately dependent on IgG, and not IgM. Immune serum reduced colonization of the spleen by LVS; however serum transfer did not confer protection against LVS infection in nude mice or mice lacking IFN-γ. Immune serum generated by a sublethal i.d. LVS infection could also confer 100% survival and reduced bacterial loads in tissue when administered i.p. 1 d prior to a lethal i.d. LVS infection in B cell-deficient mice.88 Importantly, transfer of this immune serum also dramatically reduced bacterial burdens and morbidity in wild-type mice challenged i.d. with a virulent type B strain of F. tularensis, FSC 171. Passive transfer of antibody from animals vaccinated i.d. with LVS could also confer protection in immunodeficient irradiated mice. Kubelkova et al.89 showed that serum from mice given a sublethal i.d. LVS infection (or from mice that received heat-killed LVS i.d.) could provide 100% protection against a lethal i.d. LVS challenge in irradiated mice when given 2 h prior to LVS infection.
Serum immunotherapy has also proved protective in animal models of pulmonary tularemia. Antibodies obtained from mice infected i.n. with ∆iglC F. novicida conferred 80% survival when transferred i.p. into B cell-deficient mice 8 h before i.n. challenge with wild-type F. novicida.90 The first demonstration of therapeutic protection by antibodies against pulmonary tularemia was performed by Kirimanjeswara et al.52 Serum obtained from mice intranasally (i.n.) infected with a sub-lethal dose of LVS provided 100% protection and reduced tissue burdens and organ damage following a lethal i.n. LVS challenge when serum was transferred i.p. One day prior to infection. FCγR, IFN-γ, neutrophils, and macrophages, but not complement, were required for protection. Transfer of serum could also confer survival against lethal i.n. LVS infection when administered i.p. as late as 48 h post-infection. The most potent serum to treat virulent F. tularensis infection in mice was obtained from mice that were i.n. infected with F. tularensis SchuS4 and then treated with the antibiotic levofloxacin daily for 13 d beginning on day 3 post-infection.91 Serum from these antibiotic-treated mice was able to confer up to 100% survival when transferred i.p. 4 h before or 24 h after i.n. infection with SchuS4. Transfer of immune serum can also be effective against pulmonary tularemia due to virulent F. tularensis infection in rats. Antibodies from rats vaccinated s.c. with LVS provided protection against morbidity and provided 100% survival against intratracheal (i.t.) challenge with SchuS4 when transferred i.p. to rats 1 d before challenge. This effect was shown to be dependent on IgG, and CD8+ T cells but not by IgM. Transfer of immune serum also reduced bacterial organ burdens and inflammation.92
Immunotherapy Using Serum from Animals Immunized with Inactivated Francisella or Francisella Antigens
While the majority of humoral immunotherapy studies for tularemia have utilized serum from animals infected with live Francisella, serum from animals immunized with inactivated Francisella or Francisella antigens has also proven protective. Studies by Fulop et al. showed that i.p. transfer of sera from mice immunized with LVS LPS 2 h prior to i.p. LVS challenge conferred 100% survival in mice, an effect that was independent of CD4+ or CD8+ T cells. Anti-LPS antibodies did not confer survival against i.p. challenge with virulent SchuS4, although a slight increase in time to death was observed.93 Lavine et al.94 demonstrated that serum from mice immunized with heat-killed LVS could confer 100% survival when given i.p. 8 h before a lethal i.p. LVS infection. Antibodies against LVS LPS were not required for this protection. F. tularensis outer membrane protein A (FopA) was proven to be a target of protective antibodies by Hickey et al.95 Serum from mice immunized with FopA could confer 100% protection against a lethal i.d. LVS infection when given i.p. before and after challenge. As mentioned earlier, serum therapy of F. tularensis has shown limited efficacy against virulent type A F. tularensis challenge in mice. Therefore, Sutherland et al.96 developed a model in which mice were treated i.p. with a suboptimal dose of gentamicin beginning 1 d after i.n. infection with virulent F. tularensis SchuS4 in order to extend the time to death from ~4 to ~7 d postinfection. They found that i.p. transfer of murine antibodies elicited against a Francisella membrane protein fraction (MPF) 1 d after i.n. SchuS4 challenge could reduce organ burdens and provide 100% survival when used in conjunction with suboptimal gentamicin in this model. Treatment of mice i.p. with MPF 1 d after SchuS4 challenge also conferred 100% protection when used in combination with suboptimal gentamicin.
Monoclonal Antibody Immunotherapy for Tularemia
The investigation of immunotherapy using monoclonal antibodies (MAbs) for the treatment of experimental tularemia has been limited; however, protective efficacy has been demonstrated. Using a proteome microarray Lu et al.97 identified several monoclonal antibodies (MAbs) from LVS-infected mice. The authors found that an IgG2a mAb reactive with LVS LPS conferred 100% survival when given i.p. or i.n. within an hour after a lethal LVS i.n. challenge. The relative protective efficacy of anti-LPS IgG antibodies in this study was determined to be IgG2a > IgG1 > IgG3. mAb studies performed by Savitt et al.98 showed that MAbs against LVS components could confer 100% survival and reduce organ burdens when given prior to, and after lethal i.d. challenge with LVS. When given before and after a lethal i.d. LVS challenge, anti-LVS FopA MAbs conferred partial survival from infection. Therapeutic treatment of mice with MAbs against LVS LPS, FopA and LpnA 1, 3, and 5 d after a lethal i.d. LVS infection also provided partial protection against mortality. The efficacy of mAb therapy was greatly diminished when a virulent challenge strain was used in this study; however, anti-LPS MAbs given before and after infection did modestly extend the time to death of mice challenged i.d. with type A F. tularensis SchuS4.
Perspectives and Challenges for the Study of Tularemia Immunotherapy
Immunotherapy of tularemia has received increased attention in recent years, and numerous immunotherapeutics have demonstrated protection in animal models of tularemia. However, many, if not most of these studies have utilized challenge strains that do not cause disease in humans. Virulent strains of F. tularensis possess mechanisms of immune suppression not found in attenuated strains that may interfere with immunotherapy. Also, many immunotherapy strategies reviewed here have shown impressive protective effects against F. novicida or LVS infections, but these same approaches have generally failed to provide meaningful protection against virulent strains of Francisella. These findings question the utility of F. novicida and LVS infections as models to develop immunotherapeutics against virulent F. tularensis infections. Therefore, care must be taken when translating the potential of immunotherapeutics that confer protection against attenuated strains of Francisella in animal models to their potential efficacy against Francisella strains that are pathogenic for humans. Also, it has been proposed that systemic, rather than pulmonary infection is the likely cause of death in mice following challenge with virulent F. tularensis, regardless of the route of infection.99,100 In addition, human tularemia due to inhalation of virulent Francisella often presents with systemic symptoms of illness without prominent pulmonary signs of disease.9 Therefore, while immunotherapies that target the lung have proven effective against experimental pulmonary tularemia, it is likely that immunotherapeutics will also need to reduce or block systemic colonization by Francisella in order to provide meaningful protection against tularemia.
The focus of antibody-mediated immunity against Francisella has been primarily related to the generation of prophylactic vaccines. However, the transfer of immune serum to human tularemia patients was purported to be protective in the 1940s, indicating a potential for humoral immunotherapy of tularemia. In animal models, numerous studies have shown that the transfer of immune serum prior to or shortly after infection could increase resistance to challenge. To enhance the potential clinical relevance of post-exposure treatment, future studies will need to investigate humoral immunotherapy at later timepoints post-infection. Also, due to the (as yet) limited ability of transferred immune serum to protect against mortality induced by virulent strains of F. tularensis in mice, it might be beneficial to perform these studies in conjunction with antibiotic therapy or in a more resistant host such as the rat.
The induction of protection against tularemia with innate immune agonists is exciting, particularly as several of these agonists, including Acai PS, CLDC, CLDC + MPF, CPG, MPL, Poly I:C, and yeast glucans have shown protection in animal models against bacterial and viral pathogens other than Francisella.30,71,77,101,102 Stimulation of innate immunity with these agonists may be particularly useful in an event where the etiologic agent of disease is unknown, such as a bioterror attack, as the immune responses induced by these agonists have the capability to protect against multiple pathogens. However, several mitigating factors must be considered when examining the potential of innate immune agonists. While treatment of mice with several TLR agonists prior to or at the time of infection has proven protective against tularemia in animal models, infection of mice with F. tularensis SchuS4 has been shown to suppress subsequent TLR-induced immune responses.103,104 Therefore, the targeting of TLRs for immunotherapy may have a limited window of efficacy against tularemia. Another factor that may limit the efficacy window for innate immune agonists is the hypothesis that a late upregulation of proinflammatory cytokines during pulmonary tularemia may be too late to decrease bacterial colonization, and may actually be harmful to the host.105 Therefore, enhancement of immunity may actually be detrimental to the host when agonists are given at later points after infection. To address this concern, immune agonists should be tested at later times post-infection and in conjunction with antibiotic therapy to determine their protective window of efficacy, and also to ensure that they do not exacerbate disease when endogenous host responses are also activated.
In conclusion, immunotherapy for tularemia has shown strong promise in animal models and will presumably continue to be a focus for future studies, particularly due to the potential threat of weaponized antibiotic-resistant Francisella. The elucidation of protective mechanisms of immunity elicited by tularemia immunotherapeutics may also reveal novel correlates of protection that will increase our understanding of immunity to Francisella and intracellular pathogens as a whole.
Acknowledgments
This work is supported by the Rocky Mountain Regional Center for Excellence in Bioterrorism and Emerging Infectious Diseases (NIH U54AI-65357), along with NIH P01AT-04986, and NIH Contract HHSN2662004000009/N01-AI40009.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/25454
References
- 1.Francis E. Tularemia. JAMA. 1925;278:1243–50. doi: 10.1001/jama.1925.02660430001001. [DOI] [Google Scholar]
- 2.Hong KJ, Wickstrum JR, Yeh HW, Parmely MJ. Toll-like receptor 2 controls the gamma interferon response to Francisella tularensis by mouse liver lymphocytes. Infect Immun. 2007;75:5338–45. doi: 10.1128/IAI.00561-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lake GC, Francis E. Six cases of tularemia occurring in laboratory workers. Public Health Rep. 1922;37:392–413. doi: 10.2307/4576294. [DOI] [Google Scholar]
- 4.Sewell DL. Laboratory-associated infections and biosafety. Clin Microbiol Rev. 1995;8:389–405. doi: 10.1128/cmr.8.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oyston PC. Francisella tularensis: unravelling the secrets of an intracellular pathogen. J Med Microbiol. 2008;57:921–30. doi: 10.1099/jmm.0.2008/000653-0. [DOI] [PubMed] [Google Scholar]
- 6.Aquino LL, Wu JJ. Cutaneous manifestations of category A bioweapons. J Am Acad Dermatol. 2011;65:e1–, e15. doi: 10.1016/j.jaad.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 7.Evans ME. Francisella tularensis. Infect Control. 1985;6:381–3. [PubMed] [Google Scholar]
- 8.Dennis DT. Tularemia. In: Wallace RB, ed. Maxcy-Rosenau-Last Public Health and Preventive Medicine. 14th edition. Stamford, CT: Appleton & Lange. 1998:354-57. [Google Scholar]
- 9.Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. Working Group on Civilian Biodefense Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–73. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 10.Allen LA, McCaffrey RL. To activate or not to activate: distinct strategies used by Helicobacter pylori and Francisella tularensis to modulate the NADPH oxidase and survive in human neutrophils. Immunol Rev. 2007;219:103–17. doi: 10.1111/j.1600-065X.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- 11.Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. Tularemia vaccine study. II. Respiratory challenge. Arch Intern Med. 1961;107:702–14. doi: 10.1001/archinte.1961.03620050068007. [DOI] [PubMed] [Google Scholar]
- 12.McCRUMB FR, Jr., Snyder MJ, Woodward TE. Studies on human infection with Pasteurella tularensis; comparison of streptomycin and chloramphenicol in the prophylaxis of clinical disease. Trans Assoc Am Physicians. 1957;70:74–9, discussion 79-80. [PubMed] [Google Scholar]
- 13.WHO Consultant Group. Health aspects of chemical and biological weapons. 1970; 105-7.
- 14.Kaufmann AF, Meltzer MI, Schmid GP. The economic impact of a bioterrorist attack: are prevention and postattack intervention programs justifiable? Emerg Infect Dis. 1997;3:83–94. doi: 10.3201/eid0302.970201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris S. Japanese biological warfare research on humans: a case study of microbiology and ethics. Ann N Y Acad Sci. 1992;666:21–52. doi: 10.1111/j.1749-6632.1992.tb38021.x. [DOI] [PubMed] [Google Scholar]
- 16.Alibek K, Handelman S. Biohazard. New York, NY: Random House. 1999: 29-38. [Google Scholar]
- 17.Croddy E, Krcálová S. Tularemia, biological warfare, and the battle for Stalingrad (1942-1943) Mil Med. 2001;166:837–8. [PubMed] [Google Scholar]
- 18.Christopher GW, Cieslak TJ, Pavlin JA, Eitzen EM., Jr. Biological warfare. A historical perspective. JAMA. 1997;278:412–7. doi: 10.1001/jama.1997.03550050074036. [DOI] [PubMed] [Google Scholar]
- 19.AHFS Drug Information 2006A. Streptomycin Sulfate. The American Society of Health-System Pharmacists 2006:71-73. [Google Scholar]
- 20.Woolard MD, Hensley LL, Kawula TH, Frelinger JA. Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of gamma interferon-positive T cells. Infect Immun. 2008;76:2651–9. doi: 10.1128/IAI.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rick Lyons C, Wu TH. Animal models of Francisella tularensis infection. Ann N Y Acad Sci. 2007;1105:238–65. doi: 10.1196/annals.1409.003. [DOI] [PubMed] [Google Scholar]
- 22.Elkins KL, Bosio CM, Rhinehart-Jones TR. Importance of B cells, but not specific antibodies, in primary and secondary protective immunity to the intracellular bacterium Francisella tularensis live vaccine strain. Infect Immun. 1999;67:6002–7. doi: 10.1128/iai.67.11.6002-6007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crane DD, Griffin AJ, Wehrly TD, Bosio CM. B1a cells enhance susceptibility to infection with virulent Francisella tularensis via modulation of NK/NKT cell responses. J Immunol. 2013;190:2756–66. doi: 10.4049/jimmunol.1202697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skyberg JA, Rollins MF, Samuel JW, Sutherland MD, Belisle JT, Pascual DW. IL-17 protects against the Francisella tularensis Live Vaccine Strain, but not against virulent type A F. tularensis. Infect Immun. 2013 doi: 10.1128/IAI.00203-13. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laws TR, Clark G, D’Elia RV. Differential role for Interleukin-6 during Francisella infection with virulent and vaccine strains. Infect Immun. 2013 doi: 10.1128/IAI.00234-13. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crane DD, Warner SL, Bosio CM. A novel role for plasmin-mediated degradation of opsonizing antibody in the evasion of host immunity by virulent, but not attenuated, Francisella tularensis. J Immunol. 2009;183:4593–600. doi: 10.4049/jimmunol.0901655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauler TJ, Chase JC, Bosio CM. IFN-β mediates suppression of IL-12p40 in human dendritic cells following infection with virulent Francisella tularensis. J Immunol. 2011;187:1845–55. doi: 10.4049/jimmunol.1100377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosio CM. The subversion of the immune system by francisella tularensis. Front Microbiol. 2011;2:9. doi: 10.3389/fmicb.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Troyer RM, Propst KL, Fairman J, Bosio CM, Dow SW. Mucosal immunotherapy for protection from pneumonic infection with Francisella tularensis. Vaccine. 2009;27:4424–33. doi: 10.1016/j.vaccine.2009.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rozak DA, Gelhaus HC, Smith M, Zadeh M, Huzella L, Waag D, et al. CpG oligodeoxyribonucleotides protect mice from Burkholderia pseudomallei but not Francisella tularensis Schu S4 aerosols. J Immune Based Ther Vaccines. 2010;8:2. doi: 10.1186/1476-8518-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cole LE, Mann BJ, Shirey KA, Richard K, Yang Y, Gearhart PJ, et al. Role of TLR signaling in Francisella tularensis-LPS-induced, antibody-mediated protection against Francisella tularensis challenge. J Leukoc Biol. 2011;90:787–97. doi: 10.1189/jlb.0111014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowley SC, Elkins KL. Immunity to francisella. Front Microbiol. 2011;2:26. doi: 10.3389/fmicb.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elkins KL, Cowley SC, Bosio CM. Innate and adaptive immunity to Francisella. Ann N Y Acad Sci. 2007;1105:284–324. doi: 10.1196/annals.1409.014. [DOI] [PubMed] [Google Scholar]
- 34.Edwards JA, Rockx-Brouwer D, Nair V, Celli J. Restricted cytosolic growth of Francisella tularensis subsp. tularensis by IFN-gamma activation of macrophages. Microbiology. 2010;156:327–39. doi: 10.1099/mic.0.031716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crane DD, Scott DP, Bosio CM. Generation of a convalescent model of virulent Francisella tularensis infection for assessment of host requirements for survival of tularemia. PLoS One. 2012;7:e33349. doi: 10.1371/journal.pone.0033349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elkins KL, Rhinehart-Jones TR, Culkin SJ, Yee D, Winegar RK. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect Immun. 1996;64:3288–93. doi: 10.1128/iai.64.8.3288-3293.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anthony LS, Ghadirian E, Nestel FP, Kongshavn PA. The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb Pathog. 1989;7:421–8. doi: 10.1016/0882-4010(89)90022-3. [DOI] [PubMed] [Google Scholar]
- 38.Elkins KL, Colombini SM, Krieg AM, De Pascalis R. NK cells activated in vivo by bacterial DNA control the intracellular growth of Francisella tularensis LVS. Microbes Infect. 2009;11:49–56. doi: 10.1016/j.micinf.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Melillo AA, Foreman O, Elkins KL. IL-12Rβ2 is critical for survival of primary Francisella tularensis LVS infection. J Leukoc Biol. 2013;93:657–67. doi: 10.1189/jlb.1012485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen W, Kuolee R, Shen H, Bùsa M, Conlan JW. Toll-like receptor 4 (TLR4) plays a relatively minor role in murine defense against primary intradermal infection with Francisella tularensis LVS. Immunol Lett. 2005;97:151–4. doi: 10.1016/j.imlet.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Collazo CM, Sher A, Meierovics AI, Elkins KL. Myeloid differentiation factor-88 (MyD88) is essential for control of primary in vivo Francisella tularensis LVS infection, but not for control of intra-macrophage bacterial replication. Microbes Infect. 2006;8:779–90. doi: 10.1016/j.micinf.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Henry T, Kirimanjeswara GS, Ruby T, Jones JW, Peng K, Perret M, et al. Type I IFN signaling constrains IL-17A/F secretion by gammadelta T cells during bacterial infections. J Immunol. 2010;184:3755–67. doi: 10.4049/jimmunol.0902065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cole LE, Santiago A, Barry E, Kang TJ, Shirey KA, Roberts ZJ, et al. Macrophage proinflammatory response to Francisella tularensis live vaccine strain requires coordination of multiple signaling pathways. J Immunol. 2008;180:6885–91. doi: 10.4049/jimmunol.180.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forestal CA, Malik M, Catlett SV, Savitt AG, Benach JL, Sellati TJ, et al. Francisella tularensis has a significant extracellular phase in infected mice. J Infect Dis. 2007;196:134–7. doi: 10.1086/518611. [DOI] [PubMed] [Google Scholar]
- 45.Sandström G, Löfgren S, Tärnvik A. A capsule-deficient mutant of Francisella tularensis LVS exhibits enhanced sensitivity to killing by serum but diminished sensitivity to killing by polymorphonuclear leukocytes. Infect Immun. 1988;56:1194–202. doi: 10.1128/iai.56.5.1194-1202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Löfgren S, Tärnvik A, Carlsson J. Demonstration of opsonizing antibodies to Francisella tularensis by leukocyte chemiluminescence. Infect Immun. 1980;29:329–34. doi: 10.1128/iai.29.2.329-334.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Löfgren S, Tärnvik A, Bloom GD, Sjöberg W. Phagocytosis and killing of Francisella tularensis by human polymorphonuclear leukocytes. Infect Immun. 1983;39:715–20. doi: 10.1128/iai.39.2.715-720.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stenmark S, Sjöstedt A. Transfer of specific antibodies results in increased expression of TNF-α and IL12 and recruitment of neutrophils to the site of a cutaneous Francisella tularensis infection. J Med Microbiol. 2004;53:501–4. doi: 10.1099/jmm.0.05352-0. [DOI] [PubMed] [Google Scholar]
- 49.Sjöstedt A, Conlan JW, North RJ. Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect Immun. 1994;62:2779–83. doi: 10.1128/iai.62.7.2779-2783.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conlan JW, KuoLee R, Shen H, Webb A. Different host defences are required to protect mice from primary systemic vs pulmonary infection with the facultative intracellular bacterial pathogen, Francisella tularensis LVS. Microb Pathog. 2002;32:127–34. doi: 10.1006/mpat.2001.0489. [DOI] [PubMed] [Google Scholar]
- 51.KuoLee R, Harris G, Conlan JW, Chen W. Role of neutrophils and NADPH phagocyte oxidase in host defense against respiratory infection with virulent Francisella tularensis in mice. Microbes Infect. 2011;13:447–56. doi: 10.1016/j.micinf.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 52.Kirimanjeswara GS, Golden JM, Bakshi CS, Metzger DW. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J Immunol. 2007;179:532–9. doi: 10.4049/jimmunol.179.1.532. [DOI] [PubMed] [Google Scholar]
- 53.Owen CR, Larson CL. Studies on resistance to bacterial infections in animals infected with Rickettsiae. J Exp Med. 1956;103:753–63. doi: 10.1084/jem.103.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Claflin JL, Larson CL. Infection-immunity in tularemia: specificity of cellular immunity. Infect Immun. 1972;5:311–8. doi: 10.1128/iai.5.3.311-318.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Green SJ, Nacy CA, Schreiber RD, Granger DL, Crawford RM, Meltzer MS, et al. Neutralization of gamma interferon and tumor necrosis factor alpha blocks in vivo synthesis of nitrogen oxides from L-arginine and protection against Francisella tularensis infection in Mycobacterium bovis BCG-treated mice. Infect Immun. 1993;61:689–98. doi: 10.1128/iai.61.2.689-698.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cowley SC, Myltseva SV, Nano FE. Suppression of Francisella tularensis growth in the rat by co-infection with F. novicida. FEMS Microbiol Lett. 1997;153:71–4. doi: 10.1111/j.1574-6968.1997.tb10465.x. [DOI] [PubMed] [Google Scholar]
- 57.Giron DJ, Schmidt JP, Ball RJ, Pindak FF. Effect of interferon inducers and interferon on bacterial infections. Antimicrob Agents Chemother. 1972;1:80–1. doi: 10.1128/AAC.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pammit MA, Budhavarapu VN, Raulie EK, Klose KE, Teale JM, Arulanandam BP. Intranasal interleukin-12 treatment promotes antimicrobial clearance and survival in pulmonary Francisella tularensis subsp. novicida infection. Antimicrob Agents Chemother. 2004;48:4513–9. doi: 10.1128/AAC.48.12.4513-4519.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duckett NS, Olmos S, Durrant DM, Metzger DW. Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect Immun. 2005;73:2306–11. doi: 10.1128/IAI.73.4.2306-2311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hajjar AM, Harvey MD, Shaffer SA, Goodlett DR, Sjöstedt A, Edebro H, et al. Lack of in vitro and in vivo recognition of Francisella tularensis subspecies lipopolysaccharide by Toll-like receptors. Infect Immun. 2006;74:6730–8. doi: 10.1128/IAI.00934-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dreisbach VC, Cowley S, Elkins KL. Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon. Infect Immun. 2000;68:1988–96. doi: 10.1128/IAI.68.4.1988-1996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kieffer TL, Cowley S, Nano FE, Elkins KL. Francisella novicida LPS has greater immunobiological activity in mice than F. tularensis LPS, and contributes to F. novicida murine pathogenesis. Microbes Infect. 2003;5:397–403. doi: 10.1016/S1286-4579(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 63.Cole LE, Elkins KL, Michalek SM, Qureshi N, Eaton LJ, Rallabhandi P, et al. Immunologic consequences of Francisella tularensis live vaccine strain infection: role of the innate immune response in infection and immunity. J Immunol. 2006;176:6888–99. doi: 10.4049/jimmunol.176.11.6888. [DOI] [PubMed] [Google Scholar]
- 64.Cole LE, Yang Y, Elkins KL, Fernandez ET, Qureshi N, Shlomchik MJ, et al. Antigen-specific B-1a antibodies induced by Francisella tularensis LPS provide long-term protection against F. tularensis LVS challenge. Proc Natl Acad Sci U S A. 2009;106:4343–8. doi: 10.1073/pnas.0813411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gidlund M, Orn A, Wigzell H, Senik A, Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978;273:759–61. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- 66.Pyles RB, Jezek GE, Eaves-Pyles TD. Toll-like receptor 3 agonist protection against experimental Francisella tularensis respiratory tract infection. Infect Immun. 2010;78:1700–10. doi: 10.1128/IAI.00736-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elkins KL, Rhinehart-Jones TR, Stibitz S, Conover JS, Klinman DM. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J Immunol. 1999;162:2291–8. [PubMed] [Google Scholar]
- 68.Klinman DM, Conover J, Coban C. Repeated administration of synthetic oligodeoxynucleotides expressing CpG motifs provides long-term protection against bacterial infection. Infect Immun. 1999;67:5658–63. doi: 10.1128/iai.67.11.5658-5663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gowen BB, Fairman J, Dow S, Troyer R, Wong MH, Jung KH, et al. Prophylaxis with cationic liposome-DNA complexes protects hamsters from phleboviral disease: importance of liposomal delivery and CpG motifs. Antiviral Res. 2009;81:37–46. doi: 10.1016/j.antiviral.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–5. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 71.Ireland R, Olivares-Zavaleta N, Warawa JM, Gherardini FC, Jarrett C, Hinnebusch BJ, et al. Effective, broad spectrum control of virulent bacterial infections using cationic DNA liposome complexes combined with bacterial antigens. PLoS Pathog. 2010;6:e1000921. doi: 10.1371/journal.ppat.1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lembo A, Pelletier M, Iyer R, Timko M, Dudda JC, West TE, et al. Administration of a synthetic TLR4 agonist protects mice from pneumonic tularemia. J Immunol. 2008;180:7574–81. doi: 10.4049/jimmunol.180.11.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmidt RL, Filak HC, Lemon JD, Potter TA, Lenz LL. A LysM and SH3-domain containing region of the Listeria monocytogenes p60 protein stimulates accessory cells to promote activation of host NK cells. PLoS Pathog. 2011;7:e1002368. doi: 10.1371/journal.ppat.1002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meena DK, Das P, Kumar S, Mandal SC, Prusty AK, Singh SK, et al. Beta-glucan: an ideal immunostimulant in aquaculture (a review) Fish Physiol Biochem. 2013;39:431–57. doi: 10.1007/s10695-012-9710-5. [DOI] [PubMed] [Google Scholar]
- 75.Reynolds JA, Kastello MD, Harrington DG, Crabbs CL, Peters CJ, Jemski JV, et al. Glucan-induced enhancement of host resistance to selected infectious diseases. Infect Immun. 1980;30:51–7. doi: 10.1128/iai.30.1.51-57.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holderness J, Schepetkin IA, Freedman B, Kirpotina LN, Quinn MT, Hedges JF, et al. Polysaccharides isolated from Açaí fruit induce innate immune responses. PLoS One. 2011;6:e17301. doi: 10.1371/journal.pone.0017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Skyberg JA, Rollins MF, Holderness JS, Marlenee NL, Schepetkin IA, Goodyear A, et al. Nasal Acai polysaccharides potentiate innate immunity to protect against pulmonary Francisella tularensis and Burkholderia pseudomallei Infections. PLoS Pathog. 2012;8:e1002587. doi: 10.1371/journal.ppat.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Foshay L. Tularemia: A Summary of Certain Aspects of the Disease Including Methods for Early Diagnosis and The Results of Serum Treatment in 600 Patients. Medicine. 1940;19:1–84. doi: 10.1097/00005792-194002000-00001. [DOI] [Google Scholar]
- 79.Foshay L. A comparative study of the treatment of tularemia with immune serum, hyperimmune serum and streptomycin. Am J Med. 1946;1:180–8. doi: 10.1016/0002-9343(46)90036-8. [DOI] [PubMed] [Google Scholar]
- 80.Francis E, Felton LD. Antitularemic serum. Public Health Rep. 1942;57:44–55. doi: 10.2307/4583978. [DOI] [Google Scholar]
- 81.Bell JF, Kahn OB. Efficacy of some drugs and biologic preparations as therapeutics agents for tularemia. Archives in Internal Medicine. 1945;75:155–8. doi: 10.1001/archinte.1945.00210270012003. [DOI] [Google Scholar]
- 82.Allen WP. Immunity against tularemia: passive protection of mice by transfer of immune tissues. J Exp Med. 1962;115:411–20. doi: 10.1084/jem.115.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thorpe BD, Marcus S. Phagocytotsis and intracellular fate of Pasteurella tularensis 3: in vivo studies with passively transferred cells and sera. J Immunol. 1965;94:578–85. [PubMed] [Google Scholar]
- 84.Foshay L, Ruchman I, Nicholes PS. Antitularense serum: correlation between protective capacity for white rats and precipitable antibody content. J Clin Invest. 1947;26:756–60. doi: 10.1172/JCI101858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Larson CL. A serum protection test in tularemic infections in white rats. Public Health Rep. 1947;62:1793–9. doi: 10.2307/4586386. [DOI] [PubMed] [Google Scholar]
- 86.Drabick JJ, Narayanan RB, Williams JC, Leduc JW, Nacy CA. Passive protection of mice against lethal Francisella tularensis (live tularemia vaccine strain) infection by the sera of human recipients of the live tularemia vaccine. Am J Med Sci. 1994;308:83–7. doi: 10.1097/00000441-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 87.Rhinehart-Jones TR, Fortier AH, Elkins KL. Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host gamma interferon and T cells. Infect Immun. 1994;62:3129–37. doi: 10.1128/iai.62.8.3129-3137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stenmark S, Lindgren H, Tärnvik A, Sjöstedt A. Specific antibodies contribute to the host protection against strains of Francisella tularensis subspecies holarctica. Microb Pathog. 2003;35:73–80. doi: 10.1016/S0882-4010(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 89.Kubelkova K, Krocova Z, Balonova L, Pejchal J, Stulik J, Macela A. Specific antibodies protect gamma-irradiated mice against Francisella tularensis infection. Microb Pathog. 2012;53:259–68. doi: 10.1016/j.micpath.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 90.Pammit MA, Raulie EK, Lauriano CM, Klose KE, Arulanandam BP. Intranasal vaccination with a defined attenuated Francisella novicida strain induces gamma interferon-dependent antibody-mediated protection against tularemia. Infect Immun. 2006;74:2063–71. doi: 10.1128/IAI.74.4.2063-2071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klimpel GR, Eaves-Pyles T, Moen ST, Taormina J, Peterson JW, Chopra AK, et al. Levofloxacin rescues mice from lethal intra-nasal infections with virulent Francisella tularensis and induces immunity and production of protective antibody. Vaccine. 2008;26:6874–82. doi: 10.1016/j.vaccine.2008.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mara-Koosham G, Hutt JA, Lyons CR, Wu TH. Antibodies contribute to effective vaccination against respiratory infection by type A Francisella tularensis strains. Infect Immun. 2011;79:1770–8. doi: 10.1128/IAI.00605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fulop M, Mastroeni P, Green M, Titball RW. Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine. 2001;19:4465–72. doi: 10.1016/S0264-410X(01)00189-X. [DOI] [PubMed] [Google Scholar]
- 94.Lavine CL, Clinton SR, Angelova-Fischer I, Marion TN, Bina XR, Bina JE, et al. Immunization with heat-killed Francisella tularensis LVS elicits protective antibody-mediated immunity. Eur J Immunol. 2007;37:3007–20. doi: 10.1002/eji.200737620. [DOI] [PubMed] [Google Scholar]
- 95.Hickey AJ, Hazlett KR, Kirimanjeswara GS, Metzger DW. Identification of Francisella tularensis outer membrane protein A (FopA) as a protective antigen for tularemia. Vaccine. 2011;29:6941–7. doi: 10.1016/j.vaccine.2011.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sutherland MD, Goodyear AW, Troyer RM, Chandler JC, Dow SW, Belisle JT. Post-exposure immunization against Francisella tularensis membrane proteins augments protective efficacy of gentamicin in a mouse model of pneumonic tularemia. Vaccine. 2012;30:4977–82. doi: 10.1016/j.vaccine.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu Z, Roche MI, Hui JH, Unal B, Felgner PL, Gulati S, et al. Generation and characterization of hybridoma antibodies for immunotherapy of tularemia. Immunol Lett. 2007;112:92–103. doi: 10.1016/j.imlet.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Savitt AG, Mena-Taboada P, Monsalve G, Benach JL. Francisella tularensis infection-derived monoclonal antibodies provide detection, protection, and therapy. Clin Vaccine Immunol. 2009;16:414–22. doi: 10.1128/CVI.00362-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Conlan JW, Chen W, Shen H, Webb A, KuoLee R. Experimental tularemia in mice challenged by aerosol or intradermally with virulent strains of Francisella tularensis: bacteriologic and histopathologic studies. Microb Pathog. 2003;34:239–48. doi: 10.1016/S0882-4010(03)00046-9. [DOI] [PubMed] [Google Scholar]
- 100.Anderson RV, Crane DD, Bosio CM. Long lived protection against pneumonic tularemia is correlated with cellular immunity in peripheral, not pulmonary, organs. Vaccine. 2010;28:6562–72. doi: 10.1016/j.vaccine.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goodyear A, Kellihan L, Bielefeldt-Ohmann H, Troyer R, Propst K, Dow S. Protection from pneumonic infection with burkholderia species by inhalational immunotherapy. Infect Immun. 2009;77:1579–88. doi: 10.1128/IAI.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hirano T, Kodama S, Kawano T, Maeda K, Suzuki M. Monophosphoryl lipid A induced innate immune responses via TLR4 to enhance clearance of nontypeable Haemophilus influenzae and Moraxella catarrhalis from the nasopharynx in mice. FEMS Immunol Med Microbiol. 2011;63:407–17. doi: 10.1111/j.1574-695X.2011.00866.x. [DOI] [PubMed] [Google Scholar]
- 103.Bosio CM, Bielefeldt-Ohmann H, Belisle JT. Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J Immunol. 2007;178:4538–47. doi: 10.4049/jimmunol.178.7.4538. [DOI] [PubMed] [Google Scholar]
- 104.Chase JC, Celli J, Bosio CM. Direct and indirect impairment of human dendritic cell function by virulent Francisella tularensis Schu S4. Infect Immun. 2009;77:180–95. doi: 10.1128/IAI.00879-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sharma J, Li Q, Mishra BB, Pena C, Teale JM. Lethal pulmonary infection with Francisella novicida is associated with severe sepsis. J Leukoc Biol. 2009;86:491–504. doi: 10.1189/jlb.1208728. [DOI] [PMC free article] [PubMed] [Google Scholar]


