Abstract
DNA polymerase δ (Pol δ) is a key enzyme in eukaryotic DNA replication. Human Pol δ is a heterotetramer whose p12 subunit is degraded in response to DNA damage, leading to the in vivo conversion of Pol δ4 to Pol δ3. Two E3 ubiquitin ligases, RNF8 and CRL4Cdt2, participate in the DNA damage-induced degradation of p12. We discuss how these E3 ligases integrate the formation of Pol δ3 and ubiquitinated PCNA for DNA repair processes. CRL4Cdt2 partially degrades p12 during normal cell cycle progression, thereby generating Pol δ3 during S phase. This novel finding extends the current view of the role of Pol δ3 in DNA repair and leads to the hypothesis that it participates in DNA replication. The coordinated regulation of licensing factors and Pol δ3 by CRL4Cdt2 now opens new avenues for control of DNA replication. A parallel study of Pol δ4 and Pol δ3 in Okazaki fragment processing provides evidence for a role of Pol δ3 in DNA replication. We discuss several new perspectives of the role of the 2 forms of Pol δ in DNA replication and repair, as well the significance of the integration of p12 regulation in DNA repair and cell cycle progression.
Keywords: CRL4Cdt2, DNA damage, DNA polymerase δ, DNA replication, RNF8, cell cycle, cell cycle progression, p12 subunit
Introduction
p12 degradation in response to DNA damage leads to generation of Pol δ3, which is adapted for a role in DNA repair processes
DNA polymerases that replicate genomic DNA are at the forefront of the cellular mechanisms for the maintenance of genomic stability, and synthesize DNA with great fidelity. In eukaryotes, 2 proofreading DNA polymerases, Pol δ1-3 and Pol ε,4,5 perform the polymerization steps by elongation of primers synthesized by Pol α/primase.6 Pol δ and Pol ε perform discrete roles at the replication fork: Pol ε is largely responsible for synthesis of leading-strand DNA and Pol δ largely responsible for synthesis and subsequent processing of Okazaki fragments at the lagging strand.7,8 In addition, Pol δ also participates in gap-filling processes in DNA repair.1,3 Pol δ from budding yeast is a 3-subunit enzyme that has been well studied.9 In human cells, Pol δ4 is a heterotetramer of the p125, p50, and p68 subunits, which are paralogs of their yeast counterparts, together with a fourth subunit, p12.1,2,10
While the replication machinery is highly conserved in evolution, it is to be expected that there are evolutionary adaptations in higher eukaryotes which have to deal with a larger genome, as well as a more complex chromatin organization. One of these adaptations in Pol δ is the acquisition of the p12 subunit, which has emerged as a nexus for its cellular regulation (reviewed in Lee et al.1). This concept emerged from our discovery that the p12 subunit is rapidly degraded in response to UV-induced damage, alkylating agents, and replication stress induced by aphidicolin or hydroxyurea.11 The degradation of p12 is under the control of ATR, the apical protein that regulates the intra-S checkpoint.12,13 Most importantly, we demonstrated that loss of p12 leads to the formation of Pol δ3 (the trimeric enzyme lacking the p12 subunit) in vivo, by the direct isolation of Pol δ3 by immunoaffinity chromatography from UV-treated cultured human cells.11,14 The degradation of p12 represents an unusual regulatory system that involves modification of the quaternary structure of Pol δ4.
The next question we addressed in order to advance the hypothesis that Pol δ3 is involved in DNA repair is whether Pol δ3 is present at sites of DNA damage during the period when DNA repair is underway, viz, is it in the right place at the right time? This was a technically difficult process, in the sense that we had to show that absence of p12 and the presence of the 3 other Pol δ subunits at the sites of DNA damage. This was accomplished by examining the spatiotemporal dynamics of the recruitment of all 4 Pol δ subunits to sites of UV damage as assessed by their co-localization with CPDs (cyclobutane pyrimidine dimers) by immunofluorescence microscopy.15 These studies confirmed that the p125, p50, and p68 Pol δ subunits were recruited to sites of DNA damage, i.e., for a subset of the foci (ca. 70%), only Pol δ3 was present. Moreover, the time course of recruitment of Pol δ to the CPD foci was complete within 4 h, while CPD fluorescence was detectable for periods of up to 18 h, i.e., Pol δ3 is present during the period when CPDs are being repaired. Degradation of p12 occurred in all cell cycle phases in response to UV damage,15 as was inferred from earlier studies of the effects of DNA damage on p12 levels in asynchronous cell populations.11 Essentially this meant that Pol δ3 is recruited to sites of NER (nucleotide excision repair), as well as to stalled replication forks in S-phase cells. Thus, Pol δ3 can be considered the operative form of Pol δ that undertakes Pol δ functions in DNA repair processes.
Biochemical studies of the reconstituted human tetrameric Pol δ4 holoenzyme and of its subassemblies lacking one or more of the noncatalytic subunits have contributed to our understanding of their respective contributions to Pol δ function. These studies revealed that Pol δ3 retained activity in the standard assay on poly(dA)/oligo(dT) templates, with about half the specific activity of Pol δ4, but exhibited a poorer ability to extend singly primed M13 ssDNA, which requires high processivity.2,11 More rigorous kinetic analyses of the properties of Pol δ3 and Pol δ4 revealed profound differences that are rooted in the effects of the p12 subunit on the catalytic behavior of Pol δ. These studies, in fact, supported the idea that Pol δ3 exhibits properties that represent an adaptation of Pol δ for a function in DNA repair.14,16 We compared the properties of Pol δ4 and Pol δ3 in the context of how they replicated DNA across template lesions using model oligonucleotides. Two types of lesions were examined, O6-MeG (O6-methylguanine) and 8-oxoG (7,8-dihydro-8-oxoguanine), representing small lesions that are readily bypassed by replicative DNA polymerases in a mutagenic manner, and AP (apurinic/apyrimidinic) sites and thymine–thymine dimer lesions, which pose blockages to replicative polymerases.14 Pol δ3 exhibited greater discrimination against mutagenic bypass of the smaller lesions, as well as against extension of mismatched primers, and greater 3′–5′ exonuclease proofreading activity than Pol δ4. We noted that the increased stalling at template lesions might also facilitate the promotion of checkpoint response, as well as the switching between Pol δ and TLS (translesion) polymerases at bulky adducts.14
The idea that Pol δ3 exhibits intrinsically greater proofreading abilities than its parent enzyme was provocative and counterintuitive. We established this by classical pre-steady-state kinetic analyses. Two key kinetic constants, kpol (the rate constant for the polymerization step) and kpol-exo (the rate constant for the switching or translocation of the primer terminus from the polymerase to the 3′–5′ exonuclease site), control proofreading and the fidelity of replicative DNA polymerases.17-19 These 2 parameters allow the determination of intrinsic proofreading or editing capacity of a given polymerase that can be quantitatively expressed as the ratio kpol-exo/kpol. This ratio represents the probability of the primer terminus being removed. For Pol δ4 and Pol δ3, these values are 3.5 × 10−3 and 137 × 10−3, respectively, i.e., Pol δ3 is nearly 40-fold more effective in proofreading.16 Operationally, when the polymerase encounters a primer/template, where either there is a template lesion, or where the primer terminus is not properly base paired, kpol for the unfavorable primer/template substrate is reduced, increasing the probability for the primer end to be shuttled to the exonuclease site to be excised. Thus, insertions of mismatched nucleotides or extension of mismatched primer termini are reduced. The same arguments apply with regard to the encounter of Pol δ with base lesions on the template, which can lead to mutational events. These kinetic considerations also provide insights to stalling behavior of Pol δ when it encounters template lesions. The perspective gained from these studies is that Pol δ3 possesses a gain of function that could come into play when it is present under conditions where the cell is under genotoxic stress and template lesions are generated. These classical biochemical analyses have been essential in gaining an understanding of the mechanistic basis for the behavior of Pol δ3 and also for gaining insights as well as interpretations of its possible cellular functions.
The identification and nature of the signaling pathways that regulate the degradation of p12 is an important one. Our initial report of the degradation of p12 showed that it is dependent on ATR, but not ATM. However, we noted that at high UV doses, p12 could not be stabilized in ATR-deficient cells, indicating that other damage signaling pathways might be brought into play.11 At this stage, our assessment was that p12 degradation would be regulated through known DNA damage response signaling pathways, rather than any unique pathway. Our focus turned to the identification of the E3 ligases that target p12 for degradation as these represent the proximal molecular events.
In this article, we discuss 2 recent studies that led to the identification of RNF820 and CRL4Cdt2 as E3 ligases involved in the targeting of p12 for degradation in response to DNA damage. Most importantly, the latter study21 also showed that p12 levels fall during the cell cycle progression, generating Pol δ3 in the S phase, and therefore suggesting that Pol δ3 is also involved in DNA replication. We completed a contemporaneous analysis of the reactions involved in Okazaki fragment processing by both forms of Pol δ in cooperation with Fen1.22 This study revealed that Pol δ3 possesses characteristics that are ideal for Okazaki fragment processing, further supporting the idea that it participates in DNA replication as well as in DNA repair.
In the following article, we discuss the ramifications of these studies, the broader perspectives of the integration of signals for the degradation of p12, the novel hypothesis that 2 forms of Pol δ might participate in human DNA replication, and some insights into the implications of the centralized CRL4Cdt2 control of p12 with those of other PIP-degron containing substrates. In addition, we present previously unpublished data that bear on the perspective of these 3 papers that are the subjects of the discussions below.
Results and Discussion
Identification of RNF8 as an E3 ligase that participates in the targeting of p12 for degradation: Potential integration of p12 and PCNA ubiquitination in response to UV- and IR-induced DNA damage
We isolated an E3 ligase activity for the polyubiquitination of p12 using an in vitro assay from HeLa cells, followed by proteomic methods, which led to its identification as RNF8. The role of RNF8 in vivo was confirmed by findings that UV-induced p12 degradation was attenuated by shRNA knockdown of RNF8 in A549 cells and in mouse epithelial RNF8−/− cells.20 In addition, we and others have shown that RNF8 is recruited to sites of UV damage.20,23 Thus, we proposed that RNF8 plays a role in the generation of Pol δ3 for participation in gap-filling processes in NER (Fig. 1A). However, our results showed knockdown or knockouts of RNF8 only partially stabilized p12 under UV challenge, so that it was clear that other E3 ligases were likely to be involved.
Figure 1. Role of RNF8 in targeting of p12 in response to DNA damage and its potential for integrating responses to various genotoxic stimuli. Panel (A) illustrates the role of RNF8 in p12 degradation in generating Pol δ3 at sites of NER, where it fulfils a role in gap-filling after the excision step. Panel (B) shows the special case in S phase cells, where UV damage leads to stalling of the replication fork. Here, Rad18 has been shown to monoubiquitinate PCNA and initiate the process of translesion synthesis. The gray arrows between panels (A andB) indicate the speculative possibility that RNF8 and Rad18 could both participate in the ubiquitination of PCNA and p12. (C) The presence of RNF8 at sites of DSBs suggests that it may participate in the degradation of p12, generating Pol δ3 to function in HR processes. Here, it is also possible that RNF8 may function in the monoubiquitination of PCNA for the recruitment of TLS pols or in the non-canonical polyubiquitination of PCNA for HR processes.
Another important ubiquitination reaction is that of the mono- and polyubiquitination of PCNA, an integral partner of Pol δ in DNA replication/repair. During S phase, the encounter of replicative polymerases with bulky adducts produced by UV damage leads to stalling of replication forks. To avoid cell death, the DNA damage tolerance pathways are activated to allow bypass of the lesions.24-27 The monoubiquitination of PCNA triggers the process of TLS by translesion polymerases such as Pol η, via a switching process between Pol δ and Pol η. The established mechanism for the activation of TLS is the monoubiquitination of PCNA by the E3 ligase Rad18 (Fig. 1B).28 The possibility that Rad18 also targets p12 for degradation remains an open question.
We had previously shown that RNF8 catalyzes the stoichiometric conversion of PCNA to monoubiquitinated PCNA with the E2 conjugating enzyme UbcH5c in vitro, and that mono-ub-PCNA is further polyubiquitinated via K63 isopeptide linkages by RNF8 in concert with Ubc13/Uev1a.29 These in vitro studies29 support the idea that RNF8 could participate in the ubiquitination of PCNA in vivo, although this has yet to be established. The concept that both Pol δ4 and PCNA are ubiquitinated in response to DNA damage in the context of TLS would provide for the coordination of the formation of Pol δ3 and ub-PCNA by RNF8 (Fig. 1B). This idea is also consistent with a proposal we have made that Pol δ3 may switch more readily with a TLS polymerase, based on the idea that loss of p12 would facilitate the switching of Pol δ with TLS polymerases such as Pol η.30
RNF8 has a prominent role in assembly of the repair and signaling complexes at DSBs (double-strand DNA breaks), where it participates in histone H2A ubiquitination, as well recruitment of p53BP1 and BRCA1.31-35 We have proposed that RNF8 induced p12 degradation could also generate Pol δ3 for repair processes involving HR (homologous recombination).20 Thus, there emerges an integrated system for p12 degradation in response to a spectrum of genotoxic challenges which activate the ATM and the ATR signaling pathways (Fig. 1). Such integration may also occur in the case of other E3 ligases that act on p12, as discussed later in relation to CRL4Cdt2. We also include the potential that RNF8 could ubiquitinate PCNA, bearing in mind that in vitro RNF8 is capable of noncanonical polyubiquitination of monoubiquitinated PCNA29 (Fig. 1C). The generation of monoubiquitinated PCNA carries the inference that TLS polymerases may play a role during NER or HR processes. There is evidence for the involvement of TLS pols as well as Pol δ in primer extension reactions in HR36,37 and in NER.38 The functions of polyubiquitinated PCNA in DNA repair processes are still unclear, but it has been proposed to be involved in post-replication repair, specifically the error-free pathway that involves strand invasion or template switching.25-27,39-41
The prediction that p12 is also degraded in response to IR has been recently reported.42 We have also observed that p12 is depleted by IR (6Gy) within 3 h, and returns to basal levels by 24 h in HeLa and 293T cells (Fig. S1A and B). Using ATM+/+ and ATM−/− cell lines (Fig. S1C), we observed that p12 degradation is not dependent on ATM. While much further work is needed to trace the signaling events that lead to p12 depletion in IR, it may be noted that ATR is activated during the generation of ssDNA during resection of DSB ends.43,44
Role of the CRL4Cdt2 E3 ligase in the DNA damage induced targeting of p12 for degradation
We have recently reported that CRL4Cdt2 also targets p12 for degradation in response to UV damage, and is also responsible for the partial degradation of p12 during the G1/S transition during normal cell cycle progression.21 CRL4Cdt2 targets Cdt1,45 p21,46 (p21Waf1/CIP1),47 and the histone H4 methylase Set848 for proteasomal degradation during the G1/S transition, and also in response to UV irradiation. Studies in the Xenopus egg system have revealed an elegant molecular mechanism for the common targeting of CRL4Cdt2 substrates by their possession of a specific degron. These substrates are all PCNA binding proteins that possess a PIP-box, a short motif that is bound to a hydrophobic pocket in PCNA and is present in most PCNA binding proteins.49,50 The PIP-degron is an extended variant of the PIP-box, with a TD motif within the PIP-box, and a C-terminal cluster of basic residues.51,52
Alignment of the p12 PIP-box shows that it conforms to the degron motif, so that it is a likely substrate for CRL4Cdt2. This was confirmed by ectopic expression of p12 in which the basic cluster of residues was mutated. This mutant was almost completely stabilized in cells treated with UV. The role of CRL4Cdt2 in p12 degradation was confirmed using knockdowns of the components of the CRL4Cdt2 E3 ligase.21 Thus, p12 joins the group of proteins that are regulated by CRL4Cdt2 in response to UV damage.
We did not observe complete stabilization of endogenous p12 in cells in which the CRL4Cdt2 components were depleted by knockdown experiments,21 similar to our experience with RNF8.20 This we could explain on the basis that the two pathways may be partially redundant. Thus, at least two E3 ligase systems, RNF8 and CRL4Cdt2, are involved in targeting of p12 for degradation in response to UV. It may be noted that Cdt1 and p21 are subject to regulation by multiple E3 ligases (CRL4Cdt2, SCF-Skp2, and APC/C), so that there are multiple pathways for their regulation during specific phases of the cell cycle or in response to UV damage.53-58
CRL4Cdt2 involvement in p12 degradation in response to UV irradiation, as well as in response to IR has been recently reported.42 We have used MLN4924 to confirm the role of cullin ligase activity in UV and IR degradation of p12. MLN4924 is an inhibitor of the NEDD8-activating enzyme (NAE)59 that is required for the activation of cullin ligase activity and causes stabilization of Cdt1 and re-replication.59,60 MLN4924 produced a significant stabilization of p12 and p21 levels after exposure of A549 cells to UV irradiation (Fig. S2A). MLN4924 also blocked p12 degradation by IR in H1299 cells (Fig. S2B). The effects of MLN4924 are suggestive that cullin ligase activities play a prominent role in p12 degradation in response to UV and IR. The possibility that SCF-Skp2 (CRL1Skp2), whose function is also affected by MLN4924, participates in p12 degradation should be mentioned, since it also targets p21 and Cdt1 during cell cycle progression.53,55,61 Thus, p12 degradation is subject to regulation by multiple E3 ligases in response to genotoxic agents that trigger different DNA damage signaling pathways, and CRL4Cdt2 must be added to the overall scheme shown for RNF8 above (Fig. 1) for the regulation of p12.
CRL4Cdt2 regulates p12 degradation during normal cell cycle progression to generate Pol δ3 on entry into S phase
The discovery that p12 possesses a PIP-degron for CRL4Cdt2, as well as expanding our view of the multiple regulatory mechanisms that control p12 response to DNA damage, led to the accompanying and possibly more significant discovery that Pol δ4 is converted to Pol δ3 at the G1/S transition during unperturbed cell cycle progression.21 This challenges the idea that Pol δ3 is formed solely to deal with a function in DNA repair processes, and suggests that it may also play a role in DNA replication.
It seemed reasonable to predict that p12, by virtue of its possession of a PIP-degron, would be degraded by CRL4Cdt2 during normal cell cycle progression. We therefore examined the behavior of p12 in synchronized cells, and found that p12 levels did indeed fall during entry into S phase, and that this could be partially blocked by shRNA knockdown of CUL4A or CUL4B, the 2 human isoforms of human CRL4Cdt2.21 Independent confirmation of the alteration of p12 levels was obtained by analysis of the cell cycle distribution of all four subunits of Pol δ by laser scanning cytometry. This revealed that p12 levels were relatively lower than those of the other Pol δ subunits in S phase.15,21
The salient features of the changes in p12 levels in the unperturbed cell cycle are illustrated in Figure 2A. p12 levels fall during S phase, but unlike the case with DNA damage, only partial degradation is observed. The important outcome is the alteration of the relative levels of Pol δ4 and Pol δ3, extrapolated from the fall in p12, which show that Pol δ3 levels increase during the entry into S phase, and fall again as the exit from S phase begins. This fluctuation means that during the period where DNA replication takes place, the nuclear pool of Pol δ consists of a mixture of Pol δ4 and Pol δ3. This is unlike the case with UV-induced degradation, when p12 levels are completely depleted. The question is then raised as to the functional significance of this conversion of Pol δ4 to a mixture of Pol δ4 and Pol δ3 during S phase. The most immediate hypothesis that emerges is that Pol δ3 may be a participant in the process of DNA replication (Fig. 2B), specifically at the lagging strand, as will be discussed later.
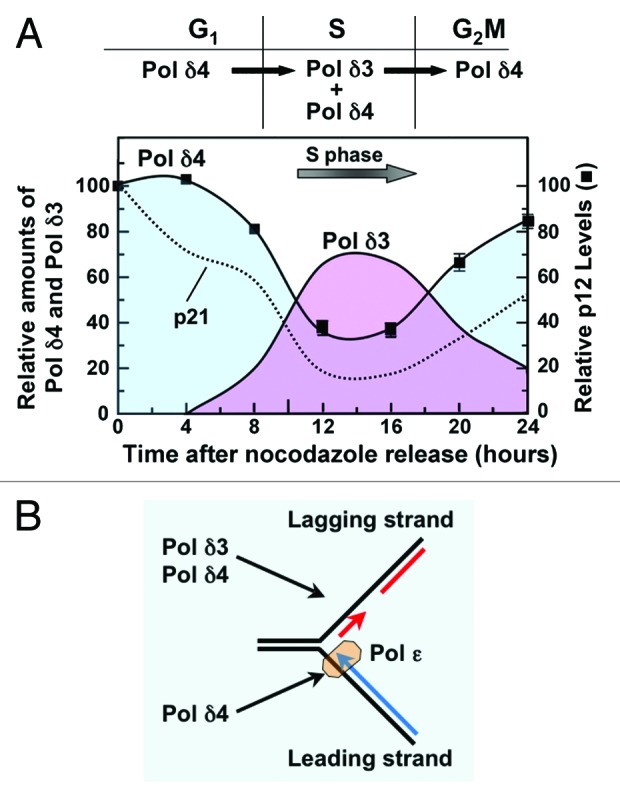
Figure 2. Regulation of Pol δ during cell cycle progression by the degradation of the p12 subunit. (A) The p12 subunit of Pol δ4 is partially degraded during S phase, leading to the formation of Pol δ3. H1299 cells were synchronized with nocodazole and allowed to progress through the cell cycle after release from nocodazole. p12 levels are shown as solid squares. The data are taken from Figure 4 in Zhang et al.21 The relative amounts of Pol δ4 and Pol δ3 are shown by the shaded areas (blue for Pol δ4 and pink for Pol δ3). The amounts of Pol δ3 are an approximation based on the loss of p12 and the assumption that Pol δ4 is converted to Pol δ3. The dotted line shows the changes in the levels of p21 in the same experiment. (B) The presence of both Pol δ4 and Pol δ3 during S phase raises questions regarding their functions at the leading and lagging strands at the replication fork. Here we show the possibility that Pol δ3 and Pol δ4 may both be involved in lagging-strand synthesis, and also that Pol δ4 may participate in leading-strand synthesis.
CRL4Cdt2 plays a crucial role in the orchestration and the initiation of the process of DNA replication and has achieved prominence as a major regulator of Cdt1 and Set8, whose activities during the G1 phase lead to the assembly and licensing of replication origins, as well as of p21.55 At the G1/S transition, the degradation of Cdt1 and Set8 is essential to prevent re-licensing of origins during S phase and the consequential loss of genomic stability, while p21 degradation is required to release its inhibition of cyclin-dependent kinase activities.53-55,62 The question now arises as to whether and how the degradation of p12 plays a role in the overall management of the global controls on DNA replication exerted by CRL4Cdt2. Given the integration of several critical regulators of the onset of DNA replication under the control of CRL4Cdt2, it seems unlikely that the generation of Pol δ3 during S phase is a trivial event without consequences for either control of DNA replication or for the replication machinery per se. The regulated or balanced formation of Pol δ3 during S phase could represent a process that is significant for the maintenance of genomic stability. Recent studies of the expression of p12 mRNA levels in lung cancer showed that p12 mRNA levels were reduced in small cell lung cancer. siRNA depletion of p12 in cultured cells led to cell cycle delay, checkpoint activation, and an increase in chromosomal gap/break formation.63 Additional studies using shRNA depletion of p12 revealed an increase in formation of karyomere-like cells.64
Comments on the mechanism for CRL4Cdt2 degradation of its substrates
CRL4Cdt2 recognizes its substrates only when they are bound to PCNA loaded onto chromatin.51,52 From the perspective of the involvement of Pol δ as a CRL4Cdt2 substrate, we have suggested that the chromatin-bound PCNA is actually PCNA loaded onto a primer terminus,21 viz., PCNADNA/primer. Formation of a primer end, either at the excision step in NER or the primer synthesis step by Pol α/primase, is then the event that leads to the loading of PCNA by RFC that is followed by recruitment of Pol δ and the elongation process, as well as the initiation of CRL4Cdt2 degradation of the licensing factors and p21. Mechanistic and structural studies of RFC function have shown that the process starts with the binding of PCNA through multiple contacts as well as the binding of ATP. RFC is structurally designed via a “notched screw-cap structure” to load PCNA onto the 3′ primer terminus, viz., at the junction of the double- and single-stranded regions.65-67 Kinetic studies show that RFC preferentially loads PCNA at the primer termini, selectively over dsDNA or ssDNA.68 In vitro assays using singly primed M13 ssDNA in which RFC, PCNA, and RPA are present show that RFC efficiently loads 1–3 molecules of PCNA per M13 ssDNA, which are efficiently acquired by Pol δ.69,70
Thus, Pol δ and the monomeric CRL4Cdt2 substrates (Cdt1, p21, and Set8) may be competing for the PCNADNA/primer platform. We have examined the degradation of p21 and Cdt1 in response to UV as well as during the cell cycle, but did not observe any great temporal preference for their degradation. The changes in p12 and p21 levels in nocadazole synchronized cells were examined, and showed that p21 declined a little faster and further than p1221 (see Fig. 2A). Thus, the question of the dynamics of the degradation p12 vis-a-vis the other CRL4Cdt2 substrates becomes important, since there is the possibility that this may impact the onset of DNA replication. The competition for PCNA by the CRL4Cdt2 substrates could provide a mechanism for delay of replication at the new primer terminus until the licensing factors are degraded. This proposition is possibly oversimplified, since at this time we do not have any information of how strongly the CRL4Cdt2 substrates compete with Pol δ4, not just for PCNA, but also for the PCNADNA/primer. As we have noted,21 Pol δ4 itself possesses a PIP-degron and is a multi-subunit protein with multivalent interactions with PCNA1 as well as a high affinity for primed DNA.16 This would greatly increase its affinity for PCNADNA/primer as against PCNA alone. Thus, the playing field is considerably leveled when considering the potential of CRL4Cdt2 degron-containing proteins (p21, Cdt1, and Set8) to compete with PCNA binding proteins in general, if the latter (such as Pol δ) also exhibit binding to DNA, viz., one must be cognizant of the distinction between competition for nucleoplasmic (free) PCNA as against chromatin-bound PCNA. A specific possibility arises from the number of observations that p21 inhibits DNA replication and competes with Pol δ for PCNA, so that p21 degradation may be a mechanism for regulating the onset of DNA synthesis.71-74 Further studies of the temporal aspects as well as of mechanistic aspects of the degradation of CRL4Cdt2 substrates in reconstituted systems could reveal new regulatory aspects of CRL4Cdt2 regulation of the temporal onset of DNA replication.
Pol δ3 is highly adapted for a role in Okazaki fragment processing by a nick translation mechanism in cooperation with Fen1
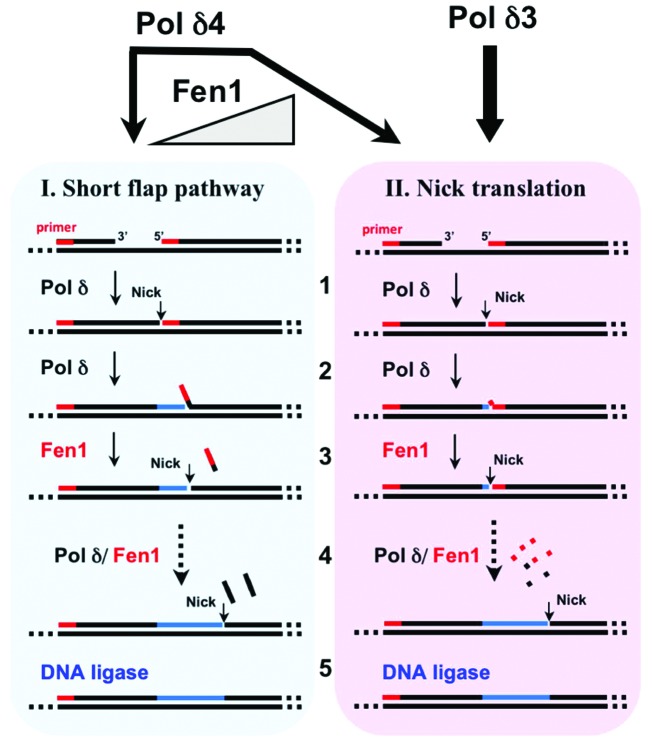
A study of the Okazaki fragment processing by human Pol δ4 and Pol δ3 is the third paper which is the subject of this Perspective.22 The processing of Okazaki fragments is essentially one of primer removal and replacement by Pol δ and Fen1.75-78 We used rigorously purified human enzymes and model substrates to compare the behavior of Pol δ4 and Pol δ3 in a reconstituted system for Okazaki fragment processing. We compared the abilities of Pol δ4 and Pol δ3 to perform strand displacement reactions that create the flap substrates for Fen1, and kinetic analysis of the distribution of products formed by Pol δ4 and Pol δ3 in the presence of increasing concentrations of Fen1. Pol δ4 exhibited strand displacement activity, and in the presence of Fen1 produced a spectrum of short products (1–10 nts), so that it functions in the short flap pathway (Fig. 3, panel I). In the presence of increasing concentrations of Fen1, this spectrum shifted to one of mainly mononucleotides, viz., the system operated as a nick translation system (Fig. 3, panel II). In contrast, Pol δ3 performed negligible strand displacement and inserted just 1 or 2 nts. In the presence of Fen1, mononucleotides were the primary products. This reaction was not affected by Fen1 concentration, so that Pol δ3 operated almost solely by a nick translation reaction and did not significantly participate in the short flap pathway (Fig. 3, panel II). Our study of the combined actions of Pol δ3 and Fen1 shows that this is predominantly a nick translation process, while that of Pol δ4 and Fen1 presents as either a short flap pathway or a nick translation process that is dependent on the Fen1 concentration22 (Fig. 3).
Figure 3. Okazaki fragment processing by Pol δ4 and Pol δ3. This diagram summarizes the reactions of Pol δ4 and Pol δ3 with Fen1 in the reconstituted system for Okazaki fragment processing.22 Panel I (blue) shows the operation of the short flap pathway. In reaction “1”, Pol δ extends the primer until it meets a 5′ downstream primer. Pol δ4 performs limited strand displacement to create short flaps of 1–10 nts (“2”) that are cleaved by Fen1 (“3”). This process is iterated to leave a nick that is sealed by DNA ligase. Panel II illustrates the operation of the nick translation pathway. In reaction “1”, Pol δ3 extends the primer until it meets a 5′ downstream primer. Pol δ inserts just 1 or 2 nts (“3”) that represent insertions due to the fraying/dissociation of the 5′ end of the blocking oligonucleotide (strand opening78). Pol δ3 does not perform significant strand displacement. The cleavage of the short flap leads to the formation of mainly 1 nt products. Multiple iterations of reactions “2” and “3” lead to the stepwise movement of the nick 1 nt at a time, viz., a nick translation reaction.
Previously, these reactions had been extensively studied in yeast, and have shown that yeast Pol δ has properties that, unlike Pol ε, promote its ability to act cooperatively with Fen1.9,79 The key attributes of yeast Pol δ that are required for its function in Okazaki fragment processing are the ability of Pol δ to idle at a nick, to prevent uncontrolled strand displacement, and the exhibition of limited strand displacement.78,79 Under conditions where Pol δ and Fen1 were in excess over the DNA substrate, the primary reaction was nick translation where mononucleotides were the primary product,78,79 similar to our findings with Pol δ4. The restriction of the combined actions of Pol δ and Fen1 to achieve nick translation both in yeast78,79 and in the human system 22 strongly supports the idea that the Pol δ and Fen1 reactions are mechanistically coupled in some manner. In the archaeal system, there is direct evidence that the polymerase, Fen1 and DNA ligase are able to form a complex on PCNA, providing a physical basis for coupling of Okazaki fragment-processing reactions.80,81
Our biochemical analyses of the properties of Pol δ4 and Pol δ3, which provided insights into the functions of Pol δ3 in gap filling in DNA repair, provide additional perspectives from several points of view. The first is that the process of removal and replacement of the 5′ end of the Okazaki fragment can be likened to a process of DNA repair that is intrinsic to the process of lagging-strand synthesis, so that the same adaptations of Pol δ3 for repair processes are ones which come into play in Okazaki fragment processing. The involvement of Pol δ3 in Okazaki fragment would also answer the conundrum that Pol δ3 is intrinsically a more accurate polymerase than Pol δ4.1,14,16
There may be advantages in the context of the maintenance of genomic stability if Okazaki fragment processing proceeds by nick translation rather than the short flap pathway. Generation of long flaps that cannot be cleaved by Fen1 requires the intervention of a separate pathway, the long flap pathway.75,82-84 Thus, utilization of the nick translation pathway would be expected to suppress the formation of long flaps. In addition, multiple reactions of the short flap pathway could result in unnecessary removal of residues beyond the primer end of the short Okazaki fragment. The conclusion from our studies is that Pol δ3 is ideally suited for Okazaki fragment processing via the nick translation system, and supports the hypothesis that Pol δ3 is a participant in lagging-strand synthesis. There are obvious questions that arise, as to what the respective roles of Pol δ4 and Pol δ3 are, either in lagging-strand synthesis, or potentially in leading-strand synthesis. Much further study is required to determine the prospective roles of Pol δ4 and Pol δ3 at leading- and lagging-strand synthesis.
Studies in yeast have provided both biochemical and genetic evidence for a division of labor at the replication fork, where Pol δ functions at the lagging strand and Pol ε at the leading strand.7,8,85 However, this division is not absolute, since yeast in which Pol ε catalytic activity is inactivated remain viable, indicating that Pol δ is capable of functioning at the leading strand, even if at a suboptimal level.86 In human cells, studies of the localization of Pol δ and Pol ε as well as analysis of their association with chromatin and lamins have suggested that they may act partly independently during S phase progression.87-89 While Pol δ3 might be excluded as a player in leading-strand synthesis, the idea that Pol δ4 could participate in leading-strand synthesis is still an open possibility. Whether and why both Pol δ3 and Pol δ4 participate in lagging-strand synthesis remain questions to be answered. The presence of 2 forms of Pol δ could be advantageous or even necessary in the replication of the complex genomes of higher eukaryotes. Such flexibility may be required in specific sequence contexts or in replication of different regions of the chromatin or at different stages of S phase. Currently, there is a trend toward a broadening of the number of DNA polymerases that may be required for DNA replication, e.g., there is evidence that Pol η may be involved in replication of microsatellite sequences90-93 and Pol κ in non-B DNA structures such as G-quadruplexes.94
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We apologize for any omissions in the literature citations, as our intent was not a comprehensive review but rather to explore perspectives and novel hypotheses that might serve to highlight areas that might lead to greater understanding of the control of Pol δ.
This work was supported by grants from the National Institutes of Health (GM31973 and ES14737) to MYWTL.
Glossary
Abbreviations:
- Cdt2
Cdc10-dependent transcript 2
- CRL4
cullin-ring ligase 4
- HR
homologous recombination
- NER
nucleotide excision repair
- PCNA
proliferating cell nuclear antigen
- Pol δ
DNA polymerase δ
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/27407
References
- 1.Lee MY, Zhang S, Lin SH, Chea J, Wang X, LeRoy C, Wong A, Zhang Z, Lee EY. Regulation of human DNA polymerase delta in the cellular responses to DNA damage. Environ Mol Mutagen. 2012;53:683–98. doi: 10.1002/em.21743. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y, Meng X, Zhang S, Lee EY, Lee MY. Characterization of human DNA polymerase delta and its subassemblies reconstituted by expression in the MultiBac system. PLoS One. 2012;7:e39156. doi: 10.1371/journal.pone.0039156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prindle MJ, Loeb LA. DNA polymerase delta in DNA replication and genome maintenance. Environ Mol Mutagen. 2012;53:666–82. doi: 10.1002/em.21745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson E, Macneill SA. The eukaryotic replicative DNA polymerases take shape. Trends Biochem Sci. 2010;35:339–47. doi: 10.1016/j.tibs.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Bermudez VP, Farina A, Raghavan V, Tappin I, Hurwitz J. Studies on human DNA polymerase epsilon and GINS complex and their role in DNA replication. J Biol Chem. 2011;286:28963–77. doi: 10.1074/jbc.M111.256289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider A, Smith RW, Kautz AR, Weisshart K, Grosse F, Nasheuer HP. Primase activity of human DNA polymerase alpha-primase. Divalent cations stabilize the enzyme activity of the p48 subunit. J Biol Chem. 1998;273:21608–15. doi: 10.1074/jbc.273.34.21608. [DOI] [PubMed] [Google Scholar]
- 7.Kunkel TA, Burgers PM. Dividing the workload at a eukaryotic replication fork. Trends Cell Biol. 2008;18:521–7. doi: 10.1016/j.tcb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavlov YI, Shcherbakova PV. DNA polymerases at the eukaryotic fork-20 years later. Mutat Res. 2010;685:45–53. doi: 10.1016/j.mrfmmm.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg P, Burgers PM. DNA polymerases that propagate the eukaryotic DNA replication fork. Crit Rev Biochem Mol Biol. 2005;40:115–28. doi: 10.1080/10409230590935433. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Mo J, Rodriguez-Belmonte EM, Lee MY. Identification of a fourth subunit of mammalian DNA polymerase delta. J Biol Chem. 2000;275:18739–44. doi: 10.1074/jbc.M001217200. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Zhou Y, Trusa S, Meng X, Lee EY, Lee MY. A novel DNA damage response: rapid degradation of the p12 subunit of dna polymerase delta. J Biol Chem. 2007;282:15330–40. doi: 10.1074/jbc.M610356200. [DOI] [PubMed] [Google Scholar]
- 12.Sirbu BM, Cortez D. DNA damage response: three levels of DNA repair regulation. Cold Spring Harb Perspect Biol. 2013;5:a012724. doi: 10.1101/cshperspect.a012724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nam EA, Cortez D. ATR signalling: more than meeting at the fork. Biochem J. 2011;436:527–36. doi: 10.1042/BJ20102162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng X, Zhou Y, Zhang S, Lee EY, Frick DN, Lee MY. DNA damage alters DNA polymerase delta to a form that exhibits increased discrimination against modified template bases and mismatched primers. Nucleic Acids Res. 2009;37:647–57. doi: 10.1093/nar/gkn1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chea J, Zhang S, Zhao H, Zhang Z, Lee EY, Darzynkiewicz Z, Lee MY. Spatiotemporal recruitment of human DNA polymerase delta to sites of UV damage. Cell Cycle. 2012;11:2885–95. doi: 10.4161/cc.21280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng X, Zhou Y, Lee EY, Lee MY, Frick DN. The p12 subunit of human polymerase delta modulates the rate and fidelity of DNA synthesis. Biochemistry. 2010;49:3545–54. doi: 10.1021/bi100042b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson KA. Conformational coupling in DNA polymerase fidelity. Annu Rev Biochem. 1993;62:685–713. doi: 10.1146/annurev.bi.62.070193.003345. [DOI] [PubMed] [Google Scholar]
- 18.Kunkel TA, Bebenek K. DNA replication fidelity. Annu Rev Biochem. 2000;69:497–529. doi: 10.1146/annurev.biochem.69.1.497. [DOI] [PubMed] [Google Scholar]
- 19.Khare V, Eckert KA. The proofreading 3′-->5′ exonuclease activity of DNA polymerases: a kinetic barrier to translesion DNA synthesis. Mutat Res. 2002;510:45–54. doi: 10.1016/S0027-5107(02)00251-8. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Zhou Y, Sarkeshik A, Yates JR, 3rd, Thomson TM, Zhang Z, Lee EY, Lee MY. Identification of RNF8 as a ubiquitin ligase involved in targeting the p12 subunit of DNA polymerase δ for degradation in response to DNA damage. J Biol Chem. 2013;288:2941–50. doi: 10.1074/jbc.M112.423392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S, Zhao H, Darzynkiewicz Z, Zhou P, Zhang Z, Lee EY, Lee MY. A novel function of CRL4Cdt2: regulation of the subunit structure of DNA polymerase delta in response to DNA damage and during the S phase. J Biol Chem. 2013;288:29950–61. doi: 10.1074/jbc.M113.490466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin SH, Wang X, Zhang S, Zhang Z, Lee EY, Lee MY. Dynamics of enzymatic interactions during short flap human Okazaki fragment processing by two forms of human DNA polymerase δ. DNA Repair (Amst) 2013;12:922–35. doi: 10.1016/j.dnarep.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marteijn JA, Bekker-Jensen S, Mailand N, Lans H, Schwertman P, Gourdin AM, Dantuma NP, Lukas J, Vermeulen W. Nucleotide excision repair-induced H2A ubiquitination is dependent on MDC1 and RNF8 and reveals a universal DNA damage response. J Cell Biol. 2009;186:835–47. doi: 10.1083/jcb.200902150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen PL, Xu F, Xiao W. Eukaryotic DNA damage tolerance and translesion synthesis through covalent modifications of PCNA. Cell Res. 2008;18:162–73. doi: 10.1038/cr.2007.114. [DOI] [PubMed] [Google Scholar]
- 25.Branzei D, Foiani M. Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol. 2008;9:297–308. doi: 10.1038/nrm2351. [DOI] [PubMed] [Google Scholar]
- 26.Ulrich HD, Walden H. Ubiquitin signalling in DNA replication and repair. Nat Rev Mol Cell Biol. 2010;11:479–89. doi: 10.1038/nrm2921. [DOI] [PubMed] [Google Scholar]
- 27.Sale JE, Lehmann AR, Woodgate R. Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat Rev Mol Cell Biol. 2012;13:141–52. doi: 10.1038/nrm3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–41. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 29.Zhang S, Chea J, Meng X, Zhou Y, Lee EY, Lee MY. PCNA is ubiquitinated by RNF8. Cell Cycle. 2008;7:3399–404. doi: 10.4161/cc.7.21.6949. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Zhang S, Lin SH, Wang X, Wu L, Lee EY, Lee MY. Structure of monoubiquitinated PCNA: implications for DNA polymerase switching and Okazaki fragment maturation. Cell Cycle. 2012;11:2128–36. doi: 10.4161/cc.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B, Elledge SJ. Ubc13/Rnf8 ubiquitin ligases control foci formation of the Rap80/Abraxas/Brca1/Brcc36 complex in response to DNA damage. Proc Natl Acad Sci U S A. 2007;104:20759–63. doi: 10.1073/pnas.0710061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolas NK, Chapman JR, Nakada S, Ylanko J, Chahwan R, Sweeney FD, Panier S, Mendez M, Wildenhain J, Thomson TM, et al. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science. 2007;318:1637–40. doi: 10.1126/science.1150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huen MS, Grant R, Manke I, Minn K, Yu X, Yaffe MB, Chen J. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell. 2007;131:901–14. doi: 10.1016/j.cell.2007.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mailand N, Bekker-Jensen S, Faustrup H, Melander F, Bartek J, Lukas C, Lukas J. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell. 2007;131:887–900. doi: 10.1016/j.cell.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 35.Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell. 2007;28:739–45. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 36.Sneeden JL, Grossi SM, Tappin I, Hurwitz J, Heyer WD. Reconstitution of recombination-associated DNA synthesis with human proteins. Nucleic Acids Res. 2013;41:4913–25. doi: 10.1093/nar/gkt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sebesta M, Burkovics P, Juhasz S, Zhang S, Szabo JE, Lee MY, Haracska L, Krejci L. Role of PCNA and TLS polymerases in D-loop extension during homologous recombination in humans. DNA Repair (Amst) 2013;12:691–8. doi: 10.1016/j.dnarep.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogi T, Lehmann AR. The Y-family DNA polymerase kappa (pol kappa) functions in mammalian nucleotide-excision repair. Nat Cell Biol. 2006;8:640–2. doi: 10.1038/ncb1417. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Bozza W, Zhuang Z. Ubiquitination of PCNA and its essential role in eukaryotic translesion synthesis. Cell Biochem Biophys. 2011;60:47–60. doi: 10.1007/s12013-011-9187-3. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W, Qin Z, Zhang X, Xiao W. Roles of sequential ubiquitination of PCNA in DNA-damage tolerance. FEBS Lett. 2011;585:2786–94. doi: 10.1016/j.febslet.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 41.Mailand N, Gibbs-Seymour I, Bekker-Jensen S. Regulation of PCNA-protein interactions for genome stability. Nat Rev Mol Cell Biol. 2013;14:269–82. doi: 10.1038/nrm3562. [DOI] [PubMed] [Google Scholar]
- 42.Terai K, Shibata E, Abbas T, Dutta A. Degradation of p12 subunit by CRL4Cdt2 E3 ligase inhibits fork progression after DNA damage. J Biol Chem. 2013;288:30509–14. doi: 10.1074/jbc.C113.505586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiotani B, Zou L. Single-stranded DNA orchestrates an ATM-to-ATR switch at DNA breaks. Mol Cell. 2009;33:547–58. doi: 10.1016/j.molcel.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomimatsu N, Mukherjee B, Deland K, Kurimasa A, Bolderson E, Khanna KK, Burma S. Exo1 plays a major role in DNA end resection in humans and influences double-strand break repair and damage signaling decisions. DNA Repair (Amst) 2012;11:441–8. doi: 10.1016/j.dnarep.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Senga T, Sivaprasad U, Zhu W, Park JH, Arias EE, Walter JC, Dutta A. PCNA is a cofactor for Cdt1 degradation by CUL4/DDB1-mediated N-terminal ubiquitination. J Biol Chem. 2006;281:6246–52. doi: 10.1074/jbc.M512705200. [DOI] [PubMed] [Google Scholar]
- 46.Abbas T, Sivaprasad U, Terai K, Amador V, Pagano M, Dutta A. PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex. Genes Dev. 2008;22:2496–506. doi: 10.1101/gad.1676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warfel NA, El-Deiry WS. p21WAF1 and tumourigenesis: 20 years after. Curr Opin Oncol. 2013;25:52–8. doi: 10.1097/CCO.0b013e32835b639e. [DOI] [PubMed] [Google Scholar]
- 48.Jørgensen S, Eskildsen M, Fugger K, Hansen L, Larsen MS, Kousholt AN, Syljuåsen RG, Trelle MB, Jensen ON, Helin K, et al. SET8 is degraded via PCNA-coupled CRL4(CDT2) ubiquitylation in S phase and after UV irradiation. J Cell Biol. 2011;192:43–54. doi: 10.1083/jcb.201009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warbrick E. PCNA binding through a conserved motif. Bioessays. 1998;20:195–9. doi: 10.1002/(SICI)1521-1878(199803)20:3<195::AID-BIES2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 50.Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–79. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Havens CG, Walter JC. Docking of a specialized PIP Box onto chromatin-bound PCNA creates a degron for the ubiquitin ligase CRL4Cdt2. Mol Cell. 2009;35:93–104. doi: 10.1016/j.molcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Havens CG, Walter JC. Mechanism of CRL4(Cdt2), a PCNA-dependent E3 ubiquitin ligase. Genes Dev. 2011;25:1568–82. doi: 10.1101/gad.2068611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–14. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jung YS, Qian Y, Chen X. Examination of the expanding pathways for the regulation of p21 expression and activity. Cell Signal. 2010;22:1003–12. doi: 10.1016/j.cellsig.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abbas T, Dutta A. CRL4Cdt2: master coordinator of cell cycle progression and genome stability. Cell Cycle. 2011;10:241–9. doi: 10.4161/cc.10.2.14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–81. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- 57.Fotedar R, Bendjennat M, Fotedar A. Role of p21WAF1 in the cellular response to UV. Cell Cycle. 2004;3:134–7. doi: 10.4161/cc.3.2.658. [DOI] [PubMed] [Google Scholar]
- 58.Bendjennat M, Boulaire J, Jascur T, Brickner H, Barbier V, Sarasin A, Fotedar A, Fotedar R. UV irradiation triggers ubiquitin-dependent degradation of p21(WAF1) to promote DNA repair. Cell. 2003;114:599–610. doi: 10.1016/j.cell.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Lin JJ, Milhollen MA, Smith PG, Narayanan U, Dutta A. NEDD8-targeting drug MLN4924 elicits DNA rereplication by stabilizing Cdt1 in S phase, triggering checkpoint activation, apoptosis, and senescence in cancer cells. Cancer Res. 2010;70:10310–20. doi: 10.1158/0008-5472.CAN-10-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milhollen MA, Narayanan U, Soucy TA, Veiby PO, Smith PG, Amidon B. Inhibition of NEDD8-activating enzyme induces rereplication and apoptosis in human tumor cells consistent with deregulating CDT1 turnover. Cancer Res. 2011;71:3042–51. doi: 10.1158/0008-5472.CAN-10-2122. [DOI] [PubMed] [Google Scholar]
- 61.Nishitani H, Sugimoto N, Roukos V, Nakanishi Y, Saijo M, Obuse C, Tsurimoto T, Nakayama KI, Nakayama K, Fujita M, et al. Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1 for proteolysis. EMBO J. 2006;25:1126–36. doi: 10.1038/sj.emboj.7601002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rizzardi LF, Cook JG. Flipping the switch from g1 to s phase with e3 ubiquitin ligases. Genes Cancer. 2012;3:634–48. doi: 10.1177/1947601912473307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang QM, Tomida S, Masuda Y, Arima C, Cao K, Kasahara TA, Osada H, Yatabe Y, Akashi T, Kamiya K, et al. Regulation of DNA polymerase POLD4 influences genomic instability in lung cancer. Cancer Res. 2010;70:8407–16. doi: 10.1158/0008-5472.CAN-10-0784. [DOI] [PubMed] [Google Scholar]
- 64.Huang QM, Akashi T, Masuda Y, Kamiya K, Takahashi T, Suzuki M. Roles of POLD4, smallest subunit of DNA polymerase delta, in nuclear structures and genomic stability of human cells. Biochem Biophys Res Commun. 2010;391:542–6. doi: 10.1016/j.bbrc.2009.11.094. [DOI] [PubMed] [Google Scholar]
- 65.Bowman GD, O’Donnell M, Kuriyan J. Structural analysis of a eukaryotic sliding DNA clamp-clamp loader complex. Nature. 2004;429:724–30. doi: 10.1038/nature02585. [DOI] [PubMed] [Google Scholar]
- 66.Bowman GD, Goedken ER, Kazmirski SL, O’Donnell M, Kuriyan J. DNA polymerase clamp loaders and DNA recognition. FEBS Lett. 2005;579:863–7. doi: 10.1016/j.febslet.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 67.Yao NY, O’Donnell M. The RFC Clamp Loader: Structure and Function. Subcell Biochem. 2012;62:259–79. doi: 10.1007/978-94-007-4572-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hingorani MM, Coman MM. On the specificity of interaction between the Saccharomyces cerevisiae clamp loader replication factor C and primed DNA templates during DNA replication. J Biol Chem. 2002;277:47213–24. doi: 10.1074/jbc.M206764200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Masuda Y, Suzuki M, Piao J, Gu Y, Tsurimoto T, Kamiya K. Dynamics of human replication factors in the elongation phase of DNA replication. Nucleic Acids Res. 2007;35:6904–16. doi: 10.1093/nar/gkm822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chilkova O, Stenlund P, Isoz I, Stith CM, Grabowski P, Lundström EB, Burgers PM, Johansson E. The eukaryotic leading and lagging strand DNA polymerases are loaded onto primer-ends via separate mechanisms but have comparable processivity in the presence of PCNA. Nucleic Acids Res. 2007;35:6588–97. doi: 10.1093/nar/gkm741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Waga S, Hannon GJ, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994;369:574–8. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- 72.Flores-Rozas H, Kelman Z, Dean FB, Pan ZQ, Harper JW, Elledge SJ, O’Donnell M, Hurwitz J. Cdk-interacting protein 1 directly binds with proliferating cell nuclear antigen and inhibits DNA replication catalyzed by the DNA polymerase delta holoenzyme. Proc Natl Acad Sci U S A. 1994;91:8655–9. doi: 10.1073/pnas.91.18.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cazzalini O, Perucca P, Riva F, Stivala LA, Bianchi L, Vannini V, Ducommun B, Prosperi E. p21CDKN1A does not interfere with loading of PCNA at DNA replication sites, but inhibits subsequent binding of DNA polymerase delta at the G1/S phase transition. Cell Cycle. 2003;2:596–603. doi: 10.4161/cc.2.6.502. [DOI] [PubMed] [Google Scholar]
- 74.Li H, Xie B, Rahmeh A, Zhou Y, Lee MY. Direct interaction of p21 with p50, the small subunit of human DNA polymerase delta. Cell Cycle. 2006;5:428–36. doi: 10.4161/cc.5.4.2425. [DOI] [PubMed] [Google Scholar]
- 75.Burgers PM. Polymerase dynamics at the eukaryotic DNA replication fork. J Biol Chem. 2009;284:4041–5. doi: 10.1074/jbc.R800062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Balakrishnan L, Bambara RA. Okazaki fragment metabolism. Cold Spring Harb Perspect Biol. 2013;5:a10173. doi: 10.1101/cshperspect.a010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng L, Shen B. Okazaki fragment maturation: nucleases take centre stage. J Mol Cell Biol. 2011;3:23–30. doi: 10.1093/jmcb/mjq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garg P, Burgers PM. How the cell deals with DNA nicks. Cell Cycle. 2005;4:221–4. doi: 10.4161/cc.4.2.1418. [DOI] [PubMed] [Google Scholar]
- 79.Garg P, Stith CM, Sabouri N, Johansson E, Burgers PM. Idling by DNA polymerase delta maintains a ligatable nick during lagging-strand DNA replication. Genes Dev. 2004;18:2764–73. doi: 10.1101/gad.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beattie TR, Bell SD. Coordination of multiple enzyme activities by a single PCNA in archaeal Okazaki fragment maturation. EMBO J. 2012;31:1556–67. doi: 10.1038/emboj.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dionne I, Nookala RK, Jackson SP, Doherty AJ, Bell SD. A heterotrimeric PCNA in the hyperthermophilic archaeon Sulfolobus solfataricus. Mol Cell. 2003;11:275–82. doi: 10.1016/S1097-2765(02)00824-9. [DOI] [PubMed] [Google Scholar]
- 82.Kang YH, Lee CH, Seo YS. Dna2 on the road to Okazaki fragment processing and genome stability in eukaryotes. Crit Rev Biochem Mol Biol. 2010;45:71–96. doi: 10.3109/10409230903578593. [DOI] [PubMed] [Google Scholar]
- 83.Balakrishnan L, Bambara RA. Flap endonuclease 1. Annu Rev Biochem. 2013;82:119–38. doi: 10.1146/annurev-biochem-072511-122603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Finger LD, Atack JM, Tsutakawa S, Classen S, Tainer J, Grasby J, Shen B. The wonders of flap endonucleases: structure, function, mechanism and regulation. Subcell Biochem. 2012;62:301–26. doi: 10.1007/978-94-007-4572-8_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nick McElhinny SA, Gordenin DA, Stith CM, Burgers PM, Kunkel TA. Division of labor at the eukaryotic replication fork. Mol Cell. 2008;30:137–44. doi: 10.1016/j.molcel.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kesti T, Flick K, Keränen S, Syväoja JE, Wittenberg C. DNA polymerase epsilon catalytic domains are dispensable for DNA replication, DNA repair, and cell viability. Mol Cell. 1999;3:679–85. doi: 10.1016/S1097-2765(00)80361-5. [DOI] [PubMed] [Google Scholar]
- 87.Fuss J, Linn S. Human DNA polymerase epsilon colocalizes with proliferating cell nuclear antigen and DNA replication late, but not early, in S phase. J Biol Chem. 2002;277:8658–66. doi: 10.1074/jbc.M110615200. [DOI] [PubMed] [Google Scholar]
- 88.Rytkönen AK, Vaara M, Nethanel T, Kaufmann G, Sormunen R, Läärä E, Nasheuer HP, Rahmeh A, Lee MY, Syväoja JE, et al. Distinctive activities of DNA polymerases during human DNA replication. FEBS J. 2006;273:2984–3001. doi: 10.1111/j.1742-4658.2006.05310.x. [DOI] [PubMed] [Google Scholar]
- 89.Vaara M, Itkonen H, Hillukkala T, Liu Z, Nasheuer HP, Schaarschmidt D, Pospiech H, Syväoja JE. Segregation of replicative DNA polymerases during S phase: DNA polymerase ε, but not DNA polymerases α/δ, are associated with lamins throughout S phase in human cells. J Biol Chem. 2012;287:33327–38. doi: 10.1074/jbc.M112.357996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rey L, Sidorova JM, Puget N, Boudsocq F, Biard DS, Monnat RJJ, Jr., Cazaux C, Hoffmann JS. Human DNA polymerase eta is required for common fragile site stability during unperturbed DNA replication. Mol Cell Biol. 2009;29:3344–54. doi: 10.1128/MCB.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hile SE, Wang X, Lee MY, Eckert KA. Beyond translesion synthesis: polymerase κ fidelity as a potential determinant of microsatellite stability. Nucleic Acids Res. 2012;40:1636–47. doi: 10.1093/nar/gkr889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baptiste BA, Eckert KA. DNA polymerase kappa microsatellite synthesis: two distinct mechanisms of slippage-mediated errors. Environ Mol Mutagen. 2012;53:787–96. doi: 10.1002/em.21721. [DOI] [PubMed] [Google Scholar]
- 93.Walsh E, Wang X, Lee MY, Eckert KA. Mechanism of replicative DNA polymerase delta pausing and a potential role for DNA polymerase kappa in common fragile site replication. J Mol Biol. 2013;425:232–43. doi: 10.1016/j.jmb.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bétous R, Rey L, Wang G, Pillaire MJ, Puget N, Selves J, Biard DS, Shin-ya K, Vasquez KM, Cazaux C, et al. Role of TLS DNA polymerases eta and kappa in processing naturally occurring structured DNA in human cells. Mol Carcinog. 2009;48:369–78. doi: 10.1002/mc.20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.