Abstract
The number and use of pharmacogenetic tests to assess a patient’s likelihood of response or risk of an adverse event is expanding across medical specialties and becoming more prevalent. During this period of development and translation, different approaches are being investigated to optimize delivery of pharmacogenetic services. In this paper, we review preemptive and point-of-care delivery approaches currently implemented or being investigated and discuss the advantages and disadvantages of each approach. The continued growth in knowledge about the genetic basis of drug response combined with development of new and cheaper testing technologies and electronic medical records will impact future delivery systems. Regardless of delivery approach, the currently limited knowledge of health professionals about genetics generally or PGx specifically will remain a major obstacle to utilization.
Keywords: Pharmacogenetic testing, clinical implementation, knowledge
Pharmacogenetic (PGx) testing entails the assessment of a patient’s genetic likelihood to respond to a given medication or develop an adverse drug response (ADR), information useful to inform therapeutic decision-making [1]. Although much debate has been published about the evidence basis for the clinical use of PGx testing [2–5], it is gradually becoming more accepted [6–8] and substantial research and commercial development continues. One of the critical issues regarding the use of PGx testing is the optimal delivery service model. Currently, several approaches are being investigated or currently used in clinical settings to deliver PGx testing services, but it is not yet clear if one model will dominate the delivery of PGx testing or if it will be case-specific or institutional-specific. The most effective model will depend on several factors including necessity for immediate treatment, severity of event potentially prevented by testing, and availability of clinical expertise to insure appropriate use of PGx information when treatment is required. In this paper, we review preemptive (or prospective) and point-of care delivery models of PGx testing – each possibly initiated or ordered by different clinicians or even the patient – and the advantages and disadvantages of each approach (Figure).
Figure.
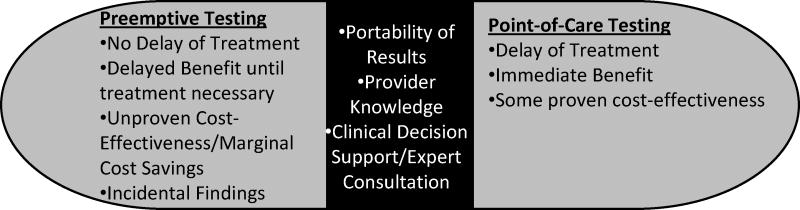
Comparison of pre-emptive testing and point-of-care testing and shared barriers (shown in middle).
Point-of-Care PGx Testing
Each time a new medication is prescribed, a PGx test can be ordered for the specific gene(s) involved in the transport, metabolism, or target of that medication. This prescription-driven approach would appear to be most logical as testing would directly inform the current treatment. However, one of the major disadvantages of doing so is the potential delay in treatment [9–12]. Major testing laboratories currently indicate that testing may take from three to 14 days to complete, a length of time for which some treatments may not be acceptably delayed. Treatment with a given medication may be delayed if there is a low risk to the patient, if the patient can be monitored when given a standard dose of the medication, or if an alternative drug is available. For example, treatment with simvastatin may be delayed to ascertain the patient’s SLCO1B1 genotype and their risk for myopathy since immediate treatment is not typically necessary [13 14]. If antiplatelet treatment is needed for patients with acute coronary syndromes (ACSs) undergoing percutaneous coronary intervention (PCI), an alternative treatment (e.g., prasugrel, ticagrelor) may be prescribed until CYP2C19 testing is completed; the patient can then be switched to clopidogrel later if found to be an ultra-rapid (*1/*17 or *17/*17) or extensive metabolizer (*1/*1) [15–18]. However, delay of treatment with clopidogrel in a hospitalized patient undergoing a stenting procedure would be unacceptable.
Alternatively, instead of testing a patient when prescribed a medication known to be associated with a PGx variant as indicated on the drug insert or recommended in a clinical guideline, some have explored testing patients after the occurrence of an ADR. Van Puijenbroek et al. [19] demonstrated the feasibility of using a pharmacovigilance center to alert physicians of potential PGx factors that may account for reported ADRs. Although test ordering was left to the physician’s discretion, 40% of physicians receiving these alerts ordered testing. The perceived value of testing may motivate providers to begin ordering tests at the time when a drug is initially prescribed rather than after an ADR has occurred.
Point-of-care PGx testing will typically involve analysis of one or two genes associated with a given medication. The limited scope of testing will reduce the potential of incidental findings, or test results unrelated to the patient’s current condition, which may be revealed when using comprehensive genomic-testing platforms or analysis of genes with multiple phenotypic associations. New advances in point-of-care testing technologies, potentially enabling office-based testing, could substantially reduce delays caused by lengthy test turnaround times [20–22]. However, technological advances in sequencing or array-based testing may eventually render single-gene testing obsolete as costs of large-scale platforms become comparable or cheaper than single-gene analysis.
Point-of-care PGx testing can be ordered in almost any clinical setting and by any clinician. In the outpatient setting, primary care physicians (PCPs) have expressed interest in using PGx testing and believe it will become more widespread in the near future [23]. However, despite their interest, PCPs’ use of PGx testing has thus far been reported to be low [24 25]. In the inpatient setting, implementation of point-of-care PGx testing has been shown to be feasible and effective for warfarin [26 27] and clopidogrel [21]. Treatment in urgent care situations and intensive care units will likely continue to be administered without the benefit of PGx testing, due in part to the lack of rapid testing platforms and evidence of the effectiveness of PGx testing on critically ill patients [28]. However, given the high rate of medications required of critically ill patients [29], testing may still be useful to optimize dosing during the patient’s stay and before discharge.
In addition to PCPs or other clinicians (outpatient setting) or hospitalists (inpatient setting), pharmacists are anticipated to play a key role in delivering point-of-care PGx testing. Several groups have suggested that including PGx testing among pharmacy clinical services would be appropriate [30–32]. Relatively early in the commercialization of PGx testing, two major pharmacy benefit managers (PBMs) in the U.S. began to provide PGx testing for interested clients. The PBM-initiated delivery model is initiated upon receipt of a patient prescription for a given drug with available PGx testing. The PBM contacts the ordering physician to alert them of the availability PGx testing and the physician decides whether to re-contact the patient to offer testing [26 33 34].
Pharmacists may assist with delivery of point-of-care PGx testing in both the inpatient and outpatient setting through patient education and professional consultation [35 36]. Pharmacists may integrate PGx testing with other pharmacist services such as medication therapy management (MTM) [37 38]. MTM aims to promote patient education and understanding, improve compliance, detect adverse drug reactions, reduce potential medication misuse, and address patient concerns [39]. Adding PGx testing to MTM may not require much additional time or resources for pharmacists specially trained in pharmacogenetics. However, additional patient face-to-face time may be required to insure that patients understand the significance of their PGx test results to current as well as future medication needs and to encourage them to share results with other treating providers who may not otherwise have access to their test report.
Community pharmacists may also be involved in the provision of PGx testing at the time they receive a prescription to be filled [37]. In the U.S., at least one study has investigated the feasibility and patient interest in offering PGx testing in a community pharmacy setting [40–42]. Potential barriers to effectively delivering PGx testing in the community pharmacy setting include discontinuity of care when medications are purchased from multiple pharmacies [43], incomplete medication histories [44], and lack of health literacy [41]. In the Netherlands, where patients generally use a single pharmacy [43 45], Swen et al [46] demonstrated the feasibility of a community pharmacist-initiated delivery of PGx testing specifically for polypharmacy patients. Another Dutch study demonstrated that recruitment of patients for PGx studies from community pharmacies utilizing a pharmacy database was feasible [47]. One major advantage of having the community pharmacist provide PGx testing services is that once a patient’s PGx profile is available in their record, the pharmacist can screen for potential interactions and communicate with physicians about adjusting medication regimens for future prescriptions. However, patients who choose to fill prescriptions at multiple pharmacies would need to share their test results with new pharmacists, as they would with new clinicians.
Preemptive/Prospective PGx Testing
Unlike many other types of clinical tests, PGx testing may be ordered prior to the need for pharmacological treatment. In contrast to point-of-care PGx testing, preemptive testing typically involves analysis of a panel of genes with known associations to drug outcomes. Certain populations may particularly benefit from preemptive testing, such as those with chronic conditions or risk factors for chronic conditions [48] as well as the elderly, both groups likely to need multiple medications, often for extended periods [6]. For example, initial dosing of warfarin, a drug commonly prescribed to older populations, could benefit from immediate access to the patient’s PGx test results for CYP2C9/VKORC1 [27 49 50]. Hospital admissions are higher for the elderly, due in part to the higher prevalence of adverse drug reactions [51]. In addition, children may also benefit from preemptive testing; on average, prescribing trends in children have increased in recent years [52–54]. Furthermore, several analyses of select medications with known PGx interactions suggest that the frequency of physician encounters support the use of preemptive testing [55]. Schildcrout et al. [11] demonstrated that patients (mostly older than 30 years of age) within a 5-year period were prescribed at least one of 56 medications known to be impacted by a genetic variant. Of six medications with severe ADRs associated with a genetic variant, it was estimated that almost 400 patients could have been identified through preemptive testing.
The use of preemptive testing is currently being evaluated in several different settings. In the outpatient setting, about 75% of physician visits (physician office visits, hospital outpatients or emergency room visits) involve drug therapy [56]. It is estimated that 86% of all ambulatory care visits involved the ordering of some type of clinical testing or screening [57]. Given these statistics and that PCPs provide the overwhelming majority of preventive care, it seems appropriate that general practitioners would order preemptive PGx testing as they will be the major group of providers to use the results for therapeutic decision-making. The 1200 Patients Project at the University of Chicago proposes to preemptively test outpatients and integrate test results into the patient’s electronic medical record to assist clinicians in interpretation and therapeutic decision-making [12]. A study at St. Jude Children’s Research Hospital currently provides a panel of PGx testing to pediatric patients in the PG4KDS study using the comprehensive microarray, Drug Metabolizing Enzymes and Transporters (DMET™) Plus array [58 59]
In the inpatient setting, the utility of preemptive PGx testing is also being assessed. Similar to ordering a comprehensive laboratory work-up upon hospital admission (e.g., complete blood cell count and electrolyte test) or liver creatine kinase to monitor skeletal muscle breakdown, a PGx panel can also be included as part of the standard laboratory order. However, given that the length of hospital stays have become progressively shorter (approximately average hospital discharge is 4.8 days) [60], the issue of test turnaround time may be problematic. PGx testing has been performed on hospitalized psychiatric patients to assess variability in plasma concentrations of antipsychotics and antidepressants [61 62]. Patients undergoing left heart catheterization at the University of Florida are prospectively genotyped for CYP2C19 as part of a standard laboratory panel [63].
The major advantage of preemptive PGx testing is the availability of results whenever treatment is needed and thus, avoidance of treatment delays. However, this is contingent upon access to test results. The results of a preemptive test ordered by a PCP in one practice setting may not be available to a specialist in another practice setting. Thus, as with point-of-care testing, insuring that PGx test results are considered by all treating providers will likely fall to the patient for the foreseeable future. Therefore, it is imperative that the ordering provider emphasize the potential applicability of PGx results to future treatments and encourage patients to disclose that they have been tested with other providers.
In addition to the patient burden of sharing PGx results with all treating prescribers, other potential disadvantages of preemptive PGx testing include the increased likelihood of incidental information and the increased liability of clinicians that may not consider the comprehensive PGx results. Incidental findings, or risks for conditions unrelated to the condition for which the patient is seeking treatment for, has been reported as a potential risk for PGx testing [64–67]. As more genes are tested, or if a comprehensive genome-wide technology is used (i.e., DMET or whole genome sequencing) to ascertain PGx information, the risk for incidental findings increases [68]. In recent years, much debate has ensued regarding the management of incidental finding and how they should be managed [69]. These incidental data may be difficult for clinicians to discuss or manage, particularly for conditions outside of their medical specialty, but may be unavoidable in some cases. Given the unsettled debate, providers may be disinclined to order a PGx test if there is a possibility of incidental findings [70]. Some have attempted to limit incidental findings by not including genes with a reported PGx association and incidental risk on PGx testing arrays (e.g., ApoE) [71]. Furthermore, physicians’ lack of awareness and inexperience with integrating PGx information into therapeutic decision-making may lead to liability risks [72–74].
The establishment of direct-to-consumer (DTC) commercial testing laboratories has provided convenient access to personal genomic information for a range of traits and conditions, including drug response. Consumers may order a single-gene PGx test or PGx gene panel, or obtain PGx results as part of a larger genome-wide risk assessment. For example, 23andMe provides genotyping for 21 genes associated with drug response in addition to 178 conditions and traits (www.23andme.com). While consumers may order PGx testing through DTC testing companies, their application to current or future treatments can only be performed by a health professional. One potential benefit is that these patrons may drive providers to learn about PGx testing to respond to questions regarding the impact of a given PGx variant for newly prescribed medication, thereby promoting provider knowledge and potentially provider-initiated PGx testing.
However, providers may dismiss such results, perhaps due to their unfamiliarity with DTC testing laboratories, beliefs that the results are inaccurate, or lack of awareness about the impact of genetic variation on drug response. Thus, for a result with potential significant impact on treatment, providers may request testing to be repeated by a reference laboratory. Such a response to patients who are motivated in obtaining as much information about their health as possible may yield adverse consequences. For example, if they find the treating provider’s response to their test results unsatisfactory, they may choose to consult with another provider for information about the significance of the results, likely delaying treatment until their concerns are addressed. Or they may seek guidance from online resources or from the company that performed the test and potentially self-adjust dosing on their own.
DISCUSSION
Several barriers to the use of PGx testing have been reported elsewhere [71 75–78]. As described in this paper, there are multiple ways in which PGx testing services may be delivered within the larger framework of preemptive and point-of-care testing. The overall success of these delivery service models will depend on how well they can overcome the reported barriers [71] (Figure). One of the major barriers to the routine use of PGx testing is clinicians’ lack of expertise and familiarity with testing [24 79–82] Presumably, providers ordering point-of-care testing have some level of expertise and familiarity with PGx testing, but this is likely to represent a relatively small proportion of providers, accounting for the relatively low reported use [24 25]. Thus, this approach may lag behind preemptive testing, particularly if preemptive testing is implemented as an institutional policy (e.g., for all hospital admissions). However, if PGx testing is routinely ordered preemptively for a clinic or hospital, it is then incumbent on the organization to also provide the necessary support to enable providers to appropriately apply the test results. One of the aims of the 1200 Patients Project at the University of Chicago is to assist clinicians with an “interactive informatics portal” to promote consideration of a patient’s results when a given medication is prescribed [12]. Clinical decision support (CDS) can also promote provider awareness about the appropriate use of the test result based on prior test results (either from prior point-of-care testing or preemptive testing) or if a PGx test is indicated in a point-of-care situation [10]. In the case of preemptive testing, this flipped educational approach may enable broader promotion of provider knowledge of PGx test use.
It is difficult to generalize how much expert knowledge is needed to integrate PGx results into therapeutic decision-making given differences between institutions and providers with respect to resources, in-house expertise, and current knowledge. For preemptive testing, some groups are implementing pharmacist-support to inform application of PGx results [9]. At St. Jude Children’s Research Hospital, results are first reviewed by clinical pharmacists and test interpretations are uploaded into the patient’s EMR [9 58]. Reported positive interactions between physicians and pharmacists in various clinical settings [83 84] could facilitate pharmacists taking a leading role in integrating PGx in clinical care. Swen and Guchalaar [85] suggested that integration of clinical guidelines into prescription databases may guide appropriate therapeutic decision-making based on PGx testing. Mills & Haga [86] proposed a novel partnership between genetic counselors and pharmacists to provide ancillary support to clinicians and patients.
Alternatively, new clinical delivery approaches may be considered to facilitate the use of PGx testing and serve as a resource for both patients and providers. One potential option is the development of a “mini-clinic” within a practice setting. Comparable to the establishment of other specialty services within outpatient settings like anticoagulation clinics, travel clinics, influenza clinics, or preventative care clinics, a PGx clinic could be established to educate patients about PGx testing, review patients’ PGx profiles and highlight results relevant to currently prescribed medications, and recommend dosing or drug adjustments as indicated by the test results. Trained pharmacists could manage this service and recommend patients to other specialists as necessary. With this model, most healthcare providers may be able to implement PGx into their everyday practice. This may be a particularly effective model for clinical practices with pharmacy services. For example, if and when rapid, office-based PGx testing becomes available, patients can see their physician, obtain a prescription and PGx testing, and have a pharmacist review the test result, recommend adjustments as needed, and dispense the medication all in one place and time. Although several professional pharmacy groups have recommended inclusion of PGx into pharmacy curricula and training [87–90], it will likely be some time before the supply of PGx-trained pharmacists become available to oversee these clinics.
Second, coverage and reimbursement will be another major barrier to the routine use of PGx testing, preemptive or point-of care. Obviously, if a PGx test is warranted for a prescribed medication, it stands a much better chance of being covered by an insurer when ordered at the point-of-care than a preemptive PGx test panel ordered for future benefit. Given the disparate coverage policies [91], clinicians may refrain from prescribing medications for which PGx testing is indicated due to the additional time needed to determine if the patient’s insurer covers the test and/or to limit inequality among their patients. If PGx testing has to be paid out-of-pocket, another potential health disparity may develop. Patients may be more inclined to pay for point-of-care testing for a newly prescribed medication if the benefits of testing for treatment outcomes are perceived to be worth the cost of the test and the long-term value is understood. However, preemptive testing paid by the patient will likely only be accessible to a limited number given the still high costs [92].
Preemptive testing is likely to be cheaper as the marginal cost-savings is anticipated to be greater due to the improved accuracy and rapidly declining costs of large-scale genome testing, [11] and development of cost-effective array-based tests [71]. With the anticipated lifetime benefits, the use of preemptive testing will likely result in greater cost-savings, though this has not been demonstrated and will only be realized if the portability and integration of PGx data improves. Indeed, the primary benefit of preemptive testing to avoid delay of treatment cannot be realized unless the results are shared with those providers providing treatment. Although the use of EMRs is being tested to facilitate use and sharing of results [93 94], they may not be compatible between clinical settings, and thus, the burden of disclosure will still fall upon the patient. However, during this early period of test use and high lack of patient familiarity with PGx testing, ‘knowledge transfer tools’ will be needed to facilitate sharing of PGx test results with new providers prescribing treatment. Like immunization records, PGx test results should be easily recorded and readily accessible by patients, such as through a PGx results app on a smartphone or PGx wallet card. In addition, new patient intake forms in physician practices or pharmacies could include questions about past PGx testing to prompt patients to disclose results, much like drug allergies.
Conclusion
During this early phase of clinical use, it remains to be seen what the optimal clinical delivery model for PGx testing will be and it may turn out that different models are appropriate for different clinical settings. Two major factors will likely significantly impact the future delivery of PGx testing. The anticipated development of rapid and comprehensive testing platforms will likely significantly impact delivery models, addressing issues of treatment delay and cost. Furthermore, with the advancement of EMRs, the integration of a patient’s PGx test results into the system may promote routine consideration by providers when prescribing medications. Regardless of delivery model, provider and patient education will be an essential part of the safe and appropriate use of PGx testing.
Acknowledgments
This work was funded by the U.S. National Institutes of Health (R01 GM081416). We thank Ms. Rachel Mills for her assistance in the preparation of this manuscript.
Footnotes
Conflicts of Interest: None to declare.
References
- 1.Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. The New England journal of medicine. 2011;364(12):1144–53. doi: 10.1056/NEJMra1010600. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lesko LJ, Zineh I, Huang SM. What Is Clinical Utility and Why Should We Care? Clinical Pharmacology & Therapeutics. 2010;88(6):729–33. doi: 10.1038/clpt.2010.229. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 3.Salari K, Watkins H, Ashley EA. Personalized medicine: hope or hype? Eur Heart J. 2012;33(13):1564–70. doi: 10.1093/eurheartj/ehs112. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodcock J, Lesko LJ. Pharmacogenetics--tailoring treatment for the outliers. The New England journal of medicine. 2009;360(8):811–3. doi: 10.1056/NEJMe0810630. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 5.Yip VL, Pirmohamed M. Expanding role of pharmacogenomics in the management of cardiovascular disorders. Am J Cardiovasc Drugs. 2013;13(3):151–62. doi: 10.1007/s40256-013-0024-5. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 6.Predazzi IM, Mango R, Norata GD, et al. Pharmacogenetics in cardiovascular disorders: an update on the principal drugs. Am J Cardiovasc Drugs. 2013;13(2):79–85. doi: 10.1007/s40256-013-0020-9. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 7.Faruki H, Lai-Goldman M. Application of a pharmacogenetic test adoption model to six oncology biomarkers. Pers Med. 2010;7(4):441–50. doi: 10.2217/Pme.10.37. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 8.Lai-Goldman M, Faruki H. Abacavir hypersensitivity: a model system for pharmacogenetic test adoption. Genetics in medicine: official journal of the American College of Medical Genetics. 2008;10(12):874–8. doi: 10.1097/GIM.0b013e31818de71c. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 9.Crews KR, Hicks JK, Pui CH, et al. Pharmacogenomics and individualized medicine: translating science into practice. Clin Pharmacol Ther. 2012;92(4):467–75. doi: 10.1038/clpt.2012.120. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulley JM, Denny JC, Peterson JF, et al. Operational implementation of prospective genotyping for personalized medicine: the design of the Vanderbilt PREDICT project. Clinical pharmacology and therapeutics. 2012;92(1):87–95. doi: 10.1038/clpt.2011.371. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schildcrout JS, Denny JC, Bowton E, et al. Optimizing drug outcomes through pharmacogenetics: a case for preemptive genotyping. Clin Pharmacol Ther. 2012;92(2):235–42. doi: 10.1038/clpt.2012.66. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Donnell PH, Bush A, Spitz J, et al. The 1200 patients project: creating a new medical model system for clinical implementation of pharmacogenomics. Clin Pharmacol Ther. 2012;92(4):446–9. doi: 10.1038/clpt.2012.117. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scarpini F, Cappellone R, Auteri A, et al. Role of genetic factors in statins side-effects. Cardiovasc Hematol Disord Drug Targets. 2012;12(1):35–43. doi: 10.2174/187152912801823138. [DOI] [PubMed] [Google Scholar]
- 14.Wilke RA, Ramsey LB, Johnson SG, et al. The clinical pharmacogenomics implementation consortium: CPIC guideline for SLCO1B1 and simvastatin-induced myopathy. Clin Pharmacol Ther. 2012;92(1):112–7. doi: 10.1038/clpt.2012.57. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry CG, Shuldiner AR. Pharmacogenomics of anti-platelet therapy: how much evidence is enough for clinical implementation? J Hum Genet. 2013;58(6):339–45. doi: 10.1038/jhg.2013.41. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorich MJ, Vitry A, Ward MB, et al. Prasugrel vs.clopidogrel for cytochrome P450 2C19-genotyped subgroups: integration of the TRITON-TIMI 38 trial data. Journal of Thrombosis and Haemostasis. 2010;8(8):1678–84. doi: 10.1111/j.1538-7836.2010.03923.x. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 17.Trenk D, Hochholzer W. Genetics of platelet inhibitor treatment. British Journal of Clinical Pharmacology. 2013 doi: 10.1111/bcp.12230. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott SA, Sangkuhl K, Gardner EE, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clinical pharmacology and therapeutics. 2011;90(2):328–32. doi: 10.1038/clpt.2011.132. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Puijenbroek E, Conemans J, van Grootheest K. Spontaneous ADR reports as a trigger for pharmacogenetic research: a prospective observational study in the Netherlands. Drug safety: an international journal of medical toxicology and drug experience. 2009;32(3):255–64. doi: 10.2165/00002018-200932030-00008. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 20.Howard R, Leathart JB, French DJ, et al. Genotyping for CYP2C9 and VKORC1 alleles by a novel point of care assay with HyBeacon(R) probes. Clin Chim Acta. 2011;412(23–24):2063–9. doi: 10.1016/j.cca.2011.07.013. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 21.Roberts JD, Wells GA, Le May MR, et al. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet. 2012;379(9827):1705–11. doi: 10.1016/S0140-6736(12)60161-5. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 22.Shahabi P, Siest G, Visvikis-Siest S. Clinical interest of point-of-care pharmacogenomic testing: clopidogrel behind warfarin. Pharmacogenomics. 2012;13(11):1215–8. doi: 10.2217/pgs.12.85. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 23.Selkirk CG, Weissman SM, Anderson A, et al. Physicians’ preparedness for integration of genomic and pharmacogenetic testing into practice within a major healthcare system. Genetic testing and molecular biomarkers. 2013;17(3):219–25. doi: 10.1089/gtmb.2012.0165. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 24.Haga SB, Burke W, Ginsburg GS, et al. Primary care physicians’ knowledge of and experience with pharmacogenetic testing. Clinical genetics. 2012;82(4):388–94. doi: 10.1111/j.1399-0004.2012.01908.x. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanek EJ, Sanders CL, Taber KA, et al. Adoption of pharmacogenomic testing by US physicians: results of a nationwide survey. Clinical pharmacology and therapeutics. 2012;91(3):450–8. doi: 10.1038/clpt.2011.306. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 26.Epstein RS, Moyer TP, Aubert RE, et al. Warfarin genotyping reduces hospitalization rates results from the MM-WES (Medco-Mayo Warfarin Effectiveness study) Journal of the American College of Cardiology. 2010;55(25):2804–12. doi: 10.1016/j.jacc.2010.03.009. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 27.Nutescu EA, Drozda K, Bress AP, et al. Feasibility of Implementing a Comprehensive Warfarin Pharmacogenetics Service. Pharmacotherapy. 2013 doi: 10.1002/phar.1329. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Empey PE. Genetic predisposition to adverse drug reactions in the intensive care unit. Crit Care Med. 2010;38(6 Suppl):S106–16. doi: 10.1097/CCM.0b013e3181de09f8. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 29.Kopp BJ, Erstad BL, Allen ME, et al. Medication errors and adverse drug events in an intensive care unit: direct observation approach for detection. Crit Care Med. 2006;34(2):415–25. doi: 10.1097/01.ccm.0000198106.54306.d7. [DOI] [PubMed] [Google Scholar]
- 30.Corkindale D, Ward H, McKinnon R. Low adoption of pharmacogenetic testing: an exploration and explanation of the reasons in Australia. Pers Med. 2007;4(2):191–99. doi: 10.2217/17410541.4.2.191. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 31.Martin P, Morrison M. Realising the Potential of Genomic Medicine. Vol. 143 London: Royal Pharmaceutical Society of Great Britain; 2006. [Google Scholar]
- 32.Deloitte. Improving the Quality Use of Medicines in Australia: Realising the Potential of Pharmacogenomics. Secondary Improving the Quality Use of Medicines in Australia. Realising the Potential of Pharmacogenomics. 2008 http://www.health.gov.au/internet/main/publishing.nsf/Content/htareview-069/$FILE/069_BUPA%20Australia%20Group%20pt%202.pdf.
- 33.Allison M. US pharmacies broaden access to pharmacogenetic tests. Nature biotechnology. 2010;28(4):299–300. doi: 10.1038/nbt0410-299. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 34.Topol EJ. Pharmacy benefit managers, pharmacies, and pharmacogenomic testing: prescription for progress? Science translational medicine. 2010;2(44):44cm22. doi: 10.1126/scitranslmed.3001067. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy MJ, Phan H, Benavides S, et al. The role of the pediatric pharmacist in personalized medicine and clinical pharmacogenomics for children: pediatric pharmacogenomics working group. J Pediatr Pharmacol Ther. 2011;16(2):118–22. doi: 10.5863/1551-6776-16.2.118. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crews KR, Cross SJ, McCormick JN, et al. Development and implementation of a pharmacist-managed clinical pharmacogenetics service. American journal of health-system pharmacy: AJHP: official journal of the American Society of Health-System Pharmacists. 2011;68(2):143–50. doi: 10.2146/ajhp100113. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Padgett L, O’Connor S, Roederer M, et al. Pharmacogenomics in a community pharmacy: ACT now. Journal of the American Pharmacists Association: JAPhA. 2011;51(2):189–93. doi: 10.1331/JAPhA.2011.10178. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 38.Reiss SM. Integrating pharmacogenomics into pharmacy practice via medication therapy management. Journal of the American Pharmacists Association: JAPhA. 2011;51(6):e64–74. doi: 10.1331/JAPhA.2011.11543. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 39.Pellegrino AN, Martin MT, Tilton JJ, et al. Medication therapy management services: definitions and outcomes. Drugs. 2009;69(4):393–406. doi: 10.2165/00003495-200969040-00001. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 40.O’Connor SK, Ferreri SP, Michaels NM, et al. Making pharmacogenetic testing a reality in a community pharmacy. Journal of the American Pharmacists Association: JAPhA. 2012;52(6):e259–65. doi: 10.1331/JAPhA.2012.12108. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 41.O’Connor SK, Ferreri SP, Michaels NM, et al. Exploratory planning and implementation of a pilot pharmacogenetic program in a community pharmacy. Pharmacogenomics. 2012;13(8):955–62. doi: 10.2217/pgs.12.67. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 42.ClinicalTrials.gov. Implementation of a Personalized Medicine (Pharmacogenomics) Service in a Community Pharmacy. 2012 [Google Scholar]
- 43.Buurma H, Bouvy ML, De Smet PA, et al. Prevalence and determinants of pharmacy shopping behaviour. Journal of clinical pharmacy and therapeutics. 2008;33(1):17–23. doi: 10.1111/j.1365-2710.2008.00878.x. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 44.Floor-Schreudering A, De Smet PA, Buurma H, et al. Documentation quality in community pharmacy: completeness of electronic patient records after patients’ first visits. The Annals of pharmacotherapy. 2009;43(11):1787–94. doi: 10.1345/aph.1M242. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 45.Monster TB, Janssen WM, de Jong PE, et al. Pharmacy data in epidemiological studies: an easy to obtain and reliable tool. Pharmacoepidemiology and drug safety. 2002;11(5):379–84. doi: 10.1002/pds.722. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 46.Swen JJ, van der Straaten T, Wessels JA, et al. Feasibility of pharmacy-initiated pharmacogenetic screening for CYP2D6 and CYP2C19. European journal of clinical pharmacology. 2012;68(4):363–70. doi: 10.1007/s00228-011-1130-4. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Wieren-de Wijer DB, Maitland-van der Zee AH, de Boer A, et al. Recruitment of participants through community pharmacies for a pharmacogenetic study of antihypertensive drug treatment. Pharmacy world & science: PWS. 2009;31(2):158–64. doi: 10.1007/s11096-008-9264-x. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 48.Libby AM, Fish DN, Hosokawa PW, et al. Patient-level medication regimen complexity across populations with chronic disease. Clin Ther. 2013;35(4):385–98.e1. doi: 10.1016/j.clinthera.2013.02.019. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 49.Horne BD, Lenzini PA, Wadelius M, et al. Pharmacogenetic warfarin dose refinements remain significantly influenced by genetic factors after one week of therapy. Thromb Haemost. 2012;107(2):232–40. doi: 10.1160/TH11-06-0388. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lenzini P, Wadelius M, Kimmel S, et al. Integration of genetic, clinical, and INR data to refine warfarin dosing. Clinical pharmacology and therapeutics. 2010;87(5):572–8. doi: 10.1038/clpt.2010.13. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Paepe P, Petrovic M, Outtier L, et al. Drug interactions and adverse drug reactions in the older patients admitted to the emergency department. Acta Clin Belg. 2013;68(1):15–21. doi: 10.2143/ACB.68.1.2062714. [DOI] [PubMed] [Google Scholar]
- 52.Cox ER, Halloran DR, Homan SM, et al. Trends in the prevalence of chronic medication use in children: 2002–2005. Pediatrics. 2008;122(5):e1053–61. doi: 10.1542/peds.2008-0214. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 53.Loy JH, Merry SN, Hetrick SE, et al. Atypical antipsychotics for disruptive behaviour disorders in children and youths. Cochrane Database Syst Rev. 2012;9:CD008559. doi: 10.1002/14651858.CD008559.pub2. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 54.Zito JM, Safer DJ, dosReis S, et al. Trends in the prescribing of psychotropic medications to preschoolers. JAMA: the journal of the American Medical Association. 2000;283(8):1025–30. doi: 10.1001/jama.283.8.1025. [DOI] [PubMed] [Google Scholar]
- 55.He YJ, McLeod HL. Ready When You Are: Easing Into Preemptive Pharmacogenetics. Clinical Pharmacology & Therapeutics. 2012;92(4):412–14. doi: 10.1038/clpt.2012.144. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 56.Statistics NCfH, editor. Health, United States, 2011: With Special Feature on Socioeconomic Status and Health. Hyattsville, MD: U.S. Government Printing Office; 2012. Prescription drug use in the past 30 days by sex, age, race, and Hispanic origin: United States, selected years 1988–1944 through 2005–2008. [Google Scholar]
- 57.Hing E, Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2004 summary. Adv Data. 2006;374:1–33. [PubMed] [Google Scholar]
- 58.Hicks JK, Crews KR, Hoffman JM, et al. A clinician-driven automated system for integration of pharmacogenetic interpretations into an electronic medical record. Clinical pharmacology and therapeutics. 2012;92(5):563–6. doi: 10.1038/clpt.2012.140. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burmester JK, Sedova M, Shapero MH, et al. DMET microarray technology for pharmacogenomics-based personalized medicine. Methods in molecular biology. 2010;632:99–124. doi: 10.1007/978-1-60761-663-4_7. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 60.CDC. Hospital Utilization (in non-Federal short-stay hospitals). Secondary Hospital Utilization (in non-Federal short-stay hospitals) 2013 http://www.cdc.gov/nchs/fastats/hospital.htm.
- 61.Kung S, Allen JD. Patients and clinicians report higher-than-average satisfaction with psychiatric genotyping for depressed inpatients. The Journal of clinical psychiatry. 2011;72(2):262–3. doi: 10.4088/JCP.10l06310blu. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 62.Mulder H, Herder A, Wilmink FW, et al. The impact of cytochrome P450-2D6 genotype on the use and interpretation of therapeutic drug monitoring in long-stay patients treated with antidepressant and antipsychotic drugs in daily psychiatric practice. Pharmacoepidemiology and drug safety. 2006;15(2):107–14. doi: 10.1002/pds.1173. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 63.Johnson JA, Elsey AR, Clare-Salzler MJ, et al. Institutional profile: University of Florida and Shands Hospital Personalized Medicine Program: clinical implementation of pharmacogenetics. Pharmacogenomics. 2013;14(7):723–6. doi: 10.2217/pgs.13.59. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Henrikson NB, Burke W, Veenstra DL. Ancillary risk information and pharmacogenetic tests: social and policy implications. The pharmacogenomics journal. 2008;8(2):85–9. doi: 10.1038/sj.tpj.6500457. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 65.Netzer C, Biller-Andorno N. Pharmacogenetic testing, informed consent and the problem of secondary information. Bioethics. 2004;18(4):344–60. doi: 10.1111/j.1467-8519.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 66.Brothers KB, Langanke M, Erdmann P. Implications of the incidentalome for clinical pharmacogenomics. Pharmacogenomics. 2013;14(11):1353–62. doi: 10.2217/pgs.13.119. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robienski J, Hoppe N. [Current medicolegal and ethical issues in pathology] Pathologe. 2013;34(1):9–15. doi: 10.1007/s00292-012-1703-8. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 68.Westbrook MJ, Wright MF, Van Driest SL, et al. Mapping the incidentalome: estimating incidental findings generated through clinical pharmacogenomics testing. Genetics in medicine: official journal of the American College of Medical Genetics. 2013;15(5):325–31. doi: 10.1038/gim.2012.147. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Green RC, Berg JS, Grody WW, et al. ACMG Recommnedations for Reporting of Incidental Findings in Clinical Exome and Genome Sequencing. American College of Medical Genetics and Genomics; 2013. pp. 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haga SB, Tindall G, O’Daniel JM. Professional perspectives about pharmacogenetic testing and managing ancillary findings. Genetic testing and molecular biomarkers. 2012;16(1):21–4. doi: 10.1089/gtmb.2011.0045. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson JA, Burkley BM, Langaee TY, et al. Implementing personalized medicine: development of a cost-effective customized pharmacogenetics genotyping array. Clin Pharmacol Ther. 2012;92(4):437–9. doi: 10.1038/clpt.2012.125. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evans BJ. Finding a liability-free space in which personalized medicine can bloom. Clinical pharmacology and therapeutics. 2007;82(4):461–5. doi: 10.1038/sj.clpt.6100335. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 73.Marchant GE, Campos-Outcalt DE, Lindor RA. Physician liability: the next big thing for personalized medicine? Pers Med. 2011;8(4):457–67. doi: 10.2217/Pme.11.33. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 74.Marchant GE, Milligan RJ, Wilhelmi B. Legal pressures and incentives for personalized medicine. Pers Med. 2006;3(4):391–97. doi: 10.2217/17410541.3.4.391. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 75.Johnson JA. Pharmacogenetics in clinical practice: how far have we come and where are we going? Pharmacogenomics. 2013;14(7):835–43. doi: 10.2217/pgs.13.52. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Veenstra DL, Roth JA, Garrison LP, Jr, et al. A formal risk-benefit framework for genomic tests: facilitating the appropriate translation of genomics into clinical practice. Genet Med. 2010;12(11):686–93. doi: 10.1097/GIM.0b013e3181eff533. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shah RR, Shah DR. Personalized medicine: is it a pharmacogenetic mirage? British journal of clinical pharmacology. 2012;74(4):698–721. doi: 10.1111/j.1365-2125.2012.04328.x. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sorich MJ, McKinnon RA. Personalized medicine: potential, barriers and contemporary issues. Curr Drug Metab. 2012;13(7):1000–6. doi: 10.2174/138920012802138615. [DOI] [PubMed] [Google Scholar]
- 79.Bartlett G, Antoun J, Zgheib NK. Theranostics in primary care: pharmacogenomics tests and beyond. Expert Rev Mol Diagn. 2012;12(8):841–55. doi: 10.1586/erm.12.115. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 80.Lunshof JE, Gurwitz D. Pharmacogenomic testing: knowing more, doing better. Clinical pharmacology and therapeutics. 2012;91(3):387–9. doi: 10.1038/clpt.2011.339. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 81.Nickola TJ, Green JS, Harralson AF, et al. The current and future state of pharmacogenomics medical education in the USA. Pharmacogenomics. 2012;13(12):1419–25. doi: 10.2217/pgs.12.113. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 82.Lesko LJ, Johnson JA. Academia at the crossroads: education and training in pharmacogenomics. Pers Med. 2012;9(5):10. doi: 10.2217/pme.12.54. [DOI] [PubMed] [Google Scholar]
- 83.Cruthirds DL, Hughes PJ, Weaver S. Value of pharmacy services to the healthcare system: an interdisciplinary assessment. Int J Pharm Pract. 2013;21(1):38–45. doi: 10.1111/j.2042-7174.2012.00239.x. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 84.Chisholm-Burns MA, Lee JK, Spivey CA, et al. US Pharmacists’ Effect as Team Members on Patient Care Systematic Review and Meta-Analyses. Med Care. 2010;48(10):923–33. doi: 10.1097/Mlr.0b013e3181e57962. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 85.Swen JJ, Guchelaar HJ. Just how feasible is pharmacogenetic testing in the primary healthcare setting? Pharmacogenomics. 2012;13(5):507–09. doi: 10.2217/Pgs.12.19. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 86.Mills R, Haga SB. Clinical delivery of pharmacogenetic testing services: a proposed partnership between genetic counselors and pharmacists. Pharmacogenomics. 2013;14(8):957–68. doi: 10.2217/pgs.13.76. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.AACP. Final Report of the 2007–2008 Bylaws and Policy Development Committee. American journal of pharmaceutical education. 2008;72(6) doi: 10.5688/ajpe7710S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cavallari LH, Overholser BR, Anderson D, et al. Recommended Basic Science Foundation Necessary to Prepare Pharmacists to Manage Personalized Pharmacotherapy. Pharmacotherapy. 2010;30(6):626–26. [Google Scholar]
- 89.Pharmacists ASoH-S. ASHP Formulary Management Policy Position: Pharmacogenomics. Secondary ASHP Formulary Management Policy Position. Pharmacogenomics. 2012 http://www.ashp.org/DocLibrary/BestPractices/FormularyPositions.aspx.
- 90.Education ACfP. Accreditation Standards and Guidelines: Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree. Secondary Accreditation Standards and Guidelines. Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree. 2011 https://www.acpe-accredit.org/standards/default.asp.
- 91.Hresko A, Haga S. Insurance Coverage Policies for Personalized Medicine. Journal of Personalized Medicine. 2012;2(4):201–16. doi: 10.3390/jpm2040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McClellan KA, Avard D, Simard J, et al. Personalized medicine and access to health care: potential for inequitable access? European journal of human genetics: EJHG. 2013;21(2):143–7. doi: 10.1038/ejhg.2012.149. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peterson JF, Bowton E, Field JR, et al. Electronic health record design and implementation for pharmacogenomics: a local perspective. Genetics in medicine: official journal of the American College of Medical Genetics. 2013 doi: 10.1038/gim.2013.109. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilke RA, Xu H, Denny JC, et al. The emerging role of electronic medical records in pharmacogenomics. Clinical pharmacology and therapeutics. 2011;89(3):379–86. doi: 10.1038/clpt.2010.260. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]


