Abstract
Flagellin is a potent immunogen that activates the innate immune system via TLR5 and Naip5/6, and generates strong T and B cell responses. The adaptor protein MyD88 is critical for signaling by TLR5, as well as IL-1 and IL-18 receptors, major downstream mediators of the Naip5/6 Nlrc4-inflammasome. Herein we define roles of known flagellin receptors and MyD88 in antibody responses generated towards flagellin. We used mice genetically deficient in flagellin recognition pathways to characterize innate immune components that regulate isotype specific antibody responses. Using purified flagellin from Salmonella, we dissected the contribution of innate flagellin recognition pathways to promote antibody responses towards flagellin and co-administered ovalbumin in C57BL/6 mice. We demonstrate IgG2c responses towards flagellin were TLR5- and inflammasome-dependent; IgG1 was the dominant isotype and partially TLR5- and inflammasome-dependent. Our data indicates a substantial flagellin-specific IgG1 response was induced through a TLR5-, inflammasome-, and MyD88-independent pathway. IgA anti-FliC responses were TLR5- & MyD88-dependent and caspase-1-independent. Unlike C57BL/6 mice, flagellin immunized A/J mice induced co-dominant IgG1 and IgG2a responses. Furthermore, MyD88-independent flagellin-induced antibody responses were even more pronounced in A/J MyD88−/− mice, and IgA anti-FliC responses were suppressed by MyD88. Flagellin also worked as an adjuvant toward co-administered ovalbumin, but it only promoted IgG1 anti-OVA responses. Our results demonstrate that a novel pathway for flagellin recognition contributes to antibody production. Characterization of this pathway will be useful for understanding immunity to flagellin and the rationale design of flagellin-based vaccines.
Introduction
Innate immunity is responsible for both sounding the alarm of pathogen invasion and directing cellular and humoral immunity. The innate immune system recognizes pathogens with germ-line encoded pattern recognition receptors (PRR) that respond to conserved pathogen associated molecular patterns (PAMPs) (1–3). Two key groups of PRRs are membrane bound Toll-like receptors (TLRs) and cytosolic NOD-like receptors (NLR). TLRs recognize structurally diverse PAMPs, including nucleic acids, glycolipids, lipoproteins, and proteins. The only known protein ligand for human TLRs is bacterial flagellin, which is recognized by TLR5 (4).
Flagellin is exposed on the surface of flagellated bacteria and is a major antigenic target of the immune system in a wide variety of hosts, ranging from plants and invertebrates to vertebrates (5–7). In Salmonella enterica serovar Typhimurium (S. Typhimurium) flagellin is encoded by the genes fliC and fljB, with fliC being the primary gene (8). FliC is a potent immunogen that is capable of inducing strong immune responses to itself (intrinsic adjuvancy) and co-administered antigens (extrinsic adjuvancy) (9–17). The intrinsic and extrinsic adjuvancy of flagellin has been attributed to conserved structures in its D0 domain, recognized by Naip5 and Naip6 (Naip5/6), and its D1 domain, recognized by TLR5 (4, 6, 18–24). Studies from several groups have established that recognition of FliC by the innate immune system leads to microbicidal activity, cytokine production, and dendritic cell (DC) activation (25–27). Immunization of mice with FliC elicits robust T cell activation and T cell-dependent antibody responses (14–16, 26, 28–33).
Flagellin, the ligand for TLR5, has been shown to induce a TH2 biased response (29, 30, 34), and is currently being developed as a vaccine adjuvant (35, 36). Because flagellin is a protein, the molecule can be engineered for vaccine development to retain immunogenicity and display foreign epitopes of interest from pathogens such as: influenza (hemagglutinin and matrix proteins) Yersinia pestis, and Helicobacter pylori (FlaA flagellin) (9, 37–41). Compared to the co-administration of flagellin with an antigen, flagellin fusion proteins elicit enhanced humoral responses and are therefore a more alluring alternative for vaccine design (35, 42–45). The greater efficacy of the flagellin-antigen fusions suggests that proximity of the antigen to the adjuvant allows for enhanced antigenicity
NLRs are cytosolic sensors that oligomerize after ligand recognition and form multi-protein complexes termed inflammasomes (46, 47). A broad range of pathogen derived and endogenous signals initiate inflammasome formation and one of its triggers, alum, has been used for decades as an adjuvant that elicits TH2 type responses towards co-administered antigens (48). The best-studied NLR, Nlrp3, is required for alum induced activation of the inflammasome (49, 50), but alum also utilizes an inflammasome-independent pathway to induce TH2 immunity (51, 52). The Naip family of NLRs activates the inflammasome in a Nlrc4-dependent manner (21, 22). Murine Naip2 recognizes the rod proteins of some bacterial type III secretory systems, whereas murine Naip5 and Naip6 recognize flagellin. Human NAIP recognizes the needle protein of some bacterial type III secretion systems (21) and has been reported to recognize flagellin (21, 53). Recognition of these protein ligands by the Naip proteins induces oligomerization with Nlrc4, leading to recruitment and activation of caspase-1 (54). Active caspase-1 processes pro-IL-1β and pro-IL-18 into mature forms for secretion, and initiates a form of cell-mediated death termed pyroptosis (55). The Nlrc4 system has been recently found to contribute to flagellin-induced antibody production in mice, in a manner that is redundant with TLR5 (16). In the absence of TLR5, Nlrc4 is required for flagellin’s immunogenicity (16). The isotype specificity of Nlrc4 inflammasome-dependent antibody responses is unknown.
TLR5 is expressed on the surface of epithelial cells, neutrophils, monocytes and dendritic cells (DCs) (4, 56, 57). Flagellin recognition by TLR5 induces its dimerization and signaling through adaptor protein MyD88 (58, 59). Activation of DCs via TLR5 leads to the upregulation of MHC class II, CD80, and CD86, and the secretion of cytokines, such as IL-23, IL-6 and Cxcl1 (14, 26, 29). TLR5 also promotes flagellin uptake and presentation that is required for efficient T cell activation (14, 33, 34, 45). Thus, TLR5 recognition of flagellin induces multiple pathways that are beneficial properties for adjuvants. In the absence of the Nlrc4 inflammasome, TLR5 is required for flagellin’s immunogenicity (16).
TLR5 and the major cytokine outputs of the Nlrc4 inflammasome, IL-1β and IL-18, require MyD88 for signaling (60). Despite this commonality antibody responses towards flagellin are maintained in MyD88-deficient mice (15). Thus, MyD88-independent pathways emanating from either TLR5, such as flagellin uptake (33, 61), or the Nlrc4 inflammasome (62) may also contribute to flagellin-specific antibody responses. Herein, we dissect the innate immune components that are responsible for flagellin’s intrinsic and extrinsic adjuvant properties, as well as the production of isotype specific antibody responses to flagellin and a model co-administered antigen, ovalbumin (OVA). These studies define the innate immune components that are required to generate robust isotype specific antibody responses towards FliC or co-administered antigens, and uncover a novel TLR5, inflammasome-, and MyD88-independent flagellin recognition pathway that contributes to flagellin’s immunogenicity.
Materials and Methods
Protein isolation and purification
Wild-type flagellin monomers were isolated from S. Typhimurium strain SL1344 (ΔflgM); purity was verified as previously described (18, 63). OVA was purchased from Sigma and ultrafiltered (Amicon) to reduce endotoxin. Removal of residual endotoxin from isolated flagellin monomers and OVA (Sigma-Aldrich) was performed by using polymyxin B columns (Thermo scientific). Endotoxin levels were <1 pg/μg of protein, as measured using the limulus colorimetric assay (Lonza). The purified flagellin was characterized biochemically and its biological activity for TLR5 and Naip5-dependent was determined prior to mouse studies (supplementary Figure 1).
NF-κB luciferease reporter assay
CHO K1 cells were stably transfected with mouse TLR5 cDNA cloned into the pEF6 V5 His TOPO vector (Invitrogen) or the empty vector, plus the ELAM-LUC plasmid; luciferase assays were performed as previously described (57, 63).
Protein transfection
Bone marrow derived macrophages (BMDMs) were prepared from femurs of C57BL/6 mice, Naip5-deficient mice, caspase-1-deficient mice, or A/J mice and cultured in RPMI 1640 supplemented with 10% FBS (ATLAS biologicals), 10% L-cell supernatant (CSF1 source), 2mM L-glutamine, and 100 U/mL penicillin, 100 μg/mL streptomycin (Gibco) (19, 64, 65). All assays were performed in triplicate and each experiment was repeated at least twice. BMDMs were primed with 10 ng/mL of ultrapure LPS (List Biologicals) for 3 hours to induce pro-IL-1β expression prior to protein transfection, using Profect-P1-lipid based protein delivery reagent (Targeting Systems) as previously described (19, 66); IL-1β secretion was determined by ELISA (Duoset R&D).
Mice and immunizations
The University of Washington Institutional Animal Care and Use Committees approved all animal protocols. Mice were bred and housed in a specific pathogen-free facility at the University of Washington. A/J and C57BL/6 animals were purchased from Jackson Laboratories and bred in-house. Naip5−/− (67), caspase-1 (Casp1−/−)(68), MyD88−/− (69) and TLR5−/− (70) mice were all generated on the C57BL/6 background and bred in-house. TLR5−/−/Casp1−/− mice were generated and bred in our animal facility. A/J MyD88−/− mice were generated by back-crossing the MyD88 deletion onto the A/J background for nine generations and then crossing to generate homozygous MyD88−/− mice. 8–14 week old matched animals were used in all experiments. Retro-orbital bleeds were performed on all animals prior to immunization to obtain naïve serum. Mice received two sequential i.p. immunizations with 30 μg FliC and 30 μg OVA separated by 21 days. Blood was drawn two weeks following each immunization.
Cytokine analysis
Mouse sera were evaluated for cytokine responses following i.p. injections of 30 μg of FliC or PBS using commercially-sourced ELISA kits according to manufacturer’s instructions (Duoset, R&D Systems). IL-18 cytokine analysis was determined by ELISA, using anti-mouse IL-18 (Clone 74; R&D Systems) as a capture antibody, and biotinylated anti-mouse IL-18 (Clone 93–10C; R&D Systems) as a detection antibody.
Antibody analysis
High binding capacity 96 well plates (COSTAR) were coated with 1 μg/mL of monomeric FliC or ovalbumin diluted in PBS (OmniPur) and allowed to incubate overnight at room temperature (RT). Plates were washed three times with PBS containing 0.05% tween-20, and blocked for 1 hour RT in PBS containing 1% BSA (Sigma). Plates were washed and serial dilutions of serum were added to the wells and incubated for 1 hour at RT. Plates were washed again and horse radish peroxidase conjugated secondary antibodies (anti-IgG1, -IgG2a, -IgG2c–HRP (Jackson Immunoresearch), or –IgA-HRP (Biolegend) were added and incubated for another hour at RT. Plates were developed with TMB substrate (Thermo), stopped with H2SO4 and absorbance was read at 450 nm (Molecular Devices). Antibody end point titer was defined by the reciprocal of the maximal serum dilution that exceeded three times the standard deviation above the mean background absorbance.
Statistical analysis
Significance was determined by one-way ANOVA with Bonferroni multiple comparison post-test or Mann-Whitney test, using Graphpad Prism 5 software. Differences were noted as significant when P <0.05.
Results
TLR5 and the Naip5 inflammasome control distinct early cytokine responses in vivo
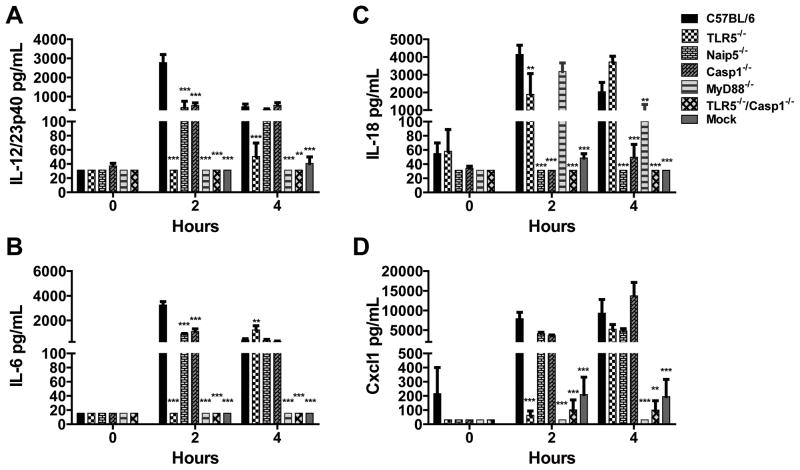
Innate recognition of FliC leads to cytokine and chemokine production that contributes to host defense and adaptive immunity. To characterize similarities and differences in early phase of innate detection of flagellin we defined the pathways necessary for triggering flagellin-dependent cytokine responses in vivo using WT, TLR5−/−, Naip5−/−, Casp1−/−, MyD88−/− and TLR5−/−/Casp1−/− (DKO) mice. Mice were injected i.p. with 30 μg of FliC, isolated and purified from S. Typhimurium (Supplementary Fig. 1), and sera were assessed at 2 and 4 hours after flagellin injection. WT mice produced IL-6, Cxcl1, IL-12/23p40, and IL-18 (Fig. 1). At the 2 hour time point serum Cxcl1, IL-6 and IL-12/23p40 were TLR5- and MyD88-dependent (Fig. 1A, C, D). Serum IL-6 and IL-12/23p40 levels were also partially dependent on Naip5 and Casp1 (Fig. 1C, D). In contrast, flagellin induction of IL-18 was TLR5- and MyD88-independent, but entirely dependent on Naip5 and Casp1 (Fig. 1B). At 4 h post-injection the role of TLR5 and the Naip5 inflammasome in flagellin induced cytokine production was more complex (Fig. 1). IL-12/23p40 and IL-18 continued to be dependent on the flagellin sensors, TLR5 and Naip5 inflammasome, respectively (Fig. 1A, B). However, IL-6 and Cxcl1 were detected at 4 h in TLR5−/−, but not MyD88−/− or TLR5/Casp1 DKO mice, suggesting a delayed cytokine cascade, where flagellin induced IL-18 or other Casp1-dependent factors induce Cxcl1 and IL-6 in a MyD88-dependent manner (Fig. 1C, D). Thus, the innate immune receptors TLR5 and Naip5 function both independently and in concert to regulate early cytokine production induced by flagellin. Flagellin has also been reported to induce low levels of TNF and IL-1β in mice (15, 16, 71). In our studies, we found no significant flagellin-dependent induction of IL-1β or TNF at 2 and 4 h post-injection in any of the mice (data not shown). For TNF, it is likely that the 2 h time point missed the early and low level induction which typically peaks around 1–1.5 h post-injection (data not shown) (71, 72). Other investigators have also had difficulty detecting IL-1β in mouse serum, suggesting that technical issues may have precluded our ability to detect the low levels reported by Bedoui and colleagues (12, 15, 71).
Figure 1. Flagellin induced cytokines are differentially regulated by TLR5, Naip5, Casp1, and MyD88.
WT (n=7–12), TLR5−/− (n=4–8), Naip5−/− (n=3), Casp1−/− (n=4–11), DKO (n=3–4), MyD88−/− (n=3–6), and mock (n=8–10) mice were injected i.p. of FliC (30 μg) or PBS (mock). Serum was collected 2 hours after injections and cytokine levels were determined by ELISA. IL-12/23p40 (A), IL-18 (B), IL-6 (C) and Cxcl1 (D). All groups have a minimum n=3. Statistical analysis was done using one-way ANOVA with Bonferroni multiple comparisons post-test: ** = P<0.01, *** = P<0.001.
IgG1 isotype specific responses are MyD88-independent
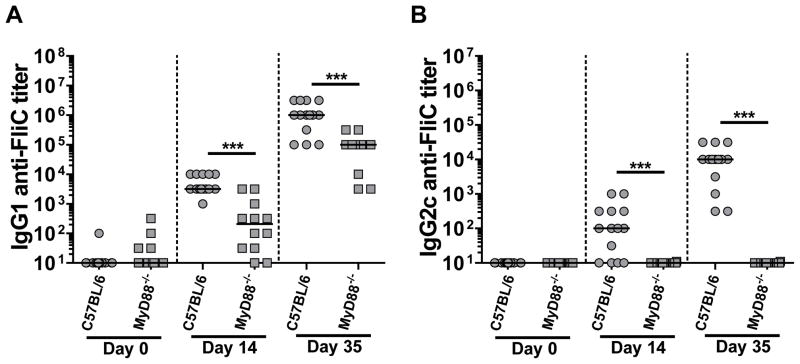
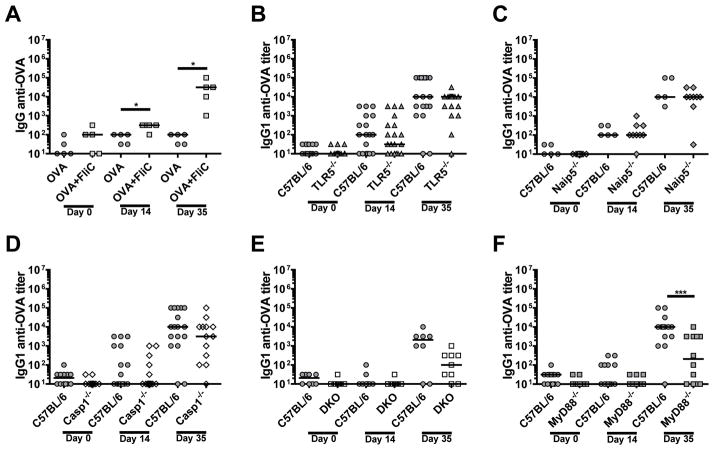
Flagellin is a major antigenic target during bacterial infections and when injected as a purified protein also induces antibodies against itself and co-administered antigens. Because flagellin is being developed as a platform for recombinant vaccines, we dissected the innate immune pathways needed to generate isotype specific antibody responses against flagellin or a co-administered antigen, OVA. C57BL/6 and MyD88−/− mice were injected i.p. with 30 μg of FliC and boosted with the same dose of flagellin 3 weeks later. The immunized animals were bled prior to immunization (naïve serum) and at two weeks post primary and secondary immunizations. We tested the sera for IgG1 and IgG2c antibodies against FliC. After primary immunizations the IgG1 anti-FliC median titer was 3160 in WT mice and of 316 in MyD88−/− mice (Fig. 2A). Following the secondary immunization, IgG1 anti-FliC median titers increased more than one hundredfold in both WT and MyD88−/−animals, and IgG1 titers remained significantly reduced in MyD88−/− compared to WT mice (Fig. 2A).
Figure 2. IgG1 anti-FliC responses are partially and IgG2c anti-FliC are entirely MyD88-dependent.
WT (n=9) and MyD88−/−(n=7) mice were immunized with 30 μg FliC on day 1 and 21 and sera collected on days 14 and 35. Naïve, day 14, and day 35 sera were analyzed for IgG1 (A) and IgG2c (B) antibody responses against FliC by ELISA. Data is a combination of two-independent experiments with n = 3–5 per experiment. Statistical analyses were done using Mann-Whitney analysis of individual groups: ** = P < 0.01.
In contrast to IgG1, FliC immunized C57BL/6 mice generated low titers of IgG2c anti-FliC, which were best detected following the secondary immunization (Fig. 2B). The IgG2c anti-FliC response was absolutely dependent on MyD88−/− (Fig. 2B). Our data demonstrates that IgG2c and a portion of IgG1 anti-flagellin responses are MyD88-dependent. Our results also support a MyD88-independent pathway for IgG1 anti-flagellin antibodies in C57BL/6 mice.
TLR5 and the inflammasome play largely redundant roles in IgG1 anti-FliC responses
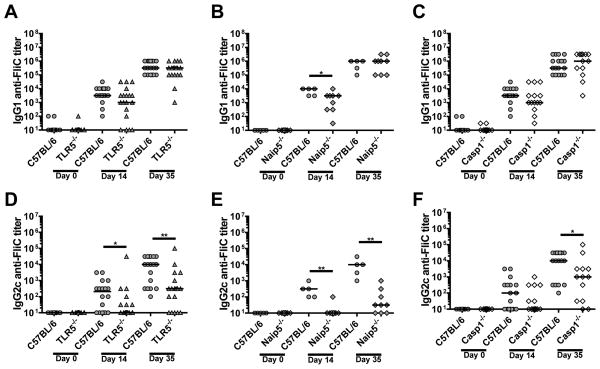
We next examined the individual components of innate flagellin recognition for their contribution to the anti-FliC antibody responses. Compared to WT mice, TLR5−/−, Naip5−/−, and Casp1−/− mice produced similar IgG1 titers towards flagellin (Fig. 3A–C). In contrast, the IgG2c anti-FliC responses were significantly reduced in TLR5−/−, Naip5−/−, and Casp1−/−mice following secondary immunizations (Fig. 3D–F). Our results suggest that each individual innate recognition pathways for flagellin contributes to the generation of IgG2c anti-FliC responses. In contrast, loss of TLR5 or the individual inflammasome molecules, Naip5 or Casp1, did not affect robust IgG1 anti-FliC responses.
Figure 3. IgG2c anti-FliC responses are partially TLR5- and inflammasome-dependent.
TLR5−/− (n=19) (A, D), Naip5−/− (n=9) (B, E), and Casp1−/− (n=14) (C, F) mice were immunized twice on day 1 and day 21 and sera collected on days 14 and 35. Naïve, day 14, and day 35 sera were analyzed for IgG1 (A–C) and IgG2c (D–F) isotype specific antibody responses against FliC by ELISA. Data is a combination of 2–3-independent experiments with n = 3–6 per experiment. Statistical analyses were done using Mann-Whitney analysis of individual groups: *= P < 0.05, ** = P < 0.01.
MyD88-independent IgG1 anti-FliC responses are also TLR5- and inflammasome-independent
To determine if TLR5 or the inflammasome contributes to MyD88-independent IgG1 anti-FliC responses, we generated TLR5 and Casp1 DKO mice. TLR5/Casp1 DKO mice had significantly reduced IgG1 anti-FliC titers, but maintained moderate titers as seen in MyD88−/− mice (Fig. 4A). As expected, the DKO animals had significantly reduced IgG2c responses towards FliC (Fig. 4B). These results strongly suggest that the MyD88-independent anti-FliC IgG1 response is mediated by a novel pathway that is distinct from the known TLR5 and inflammasome pathways for flagellin recognition.
Figure 4. IgG1 anti-FliC responses are partially TLR5- & caspase-1-independent.
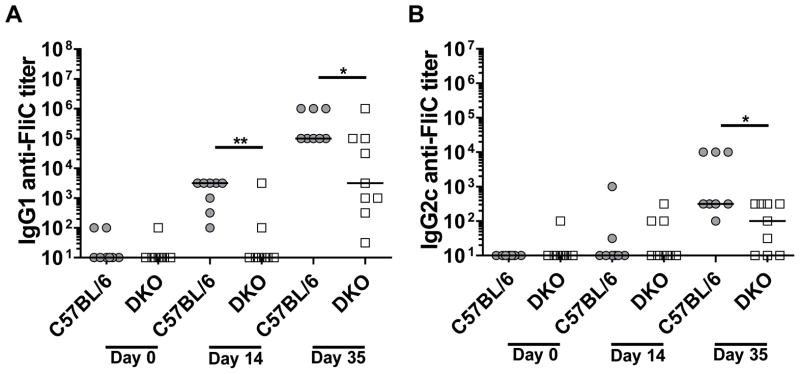
TLR5−/−/Casp1−/−, DKO (n=10) mice were immunized twice on day 1 and day 21 and sera collected on days 14 and 35. Naïve, day 14, and day 35 sera were analyzed for IgG1 (A) and IgG2c (B) isotype specific antibody responses against FliC by ELISA. Data is representative of two-independent experiments with n= 4–5 per group. Statistical analyses were done using Mann-Whitney analysis of individual groups: ** = P < 0.01.
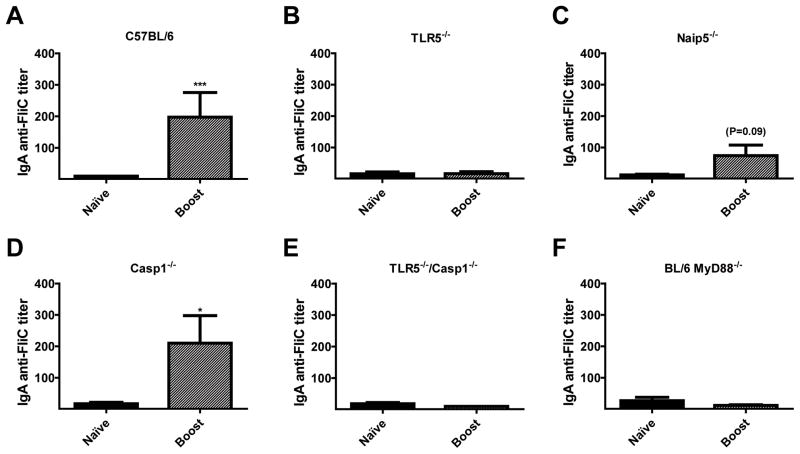
Serum IgA anti-FliC responses are TLR5 and MyD88 dependent and Casp1-independent
We tested naïve and boost sera of C57BL/6 mice and knockout mice and assessed IgA anti-FliC titers following secondary immunizations of FliC since previous in vitro and in vivo studies have shown that monomeric flagellin is capable of inducing IgA responses towards itself (73, 74). IgA anti-FliC responses were detected at low levels following the secondary immunization (Fig. 5A). Consistent with recent results of Cunningham and colleagues (73), our results with TLR5−/−, MyD88−/− and DKO mice show IgA anti-FliC responses are TLR5- and MyD88-dependent (Fig. 5B, E, F). Furthermore, the IgA anti-FliC titers were detected in Naip5−/− and Casp1−/− mice, suggesting that the inflammasome is not required for IgA anti-FliC responses (Fig. 5C, D).
Figure 5. IgA anti-FliC responses are TR5- & MyD88-dependent.
C57BL/6 (n=17) (A), TLR5−/− (n=14) (B), Naip5−/− (n=9) (C), and Casp1−/− (n=11) (D), TLR5−/−/Casp1−/−, DKO (n=9) (E), MyD88−/− (n=11) (F) mice were immunized on day 1 and day 21 and sera was collected on days 0 (naïve), 14 and 35. Naïve and day 35 sera (boost) were analyzed for IgA specific antibody responses against FliC by ELISA. Data is a combination of 2–3-independent experiments with n = 3–6 per experiment. Statistical analyses were done using Mann-Whitney analysis of individual groups: *= P < 0.05, *** = P < 0.001.
Flagellin’s adjuvancy towards extrinsic antigens is partially dependent on TLR5 and the inflammasome
Next, we examined how flagellin regulates antibody responses towards a co-administered (extrinsic) antigen, OVA. Mice immunized with OVA alone failed to generate anti-OVA responses, whereas mice co-injected with FliC and OVA generated anti-OVA antibodies (Fig. 6A). We also tested the isotype specificity of the FliC-dependent anti-OVA antibody responses and found that in all animals tested, IgG1 was the only isotype detected against OVA; no IgG2c or IgA was generated against OVA (data not shown). The IgG1 anti-OVA responses were similar in WT, TLR5−/−, Naip5−/−, and Casp1−/− mice (Fig. 6B–D). The IgG1 anti-OVA titers were significantly reduced in MyD88−/− mice and approached, but did not reach, statistical significance in DKO animals (p=0.07) (Fig. 6E, F). Thus, the IgG1 and anti-OVA and IgG1 anti-FliC responses appear to be regulated by similar MyD88-dependent & -independent mechanisms.
Figure 6. Flagellin induced IgG1 anti-OVA responses are partially MyD88-dependent.
WT, mice were immunized twice on day 1 and day 21 with OVA alone (n=5) or OVA plus FliC (n=5), and sera collected on days 14 and 35. A) Naïve, day 14, and day 35 sera were analyzed for IgG1 specific antibody responses against OVA by ELISA. B–F) WT, TLR5−/− (n=19) (B), Naip5−/− (n=9) (C), Casp1−/− (n=14) (D), MyD88−/− (n=7) (E) and TLR5−/−/Casp1−/−, DKO (n=10) (F), mice were immunized twice on day 1 and day 21 with OVA plus FliC, and sera collected on days 14 and 35. Naïve, day 14, and day 35 sera were analyzed for IgG1 specific antibody responses against OVA by ELISA. B–F) Data is a combination of 2–3-independent experiments with n=3–6 per experiment. Statistical analyses were done using Mann-Whitney analysis of individual groups: *= P < 0.05, *** = P < 0.001.
Defective flagellin-induced IL-18 in A/J mice
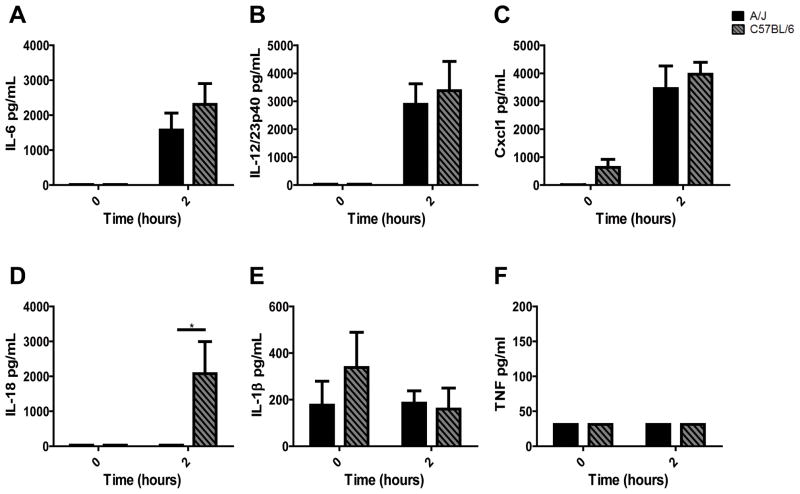
A/J mice contain a hypofunctional Naip5 that is associated with susceptibility to Legionella pneumophila (21, 22, 47, 75). We assessed the serum cytokines in response to FliC two hours post i.p. injection with 30 μg FliC. A/J and C57BL/6 mice produced equivalent amounts of IL-6, IL-12/23p40, and Cxcl1 (Fig. 7A–C). However, A/J mice failed to produce IL-18 (Fig. 7D). As with C57BL/6 mice, A/J mice did not produce detectable TNF or IL-1β (Fig. 7E, F). Thus the flagellin-induced cytokine responses in A/J mice are consistent with intact TLR5 and impaired Naip5 recognition of flagellin due to the hypofunctional Naip5A/J allele.
Figure 7. Defective flagellin-induced IL-18 in A/J mice.
C57BL/6 (n = 5) and A/J (n=5) mice were injected i.p. with FliC (30μg). Serum was collected 2 hours after injections and cytokine levels were determined by ELISA: Cxcl1 (A), IL-6 (B), IL-12/23p40 (C), IL-18 (D), IL-1β (E), and TNF (F). Statistical analysis was done using one-way ANOVA with Bonferroni multiple comparisons post-test: * = P<0.05.
Augmented IgG1 and IgG2a anti-FliC responses in A/J mice
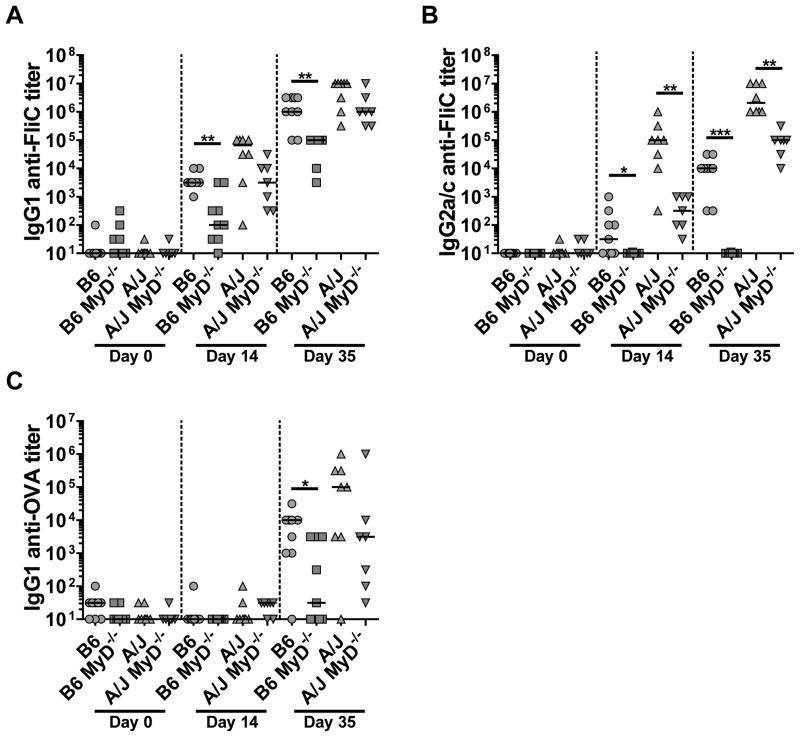
We compared isotype specific antibody responses against FliC in A/J and C57BL/6 mice and their respective MyD88 deficient strains. In contrast to C57BL/6 mice, A/J mice had more robust antibody responses to flagellin after primary and secondary immunization, with approximately 10-fold increased IgG1 anti-FliC titers following primary and secondary, and greater than 100-fold increased IgG2a/c anti-FliC titers following primary and secondary immunizations (Fig. 8A, B). The IgG2a responses in A/J mice were partially MyD88-dependent, whereas IgG1 anti-FliC titers were reduced in A/J MyD88−/− mice, trending towards significance (P=.08) (Fig. 8A, B). In contrast to C57BL/6 mice, which have a strong IgG1 biased antibody response against flagellin, A/J mice had a balanced antibody response towards flagellin, with equivalent IgG1 and IgG2a anti-FliC titers.
Figure 8. Augmented IgG1 and IgG2a anti-FliC responses in A/J mice are partially MyD88-dependent.
C57BL/6 (n=9) and A/J mice (n=7), and their MyD88-defiecient counterparts (n=7–9) were immunized twice on day 1 and day 21 with FliC plus OVA and sera collected on days 14 and 35 were analyzed for IgG1 (A), and IgG2a or IgG2c (B) responses against FliC by ELISA. C) Naïve, day 14, and day 35 sera were assessed for IgG1 anti-OVA responses by ELISA. Data is a combination of two-independent experiments with n = 3–5 per experiment. Statistical analyses were done using Mann-Whitney analysis of individual groups: *= P<0.05, ** = P<0.01.
Anti-OVA responses in A/J mice are partially dependent on MyD88
A/J and A/J MyD88−/− mice were immunized with FliC and OVA. As with IgG2c responses in C57BL/6 mice, IgG2a anti-OVA antibody responses were undetectable in A/J mice (data not shown). After primary immunizations in both A/J and A/J MyD88−/− animals, IgG1 anti-OVA antibodies were at titers of 100 or less in all mice (Fig. 8C). In A/J mice following secondary immunizations with FliC and OVA, IgG1 anti-OVA responses were robust, with titers reaching a median of 105 (Fig. 8C). As for C57BL/6 MyD88−/− and A/J MyD88−/− mice had reduced, but detectable IgG1 anti-OVA titers compared to that of their WT counterparts (Fig. 8C).
MyD88 suppresses IgA anti-FliC responses in A/J mice
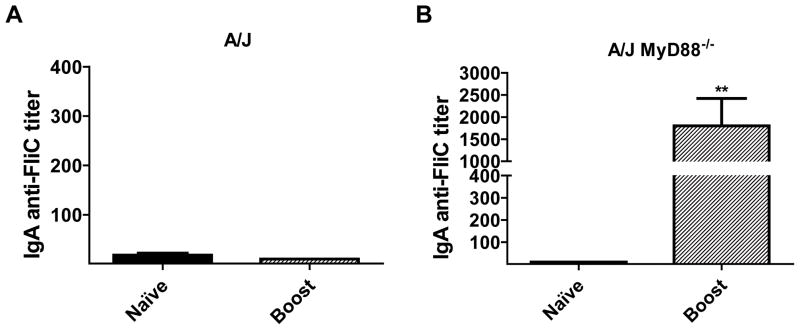
Sera from A/J and A/J MyD88−/− was also assessed for IgA anti-FliC responses. In contrast to C57BL/6 mice, A/J mice did not generate significant IgA anti-FliC following two immunizations (Fig. 9A). Conversely, the A/J MyD88−/− mice produced a substantial IgA anti-FliC titer after two immunizations (Fig. 9B). These results illuminate the fine intricacies between different strains of mice, resulting in quantitative and qualitative differences in isotype specific responses generated against FliC.
Figure 9. A/J MyD88−/− mice generate robust IgA anti-FliC responses.
A/J mice (n=8), and their MyD88-defiecient counterparts (n=7) were immunized twice on day 1 and day 21 with FliC plus OVA and sera collected on days 0 (naïve), 14 and 35. Naïve and day 35 sera were analyzed for IgA specific antibody responses against FliC by ELISA (A, B). Data is a combination of two-independent experiments with n = 3–4 per experiment. Statistical analyses were done using Mann-Whitney analysis of individual groups: ** = P<0.01.
Discussion
Dissecting the innate immune pathways that recognize flagellin’s structural properties and promote adaptive immune responses is vital for the rational design of flagellin based vaccines. Flagellin based fusion proteins are currently being developed as vaccines for infectious diseases (35, 36) and assessed in phase I and II clinical trials (38–40). Flagellin is also being developed as a therapeutic agent to treat infectious diseases, toxic exposures and cancers (76–78). Several studies have shown that flagellin’s adjuvancy is dependent on TLR5 recognition (35–37). More recently murine Naip5 and Naip6 have been demonstrated to recognize bacterial flagellin and activate the Nlrc4 inflammasome (21, 22). Additional studies have indicated that inflammasome mediated detection of flagellin also contributes to its immunogenicity (15). Vijay-Kumar et al. demonstrated that TLR5 and the Nlrc4 inflammasome play redundant roles in flagellin-induced antibody responses, with neither being necessary and either being sufficient for flagellin-induced antibody responses (16). This same group determined that MyD88 is not required for flagellin induced IgG antibody responses, suggesting that a MyD88-independent pathway emanates from either TLR5 or the Nlrc4 inflammasome, and contributes to anti-flagellin IgG production.
In our study, we dissected the role of flagellin detection by the innate immune system in generating isotype specific antibody responses to flagellin itself (intrinsic adjuvancy) and co-administered OVA (extrinsic adjuvancy). While neither deletion of TLR5 nor the Naip5 inflammasome alone were sufficient to reduce IgG1 anti-FliC antibodies, deletion of both TLR5 and Casp1 or elimination of MyD88 significantly reduced IgG1 responses towards flagellin. However, IgG1 anti-FliC responses were still detected in MyD88 and TLR5/Casp1 DKO mice, suggesting flagellin is recognized by a third uncharacterized pathway. This conclusion is supported by our studies in A/J MyD88−/− mice, which are naturally impaired in Naip5 (75). The A/J MyD88−/− mice produced reduced, but moderate IgG1 and IgG2a anti-FliC responses. The combined data from immunizations performed in C57BL/6 and A/J mice support our working hypothesis that a novel third pathway contributes to antibody responses towards flagellin and that this pathway is independent of MyD88 and known innate detection pathways for flagellin recognition, TLR5 and Naip5. Uncloaking this third pathway would help understand the complexities of innate immune recognition of flagellated pathogens, and will also be critical for the rationale design of flagellin-based vaccines. It will be critical to assess whether or not this third pathway is conserved in humans, and how this pathway may influence the generation of robust cellular and humoral responses, and the establishment of long-term immunity.
Our data also demonstrates that in C57BL/6 mice, TLR5 and the Naip5/Nlrc4/caspase-1 inflammasome work in parallel to drive IgG2c anti-FliC responses in a MyD88-dependent manner. The MyD88-dependency of the IgG2a/c antibody response is even more apparent in A/J mice, which have quantitatively greater antibody responses and markedly enhanced IgG2a response that is equivalent to the IgG1 response. The studies in A/J mice demonstrate that the IgG2a primary response has greater dependence on MyD88 than the IgG1 response, as seen in C57BL/6 mice. However, unlike C57BL/6, A/J mice also had a MyD88-independent component for the IgG2a response. This suggests that underlying genetic differences between C57BL/6 and A/J mice contribute to isotype specificity and overall quantity of anti-flagellin immune responses.
Although this MyD88-independent pathway did not contribute to IgG2c responses in C57BL/6 mice, this may be due to the low magnitude of the IgG2c responses toward flagellin observed in C57BL/6 mice. Thus, it will be important to further dissect the components of the immune systems in C57BL/6 and A/J mice that are responsible for MyD88-independent responses against flagellin. Similarly, the structural components of bacterial flagellin that dictate MyD88-independent antibody production are uncharacterized. Understanding this third pathway of flagellin is another piece to the puzzle that can be utilized to enhance flagellin based vaccine design.
Our results from C57BL/6 mice demonstrate that IgA anti-FliC responses are TLR5- & MyD88-dependent, consistent with a recently published report (73). We add to this body of work by demonstrating that the inflammasome does not impact IgA anti-FliC titers and thus does not compensate for TLR5-deficiency. Our results from the C57BL/6 mice are congruent with the data from Cunningham and colleagues, which indicate that lamina propria TLR5+ CD103+ DCs prime Foxp3+ Tregs to induce flagellin-specific IgA in the mesenteric lymph node (73). Conversely, our data from A/J and A/J MyD88−/− mice indicate that IgA anti-FliC responses may be regulated differently in different strains of mice. In A/J mice, MyD88-dependent signals suppressed IgA production. The mechanism for suppression of IgA anti-FliC responses in A/J mice is currently unknown and requires further investigation.
It is well publicized that flagellin, when used as an adjuvant, imparts an IgG1 isotype specific response towards co-administered antigens, presumably due to TLR5 recognition (9, 15, 26, 30). Our data support the conclusion that TLR5 and the Naip5/Nlrc4 inflammasome play overlapping roles for IgG1 responses towards OVA when co-administered with FliC (extrinsic adjuvancy). Because the anti-OVA IgG1 is reduced but detectable in both MyD88−/− and DKO, our data also indicate that the MyD88-independent pathway contributes to the adjuvancy of FliC towards OVA. Therefore, our immunization studies support the existence of a third pathway for flagellin recognition that also contributes to flagellin’s adjuvancy.
Interestingly, humans contain one full length Naip homolog, NAIP (79, 80). Human NAIP recognizes needle proteins from bacterial type three secretory systems (21); however, there are also reports indicating that NAIP recognizes flagellin (53, 81) and that copy number variation for the human NAIP gene may affect ligand detection (80, 82). Although flagellin recognition by human NAIP has been implicated with cellular assays (53), it still remains unsubstantiated by biochemical or genetic analysis (21, 53, 83). Further studies are required to determine the ligand specificity of human NAIP, and the contribution of NAIP to flagellin induced immune responses in humans.
In addition, it has been reported that TLR11 can recognize flagellin in mice of the C57BL/6 background (84). It is possible that TLR11 contributed to antibody responses towards FliC. However, like all other TLRs, except TLR3 (85, 86), TLR11 is MyD88-dependent (87). Because FliC immunized MyD88−/− and TLR5/Casp1 DKO mice have similar phenotypes, TLR11 does not appear to contribute significantly to flagellin-dependent antibody responses. In any case, the translational implications of TLR11 recognition of flagellin for vaccine development in humans are of little relevance, since human TLR11 is a nonfunctional pseudogene (7).
Our data indicate that flagellin is a potent adjuvant for IgG1 and IgG2a/c isotype specific responses toward itself. In addition, our data indicates that TLR5 and MyD88 contribute to the IgG2a/c immune responses in both C57BL/6 and A/J mice. Our studies revealed that the IgG2a/c vs. IgG1 bias of the anti-flagellin humoral response was strongly influenced by mouse genetic background. Because C57BL/6 mice have a weaker and IgG1 biased anti-flagellin antibody response, A/J mice may be a more relevant model for deciphering the immunogenic characteristics of flagellin and the host innate immune pathways needed for robust anti-flagellin immune responses. Since A/J animals generate a robust IgG1 and IgG2a anti-FliC responses in a Naip5-independent manner, studies in A/J mice may also be more relevant to human vaccine development.
In both A/J and C57BL/6 mice, antibody responses generated against OVA were solely IgG1, with no detectable IgG2a/c anti-OVA antibodies. It is not clear why flagellin induces distinct isotype specific responses against intrinsic (FliC) and extrinsic (OVA) antigens. It is conceivable that increased proximity of antigen to the immunogenic portions of flagellin (sites recognized by TLR5, inflammasome, and 3rd pathway) dictates a more robust IgG2a/c antibody response to intrinsic antigens. Further dissection of the cellular and molecular pathways that distinguish and differentiate responses towards intrinsic and extrinsic antigens are needed.
At present, the identity of this third pathway that promotes anti-FliC responses is unknown. The flagellin utilized in our immunizations is a monodispersed species, as expected for flagellin monomers (Supplementary Fig. 1); therefore, it is unlikely that the third pathway represents a T cell-independent response to flagellin polymers (88–90). Our results from TLR5/Casp1 DKO mice indicate that the third pathway does not utilize either TLR5 or the inflammasome. Thus, the possibility that TLR5 heterodimerizes with another TLR, such as the proposed TLR4/5 heterodimers, is an unlikely explanation for the third pathway (27, 91). Gangliosides have been implicated as receptors for bacterial flagellin (92, 93), and it is possible that flagellin binding to glycolipids may induce receptor-independent signaling in DCs, as has been proposed for monosodium urate crystals (94). It is also formally possible that TLR11 could function through a MyD88-independent mechanism, or that Naip5 or Naip6 could signal independently of Casp1. The existence of both of these novel pathways would provide a complex explanation for our results. Although we cannot exclude this possibility of multiple novel signaling pathways emerging from the known flagellin receptors, we favor the most parsimonious explanation for our data, which is a third pathway for flagellin recognition that functions independently of MyD88, TLR5 and the inflammasome.
Our results demonstrate that flagellin’s adjuvancy toward intrinsic and extrinsic antigens can be attributed to at least three pathways in our animal model: TLR5, the Naip5/Nlrc4/Casp1 inflammasome, and a novel MyD88-independent pathway. At the present time, it is unclear what constitutes the third novel pathway for flagellin-dependent antibody responses, and what structures on flagellin are required for this activity. Additionally, it is unknown whether the third pathway is evolutionarily conserved and contributes to flagellin-induced immunity in humans. These questions beg further investigation in order to enhance our understanding of the biological properties of flagellin, and how flagellin may be used as an adjuvant, a vaccine platform, and therapeutic agent.
Supplementary Material
Acknowledgments
We thank Lynn Hajjar for helpful comments. We thank Tom Hawn and his lab for technical assistance. We thank Shizuo Akira, Richard Flavell and Russel Vance for providing knockout mice.
Footnotes
Research reported in this publication was supported by National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01 AI084803 (KDS). AL-Y is supported in part by Public Health Service National Research Service Awards from the National Institutes of Health: the University of Washington STD/AIDS Research Training Program (T32AI007140, National Institute of Allergy and Infectious Diseases) and the Molecular and Cellular Biology Training Program (T32 AI027757, National Institute of General Medical Sciences)
References
- 1.Janeway C, Medzhitov R. Innate immune recognition. Annual review of immunology. 2002;20:197–413. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Pedra J, Cassel S, Sutterwala F. Sensing pathogens and danger signals by the inflammasome. Current opinion in immunology. 2009;21:10–16. doi: 10.1016/j.coi.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira S. Mammalian Toll-like receptors. Current opinion in immunology. 2003;15:5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 4.Gewirtz A, Navas T, Lyons S, Godowski P, Madara J. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. Journal of immunology (Baltimore, Md : 1950) 2001;167:1882–1887. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 5.Samatey F, Imada K, Nagashima S, Vonderviszt F, Kumasaka T, Yamamoto M, Namba K. Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature. 2001;410:331–338. doi: 10.1038/35066504. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi F, Smith K, Ozinsky A, Hawn T, Yi E, Goodlett D, Eng J, Akira S, Underhill D, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1202. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 7.Roach J, Glusman G, Rowen L, Kaur A, Purcell M, Smith K, Hood L, Aderem A. The evolution of vertebrate Toll-like receptors. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macnab R. How bacteria assemble flagella. Annual review of microbiology. 2003;57:77–177. doi: 10.1146/annurev.micro.57.030502.090832. [DOI] [PubMed] [Google Scholar]
- 9.Honko A, Sriranganathan N, Lees C, Mizel S. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infection and immunity. 2006;74:1113–1133. doi: 10.1128/IAI.74.2.1113-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bates J, Honko A, Graff A, Kock N, Mizel S. Mucosal adjuvant activity of flagellin in aged mice. Mechanisms of ageing and development. 2008;129:271–352. doi: 10.1016/j.mad.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizel S, Graff A, Sriranganathan N, Ervin S, Lees C, Lively M, Hantgan R, Thomas M, Wood J, Bell B. Flagellin-F1-V fusion protein is an effective plague vaccine in mice and two species of nonhuman primates. Clinical and vaccine immunology : CVI. 2009;16:21–29. doi: 10.1128/CVI.00333-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kupz A, Guarda G, Gebhardt T, Sander L, Short K, Diavatopoulos D, Wijburg O, Cao H, Waithman J, Chen W, Fernandez-Ruiz D, Whitney P, Heath W, Curtiss R, Tschopp J, Strugnell R, Bedoui S. NLRC4 inflammasomes in dendritic cells regulate noncognate effector function by memory CD8(+) T cells. Nature immunology. 2012;13:162–171. doi: 10.1038/ni.2195. [DOI] [PubMed] [Google Scholar]
- 13.McSorley S, Ehst B, Yu Y, Gewirtz A. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. Journal of immunology (Baltimore, Md : 1950) 2002;169:3914–3923. doi: 10.4049/jimmunol.169.7.3914. [DOI] [PubMed] [Google Scholar]
- 14.Sanders C, Yu Y, Moore D, Williams I, Gewirtz A. Humoral immune response to flagellin requires T cells and activation of innate immunity. Journal of immunology (Baltimore, Md : 1950) 2006;177:2810–2818. doi: 10.4049/jimmunol.177.5.2810. [DOI] [PubMed] [Google Scholar]
- 15.Sanders C, Franchi L, Yarovinsky F, Uematsu S, Akira S, Núñez G, Gewirtz A. Induction of adaptive immunity by flagellin does not require robust activation of innate immunity. European journal of immunology. 2009;39:359–430. doi: 10.1002/eji.200838804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vijay-Kumar M, Carvalho F, Aitken J, Fifadara N, Gewirtz A. TLR5 or NLRC4 is necessary and sufficient for promotion of humoral immunity by flagellin. European journal of immunology. 2010;40:3528–3562. doi: 10.1002/eji.201040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Kim S, Jeong B, Kim Y, Bae S, Ahn O, Lee JJ, Song HC, Kim J, Choy H, Chung S, Kweon MN, Rhee J. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infection and immunity. 2006;74:694–1396. doi: 10.1128/IAI.74.1.694-702.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith K, Andersen-Nissen E, Hayashi F, Strobe K, Bergman M, Barrett S, Cookson B, Aderem A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nature immunology. 2003;4:1247–1300. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- 19.Miao E, Alpuche-Aranda C, Dors M, Clark A, Bader M, Miller S, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nature immunology. 2006;7:569–644. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 20.Miao E, Andersen-Nissen E, Warren S, Aderem A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Seminars in immunopathology. 2007;29:275–363. doi: 10.1007/s00281-007-0078-z. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–1196. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 22.Kofoed E, Vance R. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–597. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yonekura K, Maki-Yonekura S, Namba K. Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature. 2003;424:643–693. doi: 10.1038/nature01830. [DOI] [PubMed] [Google Scholar]
- 24.Eaves-Pyles T, Wong H, Odoms K, Pyles R. Salmonella flagellin-dependent proinflammatory responses are localized to the conserved amino and carboxyl regions of the protein. Journal of immunology (Baltimore, Md : 1950) 2001;167:7009–7025. doi: 10.4049/jimmunol.167.12.7009. [DOI] [PubMed] [Google Scholar]
- 25.Jones S, Brahmakshatriya V, Huston G, Dibble J, Swain S. TLR-activated dendritic cells enhance the response of aged naive CD4 T cells via an IL-6-dependent mechanism. Journal of immunology (Baltimore, Md : 1950) 2010;185:6783–6877. doi: 10.4049/jimmunol.0901296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bates J, Uematsu S, Akira S, Mizel S. Direct stimulation of tlr5+/+ CD11c+ cells is necessary for the adjuvant activity of flagellin. Journal of immunology (Baltimore, Md : 1950) 2009;182:7539–7586. doi: 10.4049/jimmunol.0804225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honko A, Mizel S. Effects of flagellin on innate and adaptive immunity. Immunologic research. 2005;33:83–184. doi: 10.1385/IR:33:1:083. [DOI] [PubMed] [Google Scholar]
- 28.Kasahara S, Clark E. Dendritic cell-associated lectin 2 (DCAL2) defines a distinct CD8α-dendritic cell subset. Journal of leukocyte biology. 2011 doi: 10.1189/jlb.0711384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cunningham A, Khan M, Ball J, Toellner KM, Serre K, Mohr E, MacLennan I. Responses to the soluble flagellar protein FliC are Th2, while those to FliC on Salmonella are Th1. European journal of immunology. 2004;34:2986–3081. doi: 10.1002/eji.200425403. [DOI] [PubMed] [Google Scholar]
- 30.Bobat S, Flores-Langarica A, Hitchcock J, Marshall J, Kingsley R, Goodall M, Gil-Cruz C, Serre K, Leyton D, Letran S, Gaspal F, Chester R, Chamberlain J, Dougan G, López-Macías C, Henderson I, Alexander J, MacLennan I, Cunningham A. Soluble flagellin, FliC, induces an Ag-specific Th2 response, yet promotes T-bet-regulated Th1 clearance of Salmonella typhimurium infection. European journal of immunology. 2011;41:1606–1624. doi: 10.1002/eji.201041089. [DOI] [PubMed] [Google Scholar]
- 31.McSorley S, Cookson B. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. The Journal of …. 2000 doi: 10.4049/jimmunol.164.2.986. [DOI] [PubMed] [Google Scholar]
- 32.Bergman M, Cummings L, Alaniz R, Mayeda L, Fellnerova I, Cookson B. CD4+-T-cell responses generated during murine Salmonella enterica serovar Typhimurium infection are directed towards multiple epitopes within the natural antigen FliC. Infection and immunity. 2005;73:7226–7235. doi: 10.1128/IAI.73.11.7226-7235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atif S, Uematsu S, Akira S, McSorley S. CD103-CD11b+ dendritic cells regulate the sensitivity of CD4 T-cell responses to bacterial flagellin. Mucosal immunology. 2013 doi: 10.1038/mi.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Didierlaurent A, Ferrero I, Otten L, Dubois B, Reinhardt M, Carlsen H, Blomhoff R, Akira S, Kraehenbuhl J-P, Sirard J-C. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. Journal of immunology (Baltimore, Md : 1950) 2004;172:6922–6952. doi: 10.4049/jimmunol.172.11.6922. [DOI] [PubMed] [Google Scholar]
- 35.Kajikawa A, Zhang L, Long J, Nordone S, Stoeker L, LaVoy A, Bumgardner S, Klaenhammer T, Dean G. Construction and immunological evaluation of dual cell surface display of HIV-1 gag and Salmonella enterica serovar Typhimurium FliC in Lactobacillus acidophilus for vaccine delivery. Clinical and vaccine immunology : CVI. 2012;19:1374–1381. doi: 10.1128/CVI.00049-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vassilieva E, Wang BZ, Vzorov A, Wang L, Wang YC, Bozja J, Xu R, Compans R. Enhanced mucosal immune responses to HIV virus-like particles containing a membrane-anchored adjuvant. mBio. 2011;2:10. doi: 10.1128/mBio.00328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang BZ, Gill H, Kang SM, Wang L, Wang YC, Vassilieva E, Compans R. Enhanced influenza virus-like particle vaccines containing the extracellular domain of matrix protein 2 and a Toll-like receptor ligand. Clinical and vaccine immunology : CVI. 2012;19:1119–1125. doi: 10.1128/CVI.00153-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Treanor J, Taylor D, Tussey L, Hay C, Nolan C, Fitzgerald T, Liu G, Kavita U, Song L, Dark I, Shaw A. Safety and immunogenicity of a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125) in healthy young adults. Vaccine. 2010;28:8268–8342. doi: 10.1016/j.vaccine.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Taylor D, Treanor J, Strout C, Johnson C, Fitzgerald T, Kavita U, Ozer K, Tussey L, Shaw A. Induction of a potent immune response in the elderly using the TLR-5 agonist, flagellin, with a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125, STF2.HA1 SI) Vaccine. 2011;29:4897–5799. doi: 10.1016/j.vaccine.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Taylor D, Treanor J, Sheldon E, Johnson C, Umlauf S, Song L, Kavita U, Liu G, Tussey L, Ozer K, Hofstaetter T, Shaw A. Development of VAX128, a recombinant hemagglutinin (HA) influenza-flagellin fusion vaccine with improved safety and immune response. Vaccine. 2012;30:5761–5769. doi: 10.1016/j.vaccine.2012.06.086. [DOI] [PubMed] [Google Scholar]
- 41.Mori J, Vranac T, Smrekar B, Cernilec M, Serbec V, Horvat S, Ihan A, Benčina M, Jerala R. Chimeric flagellin as the self-adjuvanting antigen for the activation of immune response against Helicobacter pylori. Vaccine. 2012;30:5856–5863. doi: 10.1016/j.vaccine.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science (New York, NY) 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nature immunology. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rappuoli R, Mandl C, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nature reviews Immunology. 2011;11:865–872. doi: 10.1038/nri3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bates J, Graff A, Phipps J, Grayson J, Mizel S. Enhanced antigen processing of flagellin fusion proteins promotes the antigen-specific CD8+ T cell response independently of TLR5 and MyD88. Journal of immunology (Baltimore, Md : 1950) 2011;186:6255–6262. doi: 10.4049/jimmunol.1001855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nature immunology. 2012;13:325–357. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis B, Wen H, Ting J. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annual review of immunology. 2011;29:707–742. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marrack P, McKee A, Munks M. Towards an understanding of the adjuvant action of aluminium. Nature reviews Immunology. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eisenbarth S, Colegio O, O’Connor W, Sutterwala F, Flavell R. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H, Willingham S, Ting J, Re F. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. Journal of immunology (Baltimore, Md : 1950) 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kool M, Willart M, van Nimwegen M, Bergen I, Pouliot P, Virchow J, Rogers N, Osorio F, Reis e Sousa C, Reis C, Sousa E, Hammad H, Lambrecht B. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity. 2011;34:527–540. doi: 10.1016/j.immuni.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 52.Kuroda E, Ishii K, Uematsu S, Ohata K, Coban C, Akira S, Aritake K, Urade Y, Morimoto Y. Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasome-independent mechanisms. Immunity. 2011;34:514–526. doi: 10.1016/j.immuni.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 53.Katagiri N, Shobuike T, Chang B, Kukita A, Miyamoto H. The human apoptosis inhibitor NAIP induces pyroptosis in macrophages infected with Legionella pneumophila. Microbes and infection / Institut Pasteur. 2012 doi: 10.1016/j.micinf.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Halff E, Diebolder C, Versteeg M, Schouten A, Brondijk T, Huizinga E. Formation and Structure of a NAIP5-NLRC4 Inflammasome Induced by Direct Interactions with Conserved N- and C-terminal Regions of Flagellin. The Journal of biological chemistry. 2012;287:38460–38472. doi: 10.1074/jbc.M112.393512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kofoed E, Vance R. NAIPs: Building an innate immune barrier against bacterial pathogens: NAIPs function as sensors that initiate innate immunity by detection of bacterial proteins in the host cell cytosol. BioEssays : news and reviews in molecular, cellular and developmental biology. 2012 doi: 10.1002/bies.201200013. [DOI] [PubMed] [Google Scholar]
- 56.Shibata T, Takemura N, Motoi Y, Goto Y, Karuppuchamy T, Izawa K, Li X, Akashi-Takamura S, Tanimura N, Kunisawa J, Kiyono H, Akira S, Kitamura T, Kitaura J, Uematsu S, Miyake K. PRAT4A-dependent expression of cell surface TLR5 on neutrophils, classical monocytes and dendritic cells. International immunology. 2012 doi: 10.1093/intimm/dxs068. [DOI] [PubMed] [Google Scholar]
- 57.Smith K, Andersen-Nissen E, Hayashi F, Strobe K, Bergman M, Barrett S, Cookson B, Aderem A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nature immunology. 2003;4:1247–1300. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- 58.Mizel S, Bates J. Flagellin as an adjuvant: cellular mechanisms and potential. Journal of immunology (Baltimore, Md : 1950) 2010;185:5677–5759. doi: 10.4049/jimmunol.1002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoon S-i, Kurnasov O, Natarajan V, Hong M, Gudkov AV, Osterman AL, Wilson IA. Structural Basis of TLR5-Flagellin Recognition and Signaling. Science. 2012:335. doi: 10.1126/science.1215584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elinav E, Henao-Mejia J, Flavell R. Integrative inflammasome activity in the regulation of intestinal mucosal immune responses. Mucosal immunology. 2013;6:4–13. doi: 10.1038/mi.2012.115. [DOI] [PubMed] [Google Scholar]
- 61.Letran S, Lee SJ, Atif S, Uematsu S, Akira S, McSorley S. TLR5 functions as an endocytic receptor to enhance flagellin-specific adaptive immunity. European journal of immunology. 2011;41:29–67. doi: 10.1002/eji.201040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Moltke J, Trinidad N, Moayeri M, Kintzer A, Wang S, van Rooijen N, Brown C, Krantz B, Leppla S, Gronert K, Vance R. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–111. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andersen-Nissen E, Smith K, Strobe K, Barrett S, Cookson B, Logan S, Aderem A. Evasion of Toll-like receptor 5 by flagellated bacteria. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9247–9299. doi: 10.1073/pnas.0502040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mariathasan S, Newton K, Monack D, Vucic D, French D, Lee W, Roose-Girma M, Erickson S, Dixit V. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 65.Hawn T, Berrington W, Smith I, Uematsu S, Akira S, Aderem A, Smith K, Skerrett S. Altered inflammatory responses in TLR5-deficient mice infected with Legionella pneumophila. Journal of immunology (Baltimore, Md : 1950) 2007;179:6981–6988. doi: 10.4049/jimmunol.179.10.6981. [DOI] [PubMed] [Google Scholar]
- 66.Miao E, Mao D, Yudkovsky N, Bonneau R, Lorang C, Warren S, Leaf I, Aderem A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3076–3156. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lightfield K, Persson J, Brubaker S, Witte C, von Moltke J, Dunipace E, Henry T, Sun YH, Cado D, Dietrich W, Monack D, Tsolis R, Vance R. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nature immunology. 2008;9:1171–1179. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 69.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 70.Uematsu S, Jang M, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, Hemmi H, Coban C, Kawai T, Ishii K, Takeuchi O, Miyasaka M, Takeda K, Akira S. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nature immunology. 2006;7:868–942. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 71.Sanders C, Moore D, Williams I, Gewirtz A. Both radioresistant and hemopoietic cells promote innate and adaptive immune responses to flagellin. Journal of immunology (Baltimore, Md : 1950) 2008;180:7184–7192. doi: 10.4049/jimmunol.180.11.7184. [DOI] [PubMed] [Google Scholar]
- 72.Hajjar A, Ernst R, Fortuno E, Brasfield A, Yam C, Newlon L, Kollmann T, Miller S, Wilson C. Humanized TLR4/MD-2 mice reveal LPS recognition differentially impacts susceptibility to Yersinia pestis and Salmonella enterica. PLoS pathogens. 2012:8. doi: 10.1371/journal.ppat.1002963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flores-Langarica A, Marshall J, Hitchcock J, Cook C, Jobanputra J, Bobat S, Ross E, Coughlan R, Henderson I, Uematsu S, Akira S, Cunningham A. Systemic flagellin immunization stimulates mucosal CD103+ dendritic cells and drives Foxp3+ regulatory T cell and IgA responses in the mesenteric lymph node. Journal of immunology (Baltimore, Md : 1950) 2012;189:5745–5754. doi: 10.4049/jimmunol.1202283. [DOI] [PubMed] [Google Scholar]
- 74.Uematsu S, Fujimoto K, Jang M, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, Kiyono H, Miyasaka M, Ishii K, Akira S. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nature immunology. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 75.Diez E, Lee SH, Gauthier S, Yaraghi Z, Tremblay M, Vidal S, Gros P. Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nature genetics. 2003;33:55–60. doi: 10.1038/ng1065. [DOI] [PubMed] [Google Scholar]
- 76.Garaude J, Kent A, van Rooijen N, Blander J. Simultaneous targeting of toll- and nod-like receptors induces effective tumor-specific immune responses. Science translational medicine. 2012;4 doi: 10.1126/scitranslmed.3002868. [DOI] [PubMed] [Google Scholar]
- 77.Vijay-Kumar M, Aitken J, Sanders C, Frias A, Sloane V, Xu J, Neish A, Rojas M, Gewirtz A. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. Journal of immunology (Baltimore, Md : 1950) 2008;180:8280–8285. doi: 10.4049/jimmunol.180.12.8280. [DOI] [PubMed] [Google Scholar]
- 78.Hossain M, Jaye D, Pollack B, Farris A, Tselanyane M, David E, Roback J, Gewirtz A, Waller E. Flagellin, a TLR5 agonist, reduces graft-versus-host disease in allogeneic hematopoietic stem cell transplantation recipients while enhancing antiviral immunity. Journal of immunology (Baltimore, Md : 1950) 2011;187:5130–5140. doi: 10.4049/jimmunol.1101334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu M, Okada T, Sakai H, Miyamoto N, Yanagisawa Y, MacKenzie A, Hadano S, Ikeda J. Functional human NAIP promoter transcription regulatory elements for the NAIP and PsiNAIP genes. Biochimica et biophysica acta. 2002;1574:35–50. doi: 10.1016/s0167-4781(01)00343-8. [DOI] [PubMed] [Google Scholar]
- 80.Romanish M, Nakamura H, Lai C, Wang Y, Mager D. A novel protein isoform of the multicopy human NAIP gene derives from intragenic Alu SINE promoters. PloS one. 2009;4 doi: 10.1371/journal.pone.0005761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vinzing M, Eitel J, Lippmann J, Hocke A, Zahlten J, Slevogt H, N’Guessan P, Günther S, Schmeck B, Hippenstiel S, Flieger A, Suttorp N, Opitz B. NAIP and Ipaf control Legionella pneumophila replication in human cells. Journal of immunology (Baltimore, Md : 1950) 2008;180:6808–6823. doi: 10.4049/jimmunol.180.10.6808. [DOI] [PubMed] [Google Scholar]
- 82.Boniotto M, Tailleux L, Lomma M, Gicquel B, Buchrieser C, Garcia S, Quintana-Murci L. Population variation in NAIP functional copy number confers increased cell death upon Legionella pneumophila infection. Human immunology. 2012;73:196–200. doi: 10.1016/j.humimm.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 83.Hawn T, Verbon A, Lettinga K, Zhao L, Li S, Laws R, Skerrett S, Beutler B, Schroeder L, Nachman A, Ozinsky A, Smith K, Aderem A. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires’ disease. The Journal of experimental medicine. 2003;198:1563–1635. doi: 10.1084/jem.20031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mathur R, Oh H, Zhang D, Park SG, Seo J, Koblansky A, Hayden M, Ghosh S. A mouse model of salmonella typhi infection. Cell. 2012;151:590–602. doi: 10.1016/j.cell.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 86.O’Neill L, Bowie A. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nature reviews Immunology. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 87.Pifer R, Yarovinsky F. Innate responses to Toxoplasma gondii in mice and humans. Trends in parasitology. 2011;27:388–393. doi: 10.1016/j.pt.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feldmann M, Easten A. The relationship between antigenic structure and the requirement for thymus-derived cells in the immune response. The Journal of experimental medicine. 1971;134:103–119. doi: 10.1084/jem.134.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feldmann M. Induction of immunity and tolerance in vitro by hapten protein conjugates. I. The relationship between the degree of hapten conjugation and the immunogenicity of dinitrophenylated polymerized flagellin. The Journal of experimental medicine. 1972;135:735–753. doi: 10.1084/jem.135.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Diener E, Shortman K, Russell P. Induction of immunity and tolerance in vitro in the absence of phagocytic cells. Nature. 1970;225:731–732. doi: 10.1038/225731a0. [DOI] [PubMed] [Google Scholar]
- 91.Mizel S, Honko A, Moors M, Smith P, West A. Induction of macrophage nitric oxide production by Gram-negative flagellin involves signaling via heteromeric Toll-like receptor 5/Toll-like receptor 4 complexes. Journal of immunology (Baltimore, Md : 1950) 2003;170:6217–6223. doi: 10.4049/jimmunol.170.12.6217. [DOI] [PubMed] [Google Scholar]
- 92.Ogushi K-i, Wada A, Niidome T, Okuda T, Llanes R, Nakayama M, Nishi Y, Kurazono H, Smith K, Aderem A, Moss J, Hirayama T. Gangliosides act as co-receptors for Salmonella enteritidis FliC and promote FliC induction of human beta-defensin-2 expression in Caco-2 cells. The Journal of biological chemistry. 2004;279:12213–12219. doi: 10.1074/jbc.M307944200. [DOI] [PubMed] [Google Scholar]
- 93.Rogers T, Thorpe C, Paton A, Paton J. Role of lipid rafts and flagellin in invasion of colonic epithelial cells by Shiga-toxigenic Escherichia coli O113:H21. Infection and immunity. 2012;80:2858–2867. doi: 10.1128/IAI.00336-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ng G, Sharma K, Ward S, Desrosiers M, Stephens L, Schoel W, Li T, Lowell C, Ling CC, Amrein M, Shi Y. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008;29:807–818. doi: 10.1016/j.immuni.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.