Abstract
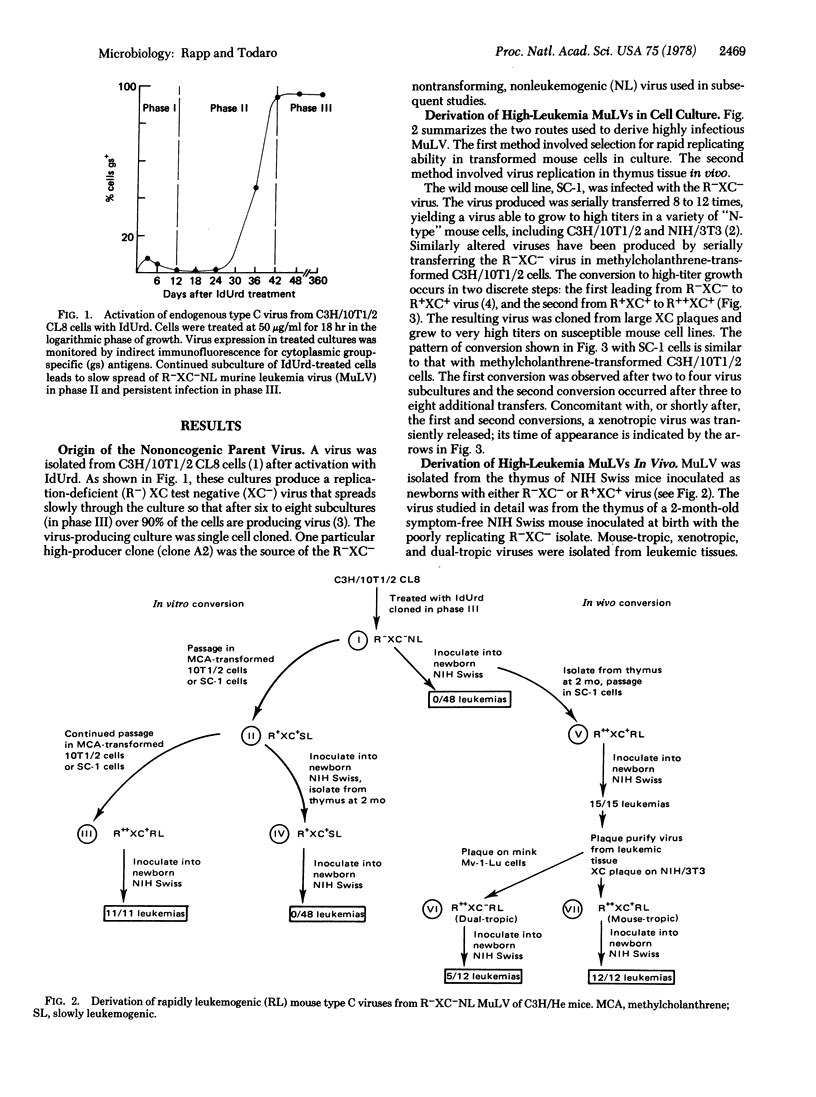
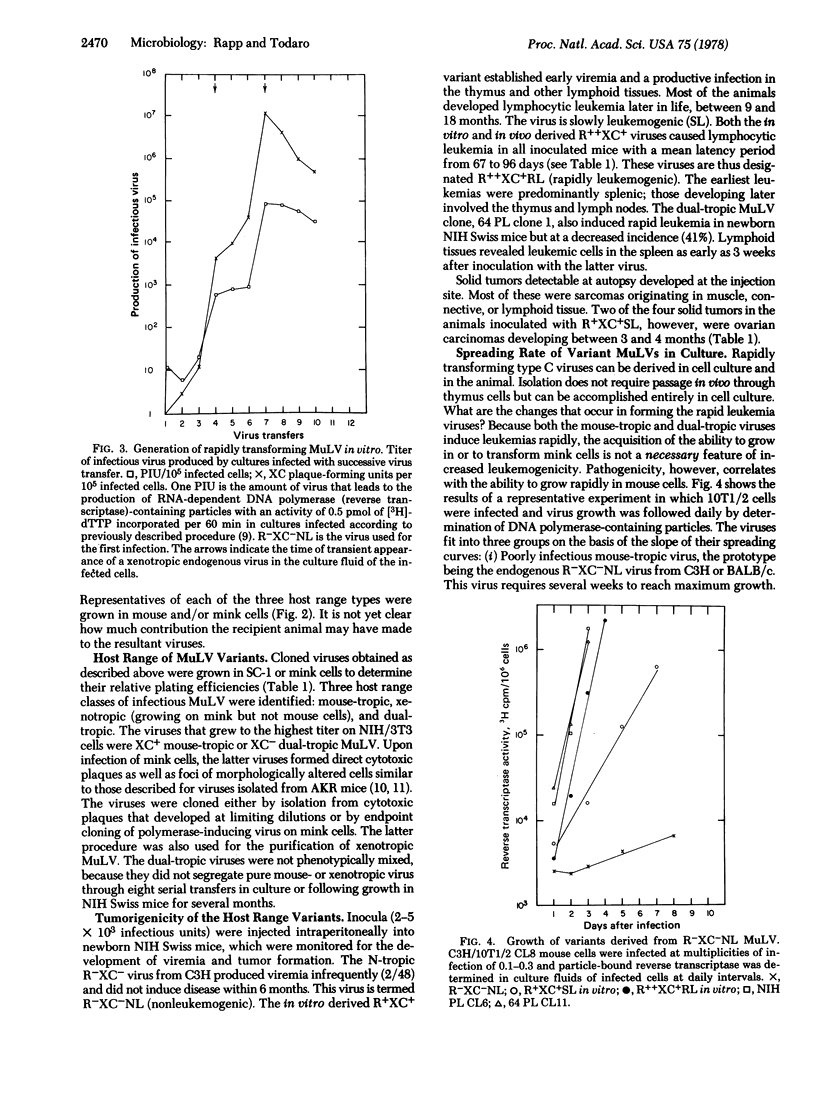
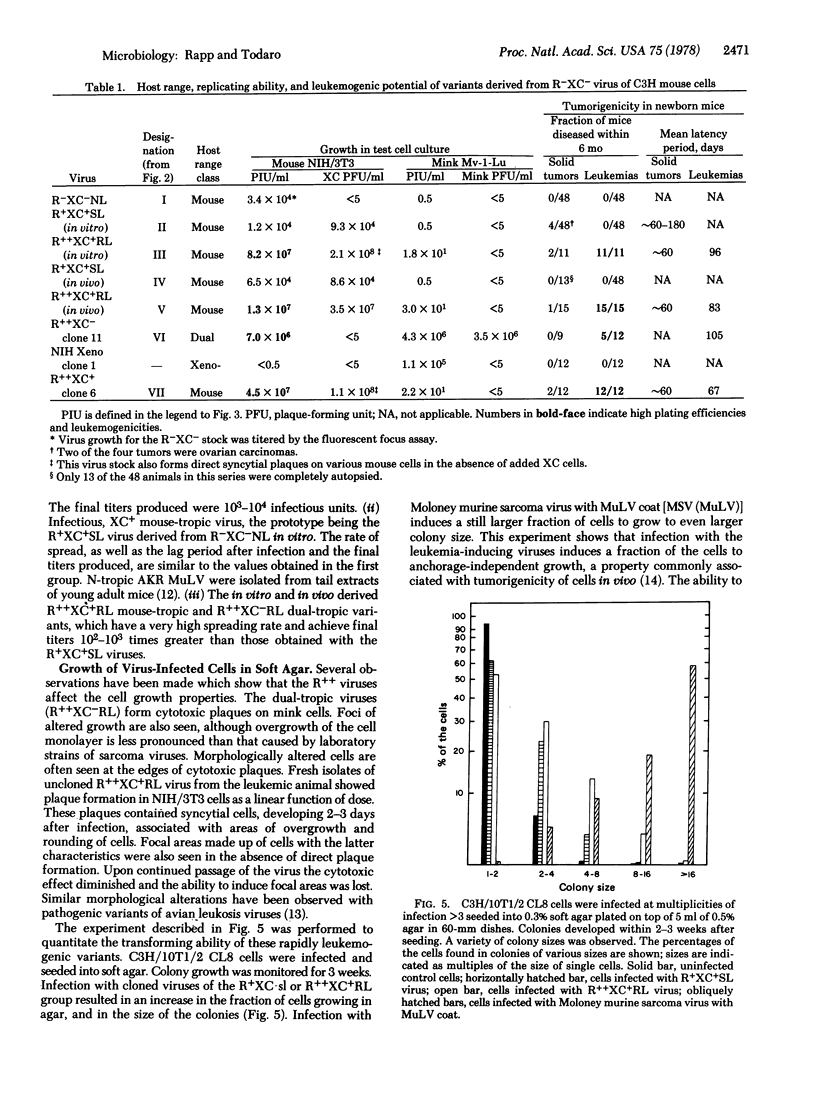
A type C virus was isolated from C3H/10T1/2 mouse cells in culture after activation with iododeoxyuridine. This virus was poorly infectious for mouse cells and did not cause tumors upon inoculation into newborn NIH Swiss mice. Variants with increased infectivity for mouse cells were then derived both in vivo and in vitro by selecting for variants able to grow to high titers. The highly infectious variants were found to induce mouse fibroblasts to grow in soft agar. When the viruses were inoculated into newborn NIH Swiss mice, 100% of the animals died of leukemia within 4 months. Solid tumors developed at the injection site. Both mouse-tropic and dualtropic viruses were isolated from the leukemic mice and plaque purified. The first group of viruses produced large syncytial plaques on rat XC cells and did not grow in mink cells. The viruses of the other group replicated well in both mouse and mink cells, producing morphologic changes similar to transformation but not XC syncytia; they, therefore, are members of the newly described MCF class of mouse type C viruses. Isolates from either group were highly leukemogenic on retesting, the mean latent period being 67 days for a mouse-tropic virus and 105 days for one of the dual-tropic viruses. The results led to the conclusion that the better a mouse type C virus grows in cell culture the more effective it is as a leukemogen. Further, it is possible to start with a weakly infectious, nonleukemogenic virus and to convert it to a rapidly replicating, highly leukemogenic virus by passage either in cell culture or in the animal. The availability of a defined series of viruses from a low-leukemia mouse strain that differ greatly in their biologic properties should facilitate studies of the molecular basis for the acquisition of type C virus oncogenicity.
Keywords: mouse leukemia, endogenous type C virus, transforming viruses, MCF viruses
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Nowinski R. C. Endogenous ecotropic mouse type C viruses deficient in replication and production of XC plaques. J Virol. 1976 May;18(2):411–417. doi: 10.1128/jvi.18.2.411-417.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Nowinski R. C., Reznikoff C. A., Heidelberger C. Endogenous oncornaviruses in chemically induced transformation. I. Transformation independent of virus production. Virology. 1975 Jun;65(2):392–409. doi: 10.1016/0042-6822(75)90045-8. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Cabradilla C. D., Stephenson J. R., Aaronson S. A. Segregation of genetic information for a B-tropic leukemia virus with the structural locus for BALB:virus-1. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2953–2957. doi: 10.1073/pnas.74.7.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Rowe W. P. Studies of genetic transmission of murine leukemia virus by AKR mice. I. Crosses with Fv-1 n strains of mice. J Exp Med. 1972 Nov 1;136(5):1272–1285. doi: 10.1084/jem.136.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal S. P., Hartley J. W., Rowe W. P. Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3065–3067. doi: 10.1073/pnas.74.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Reynolds R. K., Aaronson S. A. Isolation of temperature-sensitive mutants of murine leukemia virus. Virology. 1972 Jun;48(3):749–756. doi: 10.1016/0042-6822(72)90158-4. [DOI] [PubMed] [Google Scholar]
- Stoker M. Abortive transformation by polyoma virus. Nature. 1968 Apr 20;218(5138):234–238. doi: 10.1038/218234a0. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Kassner V. K. Replication of reticuloendotheliosis viruses in cell culture: acute infection. J Virol. 1974 Feb;13(2):291–297. doi: 10.1128/jvi.13.2.291-297.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Benveniste R. E., Lieber M. M., Sherr C. J. Characterization of a type C virus released from the porcine cell line PK(15). Virology. 1974 Mar;58(1):65–74. doi: 10.1016/0042-6822(74)90141-x. [DOI] [PubMed] [Google Scholar]



