Abstract
Preimplantation genetic diagnosis (PGD) is an innovative prenatal testing option because the determination of whether a genetic disorder or chromosomal abnormality is evident occurs prior to pregnancy. However, PGD is not covered financially under the majority of private and public health insurance institutions in the United States, leaving couples to decide whether PGD is financially feasible. The aim of this qualitative study was to understand the role of finances in the decision-making process among couples who were actively considering PGD. In-depth, semi-structured interviews were completed with 18 genetic high-risk couples (36 individual partners). Grounded theory guided the analysis, whereby three themes emerged: 1) Cost is salient, 2) Emotions surrounding affordability, and 3) Financial burden and sacrifice. Ultimately, couples determined that the opportunity to avoid passing on a genetic disorder to a future child was paramount to the cost of PGD, but expressed financial concerns and recognized financial access as a major barrier to PGD utilization.
Keywords: Cost, Decision-making, Economics, Preimplantation Genetic Diagnosis (PGD), In Vitro Fertilization (IVF), Family Planning, Genetic Counseling, Qualitative Research
Introduction
Preimplantation genetic diagnosis (PGD) is an innovative genomically-based prenatal testing option because of its ability to diagnose genetic disorders from fertilized oocytes or developing embryos prior to uterine implantation and pregnancy. This specialized procedure can only be performed in conjunction with in vitro fertilization (IVF) due to the nature of the genetic testing process. IVF allows for access to the genetic material of oocytes or developing embryos from which the PGD testing and analysis occur. PGD is designed to identify sex-linked diseases, single gene disorders, and chromosomal abnormalities prior to embryo transfer (Basille et al., 2009; Hershberger et al., 2011b; Simpson, 2012).
First successfully implemented in 1990 to prevent X-linked genetic disorders (Handyside, 1990), PGD exists as an alternative to more traditional and invasive prenatal genetic diagnosis techniques, such as amniocentesis and chorionic villus sampling. PGD is unique from other traditional prenatal testing options in that PGD circumvents the ethical dilemma of terminating a pregnancy diagnosed with a genetic abnormality (Basille et al., 2009; Soini et al., 2006). PGD is considered to be a major scientific advancement by reproductive specialists and professional groups, and worldwide use has steadily increased (Goossens et al., 2012; Practice Committee of the Society for ART & Practice Committee of the ASRM, 2008).
Although a growing number of genetic high-risk couples are drawn to PGD to avoid elective termination, the direct costs of PGD and/or IVF are usually not covered financially or reimbursed by health insurance plans in the United States. The overall finances required for PGD can present an economic barrier for prospective parents hoping to significantly reduce their chances of passing on known genetic disorders (Jae et al., 2011). Additionally, many genetic high-risk individuals are not diagnosed with infertility, which is often a prerequisite for health insurance plans in the United States that do cover costly IVF treatments.
In 2009, four percent of the over 100,000 total IVF cycles involved PGD in the United States alone (Centers for Disease Control and Prevention et al., 2011). With upwards of 4,700 phenotypes for which the molecular basis is known, and over 2,500 diseases with testing available, the applicability of PGD continues to grow (McKusick-Nathans Institute of Genetic Medicine, 2011; National Center for Biotechnology Information, 2012). While some individuals with genetic disorders can experience infertility complications, an increasing number of fertile couples in the United States prefer to undergo PGD versus natural conception when planning for a child free from a known genetic disorder. However, expenses can quickly escalate for those who choose PGD. Nationwide, 20–25% of private health insurance plans cover infertility-related IVF, yet many do not cover IVF when fertile couples opt to perform PGD, for which IVF is a prerequisite (Bitler & Schmidt, 2012; Cohen & Chen, 2010). Insurance companies in the United States do not always cover or reimburse the costs required to complete the PGD analysis, adding to the expense. Currently, only 15 of 50 states have laws requiring that health insurance companies cover or offer infertility-related treatments. Only eight states mandate coverage of IVF, and each state mandate specifies unique regulations and restrictions (Martin et al., 2011; Quinn et al., 2011).
On average, IVF cycles cost approximately US$9,226–12,513 per cycle, while PGD costs an additional US$2,500–6,000 per cycle (Chambers et al., 2009; Galpern, 2007; Martin et al., 2011; Omurtag et al., 2009; Tur-Kaspa et al., 2010). Given this level of expense, major factors limiting IVF use in developed countries have been identified as: cost, out-of-pocket expenses, and lack of insurance coverage (Ata & Seli, 2010; Collins, 2002). Chambers and colleagues (2009) demonstrated that one standard cycle of IVF could consume up to 50% of the average worker’s annual disposable income in the United States, which is a substantially higher percentage than the other developed nations studied. Furthermore, more than one assisted reproductive technology cycle is often necessary to achieve pregnancy, as 12–41% of IVF cycles in American women under the age of 42 results in a live birth (CDC et al., 2011).
Despite increasing use of PGD, very little is known about the psychological factors involved in couples’ decision-making processes and the implications of their decisions (Karatas et al., 2010a; Pivetti & Merlotti, 2013). What has been reported is that genetic high-risk couples often experience anxiety and stress during PGD procedures due to high out-of-pocket costs and pressure for an immediately successful pregnancy to avoid the long-term financial burdens associated with multiple PGD attempts (Karatas et al., 2010b). Conversely, women have indicated that undergoing PGD was empowering, as it provided renewed hope for a biological child and facilitated perception of greater control over their reproductive futures (Karatas et al., 2010c; Snowdon & Green, 1997).
Purpose of the Study
Despite the insights generated in prior investigations, we are unaware of research examining how genetic high-risk couples navigate the costs associated with PGD. Thus, the purpose of this study was to describe the role of finances in the decision-making process among couples who expressed a willingness to use PGD, or had used PGD in the prior three months to prevent the transmission of known genetic disorders. The overarching aim of our research is to improve the quality of care provided by genetic counselors and other healthcare professionals, as they provide information and facilitate decisions among genetic high-risk couples.
Methods
Sample and Procedures
Institutional Review Board members from the University of Illinois at Chicago approved the study protocol for adequate protection of human subjects. Couples were then recruited through an innovative, multi-faceted recruitment plan that included an investigator-initiated study website, advertisements and postings on websites and traditional newsletters, electronic mailing lists, and through a large reproductive center specializing in PGD. Details of the multi-faceted recruitment plan can be found elsewhere (Hershberger et al., 2011a).
After the study was fully explained to each potential participant, written informed consent was obtained. Data were collected from genetic high-risk couples who were part of a larger study examining the decision-making process among couples surrounding PGD use in the United States (Hershberger et al., 2012a). For the analysis reported here, 18 couples (n = 36 individual partners) who expressed a willingness to use PGD (n = 7), were currently undergoing PGD (n = 4), or had used PGD in the prior three months (n = 7), were selected for further in-depth analysis from the larger sample of 22 couples. We purposefully analyzed the couples who viewed PGD use favorably because we sought to explicate how costs are perceived among a homogenous sample of couples that are supportive of PGD use versus including a heterogeneous sample of couples whose reasons for declining PGD use include moral or ethical factors.
All individual partners within the couple dyads participated in a semi-structured interview that occurred separately from their partner. Individual participants were given the choice of completing the interview by phone or email based on their personal preference for communication. Allowing participants to select their preference for completing the interview is especially good for sensitive topics (Dillman et al., 2009; McCoyd, 2006) because a solid evidence base for choosing methods is still lacking at this time (Kvale & Brinkmann, 2009; Novick, 2008; Rubin & Rubin, 2012).
The phone interviews ranged from 38–89 minutes, with an average interview lasting 60 minutes. Procedures for completing the email interviews included sending an initial email that contained the primary research question followed by a series of asynchronous investigator probe-participant response cycles. The number of email cycles needed to complete the interview averaged 4.63 probe-response cycles (range 4–8 cycles) over 27.8 days per participant. One email interview participant also completed a short phone interview, per participant request, to complete the final research questions. The semi-structured interview is recommended for critical research topics and facilitates depth within the participants’ thoughts and experiences that center around a particular phenomenon (DiCicco-Bloom & Crabtree, 2006; Kvale & Brinkmann, 2009). The primary research question posed to participants and examples of probes, or follow-up questions, used during the interview process can be found in Table 1. Couples were provided with a $50 gift card honorarium after both partners completed the interviews.
Table 1.
Primary Research Question and Examples of Probes
| Primary Research Question |
|
| Examples of Probes or Follow-up Questions |
|
Interview Protocol
Data Analysis
The telephone interviews were digitally recorded, transcribed by an external transcription company, and verified for accuracy by the first author. The de-identified telephone and email data were entered into NVivo 8 software (QSR International, Pty Ltd, Doncaster, Victoria, Australia) to assist with data management, retrieval, and analysis. Using tenets of grounded theory, a thematic analysis was completed (Charmaz, 2006; Glaser & Strauss, 1967). Each participant interview was read and initially coded by the first author, who identified instances in the data where participants discussed concerns related to finances surrounding PGD use (Charmaz, 2006). The interviews were re-read several times by the first and last authors, where more detailed nuanced coding took place to develop emerging categories and sub-categories. This process of emersion in the data is typical for analysis of this type and provides trustworthiness for qualitative studies (Glaser & Strauss, 1967; Patton, 2002).
The first and last author met weekly during the coding and analysis period to discuss and refine emergent categories, sub-categories, and ultimately themes. Corroboration of the themes was accomplished by having the first author examine the content under the relevant themes using raw data and the authors reviewed the themes and selected quotations in the manuscript for fit (Burnard, 2004). In addition, spreadsheet data displays were created by the first author and assisted with the development of the emergent themes. All authors reviewed the spreadsheets and themes for consistency. Discrepancies with coding, emergent categories, or themes were discussed and resolved during the weekly team meetings to enhance rigor (Creswell & Miller, 2000). Because the use of email interviews for qualitative data collection is evolving, our email procedures and data quality comparisons between the phone and email interviews underwent additional analysis (Hershberger & Kavanaugh, 2012b; Meho, 2006). Although the individual partners participated in separate phone or email interviews, the unit of analysis consisted of the couple dyad. To ensure the dyadic nature of the analysis, individual partner interviews were linked by an assigned numeric code such as “Couple 7A” and “Couple 7B” to maintain the dyadic couple dataset.
Results
Sample Characteristics
All couples were heterosexual, married, living with their partner, and resided in 13 states within the United States. Of the 18 couples, 4 couples lived in states where IVF-inclusive infertility coverage was mandated, and 14 couples resided in non-mandated states. The mean age was 34.1 years, and the mean household income was about US$92,900, although one couple expressed a preference to forego reporting of their income. The couples in this sample were aware of their predisposition to pass on one or two of the following genetic disorders: Adrenoleukodystrophy, Charcot-Marie-Tooth, Cystic Fibrosis, Glycogen Storage Disease 1a, Hemophilia, Huntington’s disease, Hypertrophic Cardiomyopathy, Muscular Dystrophy (Duchenne, Becker, Myotonic, and Facioscapulohumeral), and Spinal Muscular Atrophy. Further details of the couples’ demographics can be found in Table 2.
Table 2.
Demographic Characteristics of the Couples by Magnitude of Barrier (n =18)
| Couple | Age (years) | State | Race/Ethnicity | Employment Status | Household Income Range | PGD Completion at Time of Interview |
|---|---|---|---|---|---|---|
| Not a barrier | ||||||
| 2 | Fe= 39 M= 39 |
MA | White | Fe= Part Time M= Full Time |
>$130,000 | Yes |
| 3 | Fe= 32 M= 33 |
IL | White | Fe= Part Time M= Full Time |
Not disclosed | No |
| 14 | Fe= 35 M= 39 |
CT | White | Fe= Part Time & Student M= Unemployed |
$30,000–49,999 | No |
| Concern, but not a barrier | ||||||
| 4 | Fe= 30 M= 28 |
SC | White | Fe= Full Time M= Student |
$50,000–69,999 | Yes |
| 10 | Fe= 37 M= 37 |
NC | White | Fe= Part Time M= Full Time |
$90,000–109,999 | Yes |
| 13 | Fe= 36 M= 38 |
GA | White | Fe= Unemployed M= Full Time |
>$130,000 | In progress |
| 15 | Fe= 33 M= 33 |
GA | White | Fe= Full Time M= Full Time |
$70,000–89,999 | No |
| Barrier for one partner | ||||||
| 7 | Fe= 35 M= 35 |
IL | White | Fe= Full Time M= Full Time |
$90,000–109,999 | Yes |
| 9 | Fe= 27 M= 30 |
MI | White | Fe= Student M= Full Time & Student |
<$29,999 | In progress |
| 11 | Fe= 34 M= 34 |
PA | White | Fe= Unemployed M= Full Time |
>$130,000 | In progress |
| Significant Barrier | ||||||
| 1 | Fe= 27 M= 30 |
AZ | White | Fe= Full Time M= Part Time |
$50,000–69,999 | In progress |
| 5 | Fe= 32 M= 28 |
CA | White | Fe= Part Time M= Full Time |
>$130,000 | No |
| 6 | Fe= 34 M= 33 |
NE | White | Fe= Unemployed M= Full Time |
<$29,999 | Yes |
| 8 | Fe= 34 M= 34 |
MI | Middle Eastern | Fe= Unemployed M= Full Time |
$80,000 | No |
| 12 | Fe= 36 M= 44 |
OR | White | Fe= Full Time M= Full Time |
$90,000–109,999 | Yes |
| 16 | Fe= 31 M= 31 |
CA | White | Fe= Full Time M= Full Time |
>$130,000 | No |
| 17 | Fe= 35 M= 42 |
WI | Hispanic | Fe= Full Time M= Full Time |
>$130,000 | No |
| 18 | Fe= 38 M= 36 |
PA | White | Fe= Full Time M= Full Time |
$110,000–129,999 | Yes |
Themes
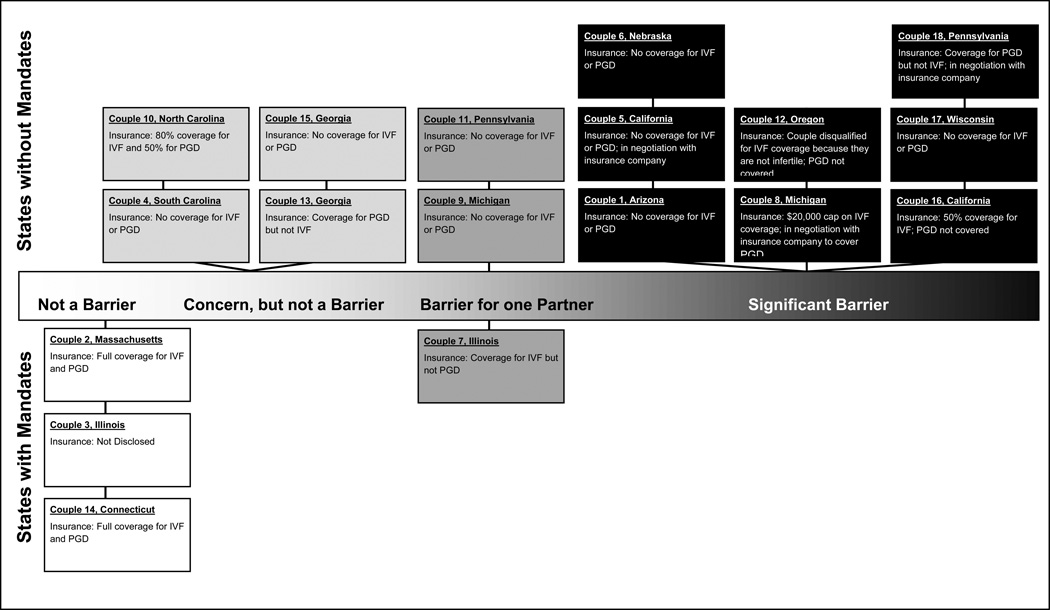
The analysis revealed nuances between the financial concerns among couples that lived in states where IVF coverage is mandated versus those couples that lived in states without mandates; however, common themes emerged that transcended state mandates for IVF and reflected many shared experiences for the couples residing within both the mandated and non-mandated states. These themes are explicated in detail below. Instances where nuances and differences were identified between the two groups (i.e., mandated and non-mandated states) are also described to provide a more thorough description. Figure 1 provides a visual that displays the financial concerns of couples on a continuum of perceived cost barriers and highlights couples that reside in mandated versus non-mandated states. Illustrative quotes corresponding to the couple number used in Table 2 and in Figure 1 are provided in the text to add context and enhance understanding. When appropriate, the gender of the partner is provided in brackets to further promote insight.
Figure 1.
Spectrum of Cost as a Barrier in PGD Decision by Couple
Genetic high-risk couples that had expressed a willingness to use PGD or had used PGD in the prior three months described three common themes related to the role of finances: 1) Cost is salient; 2) Emotions surrounding affordability; and 3) Financial burden and sacrifice.
Theme 1: Cost is salient
In this sample of couples viewing PGD favorably, cost played a vital role in the decision-making process. For the majority (n = 15) of couples, cost was the primary barrier in practical feasibility. If the couple could not immediately afford PGD or expect health insurance coverage or reimbursement for IVF and/or PGD, other factors (e.g. ethical, moral) did not appear to be as salient. Couple 16 [male] represents the thoughts of several couples when he explained, “[We] liked the idea of PGD from the start. I think the cost was what was holding us back.” He later commented, “The whole decision was really just either spending the money or not.” For four couples, the discussion of cost was intricately intertwined with the decision to move forward with PGD. Couple 8 [male] stated, “After we had the conversation with the genetics counselor and she went over the cost and the figures, I think that’s when [the decision] became easier… we didn’t even know if we could afford to go ahead and do it.”
Three couples explained that cost was not the primary barrier when deciding to use PGD. Couple 2 [female] explained that she was delaying use of PGD for a second child because the PGD process “takes over your life,” and she would prefer to “be in a place where [she is] more likely to relax and not have a lot of stress on other fronts.” Couple 3 did not allude to finances playing a large role in their decision. Couple 14 had complete financial coverage for the direct costs of IVF and PGD, and therefore they did not immediately foresee a long-term financial burden. However, Couple 14 [male] noted that their insurance coverage may change or run out in the future, and PGD procedures would then be financially out of reach. Notably, Couples 2, 3, and 14 resided in states with IVF-inclusive infertility mandates.
At least one partner of three of the four couples residing in states with IVF-inclusive mandates for infertility coverage expressed knowledge about the benefits and limitations of their state mandates and how finances impacted their decision-making process. Couple 7 [female] acknowledged, “Luckily we live in the state of Illinois… most people can get IVF covered, at least a few rounds of it.” Couples 2 and 14 also recognized the benefits of their state mandates on health insurance coverage for IVF and PGD, and neither couple felt that cost was the primary concern or barrier in their decision. Additionally, Couple 12 [female], who resided in Oregon at the time of the interview, described a relevant situation regarding the infertility prerequisite for her health insurance; she and her husband did not qualify for IVF coverage because they were not infertile, despite living in a mandated state while making their decisions about IVF and PGD. She described, “What made the situation even more infuriating was that we were in Massachusetts at the time, where IVF is covered by health insurance by state law. The loophole was that IVF was covered for patients with infertility, defined as couples that had tried to become pregnant for at least 12 months. I spoke with several representatives from my insurance company and was told we did not qualify.”
Theme 2: Emotions surrounding affordability
Couples were emotionally transparent when discussing the cost of PGD in general, or when describing insurance coverage for PGD. For example, Couple 18 expressed their determination to have a child using PGD despite the couple’s unfavorable economic situation at the time of the interview. Couple 18 [female] declared, “We could never do it [PGD] if it wasn’t paid for. Ever. And the fact that I, I mean, I did have to pay money and I put [the cost of PGD] on my credit card. My husband recently lost his job. So, there’s no way I could even fathom doing it now. So we’re taking a financial hit, our entire family… That’s a lot of stress.” Stress and frustration were common feelings expressed when speaking or writing about the expenses related to IVF and/or PGD.
Couples also expressed frustration specifically with regard to insurance coverage, and four couples explicitly stated they were “fighting” their insurance company for IVF and/or PGD coverage. Couple 7 [female] reported, “It aggravates me that the insurance companies won’t pay for PGD when people have these documented cases,” referring to families with known genetic risks. One couple’s experience highlights the aggravation resulting from inconsistent insurance coverage. For this couple, their insurance company covered PGD but not IVF. Couple 18 [female] explained, “I think it’s ridiculous that insurance companies will pay for PGD without IVF… You can’t do it [PGD] without it [IVF].” She described her situation as “horrendous,” as she spent over 100 hours at the time of the interview negotiating coverage for IVF, which was their sole reason for delaying another IVF-PGD cycle.
Although insurance companies may ultimately reimburse couples, relieving some of the financial burden, seven couples described considerable effort, including collecting letters of support from genetic counselors and other healthcare professionals, as well as formulating cost-benefit analyses arguments for their insurance companies based on personal experience. While frustration, anger, a sense of unfairness, and sometimes guilt emerged from interviews, the couples involved in negotiations with insurance companies also displayed a determined, proactive, hopeful approach to obtain coverage or reimbursement for either the cost of IVF and/or PGD. Couple 4 [female] explained, “I mean, the frustrating side might partially be due to the insurance side of things, but you have to be able handle this…I think you also have to have hope… we definitely encourage other families to definitely be proactive and approach their insurance.” Couple 18 [male] asserted, “to avoid a genetic issue… if you’re strong enough to go through what we went through to try to get things covered by insurance, then by all means I would recommend it.”
Three couples with children who have undergone major medical procedures or surgeries because of complications from their inherited disorders shared parallel exasperation because their insurance would reimburse for hundreds of thousands – or in one case – almost a million dollars for treatment, but would not pay for IVF and/or PGD to prevent the disorder in a subsequent child. Couple 18 [female] stated, “To spend $20,000 versus millions of dollars in the long run… You know, it doesn’t make any sense to me. Put the money up front, and then you don’t have to put the money in the end. So very frustrating.” These couples explained that IVF-PGD coverage often costs considerably less than a lifetime of treatment for a genetic disorder, and it would be in the best interest of insurance companies to take preventative measures based on a cost-effectiveness argument. Parents who have had a child born with a genetic disorder demonstrated greater awareness of the potential monetary savings, but other couples also expressed foresight that PGD costs are considerably less than paying for lifetime treatment of many genetic conditions.
Theme 3: Financial burden and sacrifice
For those couples acknowledging that cost was a major barrier in using PGD, financial sacrifices were made and risks were taken in order to afford PGD. Couples stated they were willing to “dig deep,” and willingly place their family in a “financial hole,” “stick [their] necks out,” accept loans from friends and family, drain their entire savings, or indefinitely delay PGD (therefore, delay starting or adding to a family) until they were able to afford it. Two couples are noteworthy for the unique sacrifices they made to afford PGD. Couple 2 uprooted their family to a state where IVF treatment was mandated for infertility coverage. Furthermore, Couple 4 decided to use their savings for PGD rather than buy a home. In this sample, the potential to have a child without the known heritable disorder was “priceless,” and couples would “do whatever it takes,” in the words of the Couple 1 [female], to ensure their child is not born with a known genetic disorder.
All the couples in this analysis acknowledged that PGD is expensive. Couple 12 [male] said that the PGD is “financially debilitating.” However, each couple’s ultimate decision and willingness to use PGD – regardless of the cost – highlights the reality for these couples: the opportunity to avoid passing on a known genetic disorder is paramount to financial concerns. Couple 16 [female] described, “Although going through IVF-PGD is expensive and difficult, you are ensuring that your child’s future is free of whatever disease you may carry.” Couple 8 [male], whose young son passed away from Myotonic Dystrophy echoed this statement: “PGD was… it’s a substantial cost, but if you look at the big picture, it’s a small cost compared to putting another life though what my son went through.”
Discussion
The decision to use PGD to prevent a genetic disorder involves a complex dynamic between cognitive appraisals, emotional responses, and moral judgments (Hershberger et al., 2010). Our findings support the idea that finances, a cognitive appraisal, intertwined with emotions, plays a significant role in the decision-making process for the majority of couples in this analysis. Couples even went to great geographic lengths, by moving or traveling hundreds or thousands of miles, to undergo affordable reproductive technology procedures, consistent with literature about access to reproductive technologies (Spar, 2005). Furthermore, we were able to demonstrate that for couples who reported full insurance coverage for IVF and PGD, the cost – although considered – was not a barrier to use. However, the majority of couples in the sample struggled with financing PGD, especially those living in non-mandated states.
Practice Implications
Increased awareness and understanding of how couples navigate the finances related to PGD use can improve the quality of care for couples that are genetic high-risk for transmitting known genetic disorders to their future offspring. Genetic counselors and other healthcare professionals can use findings from the study during counseling sessions to inform couples of how others have reacted to and addressed the financial aspects of PGD use in the United States. Furthermore, the interview data demonstrate the significant role of genetic counselors in providing information to genetic high-risk couples. As described in the case of Couple 8, it was the genetic counselor who provided foundational information about the financial cost associated with PGD that eventually allowed the couple to reach a decision about moving forward with PGD. This finding is supported by Arnold and colleagues (2005), who reported the need for pre-pregnancy counseling to discuss advantages and disadvantages of reproductive options including PGD among individuals at high-genetic risk for Charcot-Marie-Tooth disease. Thus, insight from our study, combined with the findings from Arnold and colleagues (2005), provide evidence that information about financial costs is a major concern among couples and should be included in counseling sessions when advantages and disadvantages of PGD are discussed.
Another important implication is the need for and importance of advocacy undertaken by genetic counselors and other reproductive health specialists toward ameliorating the financial hardships and economic disparities surrounding PGD use. In this study, couples described the importance of having genetic counselors and other professionals write supportive and informative letters to insurance companies with the intent of obtaining financial support for PGD from these companies. Although the role of advocacy among professionals is challenging (Fathalla et al., 2006; Powell et al., 2010; Zeiler, 2004), it is clear that couples perceived these actions (e.g. letter writing) as supportive. These findings do pose a larger question about whether, and to what degree, healthcare professionals should participate in advocacy. For example, among developed countries worldwide, there is a trend toward supporting PGD costs (Genetics Commissioning Advisory Group & UK Department of Health, 2002; Soini et al., 2006). Yet, in the United States, although there is movement toward a public health insurance program, it is unclear if PGD will be covered despite recognition that coverage for prevention or early detection of a genetic disorder should be covered (Office of the Legislative Counsel, U.S. House of Representatives, 2010; Secretary’s Advisory Committee on Genetics, Health and Society, 2006).
Advocacy and support by healthcare professionals will be needed to convince policy makers to mandate public and private health insurance companies for reimbursement for PGD to reduce economic disparities in the United States. In lieu of public policy and significant policy changes, including a national mandate for IVF and PGD coverage or reimbursement by public and private health insurance organizations, advocacy will be necessary to obtain financial support for couples who have thought deeply about the decision to use PGD, and in some cases witnessed first-hand the genetic disorder in themselves or in their own child. Additionally, cost-effectiveness research, such as two independent studies demonstrating that PGD is significantly more cost-effective than paying for treatment for cystic fibrosis, can also substantiate appeals to health insurance companies and state policy makers (Davis et al., 2010; Tur-Kaspa et al., 2010).
Research Recommendations
Our sample was composed of predominantly White, well-educated couples with a high income level who reported a difficult and emotionally-laden process to determine whether the financial cost and sacrifices to use PGD were feasible. Moreover, our in-depth interviews revealed that two couples with household incomes of less than US$30,000 per year had delayed PGD primarily because of cost, which suggests that mandated insurance could reduce economic, although perhaps not racial nor cultural disparities among couples. Current research examining the effects of state mandates on IVF use indicates that mandates increase use, but do not negate disparities (Bitler & Schmidt, 2006; Henne & Bundorf, 2008; Jain et al., 2002; Jain et al., 2005). Future comparative research across couples from a wide range of political, economic, and social contexts would provide further insight into understanding decision processes and mechanisms that may reduce cost disparities.
Noteworthy is that although the unit of analysis reported here is the couple, we did not find any major differences regarding the financial concerns among the individual partners within the couple dyads. Rather, our data indicate that couples struggled together as a unit over financial concerns and cost even when one partner perceived cost as a barrier more so than their partner. Because little research has examined couples’ perspectives, our inclusion criteria required that both partners participate in the research. This requirement may have deterred couples that were experiencing conflict from participating in the study. Future research that targets individual partners or couples experiencing conflict surrounding PGD use may provide further insight into the key concerns couples have about PGD.
Another important area of research is to understand the mechanisms of effective interactions among genetic counselors and other healthcare professionals during the counseling sessions that take place with genetic high-risk couples. Our findings provide insight into couple’s perspectives; however, there is a small but growing recognition of the benefits of understanding the professionals’ perspectives (Caldas et al., 2010; Hines et al., 2010). Additional research that determines appropriate strategies for effective information provision and communication between couples and providers would be beneficial to genetic counselors and other professionals as well as genetic high-risk individuals and couples.
Study Limitations
As this study is a qualitative investigation, the purpose is not generalization. Rather, we provide an in-depth description of the challenges that couples faced when navigating financial concerns when they either expressed intention or opted to use PGD within the prior three months. Additionally, the self-selective nature of the participants that comprise the sample may be limiting, as couples in the study expressed a desire to help other genetic high-risk couples in the future and thus, they may have a propensity for altruism that may not be reflected in a wider array of couples. In addition, we did not examine couples who opted not to use PGD, and research examining the financial concerns of these couples would also be beneficial.
Conclusion
The results of this study highlight the financial concerns of genetic high-risk couples surrounding PGD use in the United States. Notably, genetic counselors play a key role in assisting couples as they consider PGD. The findings can be used to inform genetic high-risk couples about the financial concerns and experiences surrounding PGD as described by the couples in this study. Because all couples in this sample expressed concerns regarding the affordability of the PGD process, highlighting the critical need for national discussion regarding PGD policies, advocacy by genetic counselors and other professional groups could help relieve some of the financial concerns of many couples or prospective parents. Adoption of national policies that promote the conception of healthy babies without compelling procreative couples and individuals to choose between a biological family and financial hardship would be a step toward improving access to PGD, and ultimately health equity, a broader concept that is currently of high concern for public health in the United States. Further research focusing on related populations may enhance understanding of couples’ decision-making processes and health-seeking behaviors surrounding PGD, as well as barriers to using PGD beyond financial reasons.
Acknowledgements
We graciously thank the couples who participated in the study. The research was completed in partial fulfillment of the Master of Public Health requirements for graduation at the University of Illinois at Chicago for Ms. Drazba. Support for the research was award to Dr. Hershberger by the National Institutes of Health (NIH), National Institute of Child Health and Human Development and the Office of Research on Women's Health (K12 HD055892), National Institute of Nursing Research (R03 NR010351), and the University of Illinois at Chicago College of Nursing Dean’s Fund. The content of this article is the authors’ responsibility and does not necessarily represent the official views of the NIH, the University of Illinois at Chicago, or Pennington Biomedical Research Center.
REFERENCES
- Arnold A, McEntagart M, Younger DS. Psychosocial Issues That Face Patients With Charcot-Marie-Tooth Disease: The Role of Genetic Counseling. Journal of Genetic Counseling. 2005;14(4):307–318. doi: 10.1007/s10897-005-0760-z. [DOI] [PubMed] [Google Scholar]
- Ata B, Seli E. Economics of assisted reproductive technologies. Current Opinions in Obstetrics and Gynecology. 2010;22(3):183–188. doi: 10.1097/GCO.0b013e3283373c13. [DOI] [PubMed] [Google Scholar]
- Basille C, Frydman R, El Aly A, Hesters L, Fanchin R, Tachdjian G, et al. Preimplantation genetic diagnosis: State of the art. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2009;145(1):9–13. doi: 10.1016/j.ejogrb.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Bitler M, Schmidt L. Health disparities and infertility: impacts of state-level insurance mandates. Fertility and Sterility. 2006;85(4):858–865. doi: 10.1016/j.fertnstert.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Bitler M, Schmidt L. Utilization of Infertility Treatments: The Effects of Insurance Mandates. Demography. 2012;49(1):125–149. doi: 10.1007/s13524-011-0078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnard P. Writing a qualitative research report. Nurse Education Today. 2004;24(3):174–179. doi: 10.1016/j.nedt.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Caldas GH, Caldas E, Araújo ED, Bonetti TCS, Leal CB, Costa AM. Opinions concerning pre-implantation genetic diagnosis and sex selection among gynecologist-obstetricians in Brazil. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2010;148(2):158–162. doi: 10.1016/j.ejogrb.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2009 Assisted reproductive technology success rates: National summary and fertility clinic reports. Atlanta, GA: U.S. Department of Health and Human Services; 2011. American Society for Reproductive Medicine, & Society for Assisted Reproductive Technology. Retrieved from http://www.cdc.gov/art/ARTReports.htm. [Google Scholar]
- Chambers GM, Sullivan EA, Ishihara O, Chapman MG, Adamson GD. The economic impact of assisted reproductive technology: a review of selected developed countries. Fertility and Sterility. 2009;91(6):2281–2294. doi: 10.1016/j.fertnstert.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Charmaz K. Constructing grounded theory: A practical guide through qualitative analysis. Thousand Oaks, CA: SAGE Publications; 2006. [Google Scholar]
- Cohen G, Chen DL. Trading-off reproductive technology and adoption: Does subsidizing IVF decrease adoption rates and should it matter? Minnesota Law Review. 2010;95(2):485–577. [Google Scholar]
- Creswell JW, Miller DL. Determining validity in qualitative inquiry. Theory Into Practice. 2000;39(3):124–130. [Google Scholar]
- Collins J. An international survey of the health economics of IVF and ICSI. Human Reproduction Update. 2002;8(3):265–277. doi: 10.1093/humupd/8.3.265. [DOI] [PubMed] [Google Scholar]
- Davis LB, Champion SJ, Fair SO, Baker VL, Garber AM. A cost-benefit analysis of preimplantation genetic diagnosis for carrier couples of cystic fibrosis. Fertility and Sterility. 2010;93(6):1793–1804. doi: 10.1016/j.fertnstert.2008.12.053. [DOI] [PubMed] [Google Scholar]
- DiCicco-Bloom B, Crabtree BF. Making sense of qualitative research: The qualitative interview. Medical Education. 2006;40(4):314–321. doi: 10.1111/j.1365-2929.2006.02418.x. [DOI] [PubMed] [Google Scholar]
- Dillman DA, Phelps G, Tortora R, Swift K, Kohrell J, Berck J, et al. Response rate and measurement differences in mixed-mode surveys using mail, telephone, interactive voice response (IVR) and the Internet. Social Science Research. 2009;38(1):1–18. [Google Scholar]
- Fathalla MF, Sinding SW, Rosenfield A, Fathalla MMF. Sexual and reproductive health for all: a call for action. The Lancet. 2006;368(9552):2095–2100. doi: 10.1016/S0140-6736(06)69483-X. [DOI] [PubMed] [Google Scholar]
- Galpern E. Assisted Reproductive Technologies: overview and perspective using a reproductive framework. Oakland, CA: Center for Genetics and Society; 2007. Retrieved from http://geneticsandsociety.org/downloads/ART.pdf. [Google Scholar]
- Genetics Commissioning Advisory Group & United Kingdom Department of Health. Preimplantation genetic diagnosis (PGD) – guiding principles for commissioners of NHS services. 2002 Retrieved from http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4019244.pdf.
- Glaser BG, Strauss AL. The discovery of grounded theory: Strategies for qualitative research. Chicago, IL: Aldine Publishing Company; 1967. [Google Scholar]
- Goossens V, Traeger-Synodinos J, Coonen E, De Rycke M, Moutou C, Pehlivan T, et al. ESHRE PGD Consortium data collection XI: cycles from January to December 2008 with pregnancy follow-up to October 2009. Human Reproduction. 2012;27(7):1887–1911. doi: 10.1093/humrep/des106. [DOI] [PubMed] [Google Scholar]
- Handyside A. Sex and the single cell. New Science. 1990;126(1713):34–35. [PubMed] [Google Scholar]
- Henne MB, Bundorf MK. Insurance mandates and trends in infertility treatments. Fertility and Sterility. 2008;89(1):66–73. doi: 10.1016/j.fertnstert.2007.01.167. [DOI] [PubMed] [Google Scholar]
- Hershberger PE, Pierce PF. Conceptualizing couples' decision making in PGD: Emerging cognitive, emotional, and moral dimensions. Patient Education and Counseling. 2010;81(1):53–62. doi: 10.1016/j.pec.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberger PE, Kavanaugh K, Hamilton R, Klock SC, Merry L, Olshansky E, Pierce PF. Development of an informational web site for recruiting research participants: Process, implementation, and evaluation. Computing, Informatics, Nursing. 2011a;29(10):544–551. doi: 10.1097/NCN.0b013e318224b52f. quiz 52–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberger PE, Schoenfeld C, Tur-Kaspa I. Unraveling preimplantation genetic diagnosis for high-risk couples: implications for nurses at the front line of care. Nursing for Women’s Health. 2011b;15(1):36–45. doi: 10.1111/j.1751-486X.2011.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberger PE, Gallo AM, Kavanaugh K, Olshansky E, Schwartz A, Tur-Kaspa I. The decision-making process of genetically at-risk couples considering preimplantation genetic giagnosis: Initial findings from a grounded theory study. Social Science & Medicine. 2012a;74(10):1536–1543. doi: 10.1016/j.socscimed.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberger PE, Kavanaugh K. E-mail interviewing methods: procedures, equivalency, and appropriateness in two qualitative studies. Presented at the meeting of the Midwest Nursing Research Society (MNRS) 2012 Annual Research Conference; Dearborn, MI. 2012b. [Google Scholar]
- Hines KA, McCarthy Veach P, LeRoy BS. Genetic Counselors’ Perceived Responsibilities Regarding Reproductive Issues for Patients at Risk for Huntington Disease. Journal of Genetic Counseling. 2010;19(2):131–147. doi: 10.1007/s10897-009-9265-5. [DOI] [PubMed] [Google Scholar]
- Jae GA, Lewkowitz AK, Yang JC, Shen L, Rahman A, Del Toro G. Barriers to conceiving sibling donors for sickle cell disease: Perspectives from patients and parents. Ethnicity & Health. 2011;16(4–5):431–445. doi: 10.1080/13557858.2011.558619. [DOI] [PubMed] [Google Scholar]
- Jain T, Harlow BL, Hornstein MD. Insurance coverage and outcomes of in vitro fertilization. New England Journal of Medicine. 2002;347(9):661–666. doi: 10.1056/NEJMsa013491. [DOI] [PubMed] [Google Scholar]
- Jain T, Hornstein MD. Disparities in access to infertility services in a state with mandated insurance coverage. Fertility and Sterility. 2005;84(1):221–223. doi: 10.1016/j.fertnstert.2005.01.118. [DOI] [PubMed] [Google Scholar]
- Karatas JC, Barlow-Stewart K, Meiser B, McMahon C, Strong KA, Hill W, et al. Psychological adjustment, knowledge and unmet information needs in women undergoing PGD. Human Reproduction. 2010a;25(6):1481–1489. doi: 10.1093/humrep/deq086. [DOI] [PubMed] [Google Scholar]
- Karatas JC, Barlow-Stewart K, Strong KA, Meiser B, McMahon C, Roberts C. Women's experience of pre-implantation genetic diagnosis: a qualitative study. Prenatal Diagnosis. 2010b;30(8):771–777. doi: 10.1002/pd.2542. [DOI] [PubMed] [Google Scholar]
- Karatas JC, Strong KA, Barlow-Stewart K, McMahon C, Meiser B, Roberts C. Psychological impact of preimplantation genetic diagnosis: a review of the literature. Reproductive Biomedicine Online. 2010c;20:83–91. doi: 10.1016/j.rbmo.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Kvale S, Brinkmann S. InterViews: Learning the craft of qualitative research interviewing. 2nd ed. Thousand Oaks, CA: SAGE Publications; 2009. [Google Scholar]
- Martin JR, Bromer JG, Sakkas D, Patrizio P. Insurance coverage and in vitro fertilization outcomes: A U.S. perspective. Fertility and Sterility. 2011;95(3):964–969. doi: 10.1016/j.fertnstert.2010.06.030. [DOI] [PubMed] [Google Scholar]
- McCoyd JLM, Kerson TS. Conducting intensive interviews using e-mail: A serendipitous comparative opportunity. Qualitative Social Work. 2006;5(3):389–406. [Google Scholar]
- McKusick-Nathans Institute of Genetic Medicine, John Hopkins University. Online Mendelian Inheritance in Man, OMIM®. Baltimore, MD: 2012. Retrieved from http://omim.org/statistics/geneMap. [Google Scholar]
- Meho LI. E-mail interviewing in Qualitative Research: A Methodological Discussion. Journal of the American Society for Information Science and Technology. 2006;57(10):1284–1295. [Google Scholar]
- National Center for Biotechnology Information. GeneTests: Growth of laboratory directory. 2011 Retrieved from http://www.ncbi.nlm.nih.gov/projects/GeneTests/static/whatsnew/labdirgrowth.shtml.
- Novick G. Is there a bias against telephone interviews in qualitative research? Research in Nursing & Health. 2008;31(4):391–398. doi: 10.1002/nur.20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of the Legislative Counsel, U.S. House of Representatives. Compilation of Patient Protection and Affordable Care Act. 2010 Retrieved from http://housedocs.house.gov/energycommerce/ppacacon.pdf.
- Omurtag KR, Styer AK, Session D, Toth TL. Economic implications of insurance coverage for in vitro fertilization in the United States. A review. Journal of Reproductive Medicine. 2009;54(11–12):661–668. [PubMed] [Google Scholar]
- Patton MQ. In: Enhancing the quality and credibility of qualitative analysis. 3rd ed. Patton MQ, editor. Thousand Oaks, CA: SAGE Publications; 2002. [PMC free article] [PubMed] [Google Scholar]
- Pivetti M, Melotti G. Prenatal genetic testing: An investigation of determining factors affecting the decision-making process. Journal of Genetic Counseling. 2013;22(1):76–89. doi: 10.1007/s10897-012-9498-6. [DOI] [PubMed] [Google Scholar]
- Powell KP, Hasegawa L, McWalter K. Expanding Roles: A Survey of Public Health Genetic Counselors. Journal of Genetic Counseling. 2010;19(6):593–605. doi: 10.1007/s10897-010-9313-1. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the Society for Assisted Reproductive Technology & Practice Committee of the American Society for Reproductive Medicine. Preimplantation genetic testing: A Practice Committee opinion. Fertility and Sterility. 2008;90(3 Suppl):S136–S143. doi: 10.1016/j.fertnstert.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Quinn GP, Vadaparampil ST, Lowrey KM, Eidson S, Knapp C, Bukulmez O. State laws and regulations addressing third-party reimbursement for infertility treatment: Implications for cancer survivors. Fertility and Sterility. 2011;95(1):72–78. doi: 10.1016/j.fertnstert.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Rubin HJ, Rubin IS. Qualitative interviewing: The art of hearing data. 3rd ed. Thousand Oaks, CA: SAGE Publications; 2012. [Google Scholar]
- Secretary's Advisory Committee on Genetics, Health, and Society. Coverage and reimbursement of genetic tests and services. 2006 Retrieved from http://oba.od.nih.gov/oba/sacghs/reports/CR_report.pdf.
- Simpson JL. Preimplantation genetic diagnosis at 20 years. Prenatal Diagnosis. 2010;30(7):682–695. doi: 10.1002/pd.2552. [DOI] [PubMed] [Google Scholar]
- Snowdon C, Green JM. Preimplantation diagnosis and other reproductive options: attitudes of male and female carriers of recessive disorders. Human Reproduction. 1997;12(2):341–350. doi: 10.1093/humrep/12.2.341. [DOI] [PubMed] [Google Scholar]
- Soini S, Ibarreta D, Anastasiadou V, Aymé S, Braga S, Cornel M, et al. The interface between assisted reproductive technologies and genetics: Technical, social, ethical and legal issues. European Journal of Human Genetics. 2006;14(5):588–645. doi: 10.1038/sj.ejhg.5201598. [DOI] [PubMed] [Google Scholar]
- Spar D. Reproductive tourism and the regulatory map. New England Journal of Medicine. 2005;352(6):531–533. doi: 10.1056/NEJMp048295. [DOI] [PubMed] [Google Scholar]
- Tur-Kaspa I, Aljadeff G, Rechitsky S, Grotjan HE, Verlinsky Y. PGD for all cystic fibrosis carrier couples: Novel strategy for preventive medicine and cost analysis. Reproductive BioMedicine Online. 2010;21(2):186–195. doi: 10.1016/j.rbmo.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Zeiler K. Reproductive autonomous choice--a cherished illusion? Reproductive autonomy examined in the context of preimplantation genetic diagnosis. Medicine, Health Care and Philosophy. 2004;7(2):175–183. doi: 10.1023/b:mhep.0000034323.68025.d5. [DOI] [PubMed] [Google Scholar]