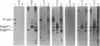
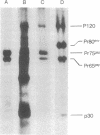
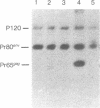
Abstract
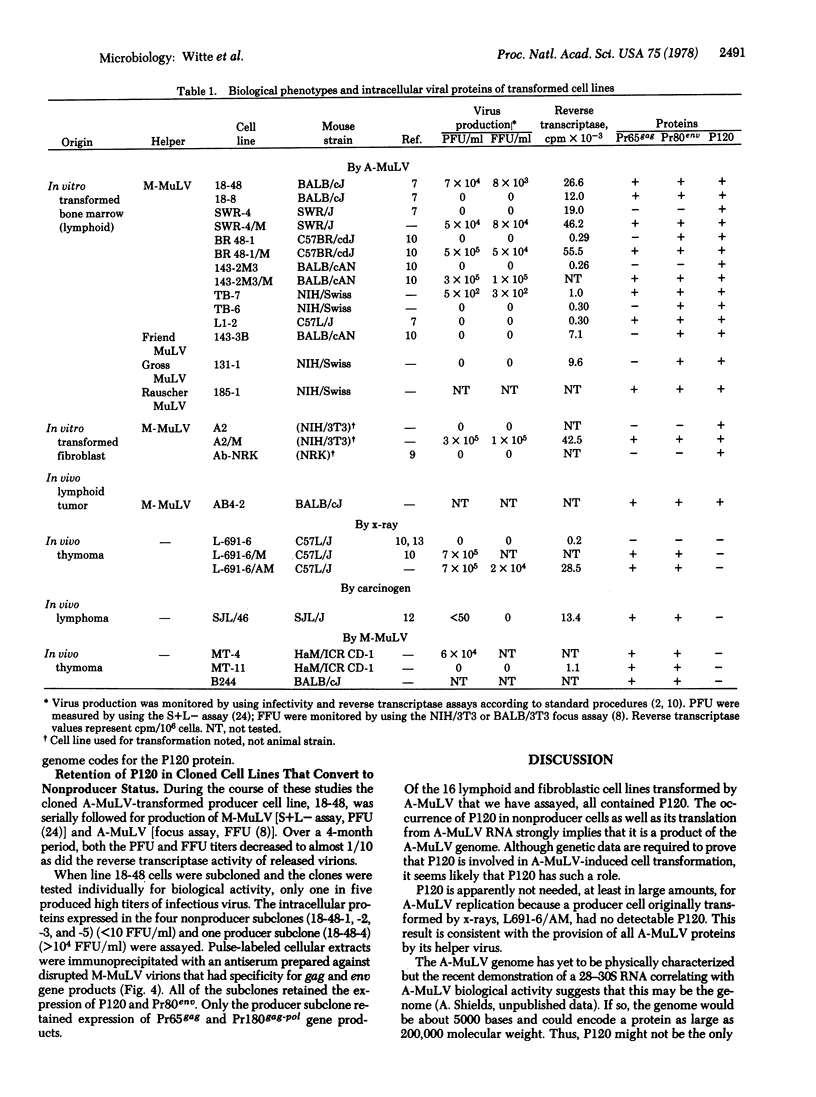
Extracts from lymphoid and fibroblast cell lines transformed by Abelson murine leukemia virus (A-MuLV) contain a protein of molecular weight 120,000 (P120). Immunoprecipitation with specific sera shows that P120 contains regions homologous to the 5'-terminal segment of the MULV gag gene complex--p15, p12, and at least part of p30--but lacks detectable determinants of p10, reverse transcriptase, and the envelope glycoprotein. P120 is phosphorylated and has an intracellular half-life of 3--6 hr. In vitro translation of virion RNA from A-MuLV, with Moloney MuLV as helper, yields a product of molecular weight 120,000 with serological reactivity similar to that of the cellular P120. Translation of the RNA from the helper gave no P120. P120 is expressed in all lymphoid and fibroblastic cell lines we have tested that were transformed by A-MuLV but is not detectable in a lymphoid line in which the A-MuLV genome was established by infection but was not responsible for the transformation. Expression of P120 is selectively retained in clones of A-MuLV-transformed lymphocytes that convert to a nonproducer state after loss of expression of helper MuLV intracellular precursors. These results suggest that the P120 product of the A-MuLV genome may be responsible for maintenance of the transformed phenotype of lymphoid and fibroblast cells transformed by the virus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Rabstein L. S. Lymphosarcoma: virus-induced thymic-independent disease in mice. Cancer Res. 1970 Aug;30(8):2213–2222. [PubMed] [Google Scholar]
- Arnstein P., Riggs J. L., Oshiro L. S., Huebner R. J., Lennette E. H. Induction of lymphoma and associated xenotropic type C virus in C57L mice by whole-body irradiation. J Natl Cancer Inst. 1976 Nov;57(5):1085–1090. doi: 10.1093/jnci/57.5.1085. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Stephenson J. R., Aaronson S. A. gag Gene of mammalian type-C RNA tumour viruses. Nature. 1976 Aug 12;262(5569):554–559. doi: 10.1038/262554a0. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Haimovich J., Bergman Y., Linker-Israeli M., Haran-Ghera N. Cell surface components of carcinogen-induced lymphoid tumors in SJL/J mice. Eur J Immunol. 1977 Apr;7(4):226–230. doi: 10.1002/eji.1830070408. [DOI] [PubMed] [Google Scholar]
- Jamjoom G. A., Naso R. B., Arlinghaus R. B. Further characterization of intracellular precursor polyproteins of Rauscher leukemia virus. Virology. 1977 May 1;78(1):11–34. doi: 10.1016/0042-6822(77)90075-7. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Howk R. S., Anisowicz A., Scolnick E. M. Deletion mapping of moloney type C virus: polypeptide and nucleic acid expression in different transforming virus isolates. J Virol. 1976 May;18(2):491–503. doi: 10.1128/jvi.18.2.491-503.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Philipson L., Andersson P., Olshevsky U., Weinberg R., Baltimore D., Gesteland R. Translation of MuLV and MSV RNAs in nuclease-treated reticulocyte extracts: enhancement of the gag-pol polypeptide with yeast suppressor tRNA. Cell. 1978 Jan;13(1):189–199. doi: 10.1016/0092-8674(78)90149-6. [DOI] [PubMed] [Google Scholar]
- Potter M., Sklar M. D., Rowe W. P. Rapid viral induction of plasmacytomas in pristane-primed BALB-c mice. Science. 1973 Nov 9;182(4112):592–594. doi: 10.1126/science.182.4112.592. [DOI] [PubMed] [Google Scholar]
- Pratt D. M., Strominger J., Parkman R., Kaplan D., Schwaber J., Rosenberg N., Scher C. D. Abelson virus-transformed lymphocytes: null cells that modulate H-2. Cell. 1977 Nov;12(3):683–690. doi: 10.1016/0092-8674(77)90268-9. [DOI] [PubMed] [Google Scholar]
- Premkumar E., Potter M., Singer P. A., Sklar M. D. Synthesis, surface deposition, and secretion of immunoglobulins by Abelson virus-transformed lymphosarcoma cell lines. Cell. 1975 Oct;6(2):149–159. doi: 10.1016/0092-8674(75)90005-7. [DOI] [PubMed] [Google Scholar]
- Robey W. G., Oskarsson M. K., Vande Woude G. F., Naso R. B., Arlinghaus R. B., Haapala D. K., Fischinger P. J. Cells transformed by certain strains of Moloney sarcoma virus contain murine p60. Cell. 1977 Jan;10(1):79–89. doi: 10.1016/0092-8674(77)90142-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976 Jun 1;143(6):1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. The effect of helper virus on Abelson virus-induced transformation of lymphoid cells. J Exp Med. 1978 Apr 1;147(4):1126–1141. doi: 10.1084/jem.147.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Donoghue D. J., Baltimore D. Analysis of a 5' leader sequence on murine leukemia virus 21S RNA: heteroduplex mapping with long reverse transcriptase products. Cell. 1978 Mar;13(3):435–451. doi: 10.1016/0092-8674(78)90318-5. [DOI] [PubMed] [Google Scholar]
- Scher C. D. Effect of pseudotype on Abelson virus and Kirsten sarcoma virus-induced leukemia. J Exp Med. 1978 Apr 1;147(4):1044–1053. doi: 10.1084/jem.147.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Sklar M. D., Shevach E. M., Green I., Potter M. Transplantation and preliminary characterisation of lymphocyte surface markers of Abelson virus-induced lymphomas. Nature. 1975 Feb 13;253(5492):550–552. doi: 10.1038/253550a0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Khan A. S., Sliski A. H., Essex M. Feline oncornavirus-associated cell membrane antigen: evidence for an immunologically crossreactive feline sarcoma virus-coded protein. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5608–5612. doi: 10.1073/pnas.74.12.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Tsukamoto-Adey A., Weissman I. L. Cellular maturation of oncornavirus glycoproteins: topological arrangement of precursor and product forms in cellular membranes. Virology. 1977 Feb;76(2):539–553. doi: 10.1016/0042-6822(77)90236-7. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Weissman I. L. Polypeptides of Moloney sarcoma-leukemia virions: their resolution and incorporation into extracellular virions. Virology. 1974 Oct;61(2):575–587. doi: 10.1016/0042-6822(74)90291-8. [DOI] [PubMed] [Google Scholar]