Abstract
Background
Sympathetic activation has been implicated in the development of left ventricular hypertrophy (LVH). However, the relationship between sympathetic activation and left ventricular mass (LVM) has not been clearly defined across a range of arterial pressure measurements. This study was planned to determine that relationship, using cardiac MRI to accurately quantify LVM, in hypertensive patients with and without LVH and normal subjects.
Methods and Results
Twenty four patients with uncomplicated and untreated essential hypertension (LVH[−]) were compared to 25 patients with essential hypertension and left ventricular hypertrophy (LVH[+]) and 24 normal control subjects (NC). Resting muscle sympathetic nerve activity was quantified from multiunit bursts (MSNA) and from single units (s-MSNA). Cardiac MRI determined LVM was indexed to body surface area (LVMI), and in the LVH[−] group, this equated to 67 ± 2.1g/m2; a value between those of the LVH[+] (91 ± 3.4g/m2) and NC (57 ± 2.2g/m2) groups respectively. The sympathetic activity in the LVH[−] group (53 ± 1.3 bursts/100 cardiac beats and 63 ± 1.6 impulses/100 cardiac beats) was between (at least P<0.001) those of LVH[+] (66 ± 1.7 bursts/100 cardiac beats and 77 ± 2.2 impulses/100 cardiac beats) and NC groups (39 ± 3.0 bursts/100 cardiac beats and 45 ± 3.4 impulses/100 cardiac beats). Importantly, there was a significant positive correlation between sympathetic activity and LVMI in the LVH[−] group and the LVH[+] group (at least r=0.76, P<0.0001), but not in the NC group. However, there was no consistent relationship between arterial blood pressure and sympathetic activity or LVMI.
Conclusions
These findings further support the hypothesis that central sympathetic activation is associated with the development of LVH in human hypertension.
Keywords: Sympathetic nervous system, hypertension, hypertrophy, action potentials
Introduction
Although the development of left ventricular hypertrophy (LVH) in essential hypertension (EHT) has been attributed to many factors including hemodynamic and humoral effects,1-5 sympathetic activation has also been implicated in the occurrence of LVH.6,7 Consistent with this implication has been the finding of augmented sympathetic drive in EHT patients with LVH as categorized by echocardiography, relative to those without LVH.8,9 However, these findings alone cannot sufficiently support the hypothesis that sympathetic activation increases left ventricular mass in human hypertension, not least because a correlation between left ventricular mass and either peripheral or cardiac sympathetic drive, has so far only been found in EHT groups having categorical LVH and not in those without LVH.8,9 Furthermore, to confound the issue, human studies have indicated that the occurrence of clinically discernible LVH can blunt sympatho-inhibitory reflexes in its own right, leading to increased sympathetic drive.10,11
The present investigation was designed therefore to determine whether or not the magnitude of sympathetic nerve hyperactivity was related to left ventricular mass (LVM) in subjects that included a broad range of both arterial blood pressures and LVM. For this purpose, we quantified central sympathetic nerve activity by microneurography and LVM by cardiac Magnetic Resonance Imaging (MRI), in essential hypertensive patients with and without LVH compared to age, body weight and body surface area matched normal control subjects (NC).
Methods
Subjects
A total of 73 Caucasian subjects were prospectively examined (Table 1). They included 49 patients with untreated essential hypertension (EHT), comprising of a group of 24 patients without left ventricular hypertrophy (LVH[−]) and a group of 25 patients with left ventricular hypertrophy (LVH[+]). In addition, a control group of 24 normal subjects (NC) were examined who were recruited from hospital staff, volunteer subjects and relatives, based upon comparable age and body weight to the hypertensive patients. All individuals had similar occupational status and dietary habits (including a sodium intake of ≈400mmol/d), and none were actively engaged in exercise training. Patients were screened by history, physical and laboratory examination. Patients were excluded if they had evidence of secondary hypertension, peripheral vascular disease, renal insufficiency, diabetes, cardiac arrhythmias or other chronic disease that may influence the autonomic nervous system or indeed any contraindication to MRI scanning such as a permanent pacemaker in situ or intra-cranial aneurysm clips.
TABLE 1. Demographic details of the 3 subject groups.
| Subjects | LVH[−] | LVH[+] | Normal controls |
|---|---|---|---|
| Number (males) | 24 (14) | 25 (17) | 24 (11) |
| Age (years) | 52 ± 1.5 | 52 ± 2.2 | 49 ± 2.5 |
| Body weight (kg) | 81 ± 2.8 | 85 ± 3.0 | 77 ± 2.4 |
| Body mass index (kg/m2) | 29 ± 0.9 | 28 ± 0.8 | 27 ± 0.7 |
| Body surface area (m2) | 1.92 ± 0.04 | 1.99 ± 0.04 | 1.90 ± 0.05 |
| Heart rate (beats/minute) | 72 ± 1.9 | 68 ± 1.8 | 64 ± 1.8 |
Data presented as mean ± SEM. Analyses were performed using ANOVA post hoc tests, except for gender difference in which Fisher’s Exact Test was used. All group comparisons were not significantly different (P>0.05).
Arterial blood pressure was defined on the basis of the average of at least 3 readings taken on separate occasions, and the occurrence of hypertension was accepted if the systolic or diastolic arterial pressures were ≥140 mmHg or ≥90 mmHg respectively.12 Both patient groups had diagnosed hypertension for no longer than 12 months and none received anti-hypertensive therapy during the study. Drug therapy was temporarily stopped for at least 4 weeks before the investigation in 5 and 6 patients respectively of the LVH[+] and LVH[−] groups. The investigation was carried out with the approval of St. James’s University Hospital Ethics Committee, and all subjects provided informed written consent.
General Protocol
Each patient underwent two independent investigative sessions within one week of each other. They comprised microneurographic and MRI assessments. The microneurographic and hemodynamic measurements were obtained in an identical manner during each session, the details of which have been published previously.8,13,14 In brief, microneurography was performed between the hours of 9AM and midday. Patients were asked to have a light breakfast and to empty their bladder before commencing the study. The patients were instructed to maintain a normal dietary intake of sodium, and to avoid nicotine, caffeine and alcohol for 12 hours prior to investigation, as well as strenuous exercise for the preceding 24 hours. During each session, subjects were studied in the semi-supine position and when the data had attained steady state for at least 30 minutes. Measurements were made in a darkened laboratory in which the temperature was constant at 22 to 24°C. Resting arterial pressure was measured from the arm using a mercury sphygmomanometer. Changes in heart rate and arterial pressure were monitored and recorded using a standard electrocardiogram and a Finometer device (Finometer Medical Systems B.V., Arnhem, Netherlands).
Microneurography
Post-ganglionic muscle sympathetic nerve activity (MSNA) was recorded from the right peroneal nerve.8,13,14 The neural signal was amplified (x50,000), and for the purpose of generating bursts representing multiunit discharge, the signal was filtered (bandwidth of 700-2000 Hz) and integrated (time constant 0.1 sec). The output of action potentials and bursts from this assembly were passed to a PC-based data-acquisition system (LabView, National Instruments Corp., Austin, TX, United States), which digitized the acquired data at 12,000 samples/second (16 bits).
Single units (s-MSNA) in the raw action potential neurogram were obtained by adjusting the electrode position whilst using fast monitor sweep and an on-line storage oscilloscope to confirm the presence of a consistent action potential morphology, as previously described.8,13-15 Only vasoconstrictor units were accepted and examined, the criteria of acceptance being appropriate responses to spontaneous changes in arterial pressure, to the Valsalva maneuver and isometric hand-grip exercise. In addition, simultaneous measurement of calf vascular resistance confirmed the vasoconstrictor function of the observed neural activity. During the Valsalva maneuver, sympathetic activity increased during the latter part of phase-II and/or phase-III and decreased during phase-IV (increase and overshoot of blood pressure). Isometric hand-grip exercise, performed using a dynamometer, produced a late increase in arterial blood pressure and sympathetic neural activity.
An electronic discriminator window was used objectively to count the spikes of s-MSNA, and these were quantified in terms of mean frequency of impulses/minute and also as impulses/100 cardiac beats to avoid any possible interference due to the length of the cardiac cycle.16 The bursts of MSNA were identified by inspection when the signal to noise ratio was greater than 3, and were quantified in a similar manner. Data were acquired over 5 minutes when the measured variables had attained steady state for at least 30 minutes. The variability of repeated measurements of s-MSNA units and MSNA bursts over a period of 30 minutes or those of two impalements performed within 60 minutes did not exceed 10%, in terms of twice the 95% confidence intervals around individual differences relative to the mean of the repeated measurements.13
Magnetic Resonance Imaging (MRI)
Quantification of LVM by MRI occurred blinded and independent of the microneurography acquisition and analysis sessions, and was performed using an identical protocol in all subjects. MRI studies were performed on a 1.5 Tesla Philips Intera CV MRI system (Philips Medical Systems, Best, The Netherlands) equipped with Master gradients (maximum gradient amplitude, 30 mT/m, maximum slew rate, 150 mT/m/msec). Patients were scanned in the supine position using a five-element cardiac phased-array coil and vectorcardiographic method for ECG-gating as in routine clinical cardiac MRI.17,18 Total scan acquisition time was short, with all data sets being obtained within 30 minutes.
Localizing survey scans were followed by breath-hold (in expiration) cine acquisitions in the ventricular long axis and horizontal long axis planes to ensure accurate planning of the true short-axis. LV volume data sets comprising multiple slices were then taken parallel to the mitral valve, covering the heart from the apex to the base in 10-14 short-axis slices. A standard steady-state free precession (SSFP) pulse sequence was used, and one for which a normal range has been established by workers in our department.18 Moreover, this sequence has been validated in animal studies.19 The parameters of this SSFP pulse sequence were as follows: TR = 3.34 msec, TE = 1.67 msec, flip angle = 55°, bandwidth = 1042 Hz/pixel, acquisition matrix =192 × 163, FOV = 360 × 288 mm, half Fourier acquisition, 6mm slice thickness, 4mm inter-slice gap, 18 phases/cardiac cycle, with two slices acquired per 10-12 second breath hold.
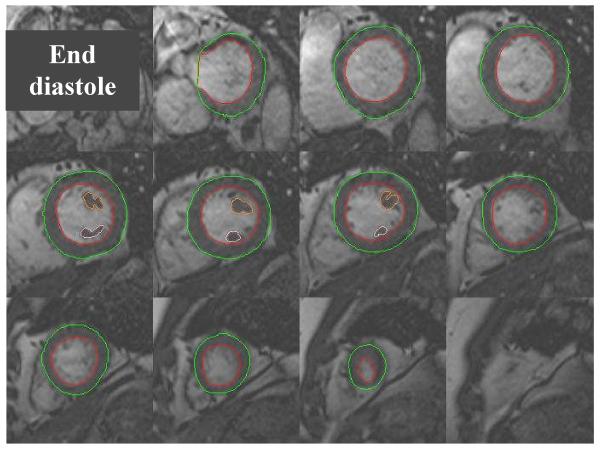
Image analysis was performed off-line using commercially available analysis software (MASS version 5.0, Medis, Leiden, The Netherlands). Standard criteria which have been previously described were used to delineate the cardiac borders (Figure 1).20 End diastolic and end systolic contours were drawn for each slice from the apex to the base of the heart. Two papillary muscles were outlined separately and included in the myocardial mass analysis. Importantly, at the base of the heart, slices were included for quantification if the blood pool was surrounded by 50% or more of ventricular myocardium as previously described.20 LVM was calculated by modified Simpson’s rule such that LVM = 1.05 × (epicardial volume – endocardial volume). Left ventricular mass index (LVMI) was subsequently calculated by dividing LVM by the body surface area (BSA), using the Mosteller equation.
Figure 1.
LV short axis images taken from the base of the heart to the apex, using a standard SSFP multi-phase, multi-slice MRI sequence. Endocardial and epicardial contours are shown outlined in end diastole enabling accurate calculation of LV mass.
Normal LVM and LVMI ranges already established by other workers in the department (using larger groups of a similar age to those used in this study) were used to categorize patients.18 These normal ranges when expressed over a range of 2 standard deviations from the mean equated to 85-181g or 46-83g/m2 for men and 66-114g or 37-67g/m2 for women respectively.18 LVH was defined as greater than two standard deviations above the mean normal range and thus these ranges were used to categorize the hypertensive patients into two groups, those either having LVH (LVH[+]) or not (LVH[−]). LVM measurements made using cardiac MRI have been shown to be more accurate and reproducible than M-mode and 2-D echocardiography measurements,21-23 and the technique is highly reproducible with extremely favorable intra- and inter-study variability of less than 3%.24
Statistics
One-way analysis of variance (ANOVA) with Newman-Keuls post hoc tests were used to compare data between the 3 groups of subjects. The least square technique was used for assessing the linear relationship between variables. Multiple regression analysis was used to examine the relationship of measured variables to left ventricular mass index. Values of P<0.05 were considered statistically significant, and data are presented as mean ± S.E.M. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
The three groups of subjects were not significantly different in respect of age, body weight, body mass index, heart rate and body surface area (Table 1). Also, there were no significant differences in the gender ratio between the three groups (χ2= 2.47; P>0.20). In the two hypertensive groups (LVH[−] and LVH[+]), the indices of arterial blood pressure (systolic, diastolic and mean) were similar (161 ± 2.4mmHg, 97 ± 4.0mmHg, 118 ± 1.7mmHg vs. 170 ± 4.7mmHg, 98 ± 2.5mmHg, 121 ± 3.0mmHg respectively), and as expected they were greater than those of the normal control group (127 ± 2.5mmHg, 79 ± 1.4mmHg, 95 ± 1.7mmHg).
Due to the nature of the study design, the measures of LVM and LVMI were greater in the LVH[+] (182 ± 8.6g; 91 ± 3.4g/m2) than in the LVH[−] group (131 ± 6.1g; 67 ± 2.1g/m2). However, the LVH[−] group also had significantly greater LVM (P<0.05) and LVMI (P<0.05) compared to the normal control group (107 ± 5.8g; 57 ± 2.2g/m2). Group analysis of the microneurographic data revealed that all indices of sympathetic nerve activity were significantly greater in the hypertensive groups relative to the control group, and were also greater in LVH[+] compared to the LVH[−] group (Table 2).
TABLE 2. LVM, blood pressure and microneurographic data from the 3 subject groups.
| Subjects | LVH[−] (A) |
LVH[+] (B) |
Normal controls (C) |
P value A vs. B |
P value A vs. C |
P value B vs. C |
|---|---|---|---|---|---|---|
| MSNA (bursts/minute) | 38 ± 1.3 | 45 ± 1.6 | 25 ± 2.1 | <0.01 | <0.001 | <0.001 |
| MSNA (bursts/100 beats) | 53 ± 1.3 | 66 ± 1.7 | 39 ± 3.0 | <0.001 | <0.001 | <0.001 |
| s-MSNA (impulses/minute) | 45 ± 1.6 | 52 ± 1.9 | 29 ± 2.2 | <0.05 | <0.001 | <0.001 |
| s-MSNA (impulses/100 beats) | 63 ± 1.6 | 77 ± 2.2 | 45 ± 3.4 | <0.001 | <0.001 | <0.001 |
| LV mass (g) | 131 ± 6.1 | 182 ± 8.6 | 107 ± 5.8 | <0.001 | <0.05 | <0.001 |
| LV mass index (g/m2) | 67 ± 2.1 | 91 ± 3.4 | 57 ± 2.2 | <0.001 | <0.05 | <0.001 |
| Systolic BP (mmHg) | 161 ± 2.4 | 170 ± 4.7 | 127 ± 2.5 | ns | <0.001 | <0.001 |
| Diastolic BP (mmHg) | 97 ± 4.0 | 98 ± 2.5 | 79 ± 1.4 | ns | <0.001 | <0.001 |
Data presented as mean ± SEM. Analyses were performed using ANOVA post hoc tests, ns=non significant.
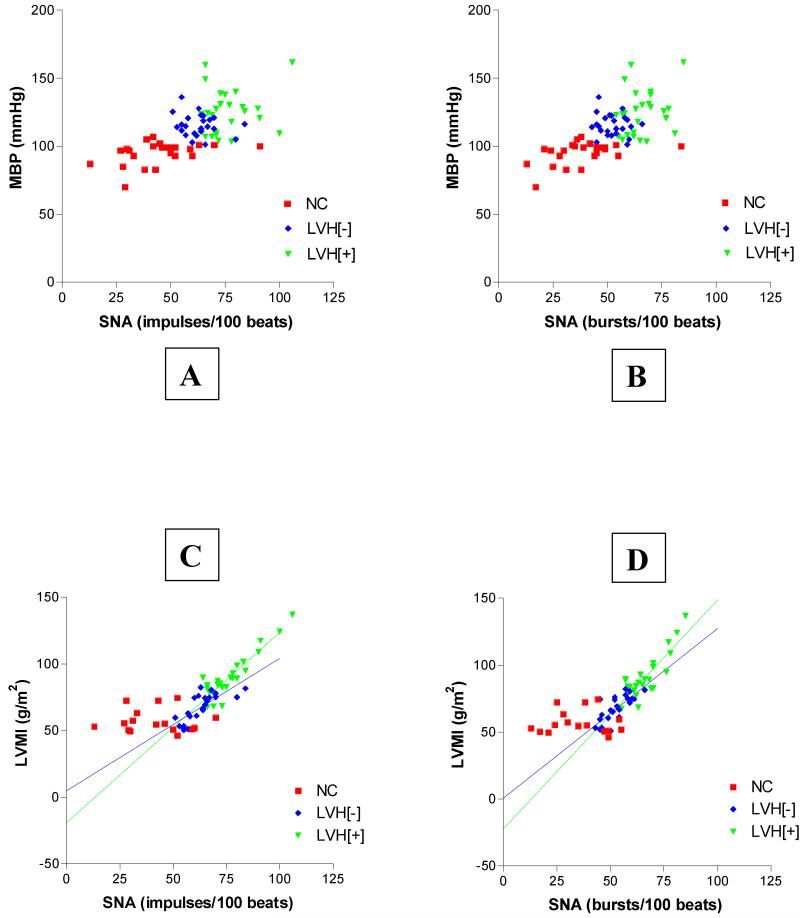
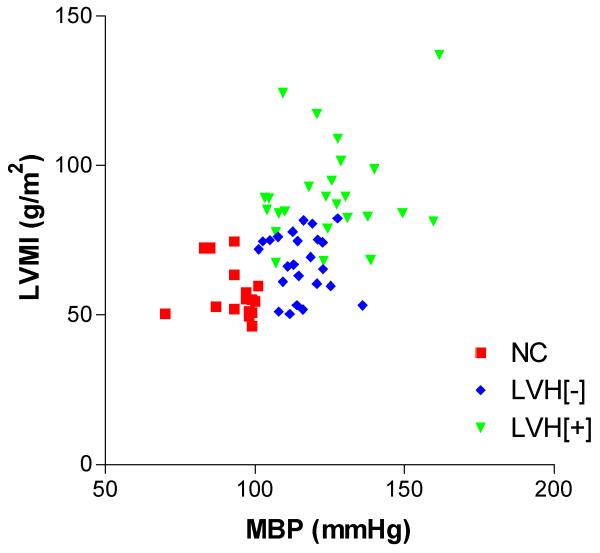
Considering the three study groups separately (NC, LVH[−], and LVH[+]) there were no significant correlations between sympathetic activity and arterial blood pressure (always P>0.05) (Figure 2, A & B). However, individual group analysis revealed that the measures of LVMI in the two hypertensive groups were significantly and positively related to measures of sympathetic nerve activity (Figure 2, C & D). Specifically, in LVH[+], correlation coefficient and linear regression analysis values were r=0.91, P<0.0001, and r2=0.83, P<0.0001 (for s-MSNA; Figure 2C) and r=0.86, P<0.0001, and r2=0.73, P<0.0001 (for MSNA; Figure 2D). In LVH[−], this significant positive relationship was also apparent, with correlation coefficient and linear regression analysis values of r=0.76, P<0.0001, and r2=0.58, P<0.0001 (for s-MSNA; Figure 2C) and r=0.76, P<0.0001, and r2=0.57, P<0.0001 (for MSNA; Figure 2D). On the contrary, in the NC group there was no relationship between LVMI and measures of sympathetic activity (s-MSNA or MSNA, r=−0.03, P>0.93 and r2=0.0009, P>0.94). Also, considering the three study groups separately (NC, LVH[−], and LVH[+]), there was no significant correlation between any of the indices of arterial blood pressure and the LVMI (always r<0.2, P>0.32) (Figure 3).
Figure 2.
Within subject groups there is no direct relationship between sympathetic nerve activity (SNA) and mean arterial pressure (MBP) (A & B). Also in normal control subjects there is no relationship between SNA and left ventricular mass index (LVMI) (C & D). However in LVH[−] and LVH[+] there is a direct linear relationship between SNA and LVMI (C & D).
Figure 3.
For any of the individual subject groups (NC, LVH[−], and LVH[+]) there are no direct relationships between mean arterial pressure (MBP) and left ventricular mass index (LVMI).
Finally, the measures of LVMI in both hypertensive groups were positively related to BSA (at least r=0.37, P<0.04), although they were not correlated to age or heart rate. In the NC group, the measures of LVMI were not related to BSA, age or heart rate. After including age, BMI, BSA, arterial pressure and sympathetic activity data into multiple regression analyses, the measures of LVMI always remained significantly related to those of sympathetic nerve activity (always P<0.005 in LVH[−] and always P<0.0001 in LVH[+]).
Discussion
The present investigation has shown for the first time in patients with essential hypertension (EHT) that left ventricular mass is significantly related to central sympathetic nerve hyperactivity regardless of whether or not there is associated clinical LVH. These findings indicate that sympathetic activation found in EHT may be an important determinant of the growth of human left ventricular myocardium, as the two variables are related to each other even in the absence of categorical LVH.
Importantly, as in any investigation of the autonomic nervous system, all subjects in this study were examined under identical laboratory conditions to avoid confounding influences related to circadian variation,25 alcohol intake,26 and visceral distension.27,28 In addition, all groups were closely matched to avoid confounding factors such as age and gender,29 race,30 body weight,31 and heart rate.16 Unavoidably, the levels of arterial pressure in the two hypertensive groups were greater than in the normal control group. However, we found no correlation between sympathetic nerve activity and indices of arterial pressure as we have reported previously in hypertensive and normotensive subjects,13 supporting the proposal that chronic sympathetic excitation was independent of baroreceptor reflex function.32 Furthermore, the two hypertensive groups were closely matched in respect of all other measured variables except for the quantified left ventricular mass and sympathetic activity. These considerations make it likely that the observed similarity in progression of LVMI and sympathetic activity from NC to LVH[−] and then to LVH[+] was not significantly affected by known confounding factors.
Whilst there are regional differences in sympathetic output,33 reported evidence indicates that resting sympathetic drive to the periphery correlates well with that to the heart in normal subjects,34 and both are increased in hypertension.35,36 Both peripheral and cardiac sympathetic drive has been shown to be abnormally increased in EHT patients with categorical LVH,8,9 and this has been confirmed by our present findings. However, unlike previous studies using echocardiography, the use of cardiac MRI, a more accurate and reproducible tool for the quantification of LVM, 21-23,37 has allowed us to show an increase in sympathetic drive in hypertensive patients without categorical LVH (LVH[−]). The previously reported absence of correlation between sympathetic drive and LVMI in LVH[−] patients, whilst not proven, could be related to limitations in accuracy of the chosen measurement technique (echocardiography).9 It is well accepted that echocardiographic estimation of LVM, calculated from equations involving LV wall thickness and cavity dimensions, can be inaccurate due to mathematical assumptions that cannot allow adequately for the complex LV geometry. There are also limitations to the present study. Our design used office arterial pressure, measured as average of at least 3 readings at rest,12 and this could be argued not to reflect the 24 hour arterial pressure profile. However, this design made it possible to match the two hypertensive groups in the resting state regarding arterial pressure values and heart rate. Although the group mean systolic arterial pressure in LVH[+] was slightly greater than that of LVH[−], this was not likely to be a significant confounding factor because firstly there were no significant correlation between arterial pressure indices and LVM. At the same time sympathetic nerve activity was significantly correlated to LVM in the two hypertensive groups. Secondly, in our group comparisons there were no significant differences in systolic arterial pressure levels between the two hypertensive groups, whilst at the same time the group values of sympathetic nerve activity and LVM were significantly different.
From the group analyses, the present investigation has shown for the first time that central sympathetic drive is closely linked to LVMI when quantified by MRI in patients without LVH. Previously in human hypertension, a link between LVMI and sympathetic drive has only been reported in patients with LVH,8,9 and this link has been re-confirmed in this study. Previous findings were therefore not able to support the hypothesis that sympathetic activation might be a significant mechanism in its own right in increasing LVM. This was even more so because LVH itself has been found to increase sympathetic drive, possibly through blunting sympatho-inhibitory reflexes from the heart.10,11 Our findings in LVH[−] patients however, are entirely consistent with the theory that central sympathetic activation in EHT is a significant mechanism leading to increased LVM in humans. The present findings then support the growing body of evidence that sympathetic activation in EHT is one mechanism instrumental to the development of target organ damage including LVH. 6,7,35,38 Indeed, the findings from this study in human hypertension are supported by the animal data, in that catecholamines have been shown experimentally to have trophic properties on both cardiac myocytes,39 and in the intact animal heart.40
That central sympathetic activation is linked to increased LVM in human hypertension, regardless of whether or not this increase is clinically categorized as LVH, could have therapeutic and prognostic implications. Hypertension, sympathetic activation and clinical LVH are recognized as independent cardiovascular risk factors,6,7,41,42 and regression of LVM in hypertension has been associated with reduction in this risk.43-45 It is reasonable therefore to expect that sympatholytic anti-hypertensive agents could help prevent the development of clinically recognized LVH. Although sympathetic activation, hypertension and LVH have complex interacting mechanisms, the data from this study show a clear relationship between sympathetic activity and LVM across a range of arterial blood pressure levels. Interestingly, there was no relationship between sympathetic activity and blood pressure, or blood pressure and LVMI, suggesting that sympathetic hyperactivity may have an independent effect on LVM in addition to that associated with raised blood pressure (after-load). Although in a human biological system it is not possible to study these interacting mechanisms in isolation during the development of LVH, it may be possible in future studies of hypertensive LVH regression to determine whether mechanisms in addition to blood pressure (after-load) lowering are important in LVM reduction.
In conclusion, the present study has shown that central sympathetic activation is associated with an increased LVM in human hypertension, regardless of whether or not this increase is categorized as definitive LVH. The findings are consistent with the theory that sympathetic activation in hypertension is a significant mechanism leading to increased left ventricular mass.
Acknowledgements
The authors wish to thank Mr. J. Bannister and Mrs. J. Corrigan for technical assistance.
Source of funding
This work was sponsored by the British Heart Foundation (Grant No. PG/03/001)
Footnotes
Disclosures
None.
References
- 1.Lauer MS, Anderson KM, Levy D. Influence of contemporary versus 30-year blood pressure levels on left ventricular mass and geometry: the Framingham Heart Study. J Am Coll Cardiol. 1991;18:1287–1294. doi: 10.1016/0735-1097(91)90549-o. [DOI] [PubMed] [Google Scholar]
- 2.Bauwens FR, Duprez DA, De Buyzere ML, De Backer TL, Kaufman JM, Van Hoecke J, Vermeulen A, Clement DL. Influence of the arterial blood pressure and nonhemodynamic factors on left ventricular hypertrophy in moderate essential hypertension. Am J Cardiol. 1991;68:925–929. doi: 10.1016/0002-9149(91)90410-m. [DOI] [PubMed] [Google Scholar]
- 3.Duprez DA, Bauwens FR, De Buyzere ML, De Backer TL, Kaufman JM, Van Hoecke J, Vermeulen A, Clement DL. Influence of arterial blood pressure and aldosterone on left ventricular hypertrophy in moderate essential hypertension. Am J Cardiol. 1993;71:17A–20A. doi: 10.1016/0002-9149(93)90240-d. [DOI] [PubMed] [Google Scholar]
- 4.Harrap SB, Dominiczak AF, Fraser R, Lever AF, Morton JJ, Foy CJ, Watt GC. Plasma angiotensin II, predisposition to hypertension, and left ventricular size in healthy young adults. Circulation. 1996;93:1148–1154. doi: 10.1161/01.cir.93.6.1148. [DOI] [PubMed] [Google Scholar]
- 5.Verdecchia P, Reboldi G, Schillaci G, Borgioni C, Ciucci A, Telera MP, Santeusanio F, Porcellati C, Brunetti P. Circulating insulin and insulin growth factor-1 are independent determinants of left ventricular mass and geometry in essential hypertension. Circulation. 1999;100:1802–1807. doi: 10.1161/01.cir.100.17.1802. [DOI] [PubMed] [Google Scholar]
- 6.Julius S. Effect of sympathetic overactivity on cardiovascular prognosis in hypertension. Eur Heart J. 1998;19(Suppl F):F14–F18. [PubMed] [Google Scholar]
- 7.Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999;34:724–728. doi: 10.1161/01.hyp.34.4.724. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood JP, Scott EM, Stoker JB, Mary DASG. Hypertensive left ventricular hypertrophy: relationship to peripheral sympathetic drive. J Am Coll Cardiol. 2001;38:1711–1717. doi: 10.1016/s0735-1097(01)01600-x. [DOI] [PubMed] [Google Scholar]
- 9.Schlaich MP, Kaye DM, Lambert E, Sommerville M, Socratous F, Esler MD. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 2003;108:560–565. doi: 10.1161/01.CIR.0000081775.72651.B6. [DOI] [PubMed] [Google Scholar]
- 10.Zanchetti A, Mancia G. Cardiovascular reflexes and hypertension. Hypertension. 1991;18(5 Suppl):III13–21. doi: 10.1161/01.hyp.18.5_suppl.iii13. [DOI] [PubMed] [Google Scholar]
- 11.Giannattasio C, Cattaneo BM, Seravalle G, Grassi G, Mancia G. Left ventricular hypertrophy and the ‘cardiogenic reflex’ in man. J Hypertens. 1991;9(Suppl 2):S43–S50. doi: 10.1097/00004872-199112002-00006. [DOI] [PubMed] [Google Scholar]
- 12.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ, National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood JP, Stoker JB, Mary DA. Single-unit sympathetic discharge: quantitative assessment in human hypertensive disease. Circulation. 1999;100:1305–1310. doi: 10.1161/01.cir.100.12.1305. [DOI] [PubMed] [Google Scholar]
- 14.Burns J, Mary DASG, Mackintosh AF, Ball SG, Greenwood JP. Arterial pressure lowering effect of chronic atenolol therapy in hypertension and vasoconstrictor sympathetic drive. Hypertension. 2004;44:454–458. doi: 10.1161/01.HYP.0000141411.94596.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macefield VG, Wallin BG, Vallbo AB. The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. J Physiol (Lond) 1994;481:799–809. doi: 10.1113/jphysiol.1994.sp020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundlöf G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol (Lond) 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chia JM, Fischer SE, Wickline SA, Lorenz CH. Performance of QRS detection for cardiac magnetic resonance imaging with a novel vectorcardiographic triggering method. J Magn Reson Imaging. 2000;12:678–688. doi: 10.1002/1522-2586(200011)12:5<678::aid-jmri4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequence. J Magn Reson Imaging. 2003;17:323–329. doi: 10.1002/jmri.10262. [DOI] [PubMed] [Google Scholar]
- 19.Fieno DS, Jaffe WC, Simonetti OP, Judd RM, Finn JP. TrueFISP: Assessment of accuracy for measurement of left ventricular mass in an animal model. J Magn Reson Imaging. 2002;15:526–531. doi: 10.1002/jmri.10107. [DOI] [PubMed] [Google Scholar]
- 20.Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP., Jr. Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson. 1999;1:7–21. doi: 10.3109/10976649909080829. [DOI] [PubMed] [Google Scholar]
- 21.Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens. 1995;8:221–228. doi: 10.1016/0895-7061(94)00178-E. [DOI] [PubMed] [Google Scholar]
- 22.Germain P, Roul G, Kastler B, Mossard JM, Bareiss P, Sacrez A. Inter-study variability in left ventricular mass measurement. Comparison between M-mode echography and MRI. Eur Heart J. 1992;13:1011–1019. doi: 10.1093/oxfordjournals.eurheartj.a060307. [DOI] [PubMed] [Google Scholar]
- 23.Myerson SG, Bellenger NG, Pennell DJ. Assessment of left ventricular mass by cardiovascular magnetic resonance. Hypertension. 2002;39:750–755. doi: 10.1161/hy0302.104674. [DOI] [PubMed] [Google Scholar]
- 24.Plein S, Bloomer TN, Ridgeway JP, Jones TR, Bainbridge GJ, Sivananthan MU. Steady-state free precession magnetic resonance imaging of the heart: comparison with segmented k-space gradient-echo imaging. J Magn Reson Imaging. 2001;14:230–236. doi: 10.1002/jmri.1178. [DOI] [PubMed] [Google Scholar]
- 25.Hartikainen J, Tarkiainen I, Tahvanainen K, Mantysaari M, Lansimies E, Pyorala K. Circadian variation of cardiac autonomic regulation during 24-h bed rest. Clin Physiol. 1993;13:185–196. doi: 10.1111/j.1475-097x.1993.tb00379.x. [DOI] [PubMed] [Google Scholar]
- 26.Grassi GM, Somers VK, Renk WS, Abboud FM, Mark AL. Effects of alcohol intake on blood pressure and sympathetic nerve activity in normotensive humans: a preliminary report. J Hypertens. 1989;7(Suppl):S20–S21. doi: 10.1097/00004872-198900076-00007. [DOI] [PubMed] [Google Scholar]
- 27.Fagius H, Karhuvaara S. Sympathetic activity and blood pressure increases with bladder distension in humans. Hypertension. 1989;14:511–517. doi: 10.1161/01.hyp.14.5.511. [DOI] [PubMed] [Google Scholar]
- 28.Cox HS, Kaye DM, Thompson JM, Turner AG, Jennings GL, Itsiopoulos C, Esler MD. Regional sympathetic nervous activation after a large meal in humans. Clin Sci (Colch) 1995;89:145–154. doi: 10.1042/cs0890145. [DOI] [PubMed] [Google Scholar]
- 29.Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- 30.Calhoun DA, Mutinga ML, Collins AS, Wyss JM, Oparil S. Normotensive blacks have heightened sympathetic response to cold pressor test. Hypertension. 1993;22:801–805. doi: 10.1161/01.hyp.22.6.801. [DOI] [PubMed] [Google Scholar]
- 31.Scherrer U, Randin D, Tappy L, Vollenweider P, Jequier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation. 1994;89:2634–2640. doi: 10.1161/01.cir.89.6.2634. [DOI] [PubMed] [Google Scholar]
- 32.Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension. 1998;31:68–72. doi: 10.1161/01.hyp.31.1.68. [DOI] [PubMed] [Google Scholar]
- 33.Esler M. Clinical application of noradrenaline spillover methodology: delineation of regional human sympathetic nervous responses. Pharmacol Toxicol. 1993;73:243–253. doi: 10.1111/j.1600-0773.1993.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 34.Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol (Lond) 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esler M, Lambert G, Jennings G. Increased regional sympathetic nervous activity in human hypertension: causes and consequences. J Hypertens. 1990;8(Suppl):S53–S57. [PubMed] [Google Scholar]
- 36.Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99S–105S. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- 37.Tse HF, Cheung BMY, Ng W, Chan JKF, Devereux RB, Lau CP. Regression of left ventricular hypertrophy after treatment of hypertension: comparison of directed M-echocardiography with magnetic resonance imaging in quantification of serial mass changes. J Card Fail. 2003;9:122–127. doi: 10.1054/jcaf.2003.12. [DOI] [PubMed] [Google Scholar]
- 38.Folkow B. Physiological aspect of primary hypertension. Physiol Rev. 1982;62:347–504. doi: 10.1152/physrev.1982.62.2.347. [DOI] [PubMed] [Google Scholar]
- 39.Simpson P. Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha-1 adrenergic response. J Clin Invest. 1983;72:732–738. doi: 10.1172/JCI111023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sen S, Tarazi RC. Cardiovascular hypertrophy in spontaneously hypertensive rats. J Hypertens Suppl. 1986;4(3):S123–S126. [PubMed] [Google Scholar]
- 41.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 42.Kannel WB. Left ventricular hypertrophy as a risk factor in arterial hypertension. Eur Heart J. 1992;13(Suppl D):82–88. doi: 10.1093/eurheartj/13.suppl_d.82. [DOI] [PubMed] [Google Scholar]
- 43.Muiesan ML, Salvetti M, Rizzoni D, Castellano M, Donato F, Agabiti-Rosei E. Association of change in left ventricular mass with prognosis during long-term antihypertensive treatment. J Hypertens. 1995;13:1091–1095. doi: 10.1097/00004872-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, Reboldi G, Porcellati C. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation. 1998;97:48–54. doi: 10.1161/01.cir.97.1.48. [DOI] [PubMed] [Google Scholar]
- 45.Verdecchia P, Angeli F, Borgioni C, Gattobigio R, de Simone G, Devereux RB, Porcellati C. Changes in cardiovascular risk by reduction of left ventricular mass in hypertension: a meta-analysis. Am J Hypertens. 2003;16:895–899. doi: 10.1016/s0895-7061(03)01018-5. [DOI] [PubMed] [Google Scholar]