Abstract
Endoscopic papillectomy (EP) is currently accepted as a viable alternative therapy to surgery in sporadic ampullary adenoma and has been reported to have high success and low recurrence rates. At present, the indications for EP are not yet fully established. The accepted criteria for EP include size (up to 5 cm), no evidence of intraductal growth, and no evidence of malignancy on endoscopic findings (ulceration, friability, and spontaneous bleeding). Endoscopic ultrasound (EUS) is the imaging modality of choice for local T staging in ampullary neoplasms. Data reported in the literature have revealed that linear EUS is superior to helical computed tomography in the preoperative assessment of tumor size, detection of regional nodal metastases and detection of major vascular invasion. Endoscopic ampullectomy is performed using a standard duodenoscope in a similar manner to snare polypectomy of a mucosal lesion. There is no standardization of the equipment or technique and broad EP methods are described. Endoscopic ampullectomy is considered a ‘‘high-risk’’ procedure due to complications. Complications of endoscopic papillectomy can be classified as early (pancreatitis, bleeding, perforation, and cholangitis) and late (papillary stenosis) complications. The appropriate use of stenting after ampullectomy may prevent post-procedural pancreatitis and papillary stenosis. Tumor recurrence of benign lesions occurs in up to 20% of patients and depends on tumor size, final histology, presence of intraductal tumor, coexisting familial adenomatous polyposis (FAP), and the expertise of the endoscopist. Recurrent lesions are usually benign and most can be retreated endoscopically.
Keywords: Endoscopic papillectomy, Papillary neoplasms, Major duodenal papilla, Endoscopic retrograde cholangiopancreatography, Endoscopic sphincterotomy
Core tip: Endoscopic papillectomy is a relatively safe and effective therapy and should be established as a first-line therapy for adenomas of the major duodenal papilla. Accurate staging of the tumor is important in the selection of patients. Performed by experienced endoscopists leads to successful tumor eradication in over 85% of patients with ampullary adenomas.
INTRODUCTION
Endoscopic papillectomy (EP) was first reported by Suzuki et al[1]. Endoscopic papillectomy is currently accepted as a viable alternative therapy to surgery in sporadic ampullary adenoma and has been reported to have high success and low recurrence rates.
In the present report, several issues relating to EP were assessed: indications, optimal papillectomy technique, complications, and results.
DEFINITION
The term ‘‘endoscopic papillectomy’’ refers to resection of the mucosa and submucosa of the duodenal wall, in the area of the anatomical attachments of the ampulla of Vater, including the tissue around the bile duct and the pancreatic-duct orifices.
Endoscopic papillectomy differs from surgical “ampullectomy” which consists of resection of the ampulla of Vater, via a duodenotomy, including resection of pancreatic-head tissue, followed by separate reinsertion of the common bile duct and main pancreatic duct into the duodenal wall.
INDICATIONS
The most critical point in EP is assessment of the indication. At present, the indications for EP are not yet fully established. These could be dictated by the collection of features that can predict complete removal of a lesion, while minimizing procedure-related morbidities.
The accepted criteria for EP include size (up to 5 cm), no evidence of intraductal growth, and no evidence of malignancy on endoscopic findings (ulceration, friability, and spontaneous bleeding)[2-9].
The indications for EP are evolving[10-16]. The application of piecemeal resection when appropriate, resulted in a gradual increase in the size of the tumor resected[17]. Intraductal extension less than 1 cm does not seem to be an absolute contraindication for EP, because the tumor can be exposed to the luminal side with balloon sweeping and, thus, resected completely[18-20]. Cancer in adenoma without invasion of the muscularis propria of the duodenum, pancreas, or extension along the bile or pancreatic duct is also a possible indication for this treatment[21-25].
It is important to note that, on some occasions, EP may be indicated as a total biopsy[26].
PRE-OPERATIVE ASSESSMENT
A common pre-operative problem is achieving a reliable distinction between benign and malignant papillary tumors.
On the basis of endoscopic appearance alone, ampullary adenomas cannot always be distinguished from ampullary carcinomas or non-adenomatous polyps (carcinoid tumors, gangliocytic paragangliomas, etc.)[27-29]. Ulceration, friability, and spontaneous bleeding are generally related to malignant lesions. The increased application of magnifying endoscopy and narrow-band imaging can assist in selecting candidates for endoscopic therapy[30,31].
A definitive tissue diagnosis is a prerequisite for appropriate management, but malignancy may be missed in up to 30% of tumors in the major duodenal papilla when forceps biopsy specimens are obtained[32-34]. Moreover, the coexistence of carcinoma within adenoma cannot be excluded by pre-procedural biopsy. Some authors advocate deeper biopsy after sphincterotomy for accurate diagnosis of endoscopic biopsy[35]. A prospective study, however, reported that sensitivity was found to be 21% before and 37% after sphincterotomy, concluding that endoscopic forceps biopsies do not allow for reliable preoperative diagnosis of ampullary tumors[36]. Thus, in some cases, endoscopic papillectomy can be recommended as a diagnostic tool prior to surgery, due to the high false-negative rate of biopsy[26].
STAGING
Endoscopic ultrasound (EUS) is the imaging modality of choice for local T staging. Data reported in the literature have revealed that linear EUS is superior to helical Computed tomography (CT) in the preoperative assessment of tumor size, detection of regional nodal metastases and detection of major vascular invasion in patients with periampullary malignancies[37-43].
Many experts agree that lesions less than 1 cm in diameter without suspicious signs of malignancy (ulceration, induration, bleeding and or biopsies showing high-grade dysplasia or carcinoma), do not require EUS, but this needs further prospective study and validation[9]. Intraductal ultrasound (IDUS) using a 20 MHz frequency probe may be more accurate in visualizing the mucosal layers compared with standard echoendoscopes[44-47].
EUS/IDUS are able to accurately detect involvement of the bile and pancreatic ducts. Tumor extension into either ductal system can also be assessed by endoscopic retrograde cholangiopancreatography (ERCP). This should be performed before ampullectomy if EUS is not available or the findings on EUS are equivocal. Although the presence of intraductal extension of tumor generally indicates the need for surgery, it has been shown that tumor extension of ± 1 cm into the common bile duct or pancreatic duct can be further resected and ablated endoscopically[18-20].
CT scan, magnetic resonance imaging (MRI), and positron emission tomographic scans are highly sensitive for the detection of distant metastases. In the assessment of nodal involvement, MRI has been found to be superior to both CT and endoscopic ultrasound[38].
TECHNIQUE
Endoscopic ampullectomy is performed using a standard duodenoscope in a similar manner to snare polypectomy of a mucosal lesion. There is no standardization of the equipment or technique and broad EP methods are described. There are also no guidelines regarding the power output and the mode of electrosurgical current (cutting or coagulation), the use of adjunctive interventions, such as submucosal injection, post-ampullectomy ablative therapy, and prophylactic stent placement. The need for prophylactic antibiotics prior to ampullectomy has not been established[48].
The procedure starts with cannulation of both the bile duct and pancreatic duct, and the ducts are partially filled with contrast to ensure easy recannulation after the major papilla is resected. To preserve access to the pancreatic duct, some experts have included methylene blue in the contrast injected into the pancreatic duct to assist in identifying the pancreatic orifice.
Once delineation of the biliary and pancreatic ducts has been made a standard polypectomy snare and blended electrosurgical current (50-60 J) are generally used. The papillary tumor is snared at the base, and constant tension is applied to the snare loop during electrosurgery until the lesion is transected. Aggressive efforts to retrieve all resected tissue in all patients for histopathologic evaluation are mandatory (Figure 1).
Figure 1.
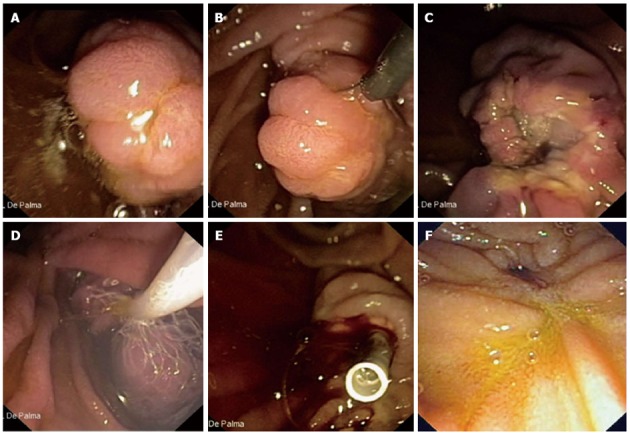
Aggressive efforts to retrieve all resected tissue in all patients for histopathologic evaluation are mandatory. A: Endoscopic view of a 3 cm neoplastic lesion of the major papilla; B: The lesion is entirely entrapped by the endoscopic snare; C: The lesion is completely resected (en-bloc resection); D: The resected specimen is retrieved by a Roth Net© device (US Endoscopy, Mentor, OH, United States); E: A plastic stent is implanted into the main pancreatic duct to prevent post-papillectomy pancreatitis; F: Duodenal view 6 mo after papillectomy. No evidence of recurrent disease is observed.
Balloon-catheter-assisted papillectomy has also been advocated to facilitate en bloc resection mainly of flat papillary tumors[18,19].
For lesions which are not resectable “en bloc”, piecemeal polypectomy is recommended. However, en bloc resection is fundamental in the treatment of neoplastic lesions, because this allows more precise histopathologic evaluation of the resection specimen[19,49].
Submucosal injection of dilute epinephrine is suggested as a means of lifting the tumor from the wall; this may also decrease the risk of bleeding. It is uncertain, however, whether epinephrine injection reduces the risk of bleeding and perforation[11,20,50].
If residual neoplastic tissue remains after snare excision this should be destroyed. Argon plasma coagulation is the most frequently used modality due to the non-contact approach that limits the depth of tissue injury[9,48,50,51].
Many authorities suggest that placement of a pancreatic stent reduces the risk of papillectomy-related pancreatitis, minimizes the risk of stenosis of the pancreatic duct orifice and allows safer use of adjunctive coagulative therapies, however, this theory is unproven. Others advocate pancreatic stent placement only if the pancreatic duct does not drain after papillectomy[52-55]. The only prospective, randomized, controlled trial to evaluate the role of prophylactic pancreatic duct stenting for the reduction of post-ERCP pancreatitis after endoscopic papillectomy showed a statistically significant decrease in the rate of post-procedure pancreatitis in the stent group[56]. There are no data on the length of the duct to be stented. Most pancreatic stents will spontaneously migrate out of the pancreatic duct within 2 wk of insertion. This is confirmed by an abdominal X-ray at 2 wk. A stent that remains in situ is removed endoscopically.
Prophylactic biliary stenting to reduce the risk of post-procedural cholangitis has not been widely performed and cannot be uniformly recommended at this time unless there is concern about inadequate biliary drainage after a papillectomy[2,8,50,57].
COMPLICATIONS
Endoscopic ampullectomy is considered a ‘‘high-risk’’ procedure due to complications. Complications of endoscopic papillectomy can be classified as early (pancreatitis, bleeding, perforation, and cholangitis) and late (papillary stenosis) complications.
The overall rate of complications after ampullectomy reported from large, tertiary care referral centers varies between 8% and 35%, with the most common complications being pancreatitis (5%-15%) and bleeding (2%-16%)[14,17,51,58-61]. Most bleeding episodes can be controlled by conservative management and endoscopic hemostasis. Most post-procedural pancreatitis episodes are mild and resolve with conservative management only. Late complications include the development of pancreatic or biliary stenosis (0%-8%) and can be treated with sphincterotomy, stents, and balloon dilation. The appropriate use of stenting after ampullectomy may prevent post-procedural pancreatitis and papillary stenosis[52-57]. As evidenced by a recent randomized trial, prophylactic rectal indomethacin significantly reduces the incidence and severity of post-ERCP pancreatitis providing an incremental benefit over temporary pancreatic stents[62].
Mortality after endoscopic ampullectomy is rare, but has been reported to be 0.4% (range 0%-7%) on average[63].
OUTCOMES
The results of endoscopic treatment of ampulla tumors reported in the literature are shown in Table 1. Outcome data of endoscopic ampullectomy are based on retrospective, heterogeneous case series. Because there is no consensus on the definition of ‘‘success’’ after endoscopic papillectomy, it is difficult to compare the outcome of the reported studies. Conventionally, ‘‘success’’ may be defined as complete resection of the tumor with endoscopic papillectomy (as the absence of endoscopically visible and histologically proven residual adenoma during a follow-up period of 3 to 6 mo).
Table 1.
Outcomes after endoscopic papillectomy
| Ref. | Patients | Successful resection | Complications | Mortality | Recurrence | Need for surgery |
| Binmoeller et al[2] | 25 | 23 | 5 | 0 | 6 | 3 |
| Vogt et al[72] | 18 | 12 | 4 | 0 | 6 | NA |
| Zádorová et al[5] | 16 | 13 | 4 | 0 | 3 | 1 |
| Desilets et al[50] | 13 | 12 | 1 | 0 | 0 | 1 |
| Norton et al[51] | 26 | 12 | 5 | 0 | 2 | 1 |
| Bohnacker et al[20] | 87 | 74 | 29 | 0 | 15 | 17 |
| Catalano et al[7] | 103 | 83 | 10 | 0 | 10 | 16 |
| Cheng et al[8] | 55 | 39 | 12 | 0 | 9 | 4 |
| Han et al[10] | 33 | 20 | 11 | 0 | 2 | 2 |
NA: Not available.
Recurrence of benign lesions occurs in up to 20% of patients and depends on tumor size, final histology, presence of intraductal tumor, coexisting FAP, and the expertise of the endoscopist[14,63-71]. Recurrent lesions are usually benign and most can be retreated endoscopically.
CONCLUSION
Endoscopic papillectomy is a relatively safe and effective therapy and should be established as a first-line therapy for adenomas of the major duodenal papilla. Accurate staging of ampullary tumors is important in the selection of appropriate candidates for endoscopic or surgical therapy. Compared with surgery, endoscopic ampullectomy is associated with lower morbidity and mortality, and appears to be a preferred treatment modality for small benign ampullary tumors that have no intraductal extension. Endoscopic ampullectomy performed by experienced endoscopists leads to successful tumor eradication in over 85% of patients with ampullary adenomas.
Footnotes
P- Reviewer: Meister T S- Editor: Gou SX L- Editor: Webster JR E- Editor: Zhang DN
References
- 1.Suzuki K, Kantou U, Murakami Y. Two cases with ampullary cancer who underwent endoscopic excision. Prog Dig Endosc. 1983;23:236–239. [Google Scholar]
- 2.Binmoeller KF, Boaventura S, Ramsperger K, Soehendra N. Endoscopic snare excision of benign adenomas of the papilla of Vater. Gastrointest Endosc. 1993;39:127–131. doi: 10.1016/s0016-5107(93)70051-6. [DOI] [PubMed] [Google Scholar]
- 3.Silvis SE. Endoscopic snare papillectomy. Gastrointest Endosc. 1993;39:205–207. doi: 10.1016/s0016-5107(93)70074-7. [DOI] [PubMed] [Google Scholar]
- 4.El Hajj II, Coté GA. Endoscopic diagnosis and management of ampullary lesions. Gastrointest Endosc Clin N Am. 2013;23:95–109. doi: 10.1016/j.giec.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Zádorová Z, Dvofák M, Hajer J. Endoscopic therapy of benign tumors of the papilla of Vater. Endoscopy. 2001;33:345–347. doi: 10.1055/s-2001-13693. [DOI] [PubMed] [Google Scholar]
- 6.Wong RF, DiSario JA. Approaches to endoscopic ampullectomy. Curr Opin Gastroenterol. 2004;20:460–467. doi: 10.1097/00001574-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Catalano MF, Linder JD, Chak A, Sivak MV, Raijman I, Geenen JE, Howell DA. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225–232. doi: 10.1016/s0016-5107(03)02366-6. [DOI] [PubMed] [Google Scholar]
- 8.Cheng CL, Sherman S, Fogel EL, McHenry L, Watkins JL, Fukushima T, Howard TJ, Lazzell-Pannell L, Lehman GA. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc. 2004;60:757–764. doi: 10.1016/s0016-5107(04)02029-2. [DOI] [PubMed] [Google Scholar]
- 9.Baillie J. Endoscopic ampullectomy. Am J Gastroenterol. 2005;100:2379–2381. doi: 10.1111/j.1572-0241.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 10.Han J, Kim MH. Endoscopic papillectomy for adenomas of the major duodenal papilla (with video) Gastrointest Endosc. 2006;63:292–301. doi: 10.1016/j.gie.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Bohnacker S, Soehendra N, Maguchi H, Chung JB, Howell DA. Endoscopic resection of benign tumors of the papilla of vater. Endoscopy. 2006;38:521–525. doi: 10.1055/s-2006-925263. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez LV, Catalano MF. Endoscopic papillectomy. Curr Opin Gastroenterol. 2008;24:617–622. doi: 10.1097/MOG.0b013e3283088e12. [DOI] [PubMed] [Google Scholar]
- 13.Ito K, Fujita N, Noda Y. Endoscopic diagnosis and treatment of ampullary neoplasm (with video) Dig Endosc. 2011;23:113–117. doi: 10.1111/j.1443-1661.2010.01101.x. [DOI] [PubMed] [Google Scholar]
- 14.Patel R, Varadarajulu S, Wilcox CM. Endoscopic ampullectomy: techniques and outcomes. J Clin Gastroenterol. 2012;46:8–15. doi: 10.1097/MCG.0b013e318233a844. [DOI] [PubMed] [Google Scholar]
- 15.Rattner DW, Fernandez-del Castillo C, Brugge WR, Warshaw AL. Defining the criteria for local resection of ampullary neoplasms. Arch Surg. 1996;131:366–371. doi: 10.1001/archsurg.1996.01430160024003. [DOI] [PubMed] [Google Scholar]
- 16.Bassan M, Bourke M. Endoscopic ampullectomy: a practical guide. J Interv Gastroenterol. 2012;2:23–30. doi: 10.4161/jig.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito K, Fujita N, Noda Y, Kobayashi G, Obana T, Horaguchi J, Koshita S, Kanno Y, Ogawa T, Kato Y, et al. Impact of technical modification of endoscopic papillectomy for ampullary neoplasm on the occurrence of complications. Dig Endosc. 2012;24:30–35. doi: 10.1111/j.1443-1661.2011.01161.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Moon JH, Choi HJ, Lee HS, Kim HK, Cheon YK, Cho YD, Lee JS, Lee MS, Shim CS. Endoscopic snare papillectomy by using a balloon catheter for an unexposed ampullary adenoma with intraductal extension (with videos) Gastrointest Endosc. 2009;69:1404–1406. doi: 10.1016/j.gie.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 19.Aiura K, Imaeda H, Kitajima M, Kumai K. Balloon-catheter-assisted endoscopic snare papillectomy for benign tumors of the major duodenal papilla. Gastrointest Endosc. 2003;57:743–747. doi: 10.1067/mge.2003.213. [DOI] [PubMed] [Google Scholar]
- 20.Bohnacker S, Seitz U, Nguyen D, Thonke F, Seewald S, deWeerth A, Ponnudurai R, Omar S, Soehendra N. Endoscopic resection of benign tumors of the duodenal papilla without and with intraductal growth. Gastrointest Endosc. 2005;62:551–560. doi: 10.1016/j.gie.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 21.Yoon SM, Kim MH, Kim MJ, Jang SJ, Lee TY, Kwon S, Oh HC, Lee SS, Seo DW, Lee SK. Focal early stage cancer in ampullary adenoma: surgery or endoscopic papillectomy? Gastrointest Endosc. 2007;66:701–707. doi: 10.1016/j.gie.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 22.Woo SM, Ryu JK, Lee SH, Lee WJ, Hwang JH, Yoo JW, Park JK, Kang GH, Kim YT, Yoon YB. Feasibility of endoscopic papillectomy in early stage ampulla of Vater cancer. J Gastroenterol Hepatol. 2009;24:120–124. doi: 10.1111/j.1440-1746.2008.05578.x. [DOI] [PubMed] [Google Scholar]
- 23.Salmi S, Ezzedine S, Vitton V, Ménard C, Gonzales JM, Desjeux A, Grimaud JC, Barthet M. Can papillary carcinomas be treated by endoscopic ampullectomy? Surg Endosc. 2012;26:920–925. doi: 10.1007/s00464-011-1968-7. [DOI] [PubMed] [Google Scholar]
- 24.Kim HK, Lo SK. Endoscopic approach to the patient with benign or malignant ampullary lesions. Gastrointest Endosc Clin N Am. 2013;23:347–383. doi: 10.1016/j.giec.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Petrone G, Ricci R, Familiari P, Inzani F, Matsuoka M, Mutignani M, Delle Fave G, Costamagna G, Rindi G. Endoscopic snare papillectomy: a possible radical treatment for a subgroup of T1 ampullary adenocarcinomas. Endoscopy. 2013;45:401–404. doi: 10.1055/s-0032-1326213. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa T, Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J, Koshita S, Kanno Y, Masu K, Ishii S. Endoscopic papillectomy as a method of total biopsy for possible early ampullary cancer. Dig Endosc. 2012;24:291. doi: 10.1111/j.1443-1661.2011.01214.x. [DOI] [PubMed] [Google Scholar]
- 27.Niido T, Itoi T, Harada Y, Haruyama K, Ebihara Y, Tsuchida A, Kasuya K. Carcinoid of major duodenal papilla. Gastrointest Endosc. 2005;61:106–107. doi: 10.1016/s0016-5107(04)02384-3. [DOI] [PubMed] [Google Scholar]
- 28.Kwon J, Lee SE, Kang MJ, Jang JY, Kim SW. A case of gangliocytic paraganglioma in the ampulla of Vater. World J Surg Oncol. 2010;8:42. doi: 10.1186/1477-7819-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Palma GD, Masone S, Siciliano S, Maione F, Falleti J, Mansueto G, De Rosa G, Persico G. Endocrine carcinoma of the major papilla: report of two cases and review of the literature. Surg Oncol. 2010;19:235–242. doi: 10.1016/j.suronc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Uchiyama Y, Imazu H, Kakutani H, Hino S, Sumiyama K, Kuramochi A, Tsukinaga S, Matsunaga K, Nakayoshi T, Goda K, et al. New approach to diagnosing ampullary tumors by magnifying endoscopy combined with a narrow-band imaging system. J Gastroenterol. 2006;41:483–490. doi: 10.1007/s00535-006-1800-7. [DOI] [PubMed] [Google Scholar]
- 31.Itoi T, Tsuji S, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Ikeuchi N, Igarashi M, Gotoda T, et al. A novel approach emphasizing preoperative margin enhancement of tumor of the major duodenal papilla with narrow-band imaging in comparison to indigo carmine chromoendoscopy (with videos) Gastrointest Endosc. 2009;69:136–141. doi: 10.1016/j.gie.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi K, Enjoji M, Kitamura K. Endoscopic biopsy has limited accuracy in diagnosis of ampullary tumors. Gastrointest Endosc. 1990;36:588–592. doi: 10.1016/s0016-5107(90)71170-4. [DOI] [PubMed] [Google Scholar]
- 33.Elek G, Gyôri S, Tóth B, Pap A. Histological evaluation of preoperative biopsies from ampulla vateri. Pathol Oncol Res. 2003;9:32–41. doi: 10.1007/BF03033712. [DOI] [PubMed] [Google Scholar]
- 34.Bellizzi AM, Kahaleh M, Stelow EB. The assessment of specimens procured by endoscopic ampullectomy. Am J Clin Pathol. 2009;132:506–513. doi: 10.1309/AJCPUZWJ8WA2IHBG. [DOI] [PubMed] [Google Scholar]
- 35.Bourgeois N, Dunham F, Verhest A, Cremer M. Endoscopic biopsies of the papilla of Vater at the time of endoscopic sphincterotomy: difficulties in interpretation. Gastrointest Endosc. 1984;30:163–166. doi: 10.1016/s0016-5107(84)72357-1. [DOI] [PubMed] [Google Scholar]
- 36.Menzel J, Poremba C, Dietl KH, Böcker W, Domschke W. Tumors of the papilla of Vater--inadequate diagnostic impact of endoscopic forceps biopsies taken prior to and following sphincterotomy. Ann Oncol. 1999;10:1227–1231. doi: 10.1023/a:1008368807817. [DOI] [PubMed] [Google Scholar]
- 37.Rivadeneira DE, Pochapin M, Grobmyer SR, Lieberman MD, Christos PJ, Jacobson I, Daly JM. Comparison of linear array endoscopic ultrasound and helical computed tomography for the staging of periampullary malignancies. Ann Surg Oncol. 2003;10:890–897. doi: 10.1245/aso.2003.03.555. [DOI] [PubMed] [Google Scholar]
- 38.Cannon ME, Carpenter SL, Elta GH, Nostrant TT, Kochman ML, Ginsberg GG, Stotland B, Rosato EF, Morris JB, Eckhauser F, et al. EUS compared with CT, magnetic resonance imaging, and angiography and the influence of biliary stenting on staging accuracy of ampullary neoplasms. Gastrointest Endosc. 1999;50:27–33. doi: 10.1016/s0016-5107(99)70340-8. [DOI] [PubMed] [Google Scholar]
- 39.Rösch T, Lorenz R, Braig C, Feuerbach S, Siewert JR, Schusdziarra V, Classen M. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc. 1991;37:347–352. doi: 10.1016/s0016-5107(91)70729-3. [DOI] [PubMed] [Google Scholar]
- 40.Azih LC, Broussard BL, Phadnis MA, Heslin MJ, Eloubeidi MA, Varadarajulu S, Arnoletti JP. Endoscopic ultrasound evaluation in the surgical treatment of duodenal and peri-ampullary adenomas. World J Gastroenterol. 2013;19:511–515. doi: 10.3748/wjg.v19.i4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J, Takasawa O, Obana T. Preoperative evaluation of ampullary neoplasm with EUS and transpapillary intraductal US: a prospective and histopathologically controlled study. Gastrointest Endosc. 2007;66:740–747. doi: 10.1016/j.gie.2007.03.1081. [DOI] [PubMed] [Google Scholar]
- 42.Will U, Bosseckert H, Meyer F. Correlation of endoscopic ultrasonography (EUS) for differential diagnostics between inflammatory and neoplastic lesions of the papilla of Vater and the peripapillary region with results of histologic investigation. Ultraschall Med. 2008;29:275–280. doi: 10.1055/s-2008-1027327. [DOI] [PubMed] [Google Scholar]
- 43.Cote GA, Edmundowicz SA. The Role of Endoscopic Ultrasonography (EUS) and Endoscopic Retrograde Cholangiopancreatography (ERCP) in the Evaluation and Management of Ampullary Adenomas. Tech Gastrointest Endosc. 2009;11:49–57. [Google Scholar]
- 44.Menzel J, Hoepffner N, Sulkowski U, Reimer P, Heinecke A, Poremba C, Domschke W. Polypoid tumors of the major duodenal papilla: preoperative staging with intraductal US, EUS, and CT--a prospective, histopathologically controlled study. Gastrointest Endosc. 1999;49:349–357. doi: 10.1016/s0016-5107(99)70012-x. [DOI] [PubMed] [Google Scholar]
- 45.Itoh A, Goto H, Naitoh Y, Hirooka Y, Furukawa T, Hayakawa T. Intraductal ultrasonography in diagnosing tumor extension of cancer of the papilla of Vater. Gastrointest Endosc. 1997;45:251–260. doi: 10.1016/s0016-5107(97)70267-0. [DOI] [PubMed] [Google Scholar]
- 46.Menzel J, Domschke W. Gastrointestinal miniprobe sonography: the current status. Am J Gastroenterol. 2000;95:605–616. doi: 10.1111/j.1572-0241.2000.01832.x. [DOI] [PubMed] [Google Scholar]
- 47.Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J. Diagnosis of ampullary cancer. Dig Surg. 2010;27:115–118. doi: 10.1159/000286607. [DOI] [PubMed] [Google Scholar]
- 48.Menees SB, Schoenfeld P, Kim HM, Elta GH. A survey of ampullectomy practices. World J Gastroenterol. 2009;15:3486–3492. doi: 10.3748/wjg.15.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charton JP, Deinert K, Schumacher B, Neuhaus H. Endoscopic resection for neoplastic diseases of the papilla of Vater. J Hepatobiliary Pancreat Surg. 2004;11:245–251. doi: 10.1007/s00534-004-0897-4. [DOI] [PubMed] [Google Scholar]
- 50.Desilets DJ, Dy RM, Ku PM, Hanson BL, Elton E, Mattia A, Howell DA. Endoscopic management of tumors of the major duodenal papilla: Refined techniques to improve outcome and avoid complications. Gastrointest Endosc. 2001;54:202–208. doi: 10.1067/mge.2001.116564. [DOI] [PubMed] [Google Scholar]
- 51.Norton ID, Gostout CJ, Baron TH, Geller A, Petersen BT, Wiersema MJ. Safety and outcome of endoscopic snare excision of the major duodenal papilla. Gastrointest Endosc. 2002;56:239–243. doi: 10.1016/s0016-5107(02)70184-3. [DOI] [PubMed] [Google Scholar]
- 52.Lee SK, Kim MH, Seo DW, Lee SS, Park JS. Endoscopic sphincterotomy and pancreatic duct stent placement before endoscopic papillectomy: are they necessary and safe procedures? Gastrointest Endosc. 2002;55:302–304. doi: 10.1067/mge.2002.120885. [DOI] [PubMed] [Google Scholar]
- 53.Baillie J. Endoscopic ampullectomy: does pancreatic stent placement make it safer? Gastrointest Endosc. 2005;62:371–373. doi: 10.1016/j.gie.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 54.Yamao T, Isomoto H, Kohno S, Mizuta Y, Yamakawa M, Nakao K, Irie J. Endoscopic snare papillectomy with biliary and pancreatic stent placement for tumors of the major duodenal papilla. Surg Endosc. 2010;24:119–124. doi: 10.1007/s00464-009-0538-8. [DOI] [PubMed] [Google Scholar]
- 55.Napoléon B, Alvarez-Sanchez MV, Leclercq P, Mion F, Pialat J, Gincul R, Ribeiro D, Cambou M, Lefort C, Rodríguez-Girondo M, Scoazec JY. Systematic pancreatic stenting after endoscopic snare papillectomy may reduce the risk of postinterventional pancreatitis. Surg Endosc. 2013;27:3377–3387. doi: 10.1007/s00464-013-2920-9. [DOI] [PubMed] [Google Scholar]
- 56.Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005;62:367–370. doi: 10.1016/j.gie.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 57.Adler DG, Qureshi W, Davila R, Gan SI, Lichtenstein D, Rajan E, Shen B, Zuckerman MJ, Fanelli RD, Van Guilder T, et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2006;64:849–854. doi: 10.1016/j.gie.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 58.Jun DW, Choi HS. [Is the endoscopic papillectomy safe procedure in periampullary tumors?] Korean J Gastroenterol. 2005;46:247–250. [PubMed] [Google Scholar]
- 59.Lee SY, Jang KT, Lee KT, Lee JK, Choi SH, Heo JS, Paik SW, Rhee JC. Can endoscopic resection be applied for early stage ampulla of Vater cancer? Gastrointest Endosc. 2006;63:783–788. doi: 10.1016/j.gie.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 60.Katsinelos P, Paroutoglou G, Kountouras J, Beltsis A, Papaziogas B, Mimidis K, Zavos C, Dimiropoulos S. Safety and long-term follow-up of endoscopic snare excision of ampullary adenomas. Surg Endosc. 2006;20:608–613. doi: 10.1007/s00464-004-2278-0. [DOI] [PubMed] [Google Scholar]
- 61.Pandolfi M, Martino M, Gabbrielli A. Endoscopic treatment of ampullary adenomas. JOP. 2008;9:1–8. [PubMed] [Google Scholar]
- 62.Elmunzer BJ, Scheiman JM, Lehman GA, Chak A, Mosler P, Higgins PD, Hayward RA, Romagnuolo J, Elta GH, Sherman S, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366:1414–1422. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heinzow HS, Lenz P, Lenze F, Domagk D, Domschke W, Meister T. Feasibility of snare papillectomy in ampulla of Vater tumors: meta-analysis and study results from a tertiary referral center. Hepatogastroenterology. 2012;59:332–335. doi: 10.5754/hge11414. [DOI] [PubMed] [Google Scholar]
- 64.Han J, Lee SK, Park DH, Choi JS, Lee SS, Seo DW, Kim MH. [Treatment outcome after endoscopic papillectomy of tumors of the major duodenal papilla] Korean J Gastroenterol. 2005;46:110–119. [PubMed] [Google Scholar]
- 65.Dittrick GW, Mallat DB, Lamont JP. Management of ampullary lesions. Curr Treat Options Gastroenterol. 2006;9:371–376. doi: 10.1007/BF02738525. [DOI] [PubMed] [Google Scholar]
- 66.Boix J, Lorenzo-Zúñiga V, Moreno de Vega V, Domènech E, Gassull MA. Endoscopic resection of ampullary tumors: 12-year review of 21 cases. Surg Endosc. 2009;23:45–49. doi: 10.1007/s00464-008-9866-3. [DOI] [PubMed] [Google Scholar]
- 67.Jung MK, Cho CM, Park SY, Jeon SW, Tak WY, Kweon YO, Kim SK, Choi YH. Endoscopic resection of ampullary neoplasms: a single-center experience. Surg Endosc. 2009;23:2568–2574. doi: 10.1007/s00464-009-0464-9. [DOI] [PubMed] [Google Scholar]
- 68.Irani S, Arai A, Ayub K, Biehl T, Brandabur JJ, Dorer R, Gluck M, Jiranek G, Patterson D, Schembre D, et al. Papillectomy for ampullary neoplasm: results of a single referral center over a 10-year period. Gastrointest Endosc. 2009;70:923–932. doi: 10.1016/j.gie.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 69.Jeanniard-Malet O, Caillol F, Pesenti C, Bories E, Monges G, Giovannini M. Short-term results of 42 endoscopic ampullectomies: a single-center experience. Scand J Gastroenterol. 2011;46:1014–1019. doi: 10.3109/00365521.2011.571711. [DOI] [PubMed] [Google Scholar]
- 70.Kim JH, Kim JH, Hwang JC, Yoo BM, Moon JH, Lee DK, Kim HG, Cho YD, Lee DH, Park SH. Management after endoscopic snare papillectomy for ampullary adenomas. Hepatogastroenterology. 2013;60:1268–1273. doi: 10.5754/hge11604. [DOI] [PubMed] [Google Scholar]
- 71.Ahn DW, Ryu JK, Kim J, Yoon WJ, Lee SH, Kim YT, Yoon YB. Endoscopic papillectomy for benign ampullary neoplasms: how can treatment outcome be predicted? Gut Liver. 2013;7:239–245. doi: 10.5009/gnl.2013.7.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vogt M, Jakobs R, Benz C, Arnold JC, Adamek HE, Riemann JF. Endoscopic therapy of adenomas of the papilla of Vater. A retrospective analysis with long-term follow-up. Dig Liver Dis. 2000;32:339–345. doi: 10.1016/s1590-8658(00)80028-6. [DOI] [PubMed] [Google Scholar]


