Abstract
AIM: To establish whether chemotherapy-induced neutropenia is predictive of better outcome in patients with metastatic colorectal cancer (mCRC).
METHODS: Survival and patient characteristics from consecutive mCRC patients treated in the Centre Georges Francois Leclerc, Dijon, France between January 2001 and December 2011 were analyzed. Patient and tumor characteristics, hematological toxicity (neutropenia, anemia, and thrombocytopenia), and type of chemotherapy received were recorded.
RESULTS: We retrospectively analyzed data from 399 consecutive patients with mCRC who received at least one line of chemotherapy. Median follow up was 6.3 years. Eighty-eight percent of the patients received more than two lines of chemotherapy. By univariate analysis, whatever their grade, neutropenia and thrombocytopenia occurring during the first two lines of chemotherapy were significantly associated with better overall survival (HR = 0.55, 95%CI: 0.43-0.70, P < 0.0001 and HR = 0.70, 95%CI: 0.56-0.88, P = 0.025 respectively). In contrast, anemia during chemotherapy was significantly associated with poorer overall survival (HR = 1.9, 95%CI: 1.22-2.97, P = 0.005). Multivariate analysis revealed that both neutropenia and thrombocytopenia were significantly associated with better overall survival: HR = 0.43, 95%CI: 0.29-0.64, P < 0.0001 and HR = 0.69, 95%CI: 0.49-0.98, P = 0.036, respectively.
CONCLUSION: These data suggest that occurrence of neutropenia or thrombocytopenia during first- or second-line chemotherapy for mCRC is associated with better survival.
Keywords: Colorectal cancer, Metastasis, Prognostic factor, Neutropenia, Chemotherapy
Core tip: Using a retrospective database of 399 patients we aimed to establish whether chemotherapy-induced cytopenia was predictive of better outcome in patients with metastatic colorectal cancer. We observed that occurrence of neutropenia and thrombocytopenia was associated with better outcome, while occurrence of anemia was associated with poorer outcome.
INTRODUCTION
In patients with unresectable metastatic colorectal cancer (mCRC), there is no curative option, but treatment with palliative systemic chemotherapy has been shown to improve overall survival (OS)[1]. Currently available therapeutic options rely on three major cytotoxic agents: fluoropyrimidine, oxaliplatin and irinotecan, associated with targeted therapies [anti-epidermal growth factor receptor (EGFR) (panitumumab and cetuximab) or anti-vascular endothelial growth factor (bevacizumab) monoclonal antibodies]. Five-year OS in patients diagnosed with unresectable distant metastases is -10%[2-4]. In contrast, when metastases can be surgically removed, 5-year OS increases to 50%[5]. OS seems to be increasing in recent clinical studies, probably because of the wider use of polychemotherapy and the routine addition of targeted therapies[6-8].
Many reports[9-14] have suggested that neutropenia occurring during anticancer cytotoxic chemotherapy could be a marker of treatment effectiveness, reflecting cytotoxic drug exposure. Accordingly, chemotherapy-induced neutropenia has previously been associated with better survival in many cancer types, both in adjuvant and metastatic settings. Indeed, myelosuppression induced by chemotherapy might be a direct reflection of cytotoxic activity, representing a biological measure of drug effectiveness, and could thus predict treatment efficacy. Usually, the recommended dosage of chemotherapy is determined on the basis of dose-finding phase I trials. Such studies determine the toxic profiles of cytotoxic agents in a small number of patients, but do not take into account the inter-individual variability of drug metabolism. In the currently available literature, only one small retrospective study observed a significant association between neutropenia and colorectal cancer survival[15].
However, most of these studies[9-14] only focused on neutropenia, and did not take in account information on other hematological toxicity such as thrombocytopenia or anemia.
In this report, we describe a retrospective analysis of 399 consecutive patients with mCRC treated by chemotherapy, in order to evaluate associations between OS and hematological toxicity (i.e., neutropenia, thrombocytopenia and anemia) occurring during the first two lines of chemotherapy.
MATERIALS AND METHODS
Patients
From January 2001 to December 2011, 399 consecutive patients with histologically proven mCRC received first-line chemotherapy at the Centre Georges-François Leclerc (Dijon, France), and were prospectively recorded in an institutional clinical database. This study was approved by our institutional review board and all data were rendered anonymous.
Statistical analysis
All patients were followed up until death or the end of data recording (December 31, 2011). The primary endpoint was OS, defined as the time from date of diagnosis until death of any cause, or December 31, 2011, whichever occurred first. Survivors were censored at last follow-up. Median follow-up with its 95%CI was calculated using the reverse Kaplan-Meier method. The association between hematological toxicity (neutropenia, thrombocytopenia, or anemia) and patient or disease characteristics were examined using the χ2 test or Fisher’s exact test for qualitative variables, and the Student t or Mann-Whitney test for continuous variables, as appropriate. Hematological toxicities were only considered if they occurred during the first two lines of chemotherapy, because data obtained thereafter could be biased due to inherent longer survival of these patients, and the classical problem of immortal time bias[16].
Survival probabilities were estimated using the Kaplan-Meier method and survival curves were compared using the log-rank test. Hazard ratio (HR) and 95%CI for univariate and multivariate analyses of OS were estimated using Cox’s proportional hazards regression with a backward elimination procedure. All predictors with P < 0.10 by univariate analysis were retained in the multivariate models. To prevent colinearity, when two variables were significantly correlated, only the more informative of the two was retained according to its clinical relevance, or according to the value of the likelihood ratio. Variables included in uni- and multivariate analyses were: neutropenia, thrombocytopenia and anemia during the two first lines of chemotherapy (occurrence or no occurrence), location of the primary tumor (colon or rectum), bevacizumab use, anti-EGFR use, number of metastatic sites (1 vs ≥ 2), age (< 75, vs ≥ 75 years), sex, serum carcinoembryonic antigen (CEA) (< 200 vs ≥ 200 ng/mL), leukocyte count, serum level of alkaline phosphatase (ALP) (< 300 vs ≥ 300 IU/L) and lactate dehydrogenase (LDH) (median value used as cutoff value), WHO performance status (0 vs 1-4), primary tumor surgery, metastases resection, number of chemotherapy lines (1 or 2 vs ≥ 3) and molecules (1-3). Multivariate Cox models were constructed with all predictors with P < 0.10 in univariate analysis, carried out by the backward elimination procedure. To handle missing data, we performed Cox regression using multiple imputations[17].
Routine blood counts were taken during every chemotherapy cycle, usually the day before treatment. Hematologic toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 [in detail anemia grade 1 (hemoglobin; Hb < 10 g/dL), grade 2 (Hb: 8-10 g/dL), grade 3 (Hb < 8 g/dL), grade 4 (life-threatening consequences); thrombocytopenia grade 1 (150000-75000/mm3), grade 2 (75000-50000/mm3), grade 3 (50000-10000/mm3), grade 4 (< 10000/mm3); neutropenia grade 1 (2000-1500/mm3), grade 2 (1500-1000/mm3), grade 3 (1000-500/mm3), grade 4 (< 500/mm3)]. For each type of cytopenia, patients were divided into three categories: absent (grade 0), mild (grades 1-2), and severe (grades 3-4).
Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, United States). All tests were two sided, and P < 0.05 was considered statistically significant.
RESULTS
Patients
The median follow-up at the time of this analysis was 6.3 years (95%CI: 5.6-7.4 years). Patient and tumor characteristics are described in Table 1. Treatment characteristics are presented in Table 2. Most patients (88%) received more than two lines of chemotherapy. Only 3.5% received one line of chemotherapy (fluropyrimidine alone), 13.5% received only fluoropyrimidine and oxaliplatinum, and 4.5% received only fluorpyrimidine and irinotecan. In total, 78.5% received the three major drugs (oxaliplatin, irinotecan and fluoropyrimidine). The median number of chemotherapy lines was four (range: 1-8). Seventy-two percent of patients received targeted therapy (35% received only anti-EGFR, 40% received only bevacizumab, and 25% received both). Thirty-eight percent of the patients had metachronous and 62% had synchronous disease. We noted that 83% of patients had liver metastases, 35% had lung metastases, and 12% had peritoneal carcinomatosis. Although 60% of patients had only one tumor, most of these had diffuse liver or lung involvement and less than half could benefit from metastases surgery.
Table 1.
Patient characteristics according to hematological toxicity in 399 consecutive patients with metastatic colorectal cancer n (%)
| All (n = 399) |
Anemia (n
= 397) |
Neutropenia (n
= 396) |
Thrombopenia (n
= 396) |
||||||||||||||
| No (n = 33) | Yes (n = 364) | P | No (n = 114) | Yes (n = 282) | P | No (n = 163) | Yes (n = 233) | P | |||||||||
| Mean ± SD | 65 ± 11 | 66.5 ± 10 | 64.9 ± 11.1 | 0.508 | 69 ± 11.7 | 63.5 ± 10.4 | < 10-4 | 67.4 ± 11.2 | 63.4 ± 10.7 | < 10-3 | |||||||
| Median (min-max) | 65.5 (24.7-90.7) | 67.8 (50-85.8) | 65.5 (24.7-90.7) | 69.6 (44.4-90.7) | 63.5 (24.7-85.8) | 67.5 (24.7-90.7) | 63.5 (28.2-88.2) | ||||||||||
| Origin | 0.994 | 0.622 | 0.392 | ||||||||||||||
| Colon | 314 | (79.1) | 26 | (78.8) | 287 | (78.8) | 88 | (77.2) | 224 | (79.4) | 125 | (76.7) | 187 | (80.3) | |||
| Rectum | 85 | (21.4) | 7 | (21.2) | 77 | (21.2) | 26 | (22.8) | 58 | (20.6) | 38 | (23.3) | 46 | (19.7) | |||
| Gender | 0.554 | 0.106 | 0.559 | ||||||||||||||
| Male | 213 | (53.4) | 16 | (48.5) | 196 | (53.8) | 68 | (59.6) | 143 | (50.7) | 84 | (51.5) | 127 | (54.5) | |||
| Female | 186 | (46.6) | 17 | (51.5) | 168 | (46.2) | 46 | (40.4) | 139 | (49.3) | 79 | (48.5) | 106 | (45.5) | |||
| WHO PS | 0.233 | 0.024 | 0.139 | ||||||||||||||
| 0 | 134 | (47.9) | 16 | (72.7) | 117 | (45.5) | 26 | (32.1) | 107 | (54.0) | 49 | (40.2) | 84 | (53.5) | |||
| 1 | 102 | (36.4) | 5 | (22.7) | 97 | (37.7) | 34 | (42.0) | 68 | (34.3) | 52 | (42.6) | 50 | (31.8) | |||
| 2 | 33 | (11.8) | 1 | (4.5) | 32 | (12.5) | 16 | (19.8) | 17 | (8.6) | 14 | (11.5) | 19 | (12.1) | |||
| 3 | 8 | (2.9) | 0 | (0) | 8 | (3.1) | 3 | (3.7) | 5 | (2.5) | 5 | (4.1) | 3 | (1.9) | |||
| 4 | 3 | (1.1) | 0 | (0) | 3 | (1.2) | 2 | (2.5) | 1 | (0.5) | 2 | (1.6) | 1 | (0.6) | |||
| Missing | 119 | 11 | 107 | 33 | 84 | 41 | 76 | ||||||||||
| Metastatic sites | 0.264 | 0.646 | 0.774 | ||||||||||||||
| Single | 241 | (60.6) | 23 | (69.7) | 217 | (59.8) | 71 | (62.3) | 168 | (59.8) | 100 | (61.3) | 139 | (59.9) | |||
| Multiple | 157 | (39.4) | 10 | (30.3) | 146 | (40.2) | 43 | (37.7) | 113 | (40.2) | 63 | (38.7) | 93 | (40.1) | |||
| Missing | 1 | 0 | 1 | 0 | 1 | 0 | 1 | ||||||||||
| Leucocyte count | 0.151 | 0.337 | 0.501 | ||||||||||||||
| < 10000 | 219 | (74.2) | 20 | (87.0) | 198 | (73.3) | 60 | (70.6) | 158 | (76.0) | 89 | (72.4) | 129 | (75.9) | |||
| ≥ 10000 | 76 | (25.8) | 3 | (13.0) | 72 | (26.7) | 25 | (29.4) | 50 | (24.0) | 34 | (27.6) | 41 | (24.1) | |||
| Missing | 104 | 10 | 94 | 29 | 74 | 40 | 63 | ||||||||||
| ALP | 0.138 | 0.023 | 0.344 | ||||||||||||||
| < 300 | 217 | (81.0) | 19 | (95.0) | 197 | (79.8) | 53 | (71.6) | 163 | (84.5) | 85 | (78.0) | 131 | (82.9) | |||
| ≥ 300 | 51 | (19.0) | 1 | (5.0) | 50 | (20.2) | 21 | (28.4) | 30 | (15.5) | 24 | (22.0) | 27 | (17.1) | |||
| Missing | 131 | 13 | 117 | 40 | 89 | 54 | 75 | ||||||||||
| Prior anemia | < 10-4 | 0.103 | 0.763 | ||||||||||||||
| Grade 0 | 175 | (44.8) | 28 | (84.8) | 145 | (40.7) | 41 | (38.0) | 132 | (47.1) | 69 | (42.3) | 104 | (45.2) | |||
| Grade 1-2-3-4 | 216 | (55.2) | 5 | ( 15.2) | 211 | (59.3) | 67 | (62.0) | 148 | (52.9) | 89 | (54.6) | 126 | (54.8) | |||
| Missing | 8 | 0 | 8 | 6 | 2 | 5 | 3 | ||||||||||
| CEA | |||||||||||||||||
| n | 307 | 23 | 282 | 90 | 215 | 126 | 179 | ||||||||||
| Mean ± SD | 455.3 ± 1979.3 | 43.7 ± 116.3 | 491.1 ± 2061.4 | 0.008 | 714.8 ± 2643 | 349.6 ± -1629.8 | 0.389 | 487.6 ± 1736.2 | 436.1 ± 2148.4 | 0.219 | |||||||
| Median (min-max) | 18 (0.1-20820) | 5.7 (0.3-508.9) | 19.4 (0.1-20820) | 19.2 (0.1-18380) | 15.6 (0.2-20820) | 21.1 (0.4-14660) | 15.4 (0.1-20820) | ||||||||||
| LDH | |||||||||||||||||
| n | 261 | 20 | 240 | 73 | 187 | 103 | 157 | ||||||||||
| Mean ± SD | 496.4 ± 860.1 | 299.6 ± 225.4 | 513.3 ± 892.8 | 0.282 | 506.7 ± 837.6 | 493 ± 873.1 | 0.374 | 530.8 ± 982.5 | 474.5 ± 774.8 | 0.244 | |||||||
| Median (min-max) | 233 (82-7230) | 224 (117-878) | 234 (82-7230) | 249 (113-6190) | 227 (82-7230) | 248 (83-7230) | 227 (82-6450) | ||||||||||
| Vital status | 0.04 | 0.015 | 0.842 | ||||||||||||||
| Death | 311 | (77.9) | 21 | (63.6) | 288 | (79.1) | 98 | (86.0) | 211 | (74.8) | 128 | (78.5) | 181 | (77.7) | |||
| Censored | 88 | (22.1) | 12 | (36.4) | 76 | (20.9) | 16 | (14.0) | 71 | (25.2) | 35 | (21.5) | 52 | (22.3) | |||
WHO PS: WHO performance status; ALP: Alkaline phosphatase; CEA: Carcinoembryonic antigen; LDH: Lactate dehydrogenase.
Table 2.
Treatment characteristics according to hematological toxicity in 399 consecutive patients with metastatic colorectal cancer n (%)
| All (n = 399) |
Anemia (n
= 397) |
Neutropenia (n
= 396) |
Thrombopenia (n
= 396) |
||||||||||||||
| No (n = 33) | Yes (n = 364) | P | No (n = 114) | Yes (n = 282) | P | No (n = 163) | Yes (n = 233) | P | |||||||||
| Lines of chemotherapy | 0.3 | < 10-4 | 0.053 | ||||||||||||||
| < 2 | 46 | (11.5) | 2 | (6.1) | 44 | (12.1) | 25 | (21.9) | 21 | (7.4) | 25 | (15.3) | 21 | (9) | |||
| ≥ 2 | 353 | (88.5) | 31 | (93.9) | 320 | (87.9) | 89 | (78.1) | 261 | (92.6) | 138 | (84.7) | 212 | (91) | |||
| Lines of chemotherapy | |||||||||||||||||
| n | 399 | 33 | 364 | 114 | 282 | 163 | 233 | ||||||||||
| Mean ± SD | 4.4 ± 2.5 | 5 ± 2.8 | 4.4 ± 2.5 | 0.266 | 3.4 ± 2.1 | 4.9 ± 2.6 | < 10-4 | 3.9 ± 2.4 | 4.9 ± 2.6 | < 10-4 | |||||||
| Median (min-max) | 4 (1-13) | 4 (1-11) | 4 (1-13) | 3 (1-9) | 5 (1-13) | 3 (1-12) | 5 (1-13) | ||||||||||
| Number of drugs | 0.760 | < 10-4 | < 10-3 | ||||||||||||||
| 1 | 20 | (5) | 2 | (6.1) | 18 | (4.9) | 13 | (11.4) | 7 | (2.5) | 15 | (9.2) | 5 | (2.1) | |||
| 2 | 79 | (19.8) | 5 | (15.2) | 74 | (20.3) | 34 | (29.8) | 45 | (16.0) | 42 | (25.8) | 37 | (15.9) | |||
| 3 | 300 | (75.2) | 26 | (78.8) | 272 | (74.7) | 67 | (58.8) | 230 | (81.6) | 106 | (65.0) | 191 | (82.0) | |||
| Bevacizumab | 0.613 | 0.022 | 0.245 | ||||||||||||||
| Yes | 226 | (57.1) | 17 | (53.1) | 209 | (57.7) | 54 | (48.2) | 171 | (60.9) | 86 | (53.8) | 139 | (59.7) | |||
| No | 170 | (42.9) | 15 | (46.9) | 153 | (42.3) | 58 | (51.8) | 110 | (39.1) | 74 | (46.3) | 94 | (40.3) | |||
| Missing | 3 | 1 | 2 | 2 | 1 | 3 | 0 | ||||||||||
| Anti- EGFR mab | 0.789 | 0.004 | 0.096 | ||||||||||||||
| Yes | 165 | (41.6) | 14 | (43.8) | 150 | (41.3) | 34 | (30.4) | 130 | (46.1) | 59 | (36.6) | 105 | (45.1) | |||
| No | 232 | (58.4) | 18 | (56.3) | 213 | (58.7) | 78 | (69.6) | 152 | (53.9) | 102 | (63.4) | 128 | (54.9) | |||
| Missing | 2 | 1 | 1 | 2 | 0 | 2 | 0 | ||||||||||
| Granulocyte-colony stimulating factor use | 0.838 | < 10-4 | 0.003 | ||||||||||||||
| Yes | 105 | (26.7) | 9 | (28.1) | 95 | (26.5) | 10 | (9.2) | 94 | (33.5) | 30 | (18.8) | 74 | (32.2) | |||
| No | 288 | (73.3) | 23 | (71.9) | 264 | (73.5) | 99 | (90.8) | 187 | (66.5) | 130 | (81.3) | 156 | (67.8) | |||
| Missing | 6 | 1 | 5 | 5 | 1 | 3 | 3 | ||||||||||
| EPO use | 0.049 | 0.57 | 0.41 | ||||||||||||||
| Yes | 76 | (19.4) | 2 | (6.3) | 74 | (20.7) | 23 | (21.1) | 52 | (18.6) | 34 | (21.3) | 41 | (17.9) | |||
| No | 316 | (80.6) | 30 | (93.8) | 284 | (79.3) | 86 | (78.9) | 228 | (81.4) | 126 | (78.8) | 188 | (82.1) | |||
| Missing | 7 | 1 | 6 | 5 | (4.6) | 2 | 3 | 4 | |||||||||
| Surgery of primary tumor | 0.052 | 0.059 | 0.487 | ||||||||||||||
| Yes | 323 | (81.2) | 31 | (93.9) | 291 | (80.2) | 86 | (75.4) | 235 | (83.6) | 129 | (79.6) | 192 | (82.4) | |||
| No | 75 | (18.8) | 2 | (6.1) | 72 | (19.8) | 28 | (24.6) | 46 | (16.4) | 33 | (20.4) | 41 | (17.6) | |||
| Missing | 1 | 0 | 1 | 0 | 1 | 1 | 0 | ||||||||||
| Surgery of metastatic site | 0.148 | 0.007 | 0.001 | ||||||||||||||
| Yes | 102 | (25.7) | 13 | (40.6) | 103 | (28.4) | 22 | (19.6) | 94 | (33.3) | 33 | (20.4) | 83 | (35.8) | |||
| No | 295 | (74.3) | 19 | (59.4) | 260 | (71.6) | 90 | (80.4) | 188 | (66.7) | 129 | (79.6) | 149 | (64.2) | |||
| Missing | 2 | 1 | 1 | 2 | 0 | 1 | 1 | ||||||||||
GCSF: Granulocyte-colony stimulating factor; EPO: Erythropoietin.
Hematological toxicity
In the whole population, neutropenia occurred in 71.2% of the patients: 36.1% experienced grade 1-2 (mild) neutropenia, and 35.1% had grade 3-4 (severe) neutropenia during the first two lines of chemotherapy. Most patients (91.7%) developed anemia. Among these, 71.3% developed grade 1-2 anemia and only 20.4% had grade 3-4 anemia during the first two lines of chemotherapy. Comparatively few patients developed thrombocytopenia during treatment (58.8%). Grade 1-2 thrombocytopenia occurred in 50.7% of patients, and only 8.1% of patients experienced grade 3-4 thrombocytopenia. Most classical prognostic variables did not differ significantly between patients who had hematological toxicity and those who did not (Table 1). However, patients who experienced thrombocytopenia were significantly younger than those who did not (P < 0.001). Similarly, patients who experienced neutropenia were more frequently younger (P < 0.0001), had better performance status (P = 0.024), and more frequently had low alkaline phosphatase serum level at diagnosis (P = 0.023), compared with those without neutropenia (Table 1). Figure 1 shows the worst grade of neutropenia, anemia and thrombocytopenia recorded at each cycle of chemotherapy during the two first lines of treatment in the 399 patients analyzed. Table 2 represents hematological toxicity in function of treatment received. Except for neutropenia, the use of anti-EGFR or bevacizumab was not associated with significantly higher hematological toxicity. Neutropenia, but not thrombocytopenia or anemia, was more frequently experienced by patients who received all three major drugs (P < 0.0001, P = 0.053, P = 0.3), as compared to patients who received one or two drugs, or fewer than two lines of chemotherapy (Table 2).
Figure 1.
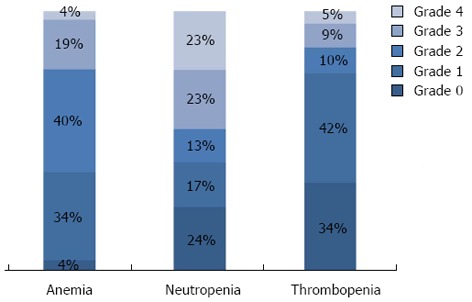
Worst grade of hematological toxicity (neutropenia, anemia, and thrombocytopenia) recorded during the first two lines of chemotherapy in metastatic colorectal cancer patients (n = 399).
Relationship between hematological toxicity and prognosis
At the time of analysis, 311 patients had died (77.9%). Patients who experienced at least one episode of neutropenia had significantly better outcome compared to those with no neutropenia in terms of OS (log-rank test P < 0.0001) (Figure 2A). Similarly, patients who experienced thrombocytopenia also had significantly better OS compared to patients that did not present with thrombocytopenia during chemotherapy (log-rank test P = 0.008) (Figure 2B). In contrast, occurrence of anemia was associated with poorer outcome (log-rank test P = 0.038) (Figure 2C). Mild (grade 1-2) or severe (grade 3-4) toxicity had the same prognostic role for each hematological toxicity. Two hundred and four patients received FOLFOX (5-fluorouracil plus oxaliplatin combination) alone or with targeted therapies; 113 patients received FOLFIRI (5-fluorouracil plus irinotecan combination) alone or with targeted therapies; and 82 patients received monotherapy by fluoropyrimidine alone or with targeted therapies as first-line treatment. We observed that neutropenia was a predictor of better outcome in patients that received FOLFOX alone or with targeted therapies (mean OS of 27 mo in patients without neutropenia vs 47 mo in patients with neutropenia, P = 0.004), or in patients that received FOLFIRI alone or with targeted therapies as first-line therapy (mean OS of 28 mo in patients without neutropenia vs 41 mo in patients with neutropenia, P = 0.04). Similar results were observed in patients treated with fluoropyrimidine alone or with targeted therapies as first-line therapy (mean OS of 22 mo in patients without neutropenia vs 34 mo in patients with neutropenia, P = 0.0005).
Figure 2.
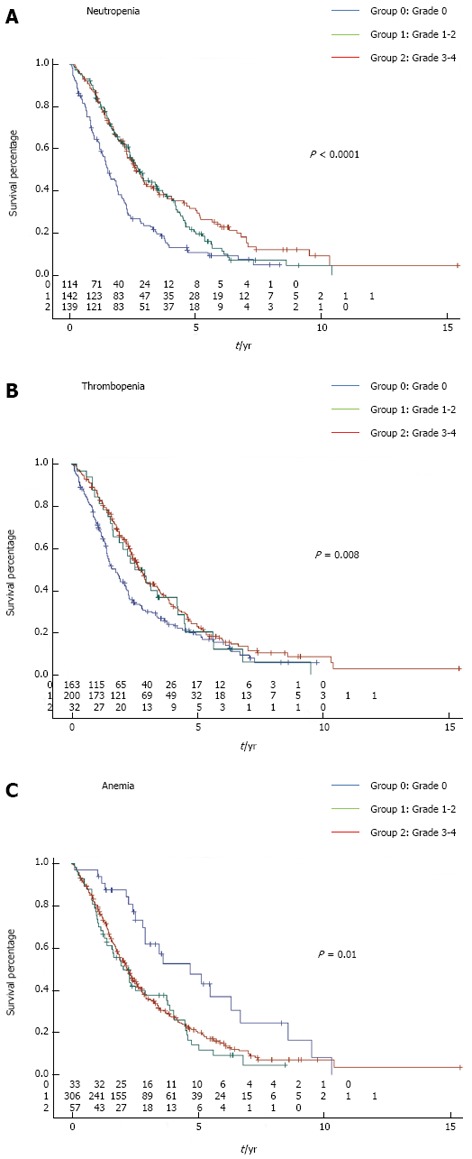
Kaplan-Meier curves for overall survival stratified according to the occurrence or not of neutropenia (A), thrombocytopenia (panel B), and anemia (C) recorded during the first two lines of chemotherapy in metastatic colorectal cancer patients (n = 399). P values were calculated using the log-rank test.
Univariate analysis of factors associated with OS indicated that among the classical prognostic factors, age > 75 years, primitive colon (vs rectal) tumor, poor WHO status, CEA level > 200 ng/mL, multiple metastatic sites, initial anemia, high ALP or LDH serum levels, and high leukocyte count were also significantly associated with poorer OS (Table 3). As regards treatment-related variables, bevacizumab use, anti-EGFR use, resection of the primary tumor, resection of metastases, and use of the three major cytotoxic drugs were also significantly associated with better survival (Table 3).
Table 3.
Univariate and multivariate analysis (Cox regression) of factors associated with overall survival in 399 consecutive patients with metastatic colorectal cancer
|
Univariate analysis
n
= 399 |
Multivariate analysis
n
= 214 |
||||||||
| HR | 95%CI | P | HR | 95%CI | P | ||||
| Age | < 10-4 | ||||||||
| ≥ 75 | 1.77 | 1.33 | 2.35 | ||||||
| Sex | 0.615 | ||||||||
| Male | 1 | ||||||||
| Female | 1.06 | 0.85 | 1.33 | ||||||
| Origin | 0.04 | 0.0767 | |||||||
| Colon | 1 | 1 | |||||||
| Rectum | 0.75 | 0.57 | 0.99 | 0.68 | 0.44 | 1.04 | |||
| WHO PS | < 10-4 | 0.021 | |||||||
| 0 | 1 | 1 | |||||||
| 1-2-3-4 | 2.15 | 1.63 | 2.82 | 1.53 | 1.07 | 2.19 | |||
| CEA | < 10-4 | 0.143 | |||||||
| < 200 | 1 | 1 | |||||||
| ≥ 200 | 2.31 | 1.72 | 3.09 | 1.37 | 0.90 | 2.09 | |||
| Prior anemia | < 10-4 | 0.188 | |||||||
| No | 1 | 1 | |||||||
| Yes | 1.83 | 1.45 | 2.31 | 0.79 | 0.55 | 1.13 | |||
| Anemia | 0.005 | 0.029 | |||||||
| No | 1 | 1 | |||||||
| Yes | 1.91 | 1.22 | 2.98 | 2.19 | 1.08 | 4.41 | |||
| Thrombopenia | 0.003 | 0.036 | |||||||
| No | 1 | 1 | |||||||
| Yes | 0.70 | 0.56 | 0.88 | 0.69 | 0.49 | 0.98 | |||
| Neutropenia | < 10-4 | < 10-4 | |||||||
| No | 1 | 1 | |||||||
| Yes | 0.55 | 0.44 | 0.705 | 0.43 | 0.29 | 0.64 | |||
| Metastatic sites | 0.005 | 0.026 | |||||||
| 1 | 1 | 1 | |||||||
| > 1 | 1.40 | 1.11 | 1.77 | 1.49 | 1.05 | 2.11 | |||
| Surgery of the primary tumor | < 10-4 | < 10-3 | |||||||
| No | 1 | 1 | |||||||
| Yes | 0.33 | 0.25 | 0.44 | 0.46 | 0.30 | 0.70 | |||
| Surgery of metastatic site | < 10-4 | < 10-3 | |||||||
| No | 1 | 1 | |||||||
| Yes | 0.27 | 0.21 | 0.36 | 0.48 | 0.31 | 0.74 | |||
| ALP | < 10-4 | 0.007 | |||||||
| < 300 | 1 | 1 | |||||||
| ≥ 300 | 3 | 2.16 | 4.18 | 1.81 | 1.18 | 2.78 | |||
| LDH | < 10-4 | < 10-3 | |||||||
| < 233 | 1 | 1 | |||||||
| ≥ 233 | 2.15 | 1.62 | 2.85 | 2.03 | 1.38 | 2.97 | |||
| Leucocyte count | < 10-4 | ||||||||
| < 10000 | 1 | ||||||||
| ≥ 10000 | 2.04 | 1.53 | 2.73 | ||||||
| Anti-EGFR Mab | 0.006 | ||||||||
| No | 1 | ||||||||
| Yes | 0.73 | 0.58 | 0.91 | ||||||
| Bevacizumab | < 10-4 | 0.107 | |||||||
| No | 1 | 1 | |||||||
| Yes | 0.61 | 0.49 | 0.76 | 0.76 | 0.55 | 1.06 | |||
| Chemotherapy lines | < 10-4 | ||||||||
| < 2 | 1 | ||||||||
| ≥ 2 | 0.46 | 0.32 | 0.66 | ||||||
| Chemotherapy molecules | < 10-4 | ||||||||
| 1 | 1 | ||||||||
| 2 | 0.5 | 0.29 | 0.87 | ||||||
| 3 | 0.35 | 0.21 | 0.58 | ||||||
WHO PS: WHO performance status; ALP: Alkaline phosphatase; LDH: Lactate dehydrogenase.
Multivariate analysis revealed that poor WHO status (P = 0.021), anemia during the first two chemotherapy lines (P = 0.029), multiple metastases locations (P = 0.026), and high serum levels of ALP (P = 0.007), or LDH (P < 0.001), were independently associated with poorer OS. On the contrary, resection of the primary tumor (P < 0.001), resection of metastases (P < 0.001), occurrence of thrombocytopenia (P = 0.036) and neutropenia (P < 0.001) were all independently associated with better OS (Table 3). Thrombocytopenia and neutropenia were better correlated (r = 0.35) than anemia and neutropenia (r = 0.03), or anemia and thrombocytopenia (r = 0.04).
Analysis with multiple imputations confirmed that neutropenia during chemotherapy for mCRC was associated with increased survival (P = 0.004), while thrombocytopenia was no longer significant (P = 0.079). To avoid the bias of longer survival in patients with hematological toxicity, a landmark analysis using a time point of 6 mo from the diagnosis was performed because > 90% of patients developed hematological toxicity during the first 6 mo follow-up[9]. Patients who died before the landmark time were excluded from the analysis regardless of the presence or absence of hematological toxicity. Thirty-five patients were excluded from the analysis because they died before the landmark time. Using such analysis, univariate and multivariate analyses confirm that neutropenia and thrombocytopenia during chemotherapy for mCRC were associated with increased survival (data not shown).
DISCUSSION
This study shows that both chemotherapy-induced thrombocytopenia and neutropenia are associated with better OS in patients treated for mCRC. In contrast, the occurrence of anemia during chemotherapy is associated with poorer survival. Surprisingly, each type of hematological toxicity had the same prognostic role, regardless of the severity [mild (grade 1-2) or severe (grade 3-4) toxicity], suggesting that there is no relation between the severity of the toxicity and the prognostic effect.
Several studies have previously focused on the prognostic role of neutropenia occurring in patients receiving chemotherapy for various tumor types. Chemotherapy-induced neutropenia was recognized as a prognostic factor of better survival in patients with metastatic lung, gastric, or ovarian cancer[9-11], and also in breast or esophageal cancer patients treated in a neoadjuvant setting[12,13]. In the context of CRC, a retrospective Japanese study recently evaluated the impact of neutropenia in a cohort of 153 patients treated by fluorouracil and oxaliplatin as first-line treatment for mCRC[15]. The occurrence of both mild and severe neutropenia was associated with better outcome. Of note, as in our study, both mild and severe neutropenia had a favorable impact on patient outcome. However, we extended this finding in a larger population that received different chemotherapy regimens, thus suggesting that the favorable prognostic role of neutropenia during chemotherapy for mCRC is a general phenomenon that may not depend on the type of chemotherapy. In addition, our study involved Caucasian patients, suggesting that the observations of Shitara et al[15] may be generalized to different ethnic populations, and may not rely on a particular drug metabolism in patients of Japanese origin.
Thrombocytopenia at baseline is known to be a factor of poor prognosis in patients with hematological malignancy[18,19]. However, to the best of our knowledge, no information has been reported in the literature regarding the prognostic role of chemotherapy-induced thrombocytopenia. In our study, thrombocytopenia was also found to be an independent prognostic factor of better survival. The prognostic role of both neutropenia and thrombocytopenia strongly suggests that these hematological toxicities could be used a surrogate maker of the efficacy of cytotoxic chemotherapy.
Hematological toxicity may reflect the biological activity of cytotoxic drugs, while the absence of toxicity may, on the contrary, indicate possible underdosing. Such underdosing may result from the use of body surface area (BSA) to determine the dose of chemotherapy. In the case of fluoropyrimidine, it is well known that using the BSA may result in either under- or overdosing. Personal dose adaptation could limit toxicity, and enhance efficacy[20-22]. Similarly, BSA is not a valuable tool to adapt irinotecan-based regimens. The degradation of the irinotecan bioactive metabolite SN38 is dependent on UGT1A1 polymorphism. UGT1A1*28 variant is a common allele with seven TA repeats in the promoter, compared with the wild-type allele (UGT1A1*1) with six TA repeats[23]. UGT1A1*28 variant is associated with decreased gene transcription and expression, as well as reduced enzyme activity, which leads to higher exposure of SN38, and thus higher hematological and digestive toxicity[24,25]. Concerning oxaliplatin, pharmacokinetic data indicate that plasma clearance of oxaliplatin is dependent not only on BSA, but also on age, sex and serum creatinine level[26]. In addition, it is also possible that underdosing of chemotherapy in everyday clinical practice may be a result of the methods used for phase I studies to determine maximum tolerated dose. A low number of patients are included in phase I trials, and these studies do not take into account inter-individual variation in drug pharmacokinetics[27].
Surprisingly, in our study, multivariate analysis revealed that anemia occurring during chemotherapy was associated with poorer outcome, while prior anemia was not. Previous studies have shown that initial anemia is a factor for poor prognosis in localized[28,29] and metastatic CRC[30]. However, in our study, the anemia variables (i.e., anemia existing before and occurring during chemotherapy) were strongly correlated (r² = 0.25).
Some limitations of our study include the single-center design, and the use of different chemotherapeutic schedules, which could affect survival. In addition, hematological toxicity could be associated with more intensive chemotherapeutic regimens, or longer treatment duration. Thus, we cannot exclude the possibility that the prognostic role of hematological toxicity could be linked to an immortal time bias[31]. However, to limit this problem, we decided to focus only on hematological toxicity occurring during the two first lines of chemotherapy. Another potential limit is the number of missing data. Despite the missing data, we chose to include in the multivariate analysis the WHO performance status, ALP and CEA levels and the leukocyte count, because they are known prognostic factors, and were strongly associated with OS by univariate analysis. To handle the missing data issue, we performed Cox regression using multiple imputations. This analysis strengthened our findings that neutropenia is an independent prognostic factor of better survival. As regards thrombocytopenia, the result was less clear, but there was a trend towards better survival in patients experiencing thrombocytopenia.
In conclusion, neutropenia and thrombocytopenia occurring during the two first lines of chemotherapy for mCRC are associated with better survival. One hypothesis could be that using BSA to calculate the dose of chemotherapy in mCRC patients is not ideal. However, other mechanisms could explain our observation. Further clinical trials are warranted to determine whether adaptation of drug doses based on hematological toxicity could improve the efficacy of standard regimens in mCRC patients.
ACKNOWLEDGMENTS
We thank Fiona Ecarnot for assistance with writing English.
COMMENTS
Background
During metastatic colorectal cancer, cytotoxic agents are the major anticancer agents. Currently, the dosage schedule of chemotherapy remains empirical. Further research is warranted to define better the optimal schedules.
Research frontiers
Usually, the recommended dosage of chemotherapy is determined on the basis of dose-finding phase I trials. Such studies determine the toxic profiles of cytotoxic agents in a small number of patients, but do not take into account the inter-individual variability of drug metabolism. Some reports suggests that hematological toxicity may reflect the bioactive dosage of chemotherapy.
Innovations and breakthroughs
Neutropenia and thrombocytopenia occurring during the first two lines of chemotherapy were significantly associated with better overall survival (OS) (HR = 0.55, 95%CI: 0.43-0.70, P < 0.0001 and HR = 0.70, 95%CI: 0.56-0.88, P = 0.025 respectively). In contrast, anemia during chemotherapy was significantly associated with poorer OS (HR = 1.9, 95%CI: 1.22-2.97, P = 0.005).
Applications
This study underlines the possible prognostic role of neutropenia and thrombocytopenia occurring during chemotherapy for metastatic colorectal cancer (mCRC).
Terminology
Hematological toxicity, namely neutropenia, anemia and thrombocytopenia, was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. For each type of cytopenia, patients were divided into three categories: absent (grade 0), mild (grades 1-2), and severe (grades 3-4).
Peer review
This is a well-written, interesting article showing an association between neutropenia and thrombocytopenia occurring during chemotherapy and better survival for mCRC. It would be of interest to know where the metastases were, if they were metachronous or synchronous, why 60% of the patients had only one metastatic site, and only 25% had surgery for the metastases.
Footnotes
P- Reviewers: Muraro MG, Nedrebo BS, Sorbye H S- Editor: Zhai HH L- Editor: Kerr C E- Editor: Liu XM
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Manfredi S, Bouvier AM, Lepage C, Hatem C, Dancourt V, Faivre J. Incidence and patterns of recurrence after resection for cure of colonic cancer in a well defined population. Br J Surg. 2006;93:1115–1122. doi: 10.1002/bjs.5349. [DOI] [PubMed] [Google Scholar]
- 3.McArdle C. ABC of colorectal cancer: effectiveness of follow up. BMJ. 2000;321:1332–1335. doi: 10.1136/bmj.321.7272.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venderbosch S, de Wilt JH, Teerenstra S, Loosveld OJ, van Bochove A, Sinnige HA, Creemers GJ, Tesselaar ME, Mol L, Punt CJ, et al. Prognostic value of resection of primary tumor in patients with stage IV colorectal cancer: retrospective analysis of two randomized studies and a review of the literature. Ann Surg Oncol. 2011;18:3252–3260. doi: 10.1245/s10434-011-1951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemeny N, Niedzwiecki D, Shurgot B, Oderman P. Prognostic variables in patients with hepatic metastases from colorectal cancer. Importance of medical assessment of liver involvement. Cancer. 1989;63:742–747. doi: 10.1002/1097-0142(19890215)63:4<742::aid-cncr2820630423>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 8.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 9.Di Maio M, Gridelli C, Gallo C, Shepherd F, Piantedosi FV, Cigolari S, Manzione L, Illiano A, Barbera S, Robbiati SF, et al. Chemotherapy-induced neutropenia and treatment efficacy in advanced non-small-cell lung cancer: a pooled analysis of three randomised trials. Lancet Oncol. 2005;6:669–677. doi: 10.1016/S1470-2045(05)70255-2. [DOI] [PubMed] [Google Scholar]
- 10.Shitara K, Matsuo K, Takahari D, Yokota T, Shibata T, Ura T, Ito S, Sawaki A, Tajika M, Kawai H, et al. Neutropenia as a prognostic factor in advanced gastric cancer patients undergoing second-line chemotherapy with weekly paclitaxel. Ann Oncol. 2010;21:2403–2409. doi: 10.1093/annonc/mdq248. [DOI] [PubMed] [Google Scholar]
- 11.Kim YH, Chung HH, Kim JW, Park NH, Song YS, Kang SB. Prognostic significance of neutropenia during adjuvant concurrent chemoradiotherapy in early cervical cancer. J Gynecol Oncol. 2009;20:146–150. doi: 10.3802/jgo.2009.20.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyoshi N, Yano M, Takachi K, Kishi K, Noura S, Eguchi H, Yamada T, Miyashiro I, Ohue M, Ohigashi H, et al. Myelotoxicity of preoperative chemoradiotherapy is a significant determinant of poor prognosis in patients with T4 esophageal cancer. J Surg Oncol. 2009;99:302–306. doi: 10.1002/jso.21235. [DOI] [PubMed] [Google Scholar]
- 13.Han Y, Yu Z, Wen S, Zhang B, Cao X, Wang X. Prognostic value of chemotherapy-induced neutropenia in early-stage breast cancer. Breast Cancer Res Treat. 2012;131:483–490. doi: 10.1007/s10549-011-1799-1. [DOI] [PubMed] [Google Scholar]
- 14.Saarto T, Blomqvist C, Rissanen P, Auvinen A, Elomaa I. Haematological toxicity: a marker of adjuvant chemotherapy efficacy in stage II and III breast cancer. Br J Cancer. 1997;75:301–305. doi: 10.1038/bjc.1997.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shitara K, Matsuo K, Takahari D, Yokota T, Inaba Y, Yamaura H, Sato Y, Najima M, Ura T, Muro K. Neutropaenia as a prognostic factor in metastatic colorectal cancer patients undergoing chemotherapy with first-line FOLFOX. Eur J Cancer. 2009;45:1757–1763. doi: 10.1016/j.ejca.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Ng WW, Cheung YS, Wong J, Lee KF, Lai PB. A preliminary analysis of combined liver resection with new chemotherapy for synchronous and metachronous colorectal liver metastasis. Asian J Surg. 2009;32:189–197. doi: 10.1016/S1015-9584(09)60394-8. [DOI] [PubMed] [Google Scholar]
- 17.Pan W. A multiple imputation approach to Cox regression with interval-censored data. Biometrics. 2000;56:199–203. doi: 10.1111/j.0006-341x.2000.00199.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen LP, Lin SJ, Yu MS. Prognostic value of platelet count in diffuse large B-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2012;12:32–37. doi: 10.1016/j.clml.2011.09.215. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Porras JR, Cordoba I, Such E, Nomdedeu B, Vallespi T, Carbonell F, Luño E, Ardanaz M, Ramos F, Pedro C, et al. Prognostic impact of severe thrombocytopenia in low-risk myelodysplastic syndrome. Cancer. 2011;117:5529–5537. doi: 10.1002/cncr.26173. [DOI] [PubMed] [Google Scholar]
- 20.Gamelin E, Delva R, Jacob J, Merrouche Y, Raoul JL, Pezet D, Dorval E, Piot G, Morel A, Boisdron-Celle M. Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: results of a multicenter randomized trial of patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2099–2105. doi: 10.1200/JCO.2007.13.3934. [DOI] [PubMed] [Google Scholar]
- 21.Kaldate RR, Haregewoin A, Grier CE, Hamilton SA, McLeod HL. Modeling the 5-fluorouracil area under the curve versus dose relationship to develop a pharmacokinetic dosing algorithm for colorectal cancer patients receiving FOLFOX6. Oncologist. 2012;17:296–302. doi: 10.1634/theoncologist.2011-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capitain O, Asevoaia A, Boisdron-Celle M, Poirier AL, Morel A, Gamelin E. Individual fluorouracil dose adjustment in FOLFOX based on pharmacokinetic follow-up compared with conventional body-area-surface dosing: a phase II, proof-of-concept study. Clin Colorectal Cancer. 2012;11:263–267. doi: 10.1016/j.clcc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Miners JO, McKinnon RA, Mackenzie PI. Genetic polymorphisms of UDP-glucuronosyltransferases and their functional significance. Toxicology. 2002;181-182:453–456. doi: 10.1016/s0300-483x(02)00449-3. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Cheng D, Kuang Q, Liu G, Xu W. Association between UGT1A1*28 polymorphisms and clinical outcomes of irinotecan-based chemotherapies in colorectal cancer: a meta-analysis in Caucasians. PLoS One. 2013;8:e58489. doi: 10.1371/journal.pone.0058489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Cheng D, Kuang Q, Liu G, Xu W. Association of UGT1A1*28 polymorphisms with irinotecan-induced toxicities in colorectal cancer: a meta-analysis in Caucasians. Pharmacogenomics J. 2013:Epub ahead of print. doi: 10.1038/tpj.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delord JP, Umlil A, Guimbaud R, Grégoire N, Lafont T, Canal P, Bugat R, Chatelut E. Population pharmacokinetics of oxaliplatin. Cancer Chemother Pharmacol. 2003;51:127–131. doi: 10.1007/s00280-002-0550-3. [DOI] [PubMed] [Google Scholar]
- 27.Gurney H. How to calculate the dose of chemotherapy. Br J Cancer. 2002;86:1297–1302. doi: 10.1038/sj.bjc.6600139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhen L, Zhe S, Zhenning W, Zhifeng M, Zhidong L, Xiaoxia L, Jianguang Y, Huimian X. Iron-deficiency anemia: a predictor of diminished disease-free survival of T3N0M0 stage colon cancer. J Surg Oncol. 2012;105:371–375. doi: 10.1002/jso.22032. [DOI] [PubMed] [Google Scholar]
- 29.Qiu MZ, Yuan ZY, Luo HY, Ruan DY, Wang ZQ, Wang FH, Li YH, Xu RH. Impact of pretreatment hematologic profile on survival of colorectal cancer patients. Tumour Biol. 2010;31:255–260. doi: 10.1007/s13277-010-0024-x. [DOI] [PubMed] [Google Scholar]
- 30.Zacharakis M, Xynos ID, Lazaris A, Smaro T, Kosmas C, Dokou A, Felekouras E, Antoniou E, Polyzos A, Sarantonis J, et al. Predictors of survival in stage IV metastatic colorectal cancer. Anticancer Res. 2010;30:653–660. [PubMed] [Google Scholar]
- 31.Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. doi: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]


