Abstract
AIM: To evaluate transjugular intrahepatic portosystemic shunt (TIPS) with covered stents for hepatocellular carcinoma (HCC) with main portal vein tumor thrombus (PVTT).
METHODS: Eleven advanced HCC patients (all male, aged 37-78 years, mean: 54.3 ± 12.7 years) presented with acute massive upper gastrointestinal bleeding (n = 9) or refractory ascites (n = 2) due to tumor thrombus in the main portal vein. The diagnosis of PVTT was based on contrast-enhanced computed tomography and color Doppler sonography. The patients underwent TIPS with covered stents. Clinical characteristics and average survival time of 11 patients were analyzed. Portal vein pressure was assessed before and after TIPS. The follow-up period was 2-18 mo.
RESULTS: TIPS with covered stents was successfully completed in all 11 patients. The mean portal vein pressure was reduced from 32.0 to 11.8 mmHg (t = 10.756, P = 0.000). Gastrointestinal bleeding was stopped in nine patients. Refractory ascites completely disappeared in one patient and was alleviated in another. Hepatic encephalopathy was observed in six patients and was resolved with drug therapy. During the follow-up, ultrasound indicated the patency of the shunt and there was no recurrence of symptoms. Death occurred 2-14 mo (mean: 5.67 mo) after TIPS in nine cases, which were all due to multiple organ failure. In the remaining two cases, the patients were still alive at the 16- and 18-mo follow-up, respectively.
CONCLUSION: TIPS with covered stents for HCC patients with tumor thrombus in the main portal vein is technically feasible, and short-term efficacy is favorable.
Keywords: Transjugular intrahepatic portosystemic shunt, Covered stent, Portal hypertension, Main portal vein tumor thrombus, Hepatocellular carcinoma
Core tip: Tumor thrombus invasion of portal veins is very common in advanced hepatocellular carcinoma patients, especially in the patients presenting with upper gastrointestinal hemorrhage. Transjugular intrahepatic portosystemic shunt (TIPS) with bare stents has been attempted to treat those patients. We choose Fluency stent (Bard Inc, Germany) in this study, and our primary experience showed that the efficacy of TIPS with covered stents is favorable for treatment of complications related to portal hypertension due to tumor thrombus invasion in the main portal vein. Significant improvement of symptoms was observed in all the patients. The patency rate was 100%.
INTRODUCTION
Transjugular intrahepatic portosystemic shunt (TIPS) with covered stents has been widely adopted as a minimally invasive treatment for complications related to portal hypertension, such as variceal bleeding and refractory ascites, and the effectiveness has been confirmed by several clinical studies[1-9]. Patients with advanced hepatocellular carcinoma (HCC) may present with fatal massive gastrointestinal bleeding or refractory ascites due to occlusion of the main portal vein by tumor thrombus[10-13]. Studies on TIPS with covered stents in this clinical setting have been limited and its therapeutic role has not been established[14-16].
Here, we report a case series using TIPS with covered stents for the treatment of complications related to portal hypertension due to tumor thrombus invasion in the main portal vein.
MATERIALS AND METHODS
Eleven patients (all male, aged 37-78 years, mean: 54.3 ± 12.7 years) with advanced HCC with main portal vein tumor thrombus were admitted to our department because of acute massive upper gastrointestinal bleeding (volume > 500 mL, n = 9) or refractory ascites (n = 2). The characteristics of the patients are summarized in Table 1. All the patients had coexisting chronic type B hepatitis and cirrhosis. Seven patients underwent transarterial chemoembolization or radiofrequency ablation. The Child-Pugh score was 5, 6 or 7 in three patients each and 8 in two. The Eastern Cooperative Oncology Group performance status was 0-3. Preoperative enhanced computed tomography (CT) scans demonstrated tumor thrombus invasion of the main portal vein and its branches, but the superior mesenteric vein remained patent. Severe gastric varices were found in the nine patients with acute hemorrhage. Sclerotherapy and band ligation under endoscopy were performed but failed to provide any clinical improvement. In the two patients with refractory ascites, the effectiveness of paracentesis and intravenous albumin injection was poor. TIPS placement was then undertaken in all 11 patients for the purpose of saving or prolonging life. Ethics Committee approval was obtained, along with written informed consent from the patients and their family members, before the procedures.
Table 1.
Clinical data of the 11 patients
| Case No. | Age (yr) | Localization of tumor | Localization of portal vein tumor thrombus | Treatment before TIPS | Eextrahepatic metastasis | Symptoms before TIPS | Gastroscopic findings before TIPS | Child-Pugh score |
| 1 | 59 | S2, 3, 4 | Main, left branch | TACE once | Metastasis in both lungs | Vomiting blood for 3 d | Severe varicosis | 6 |
| 2 | 78 | S5, 6 | Main, both left and right branches | None | None | Massive refractory ascites for 3 mo | Not performed | 8 |
| 3 | 41 | S7, 8 | Main, both left and right branches | TACE 3 times | Lymphatic metastasis in hepatic hilus and cardio- diaphragmatic angle | Vomiting blood for 5 d | Severe varicosis | 6 |
| 4 | 66 | S2, 3 | Main, left branch | TACE twice, RF once | None | Massive refractory ascites for 6 mo | Not performed | 7 |
| 5 | 37 | S2, 3, 4, 7 | Main, both left and right branches | None | Metastasis in both lungs | Vomiting blood for 5 d | Severe varicosis | 7 |
| 6 | 46 | S2, 3, 5, 6 | Main, left branch | None | None | Vomiting blood for 6 d | Severe varicosis | 6 |
| 7 | 43 | S5, 6 | Main, right branch | None | None | Vomiting blood for 3 d | Severe varicosis | 5 |
| 8 | 55 | S5, 6, 7 | Main, both left and right branches | TACE 3 times, RF once | Metastasis in both lungs | Vomiting blood for 2 d | Severe varicosis | 8 |
| 9 | 60 | S2, 5, 6 | Main, both left and right branches | TACE once | Metastasis in both lungs | Vomiting blood for 3 d | Severe varicosis | 5 |
| 10 | 57 | S2, 3, 4 | Main, left branch | TACE 2 times | None | Vomiting blood for 7 d | Severe varicosis | 5 |
| 11 | 55 | S5, 8 | Main, both left and right branches | TACE once, RF once | Metastasis in both lungs | Vomiting blood for 1 d | Severe varicosis | 7 |
TIPS: Transjugular intrahepatic portosystemic shunt; TACE: Transcatheter arterial chemoembolization; RF: Radio frequency.
Prior to TIPS, enhanced CT was performed to localize the hepatic vein and the main portal vein and its branches, and to identify their spatial relationship using 3D reconstruction. The right jugular vein was punctured, and a 10-Fr Ring Transjugular Intrahepatic Access Set (Arrow, Reading, PA, United States) was advanced into the vessel. A 16-gauge Colapinto needle (Optimed, Germany) was used to puncture into the portal system. The needle direction was estimated according to the location of the tumor thrombus. For the patients with tumor thrombus in the main portal vein and its left or right branch, we attempted puncture of the patency branch (Figure 1). For the patients with tumor thrombus in the main portal vein and both right and left branches, we attempted to puncture the bifurcation of the main branches. Simultaneous needle withdrawal and contrast agent injection were performed to identify the portal system. Successful access to the portal system was confirmed based on the outline of the tumor thrombus in the portal vein (Figure 2A). The appearance of a “grid-like outline” in the tumor thrombus interspaces, or occasionally, small branches (it belongs to a branch of the left or right stem of hepatic portal vein), was considered to indicate successful puncture of the portal system (Figure 3). Then a hydrophilic guidewire and a catheter (Terumo, Japan) were manipulated through the tumor thrombus to the superior mesenteric vein (Figure 2B). After measurement of the portosystemic pressure gradient (PPG), the 10-Fr sheath was advanced along the guidewire across the tumor thrombus to the superior mesenteric vein. The covered stent (Fluency; Bard, Germany) was advanced. Contrast agent was injected via the 10-Fr sheath to make sure that the tumor thrombus was completely covered. After that, the 10-Fr sheath was withdrawn and the covered stent was released. Shunt venography was performed (Figure 2C). All patients received medical treatment after the TIPS.
Figure 1.
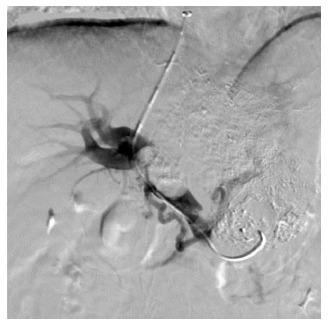
Portal venogram in a 59-year-old man with hepatocellular carcinoma and lung metastasis, who was treated with transjugular intrahepatic portosystemic shunt for severe varicosis and vomiting blood for 3 d. Puncture was successfully performed in the right branch with tumor thrombus in the trunk and left branch of the portal vein.
Figure 2.
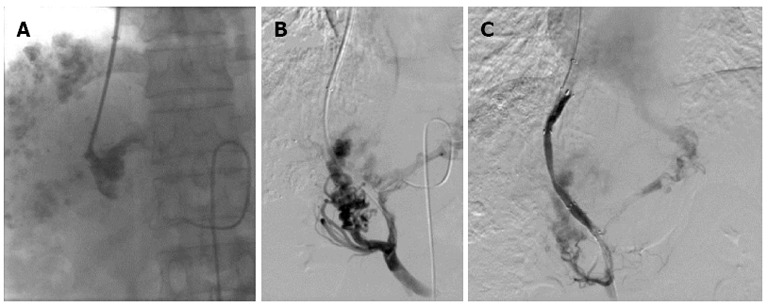
Portal venograms in a 41-year-old man with hepatocellular carcinoma and lymphatic metastasis, who was treated with transjugular intrahepatic portosystemic shunt for severe varicosis and vomiting blood for 5 d. A: Injection of a small amount of contrast agent demonstrated tubular and slow blood flow with no dissipation and the portal vein was clearly defined; B: After a catheter was introduced, the portal vein image was confirmed by 30° right anterior oblique angiography; C: After two Fluency stent grafts with a length of 80 mm and diameter of 8 mm were implanted, the shunt was shown to have smooth blood flow by postoperative angiography.
Figure 3.
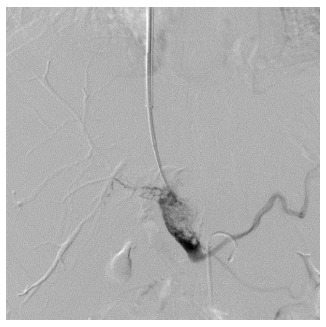
Portal venogram in a 37-year-old man with hepatocellular carcinoma and lung metastasis, who was treated with transjugular intrahepatic portosystemic shunt for severe varicosis and vomiting blood for 5 d. The tumor thrombus was located in the trunk and both left and right branches of the portal vein, and needle puncture was performed in the portal bifurcation. After injection of contrast agent, tumor thrombus was seen as an expansive growth with loose texture, with clear imaging of the portal vein branches.
Statistical analysis
The results are expressed as the mean ± SD. The results of portal vein pressure were assessed before and after TIPS by the paired comparisons t-test. A P-value < 0.05 was considered statistically significant. All calculations were performed using SPSS version 13.0 software for windows.
RESULTS
TIPS was successfully performed in all 11 patients. In the five patients with tumor thrombus in the main portal vein and its left or right branch, the patent branch was successfully punctured. In the six patients with tumor thrombus in the main portal vein and both branches, the bifurcation of the main branches was successfully punctured. Twenty-one Fluency stent grafts (length 4-8 cm) were implanted in all cases, with a diameter 8 mm in 20 and 7 mm in one. The mean PPG was 32.0 ± 4.8 mmHg (range: 26-37 mmHg) before the procedure and decreased to 11.8 ± 3.8 mmHg (range: 7-19 mmHg) after the procedure (t = 10.756, P = 0.000). Gastrointestinal tract bleeding stopped in nine cases after TIPS, without recurrence during follow-up. Refractory ascites completely disappeared in one patient and was alleviated in another. Six patients experienced hepatic encephalopathy (HE; 2 grade I, 3 grade II, and 1 grade III) about 1 wk after TIPS and recovered after administration of lactulose and protein restriction. Color Doppler ultrasound was used for monitoring the patency of the TIPS and demonstrated that the stent grafts were patent during 2-18 mo of follow-up. No patients had recurrence of bleeding or worsening of ascites. Death occurred 2-14 mo (mean: 5.67 mo) after TIPS in nine cases; all due to multiple organ failure. However, the other two cases were still alive at the 16- and 18-mo follow-up, respectively (Table 2).
Table 2.
Transjugular intrahepatic portosystemic shunt procedures and outcomes
| Case No. | Covered stent (diameter × length mm) | Hepatic arterial angiography | PPG before TIPS (mmHg) | PPG after TIPS (mmHg) | Outcomes | Postoperative HE | Follow-up |
| 1 | 8 × 40, 8 × 80 | No obvious APF | 33 | 10 | Hemostasis | Grade II HE | Death after 4 mo |
| 2 | 8 × 80, 8 × 60 | No obvious APF | 29 | 10 | Cured ascites | Grade II HE | Death after 4 mo |
| 3 | 8 × 80, 8 × 80 | No obvious APF | 36 | 12 | Hemostasis | No | Death after 2 mo |
| 4 | 7 × 80 | Obvious APF | 26 | 7 | Alleviated ascites | No | Survived the 16 mo |
| 5 | 8 × 80, 8 × 80 | No obvious APF | 37 | 19 | Hemostasis | Grade I HE | Death after 2 mo |
| 6 | 8 × 80, 8 × 60 | No obvious APF | 32 | 14 | Hemostasis | Grade I HE | Death after 6 mo |
| 7 | 8 × 80, 8 × 60 | No obvious APF | 27 | 12 | Hemostasis | No | Death after 14 mo |
| 8 | 8 × 80, 8 × 60 | No obvious APF | 37 | 13 | Hemostasis | Grade III HE | Death after 3 mo |
| 9 | 8 × 80, 8 × 80 | No obvious APF | 31 | 12 | Hemostasis | No | Death after 10 mo |
| 10 | 8 × 80, 8 × 60 | No obvious APF | 28 | 9 | Hemostasis | No | Survived the 18 mo |
| 11 | 8 × 80, 8 × 60 | No obvious APF | 36 | 12 | Hemostasis | Grade II HE | Death after 6 mo |
TIPS: Transjugular intrahepatic portosystemic shunt; PPG: Portosystemic pressure gradient; APF: Active power filter.
DISCUSSION
Tumor thrombus invasion of the portal veins is common in patients with advanced HCC, especially in those with upper gastrointestinal hemorrhage[9]. TIPS with bare stents has been attempted to treat the complications related to portal hypertension due to tumor thrombus invasion (Table 3)[17-20]. Our study was markedly different from previous ones. First, all the patients were diagnosed with HCC, tumor thrombus in the main portal vein, and complete portal vein occlusion. Second, all portosystemic shunts were created with an expanded polytetrafluoroethylene (ePTFE)-covered stent.
Table 3.
Brief summary of published studies: Transjugular intrahepatic portosystemic shunt for malignant portal vein obstruction n (%)
| Ref. | Type of paper | Technical success rate | Stent used in TIPS | Shunt dysfunction | Indication of TIPS | Localization of PVTT | Prognosis |
| Chung et al[17] | Case report | 6 (100) | Bare stent | 2 (33) | Recurrent variceal bleeding (n = 6) | Portal trunk (n = 6) | 3 patients died at postoperative month 4 |
| Wallace et al[18] | Case series | 37 (97) | Bare stent | 44% | Variceal hemorrhage (n = 16), BCS (n = 3), refractory ascites/hydrothorax (n = 14), other (n = 5) | Portal trunk (n = 6), right branch (n = 5), left branch (n = 2) | 30- and 90-d survival rates were 84% and 60%, respectively |
| Jiang et al[19] | Case series | 10 (71.4) | Bare stent | NA | Intractable ascites (n = 3), simple hemorrhage (n = 1), hemorrhage and ascites (n = 10) | Portal trunk (n = 14), right branch (n = 11), left branch (n = 4) | Mean survival time 132.3 d |
| Burger et al[20] | Case report | 2 (100) | Bare stent | 0% | Recurrent variceal bleeding (n = 1), refractory ascites (n = 1) | Portal trunk (n = 2) | One patient died at postoperative month 3 |
BCS: Budd-Chiari syndrome; NA: Not available; PVTT: Portal vein tumor thrombosis; TIPS: Transjugular intrahepatic portosystemic shunt.
Several problems such as shedding of cancer cells into the circulation, or shunt occlusion by tumor ingrowth into the mesh of the stent may occur after TIPS with bare stents[21-24]. We speculated that creation of TIPS with covered stents may overcome these shortcomings. The most commonly used Viatorr stent (Gore, United States) is not commercially available in China[25,26], therefore, we chose the Fluency stent (Bard) in this study. The Fluency stent is ePTFE-coated throughout the endograft with a 2-mm bare segment[25-27].
Our primary experience showed that the efficacy of TIPS with covered stents is favorable for complications related to portal hypertension due to tumor thrombus invasion in the main portal vein. Significant improvement of symptoms was observed in all 11 patients. The patency rate was 100% during follow-up.
Creation of TIPS in this clinical setting is technically feasible and obviously challenging. How to puncture the portal system remains the greatest challenge. In our study, five patients had tumor thrombus in the trunk of the portal vein and its left or right branch, and the patent branches were successfully accessed with the conventional TIPS method[28,29]. However, for the patients with tumor thrombus in the main portal vein and both its branches, simultaneous needle withdrawal and contrast agent injection were helpful for successful puncture of the portal vein. We preferred the bifurcation of the portal vein as the puncture site, because the portal vein was enlarged due to expansion of the tumor thrombus. Contrast material is injected to determine whether access to a portal vein has been gained. When it displayed like vascular structure and the contrast materials flowed slowly with no dissipation, the portal vein could be clearly defined. The appearance of a “grid-like outline” in the tumor thrombus interspaces, or occasionally, small branches, was considered to indicate successful puncture of the portal system.
In order to achieve long-term shunt patency, the tumor thrombus in the main portal vein should be completely covered. After the procedure, the portal blood flow was redirected to the systemic circulation. Subsequent HE and liver failure are two problems to which one needs to pay attention. In our study, only one patient experienced grade III HE, three grade II, and two grade I; all of whom recovered after administration of lactulose and protein restriction. No death resulted from hepatic failure in our 11 patients. We speculate that the blood supply to the liver from the peripheral portal branches and hepatic artery increased in a compensatory manner during occlusion of the main portal veins[30].
This study had some limitations. First, the sample size in this case series was small, and the effectiveness and safety of TIPS in this clinical setting need to be evaluated in prospective studies with larger samples. Second, the long-term patency of the shunt could not be observed, because the patients had end-stage HCC.
Undoubtedly, the prognosis of advanced HCC is poor. The main purpose of TIPS was to save or prolong life, and this should be explained clearly to the patients and their relatives.
In conclusion, TIPS with covered stents for HCC with tumor thrombus in the main portal vein is technically feasible, challenging, and has favorable short-term efficacy.
COMMENTS
Background
Transjugular intrahepatic portosystemic shunt with covered stents has been widely adopted as a minimally invasive treatment for complications related to portal hypertension, such as variceal bleeding and refractory ascites, and its effectiveness has been confirmed by several clinical studies. Advanced hepatocellular carcinoma patients may present with fatal massive gastrointestinal bleeding or refractory ascites due to occlusion of the main portal vein by tumor thrombus. Transjugular intrahepatic portosystemic shunt with covered stents for those patients is technically feasible, challenging, and with favorable short-term efficacy.
Research frontiers
The authors demonstrated that transjugular intrahepatic portosystemic shunt (TIPS) with the expanded polytetrafluo- roethylene (ePTFE)-covered Fluency stent has favorable short-term efficacy compared with the bare stent. Significant improvement of symptoms was observed in all the patients. The patency rate was 100% during the follow-up.
Innovations and breakthroughs
This study was markedly different from previous ones. First, all the patients were diagnosed with hepatocellular carcinoma, tumor thrombus in the main portal vein, and complete portal vein occlusion. Second, all portosystemic shunts were created with an ePTFE-covered stent.
Applications
TIPS with covered stents is a feasible procedure for hepatocellular carcinoma patients with tumor thrombus in the main portal vein. It is beneficial for advanced hepatocellular carcinoma patients with severe clinical symptoms.
Terminology
The Fluency stent is a polytetrafluoroethylene-encapsulated grid-like cylinder composed of a biocompatible nickel-titanium alloy.
Peer review
In the present manuscript, the authors showed that TIPS with covered stents for hepatocellular carcinoma patients with tumor thrombus in the main portal vein is a feasible procedure.
Footnotes
Supported by Science and Technology Planning Project of Guangdong Province, China, No. 2012B010200027; The Key Technologies RD Program of Guangzhou, China; The Presidential Foundation of the Nanfang Hospital, Southern Medical University, Guangzhou, China, No. 2011B006
P- Reviewers: Cerwenka HR, Hasegawa K, Pirisi M, Wang DS S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 2.Riggio O, Ridola L, Angeloni S, Cerini F, Pasquale C, Attili AF, Fanelli F, Merli M, Salvatori FM. Clinical efficacy of transjugular intrahepatic portosystemic shunt created with covered stents with different diameters: results of a randomized controlled trial. J Hepatol. 2010;53:267–272. doi: 10.1016/j.jhep.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 3.Bureau C, Garcia-Pagan JC, Otal P, Pomier-Layrargues G, Chabbert V, Cortez C, Perreault P, Péron JM, Abraldes JG, Bouchard L, Bilbao JI, Bosch J, Rousseau H, Vinel JP. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized study. Gastroenterology. 2004;126:469–475. doi: 10.1053/j.gastro.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Bureau C, Pagan JC, Layrargues GP, Metivier S, Bellot P, Perreault P, Otal P, Abraldes JG, Peron JM, Rousseau H, Bosch J, Vinel JP. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre study. Liver Int. 2007;27:742–747. doi: 10.1111/j.1478-3231.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- 5.Heinzow HS, Lenz P, Köhler M, Reinecke F, Ullerich H, Domschke W, Domagk D, Meister T. Clinical outcome and predictors of survival after TIPS insertion in patients with liver cirrhosis. World J Gastroenterol. 2012;18:5211–5218. doi: 10.3748/wjg.v18.i37.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng M, Chen Y, Bai J, Zeng Q, You J, Jin R, Zhou X, Shen H, Zheng Y, Du Z. Transjugular intrahepatic portosystemic shunt versus endoscopic therapy in the secondary prophylaxis of variceal rebleeding in cirrhotic patients: meta-analysis update. J Clin Gastroenterol. 2008;42:507–516. doi: 10.1097/MCG.0b013e31815576e6. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823–832. doi: 10.1056/NEJMra0901512. [DOI] [PubMed] [Google Scholar]
- 8.Merli M, Salerno F, Riggio O, de Franchis R, Fiaccadori F, Meddi P, Primignani M, Pedretti G, Maggi A, Capocaccia L, et al. Transjugular intrahepatic portosystemic shunt versus endoscopic sclerotherapy for the prevention of variceal bleeding in cirrhosis: a randomized multicenter trial. Gruppo Italiano Studio TIPS (G.I.S.T.) Hepatology. 1998;27:48–53. doi: 10.1002/hep.510270109. [DOI] [PubMed] [Google Scholar]
- 9.Englesbe MJ, Kubus J, Muhammad W, Sonnenday CJ, Welling T, Punch JD, Lynch RJ, Marrero JA, Pelletier SJ. Portal vein thrombosis and survival in patients with cirrhosis. Liver Transpl. 2010;16:83–90. doi: 10.1002/lt.21941. [DOI] [PubMed] [Google Scholar]
- 10.Qi X, Han G, He C, Yin Z, Zhang H, Wang J, Xia J, Cai H, Yang Z, Bai M, et al. Transjugular intrahepatic portosystemic shunt may be superior to conservative therapy for variceal rebleeding in cirrhotic patients with non-tumoral portal vein thrombosis: a hypothesis. Med Sci Monit. 2012;18:HY37–HY41. doi: 10.12659/MSM.883252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosch J, Abraldes JG, Berzigotti A, Garcia-Pagan JC. Portal hypertension and gastrointestinal bleeding. Semin Liver Dis. 2008;28:3–25. doi: 10.1055/s-2008-1040318. [DOI] [PubMed] [Google Scholar]
- 12.European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 13.de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56 Suppl 1:S75–S87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- 14.Hur J, Lee KH, Lee JH, Yu JS, Won JY, Lee DY. Stent-graft for TIPS in a hepatocellular carcinoma patient with main portal vein invasion. AJR Am J Roentgenol. 2004;182:1301–1304. doi: 10.2214/ajr.182.5.1821301. [DOI] [PubMed] [Google Scholar]
- 15.Serafini FM, Zwiebel B, Black TJ, Carey LC, Rosemurgy AS. Transjugular intrahepatic portasystemic stent shunt in the treatment of variceal bleeding in hepatocellular cancer. Dig Dis Sci. 1997;42:59–65. doi: 10.1023/a:1018876803292. [DOI] [PubMed] [Google Scholar]
- 16.Wallace MJ, Madoff DC. Transjugular intrahepatic portosystemic shunts in patients with hepatic malignancy. Semin Intervent Radiol. 2005;22:309–315. doi: 10.1055/s-2005-925557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung WJ, Jang BK, Park KS, Cho KB, Hwang JS, Ahn SH, Kim YH, Kim YH, Kim YJ. [Effect of transjugular intrahepatic portosystemic shunt for variceal bleeding in hepatocellular carcinoma patients with portal vein thrombosis] Korean J Hepatol. 2005;11:157–163. [PubMed] [Google Scholar]
- 18.Wallace MJ, Madoff DC, Ahrar K, Warneke CL. Transjugular intrahepatic portosystemic shunts: experience in the oncology setting. Cancer. 2004;101:337–345. doi: 10.1002/cncr.20367. [DOI] [PubMed] [Google Scholar]
- 19.Jiang ZB, Shan H, Shen XY, Huang MS, Li ZR, Zhu KS, Guan SH. Transjugular intrahepatic portosystemic shunt for palliative treatment of portal hypertension secondary to portal vein tumor thrombosis. World J Gastroenterol. 2004;10:1881–1884. doi: 10.3748/wjg.v10.i13.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burger JA, Ochs A, Wirth K, Berger DP, Mertelsmann R, Engelhardt R, Roessle M, Haag K. The transjugular stent implantation for the treatment of malignant portal and hepatic vein obstruction in cancer patients. Ann Oncol. 1997;8:200–202. doi: 10.1023/a:1008219307810. [DOI] [PubMed] [Google Scholar]
- 21.Saxon RR. A new era for transjugular intrahepatic portosystemic shunts? J Vasc Interv Radiol. 2004;15:217–219. doi: 10.1097/01.rvi.0000116862.34422.a5. [DOI] [PubMed] [Google Scholar]
- 22.Moszura T, Zubrzycka M, Michalak KW, Rewers B, Dryzek P, Moll JJ, Sysa A, Burczynski P. Acute and late obstruction of a modified Blalock-Taussig shunt: a two-center experience in different catheter-based methods of treatment. Interact Cardiovasc Thorac Surg. 2010;10:727–731. doi: 10.1510/icvts.2009.219741. [DOI] [PubMed] [Google Scholar]
- 23.Artifon EL, Coelho F, Frazao M, Marques S, Paione JB, Takada J, Boaventura P, Rebello C, Pinhata Otoch J. A prospective randomized study comparing partially covered metal stent versus plastic multistent in the endoscopic management of patients with postoperative benign bile duct strictures: a follow-up above 5 years. Rev Gastroenterol Peru. 2012;32:26–31. [PubMed] [Google Scholar]
- 24.Hausegger KA, Karnel F, Georgieva B, Tauss J, Portugaller H, Deutschmann H, Berghold A. Transjugular intrahepatic portosystemic shunt creation with the Viatorr expanded polytetrafluoroethylene-covered stent-graft. J Vasc Interv Radiol. 2004;15:239–248. doi: 10.1097/01.rvi.0000116194.44877.c1. [DOI] [PubMed] [Google Scholar]
- 25.ter Borg PC, Hollemans M, Van Buuren HR, Vleggaar FP, Groeneweg M, Hop WC, Laméris JS. Transjugular intrahepatic portosystemic shunts: long-term patency and clinical results in a patient cohort observed for 3-9 years. Radiology. 2004;231:537–545. doi: 10.1148/radiol.2312021797. [DOI] [PubMed] [Google Scholar]
- 26.Luo XF, Nie L, Wang Z, Tsauo J, Liu LJ, Yu Y, Zhou B, Tang CW, Li X. Stent-grafts for the treatment of TIPS dysfunction: fluency stent vs Wallgraft stent. World J Gastroenterol. 2013;19:5000–5005. doi: 10.3748/wjg.v19.i30.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haskal ZJ. Improved patency of transjugular intrahepatic portosystemic shunts in humans: creation and revision with PTFE stent-grafts. Radiology. 1999;213:759–766. doi: 10.1148/radiology.213.3.r99dc28759. [DOI] [PubMed] [Google Scholar]
- 28.Fidelman N, Kwan SW, LaBerge JM, Gordon RL, Ring EJ, Kerlan RK. The transjugular intrahepatic portosystemic shunt: an update. AJR Am J Roentgenol. 2012;199:746–755. doi: 10.2214/AJR.12.9101. [DOI] [PubMed] [Google Scholar]
- 29.Han G, Qi X, He C, Yin Z, Wang J, Xia J, Yang Z, Bai M, Meng X, Niu J, et al. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with symptomatic portal hypertension in liver cirrhosis. J Hepatol. 2011;54:78–88. doi: 10.1016/j.jhep.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 30.Patel NH, Sasadeusz KJ, Seshadri R, Chalasani N, Shah H, Johnson MS, Namyslowski J, Moresco KP, Trerotola SO. Increase in hepatic arterial blood flow after transjugular intrahepatic portosystemic shunt creation and its potential predictive value of postprocedural encephalopathy and mortality. J Vasc Interv Radiol. 2001;12:1279–1284. doi: 10.1016/s1051-0443(07)61552-8. [DOI] [PubMed] [Google Scholar]


