Abstract
AIM: To investigate the role of the hydrogen-rich water (HRW) in the prevention of aspirin-induced gastric mucosal injury in rats.
METHODS: Forty male rats were allocated into four groups: normal control group, HRW group, aspirin group, and HRW plus aspirin group. The protective efficacy was tested by determining the gastric mucosal damage score. Malondialdehyde (MDA), superoxide dismutase (SOD), myeloperoxidase (MPO), interleukin (IL)-06 and tumor necrosis factor (TNF)-α in gastric tissues were evaluated. The serum levels of IL-1β and TNF-α were also detected. Histopathology of gastric tissues and localization of Cyclooxygenase 2 (COX-2) were detected using hematoxylin and eosin staining and immunohistochemistry, respectively.
RESULTS: Pretreatment with HRW obviously reduced aspirin-induced gastric damage scores (4.04 ± 0.492 vs 2.10 ± 0.437, P < 0.05). The oxidative stress levels of MDA and MPO in the gastric tissues increased significantly in the aspirin-treated group compared with the HRW group (2.43 ± 0.145 vs 1.79 ± 0.116 nmol/mg prot, P < 0.05 and 2.53 ± 0.238 vs 1.40 ± 0.208 U/g tissue, P < 0.05, respectively). HRW could obviously elevated the SOD levels in the gastric tissues (37.94 ± 8.44 vs 59.55 ± 9.02 nmol/mg prot, P < 0.05). Pretreatment with HRW significantly reduced IL-06 and TNF-α in the gastric tissues (46.65 ± 5.50 vs 32.15 ± 4.83 pg/mg, P < 0.05 and 1305.08 ± 101.23 vs 855.96 ± 93.22 pg/mg, P < 0.05), and IL-1β and TNF-α in the serum (505.38 ± 32.97 vs 343.37 ± 25.09 pg/mL, P < 0.05 and 264.53 ± 28.63 vs 114.96 ± 21.79 pg/mL, P < 0.05) compared to treatment with aspirin alone. HRW could significantly decrease the COX-2 expression in the gastric tissues (staining score: 8.4 ± 2.1 vs 2.9 ± 1.5, P < 0.05).
CONCLUSION: HRW pretreatment alleviated the aspirin-induced gastric lesions by inhibiting the oxidative stress, inflammatory reaction and reducing the COX-2 in the gastric tissues.
Keywords: Hydrogen, Aspirin, Gastric lesion, Oxidative stress, Cytokines, Cyclooxygenase 2
Core tip: Aspirin is one of the most widely used medicines, but can cause adverse side effects of gastric injury. Hydrogen has been found to have powerful anti-oxidant and anti-inflammatory effects. We investigated the protective role of hydrogen-rich water on aspirin-induced gastric mucosal damage in rats. We found that hydrogen could alleviate the aspirin-induced gastric lesions by inhibiting the oxidative stress, inflammatory reaction and reducing the Cyclooxygenase 2 in the gastric tissues. This may provide a potential therapy for the adverse effects of aspirin.
INTRODUCTION
Non-steroidal anti-inflammatory drugs (NSAIDs), including the anti-inflammatory and analgesic agents, are the most common prescription medicines for the treatment of many diseases[1]. Aspirin, in particular, is widely used in cardiovascular disorders and even in the treatment of cancer because of its multiple actions[2,3]. However, there are adverse effects even it is used at a low and safe dose, especially the side effects in the digestive tract, such as the dyspeptic symptoms, gastrointestinal erosions, peptic ulcers, overt bleeding or perforation[4,5]. Hence, attempts have been made to develop a risk-free dose, or coated and buffered aspirin to mitigate the injury. However, little progress has been made[6].
Research has revealed that various factors, such as endogenous prostaglandin (PG), neutrophil-dependent microvascular injury, oxygen-derived free radicals, inflammatory cytokines[7-10], are associated with the aspirin-induced gastric mucosal damage. tumor necrosis factor (TNF)-α is a proinflammatory cytokine and can augment the neutrophil-derived superoxide generation, leading to oxygen radical-mediated tissue injury. Pretreatment of TNF-α inhibitors have been reported to suppress the gastric mucosal injury[11,12]. Moreover, the tissue enzymatic activity, such as dismutase (SOD), myeloperoxidase (MPO) and malondialdehyde (MDA), are also considered to be related to aspirin-induced gastric mucosal injury[13-16]. Among the multiple mechanisms, the function of cyclooxygenase (COX) is one of the biggest concerns for the scientists. COX-1 plays a definite protective role in the NSAIDs-induced gastric injury[17]. Nevertheless, the COX-2 plays a perplexing role in this pathological process. First, COX-2 can be induced by the damaging agents such as luminal acid and aspirin in the gastric mucosa, and inhibition of COX-2 can alleviate the damage[18-20]. It involves the maintenance of gastric mucosal integrity by preventing exogenous injury and by promoting gastric mucosal healing[21]. However, there are also studies reporting that COX-2 inhibitors exacerbate gut injury and attenuate the tissue’s ability to respond to mild damaging agents[22-24]. The possible mechanism is that COX-2 expression may be a compensatory response to increase the levels of gastroprotective PG in the process of gastric injury[17].
Hydrogen therapy is a new method which has gained much appreciation recently and shown efficacy in many diseases. Hydrogen has anti-oxidant, anti-inflammatory, anti-apoptotic, anti-allergy, and anti-cancer effects. Several methods, including inhalation, drinking hydrogen-rich water (HRW) and injection of hydrogen-saturated saline, have been developed and proved to be valid and reliable[25,26]. Oral intake of HRW is an effective and convenient way to deliver the molecular hydrogen, which is more suitable for application. Some researches showed that oral intake of HRW could protect cardiac allografts from inflammation-associated deterioration, kidney allografts from chronic rejection and so on[27-29]. Although the research of hydrogen as a medical therapy has been extensively investigated, its effect on the aspirin-induced gastric mucosal injury has not been reported.
The aim of this study was to assess the protective role of HRW on aspirin-induced gastric mucosal damage in rats mainly through measurement of oxidative stress indicators and cytokines, including levels of MDA, SOD, MPO, interleukin (IL)-6 and TNF-α in the gastric tissues, IL-1β and TNF-α in the serum, and expression of COX-2 in gastric mucosa.
MATERIALS AND METHODS
Experimental animals and preparation of hydrogen-rich water
The study was conducted using male Sprague Dawley rats (200-250 g) (Animal Feeding Center of Xi’an Jiaotong University Health Science Center, Xi’an, China). All rats were housed (5 per cage) in clear, pathogen-free polycarbonate cages in the animal care facility, and were fed a standard animal diet and water ad libitum under controlled temperature with 12-h light-dark cycles. They were cared in accordance with the guidelines of the Ethical Committee, Xi’an Jiaotong University Health Science Center. The HRW was produced by Naturally Plus Japan International Co, Ltd (Japan), which was stored under atmospheric pressure at 4 °C in an aluminum bag with no dead volume. The gas chromatography was used to confirm the content of hydrogen by the method described by Ohsawa et al[25] The hydrogen concentration of the HRW we used in this study was 0.63-0.82 mmol/L.
Study design
Forty rats were divided into four groups randomly, each consisting of 10 animals, with different pre-treatments for 7 d, as follows: (1) normal control group received oral saline; (2) HRW control group received oral HRW (changed every 3 h, at about 80 mL/d); (3) aspirin group took oral saline; and (4) HRW plus aspirin group received HRW (80 mL/d per rat). On the 8th day, gastric injury was induced by administration of aspirin (400 mg/kg) (Sigma Chemical Co., St. Louis, MO, United States) in the aspirin and HRW plus aspirin groups. Normal control and HRW control groups were given saline (1 mL) following overnight fast. After 8 h of aspirin treatment, rats were anesthetized under mild ether, and sacrificed via cervical decapitation. Gastric mucosa was harvested by gently scraping the mucosa off the underlying muscularis mucosa and serosal layers with a microscope slide and were frozen in liquid nitrogen and stored at -80 °C until assayed. The serum was separated by centrifugation at 3000 × g for 15 min at -4 °C to obtain clear serum, aliquoted, and stored at -80 °C until assayed.
Macroscopic analysis
Stomach was opened along the greater curvature, and mucosae were rinsed with cold PBS to remove blood impurities. Gastric mucosal changes were evaluated by two authors who were blinded to the treatment regimen. A scoring system to grade the degree of gastric mucosa was applied using a scale of 0 to 6 as described by Coleman et al[30] (Table 1).
Table 1.
Scale for grading gastric mucosal damage[30]
| Grade | Appearance of the gastric mucosa |
| 0 | Normal |
| 1 | Slight edema and congestion |
| 2 | Edema, congestion, and bleeding |
| 3 | One or two spot erosions |
| 4 | One or two linear erosions |
| 5 | Many small and a few large erosions |
| 6 | Extensive erosions over entire mucosa |
Histological study
Samples from the gastric mucosa were excised from the gastric glandular epithelium at a region located 2 mm below the limiting ridge that separates the forestomach from the glandular epithelium along the greater curvature of the stomach. They were fixed in 10% formalin solution and embedded in paraffin after completion of the routine procedure. Serial sections of 5-μm thickness were obtained and stained with hematoxylin/eosin (HE) to evaluate gastric morphology. The results were evaluated in a blinded fashion by two researchers. The mucosa was considered injured if one or more of the following criteria were met: discontinuous surface, dilated gland, hemorrhage, or damage to superficial cells[31].
Gastric mucosal enzymatic activity assay
The gastric mucosal tissue was homogenized, and tissue MPO, MDA and SOD activities were measured using the activity assay kits from Nanjing Jian Cheng Bioengineering Institute (Nanjing, China) according to the manufacturer’s instructions.
Gastric mucosal and serum cytokine assay
Gastric tissue and blood samples were homogenized and the supernatant was used for the determination of cytokines. The levels of cytokines (IL-6 and TNF-α) in the gastric tissue samples and IL-1β and TNF-α in the blood samples were evaluated using the ELISA kit reagent from Dakewe Biotech Co. (Shenzhen, China) according to the manufacturer’s instructions. A standard curve was run on each assay plate using recombinants of the respective cytokines in serial dilutions.
Immunohistochemistry
To establish the immunolocalization of COX-2, a mouse polyclonal antibody (Beijing Biosynthesis Biotechnology Co., Ltd, Beijing, China), was used at a working dilution of 1:20. The antibody was applied directly to sections, and slides were incubated overnight at 4 °C in a humidified chamber. Immune complexes were subsequently treated with the secondary antibody (containing anti-rabbit and anti-mouse immunoglobulins) and detected after streptavidin peroxidase treatment for 20 min at room temperature. After rinsing sections with three changes of PBS, immunoreactivity was visualized with diamine benzidine (DAB)-hydrogen peroxide (20 min). Sections were gently rinsed in distilled water, counterstained with HE, and photomicrographs were taken under a microscope (Olympus Optical Co., Tokyo, Japan). The results of immunohistochemistry were obtained by two researchers under blinded conditions. The intensity of immunohistochemical staining was scored as 0 (negative), 1 (weak), 2 (moderate strong) or 3 (strong). The extent of staining was assessed based on the percentage of positive cells: 0 (negative), 1 (1%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (76%-100%). The final staining score for each sample was the mean of the sum of the intensity and extent scores from five fields. The expression was considered as low if the final scores was 1-5 and as high if the final scores were 6-12.
Statistical analysis
All data were presented as mean ± SD for 10 rats in each group. To compare data among all groups of animals, one-way analysis of variance (one-way ANOVA) and Duncan comparisons were employed. All statistical tests were performed using SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, United States). Differences were considered statistically significant at P < 0.05.
RESULTS
Histopathological changes
Histopathological examination revealed aspirin-induced severe congestion and multiple hemorrhagic erosions in the stomach tissue, particularly in mucus-secreting cells, characterized by gastric pit damage and vacuolization of the glandular portion. Pretreatment with HRW considerably attenuated, but did not completely prevent the severity of these histopathological changes, while some erosion in sub-glandular and epithelial necks was evident (Figure 1A). In the aspirin group, the mean gastric mucosal damage score was 4.04 ± 0.492, while HRT pretreatment could reduce the damage to 2.10 ± 0.437 (P < 0.05) (Figure 1B).
Figure 1.
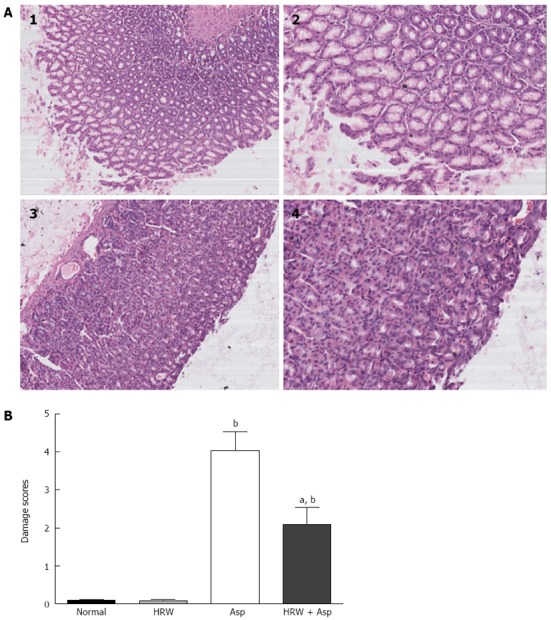
Histopathological examination of stomach sections. A: Hematoxylin-eosin stained results showed severe degenerative changes in glandular region, epithelial folds and connective septa in aspirin-induced mucosal tissue (1, × 100 and 2, × 200), while hydrogen-rich water (HRW) pretreatment displayed slight changes (3, × 100 and 4, × 200); B: The gastric mucosal damage score in four groups. The mean scores are significantly higher in the aspirin group and HRW plus aspirin group (HRW + Asp) when compared with the normal control group and HRW alone group (bP < 0.01). Pretreatment with HRW could significantly decrease the damage score in HRW + aspirin group (Asp) group when compared with aspirin group (aP < 0.05).
Effects of HRW on MDA, MPO, SOD and cytokine levels in aspirin-induced gastric mucosal injury
The oxidative stress parameters including MDA and MPO in the gastric mucosa increased significantly in the aspirin-treated group compared with the HRW pretreatment group (2.43 ± 0.145 vs 1.79 ± 0.116 nmol/mg prot, P < 0.05 and 2.53 ± 0.238 vs 1.40 ± 0.208 U/g tissue, P < 0.05). And the protective indicator SOD could increase significantly from 37.94 ± 8.44 nmol/mg prot to 59.55 ± 9.02 nmol/mg prot by the use of HRW (P < 0.05). Pretreatment with HRW could also significantly decrease the elevation of IL-06 and TNF-α in the gastric tissue (46.65 ± 5.50 vs 32.15 ± 4.83 pg/mg, P < 0.05 and 1305.08 ± 101.23 vs 855.96 ± 93.22 pg/mg, P < 0.05), (Figure 2).
Figure 2.
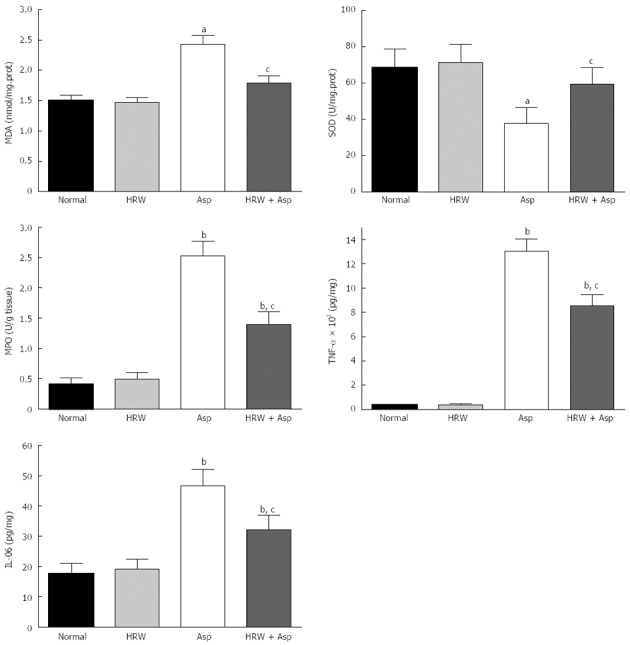
Levels of oxidative stress indicators and cytokines in all groups. Malonaldehyde (MDA), myeloperoxidase (MPO), tumor necrosis factor (TNF)-α, and interleukin (IL)-06 in the gastric mucosal tissues are significantly higher, and superoxide dismutase (SOD) levels is obviously lower in aspirin group and hydrogen-rich water (HRW) plus aspirin group [HRW + aspirin group (Asp)] when compared with the normal control group and/or HRW alone group (bP < 0.01, aP < 0.05 when compared with the control and HRW groups). Pretreatment with HRW could significantly decrease the MDA, MPO, TNF-α, and IL-06 levels and increase the SOD activity in HRW + Asp group when compared with aspirin group (cP < 0.05).
Effects of HRW on cytokine levels in the peripheral blood
The levels of IL-1β and TNF-α in the peripheral blood were markedly increased in the aspirin-treated group compared with the normal control and HRW control groups (P < 0.01). The increase in IL-1β and TNF-α concentration in the gastric mucosa elicited by aspirin were significantly suppressed by HRW pretreatment (505.38 ± 32.97 vs 343.37 ± 25.09 pg/mL, P < 0.05 and 264.53 ± 28.63 vs 114.96 ± 21.79 pg/mL, P < 0.05, respectively) (Figure 3).
Figure 3.
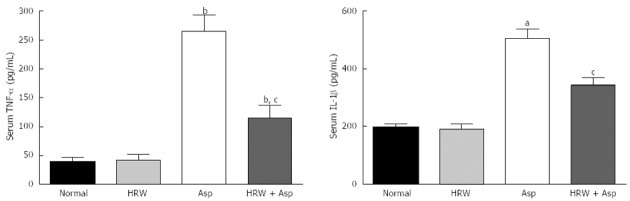
Serum tumor necrosis factor-alpha and interleukin-Iβ levels in all groups. All data are expressed as mean ± SD. The mean tumor necrosis factor (TNF)-α and interleukin (IL)-Iβ levels are significantly higher in the aspirin group (Asp) and HRW plus aspirin group (HRW + Asp) when compared with the normal control group and/or HRW alone group (bP < 0.01, aP < 0.05 when compared with the control and HRW groups). And pretreatment with HRW could significantly decrease serum TNF-α and IL-Iβ levels in HRW + Asp group when compared with aspirin group (cP < 0.05). HRW: Hydrogen-rich water.
Immunohistochemical analysis of COX-2 in gastric mucosal tissues
Immunohistochemical analysis was performed to ascertain the localization of COX-2 in gastric mucosal tissues. Rats treated with aspirin displayed significant immunoreactivity for COX-2 around the glandular regions, mucoid cells and neck gland cells of gastric tissues (staining score: 8.4 ± 2.1). In contrast, the group of rats pretreated with HRW presented lower COX-2 immunoreactivities (staining score: 2.9 ± 1.5) (P < 0.05) (Figure 4).
Figure 4.
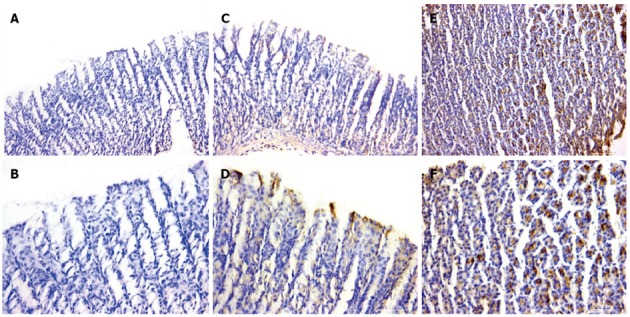
Immunolocalization of cyclooxygenase 2 in gastric tissue. A, B: The results in the control group; C, D: A scarce cyclooxygenase 2 (COX-2) immunoreactivity in sub-glandular region of hydrogen plus aspirin-treated rats; E, F: An apparent COX-2 immunoreactivity in the glandular region and epithelial necks of stomach tissues of rats treated with aspirin (A, C, E: × 200 and B, D, F: × 400).
DISCUSSION
Aspirin is widely used as an anti-inflammatory and analgesic drug, but it often induces gastrointestinal adverse effects, which limits its clinical use. Studies focusing on reduction of the aspirin-induced gastric mucosal damages is urgently needed[32-34]. Here, we examined the effect of HRW on the aspirin-induced gastric mucosal injury. Although hydrogen is the lightest gas in nature, it has an enormous anti-oxidation and anti-inflammatory capacity. In fact, there are few studies investigating the preventive or therapeutic effects of gas on gastric mucosal damages. In one study, hydrogen sulfide was found to protect against aspirin-induced gastric injury via reducing oxidative stress[35]. In another study, Liu et al[36] found that hydrogen treatment successfully ameliorated stress-associated gastric ulceration via its anti-oxidant, anti-inflammatory and anti-apoptotic effects. Our research shows that hydrogen can reduce aspirin-induced gastric injury by reducing MDA, MPO, IL-06 and TNF-α levels and increasing SOD activity in gastric tissues. Simultaneously, hydrogen could decrease the elevation of IL-1β and TNF-α in the serum compared to treatment with aspirin alone. We also found that hydrogen can decrease COX-2 expression in the gastric tissues, which may be the key mechanism of hydrogen action because of its importance in the aspirin-induced gastric injury. The present study demonstrated that hydrogen may prevent against aspirin-induced gastric mucosal injury, mainly depending on the modulation of anti-inflammatory cytokines, anti-oxidative stress and activation of COX-2.
In the past few years, the research of hydrogen therapy attracted wide attention from scientists and physicians[26,37]. Hydrogen molecules have been proven to act as an important physiological regulatory factor to cells and organs by anti-oxidant, anti-inflammatory, anti-apoptotic and other protective effects, which can be applied in the treatment of various diseases[26]. Compared with other studies on hydrogen therapy, a distinctive experimental design in our research was the delivery of hydrogen molecule. We designed and used the random oral intake of HRW to deliver the hydrogen molecule, which was similar to the human physiological status. This special and convenient delivery method implies that the HRW can be used as a kind of drink, which can immensely expand its applications.
According to a previous study, redox imbalance plays a major pathogenic role in aspirin gastropathy[5]. The over oxidative stress in the gastric mucosa can increase the production of oxygen radicals or decrease the capability of antioxidant defenses[38]. We found in the present study that hydrogen can significantly reduce the Asp-induced elevation of MDA and MPO, the most typical markers of free radical species-related injury. In addition, hydrogen also reversed the aspirin-reduced elevation of SOD, which is responsible for converting superoxide radicals to molecular oxygen and hydrogen peroxide within cytoplasm and mitochondria[39]. The cytokines, such as TNF-α, IL-1β and IL-06, induced by lymphocytes and macrophages that infiltrate the gastric mucosa, are associated with the tissue injury[40]. They are responsible for the neutrophil adherence in the microcirculation of gastric mucosa, and the release from activated macrophages with parallel accumulation of neutrophils within the gastric pits[41]. In the present study, we observed that pretreatment with hydrogen resulted in significantly decreased TNF-α, IL-1β and IL-06 production which could prevent subsequent neutrophils infiltration and alleviate injury.
On a deeper level, we focused on COX-2, which was the rate-limiting enzyme to regulate the synthesis of PG. This is a key factor in response to stress. It was reported that aspirin can rapidly up-regulate COX-2 mRNA expression in rat gastric mucosa, probably as a compensatory response to inhibition of COX-2 activity and gastrin PG synthesis[42,43]. COX-2 products play a relevant role in the maintenance of gastric mucosal integrity by preventing exogenous injury to the stomach and accelerating gastric mucosal healing[44]. Interestingly, HRW pre-treatment counteracted the increased expression of COX-2 induced by aspirin treatment, which promote mucosal healing. According to the description in the introduction section that the COX-2 plays a controversial role in the aspirin-induced gastric injury, we speculate that the COX-2 can act as the double-edged sword. First, COX-2 is induced by cytokines and then promotes the release of cytokines to exacerbate inflammation[20]; meanwhile, as a compensatory mechanism, the expression of the COX-2 can accelerate the prostaglandin production to protect the gastric mucosa[21]. Thus, when the noxious stimuli are strong enough to cover the protective role of the COX-2, high expression of COX-2 is a harmful indicator in the pathological process of aspirin-induced gastric injury. In this study, a high dosage of 400 mg/kg of aspirin was used for the rats and the high expression of COX-2 was induced by the cytokines. The hydrogen can suppress its expression to protect the gastric mucosa. Although the different molecular mechanism between hydrogen and COX-2 is not clear, COX-2 as a definite target of hydrogen can be confirmed. A clue of “hydrogen → mitigating oxidative stress → alleviating inflammatory reaction → suppressing COX-2 expression → protecting aspirin-induced gastric injury” can be used to summarize the research.
In conclusion, the present study shows that: (1) hydrogen is able to prevent aspirin-induced injury to the rat gastric mucosa through a mechanism, which is in part contributed by the anti-oxidant and anti-inflammatory activities; (2) aspirin causes up-regulation of COX-2 expression in the gastric mucosa; and (3) hydrogen significantly counteracts aspirin-induced up-regulation of COX-2 expression, probably as a consequence of the reduction in the extent of aspirin-induced injury. Therefore, hydrogen therapy might be safe and effective in the prevention of the stomach injury due to NSAIDs. Future clinical studies are necessary to determine whether it can be applied as preventive or therapeutic agents as shown in this pre-clinical study.
ACKNOWLEDGMENTS
We thank UNIVA Guangzhou Trading Co., Ltd, China for providing the hydrogen-rich water.
COMMENTS
Background
Aspirin is widely used as an anti-inflammatory and analgesic drug, but it often induces gastrointestinal adverse effects which include dyspeptic symptoms, gastrointestinal erosions, peptic ulcers, overt bleeding or perforation. Hence, efforts have been made to develop the risk-free dose or coated and buffered aspirin to mitigate the injury, but little progress has been made. This limits the clinical use of aspirin, and studies aimed to reduce the aspirin-induced gastric mucosal damages are urgently needed.
Research frontiers
Hydrogen therapy is a new medical method which has gained much attention recently. It has been shown that hydrogen has anti-oxidant, anti-inflammatory, anti-apoptotic, anti-allergy, and anti-cancer effects. Several methods used to deliver hydrogen, including inhalation, drinking hydrogen-rich water (HRW) and injection with hydrogen-saturated saline, have been proved to be valid and reliable.
Innovations and breakthroughs
Compared with other researches on hydrogen therapy, this study is characterized by a distinctive experimental design in the delivery of hydrogen molecule. The authors used the random oral intake of HRW to deliver hydrogen molecule, which was similar to human physiological status. This special and convenient delivery method implies that the HRW can be used as a kind of drink, which can immensely expand its applications. The authors also found that the hydrogen did have a protective role in the aspirin-induced gastric injury, which is in part contributed by the anti-oxidant and anti-inflammation activities. Hydrogen can significantly counteract aspirin-induced up-regulation of Cyclooxygenase 2 (COX-2) expression, probably as a consequence of the reduction in the extent of aspirin-induced injury.
Applications
Hydrogen therapy is safe and effective in prevention of the stomach injury derived from non-steroidal anti-inflammatory drugs administration. The special and convenient delivery method used in this study implies that HRW can be used as a kind of drink, which can immensely expand its applications.
Terminology
Hydrogen is the lightest gas in the nature, but it has huge anti-oxidant, anti-inflammatory, anti-apoptotic, anti-allergy, and anti-cancer effects. HRW is produced by pressing the hydrogen gas into the water by a specific device under high pressure.
Peer review
This study demonstrates the protective effects of hydrogen therapy (hydrogen rich water) on acute aspirin gastropathy in rats, which are probably dependent on the modulation of COX-2, cytokines and antioxidant activities. This is an interesting paper, with a new clinical application of a new therapy, in an experimental model of gastropathy. The results are well presented and provide sufficient experimental evidence to support the conclusions.
Footnotes
P- Reviewers: Auricchio S, Decorti G, Han X, Hassan M S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Whittle BJ. Mechanisms underlying intestinal injury induced by anti-inflammatory COX inhibitors. Eur J Pharmacol. 2004;500:427–439. doi: 10.1016/j.ejphar.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 2.Kong DF. Aspirin in cardiovascular disorders. What is the optimum dose? Am J Cardiovasc Drugs. 2004;4:151–158. doi: 10.2165/00129784-200404030-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol. 2012;23:1403–1415. doi: 10.1093/annonc/mds113. [DOI] [PubMed] [Google Scholar]
- 4.Jainu M, Mohan KV. Protective role of ascorbic acid isolated from Cissus quadrangularis on NSAID induced toxicity through immunomodulating response and growth factors expression. Int Immunopharmacol. 2008;8:1721–1727. doi: 10.1016/j.intimp.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Becker JC, Domschke W, Pohle T. Current approaches to prevent NSAID-induced gastropathy--COX selectivity and beyond. Br J Clin Pharmacol. 2004;58:587–600. doi: 10.1111/j.1365-2125.2004.02198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niv Y, Battler A, Abuksis G, Gal E, Sapoznikov B, Vilkin A. Endoscopy in asymptomatic minidose aspirin consumers. Dig Dis Sci. 2005;50:78–80. doi: 10.1007/s10620-005-1281-1. [DOI] [PubMed] [Google Scholar]
- 7.Wang JY, Yamasaki S, Takeuchi K, Okabe S. Delayed healing of acetic acid-induced gastric ulcers in rats by indomethacin. Gastroenterology. 1989;96:393–402. doi: 10.1016/0016-5085(89)91563-1. [DOI] [PubMed] [Google Scholar]
- 8.Naito Y, Yoshikawa T, Yagi N, Matsuyama K, Yoshida N, Seto K, Yoneta T. Effects of polaprezinc on lipid peroxidation, neutrophil accumulation, and TNF-alpha expression in rats with aspirin-induced gastric mucosal injury. Dig Dis Sci. 2001;46:845–851. doi: 10.1023/a:1010716804594. [DOI] [PubMed] [Google Scholar]
- 9.Salim AS. Use of scavenging oxygen-derived free radicals to protect the rat against aspirin- and ethanol-induced erosive gastritis. J Pharm Sci. 1992;81:943–946. doi: 10.1002/jps.2600810921. [DOI] [PubMed] [Google Scholar]
- 10.Sener-Muratoğlu G, Paskaloğlu K, Arbak S, Hürdağ C, Ayanoğlu-Dülger G. Protective effect of famotidine, omeprazole, and melatonin against acetylsalicylic acid-induced gastric damage in rats. Dig Dis Sci. 2001;46:318–330. doi: 10.1023/a:1005652815921. [DOI] [PubMed] [Google Scholar]
- 11.Santucci L, Fiorucci S, Di Matteo FM, Morelli A. Role of tumor necrosis factor alpha release and leukocyte margination in indomethacin-induced gastric injury in rats. Gastroenterology. 1995;108:393–401. doi: 10.1016/0016-5085(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 12.Santucci L, Fiorucci S, Giansanti M, Brunori PM, Di Matteo FM, Morelli A. Pentoxifylline prevents indomethacin induced acute gastric mucosal damage in rats: role of tumour necrosis factor alpha. Gut. 1994;35:909–915. doi: 10.1136/gut.35.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 14.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 15.Mateos R, Lecumberri E, Ramos S, Goya L, Bravo L. Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress. Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:76–82. doi: 10.1016/j.jchromb.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 16.Raghavendran HR, Srinivasan P, Rekha S. Immunomodulatory activity of fucoidan against aspirin-induced gastric mucosal damage in rats. Int Immunopharmacol. 2011;11:157–163. doi: 10.1016/j.intimp.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Darling RL, Romero JJ, Dial EJ, Akunda JK, Langenbach R, Lichtenberger LM. The effects of aspirin on gastric mucosal integrity, surface hydrophobicity, and prostaglandin metabolism in cyclooxygenase knockout mice. Gastroenterology. 2004;127:94–104. doi: 10.1053/j.gastro.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993;268:6610–6614. [PubMed] [Google Scholar]
- 19.Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci USA. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flower RJ. The development of COX2 inhibitors. Nat Rev Drug Discov. 2003;2:179–191. doi: 10.1038/nrd1034. [DOI] [PubMed] [Google Scholar]
- 21.Brzozowski T, Konturek PC, Konturek SJ, Pajdo R, Schuppan D, Drozdowicz D, Ptak A, Pawlik M, Nakamura T, Hahn EG. Involvement of cyclooxygenase (COX)-2 products in acceleration of ulcer healing by gastrin and hepatocyte growth factor. J Physiol Pharmacol. 2000;51:751–773. [PubMed] [Google Scholar]
- 22.Mizuno H, Sakamoto C, Matsuda K, Wada K, Uchida T, Noguchi H, Akamatsu T, Kasuga M. Induction of cyclooxygenase 2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology. 1997;112:387–397. doi: 10.1053/gast.1997.v112.pm9024292. [DOI] [PubMed] [Google Scholar]
- 23.Reuter BK, Asfaha S, Buret A, Sharkey KA, Wallace JL. Exacerbation of inflammation-associated colonic injury in rat through inhibition of cyclooxygenase-2. J Clin Invest. 1996;98:2076–2085. doi: 10.1172/JCI119013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gretzer B, Ehrlich K, Maricic N, Lambrecht N, Respondek M, Peskar BM. Selective cyclo-oxygenase-2 inhibitors and their influence on the protective effect of a mild irritant in the rat stomach. Br J Pharmacol. 1998;123:927–935. doi: 10.1038/sj.bjp.0701673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 26.Zhang JY, Liu C, Zhou L, Qu K, Wang R, Tai MH, Lei Lei JC, Wu QF, Wang ZX. A review of hydrogen as a new medical therapy. Hepatogastroenterology. 2012;59:1026–1032. doi: 10.5754/hge11883. [DOI] [PubMed] [Google Scholar]
- 27.Noda K, Tanaka Y, Shigemura N, Kawamura T, Wang Y, Masutani K, Sun X, Toyoda Y, Bermudez CA, Nakao A. Hydrogen-supplemented drinking water protects cardiac allografts from inflammation-associated deterioration. Transpl Int. 2012;25:1213–1222. doi: 10.1111/j.1432-2277.2012.01542.x. [DOI] [PubMed] [Google Scholar]
- 28.Cardinal JS, Zhan J, Wang Y, Sugimoto R, Tsung A, McCurry KR, Billiar TR, Nakao A. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int. 2010;77:101–109. doi: 10.1038/ki.2009.421. [DOI] [PubMed] [Google Scholar]
- 29.Sun Q, Kawamura T, Masutani K, Peng X, Sun Q, Stolz DB, Pribis JP, Billiar TR, Sun X, Bermudez CA, et al. Oral intake of hydrogen-rich water inhibits intimal hyperplasia in arterialized vein grafts in rats. Cardiovasc Res. 2012;94:144–153. doi: 10.1093/cvr/cvs024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coleman JC, Lacz JP, Browne RK, Drees DT. Effects of sucralfate or mild irritants on experimental gastritis and prostaglandin production. Am J Med. 1987;83:24–30. doi: 10.1016/0002-9343(87)90823-0. [DOI] [PubMed] [Google Scholar]
- 31.Graziani G, D’Argenio G, Tuccillo C, Loguercio C, Ritieni A, Morisco F, Del Vecchio Blanco C, Fogliano V, Romano M. Apple polyphenol extracts prevent damage to human gastric epithelial cells in vitro and to rat gastric mucosa in vivo. Gut. 2005;54:193–200. doi: 10.1136/gut.2004.046292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blandizzi C, Tuccori M, Colucci R, Fornai M, Antonioli L, Ghisu N, Del Tacca M. Role of coxibs in the strategies for gastrointestinal protection in patients requiring chronic non-steroidal anti-inflammatory therapy. Pharmacol Res. 2009;59:90–100. doi: 10.1016/j.phrs.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Sørensen HT, Mellemkjaer L, Blot WJ, Nielsen GL, Steffensen FH, McLaughlin JK, Olsen JH. Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. Am J Gastroenterol. 2000;95:2218–2224. doi: 10.1111/j.1572-0241.2000.02248.x. [DOI] [PubMed] [Google Scholar]
- 34.García Rodríguez LA, Hernández-Díaz S, de Abajo FJ. Association between aspirin and upper gastrointestinal complications: systematic review of epidemiologic studies. Br J Clin Pharmacol. 2001;52:563–571. doi: 10.1046/j.0306-5251.2001.01476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu L, Cui J, Song CJ, Bian JS, Sparatore A, Soldato PD, Wang XY, Yan CD. H(2)S-releasing aspirin protects against aspirin-induced gastric injury via reducing oxidative stress. PLoS One. 2012;7:e46301. doi: 10.1371/journal.pone.0046301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Chen Z, Mao N, Xie Y. The protective of hydrogen on stress-induced gastric ulceration. Int Immunopharmacol. 2012;13:197–203. doi: 10.1016/j.intimp.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Hong Y, Chen S, Zhang JM. Hydrogen as a selective antioxidant: a review of clinical and experimental studies. J Int Med Res. 2010;38:1893–1903. doi: 10.1177/147323001003800602. [DOI] [PubMed] [Google Scholar]
- 38.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 39.Vacek TP, Gillespie W, Tyagi N, Vacek JC, Tyagi SC. Hydrogen sulfide protects against vascular remodeling from endothelial damage. Amino Acids. 2010;39:1161–1169. doi: 10.1007/s00726-010-0550-2. [DOI] [PubMed] [Google Scholar]
- 40.Zhang XW, Liu Q, Thorlacius H. Inhibition of selectin function and leukocyte rolling protects against dextran sodium sulfate-induced murine colitis. Scand J Gastroenterol. 2001;36:270–275. doi: 10.1080/003655201750074555. [DOI] [PubMed] [Google Scholar]
- 41.Choi JI, Raghavendran HR, Sung NY, Kim JH, Chun BS, Ahn DH, Choi HS, Kang KW, Lee JW. Effect of fucoidan on aspirin-induced stomach ulceration in rats. Chem Biol Interact. 2010;183:249–254. doi: 10.1016/j.cbi.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Fiorucci S, de Lima OM, Mencarelli A, Palazzetti B, Distrutti E, McKnight W, Dicay M, Ma L, Romano M, Morelli A, et al. Cyclooxygenase-2-derived lipoxin A4 increases gastric resistance to aspirin-induced damage. Gastroenterology. 2002;123:1598–1606. doi: 10.1053/gast.2002.36558. [DOI] [PubMed] [Google Scholar]
- 43.Davies NM, Sharkey KA, Asfaha S, Macnaughton WK, Wallace JL. Aspirin causes rapid up-regulation of cyclo-oxygenase-2 expression in the stomach of rats. Aliment Pharmacol Ther. 1997;11:1101–1108. doi: 10.1046/j.1365-2036.1997.00247.x. [DOI] [PubMed] [Google Scholar]
- 44.Hatazawa R, Tanaka A, Tanigami M, Amagase K, Kato S, Ashida Y, Takeuchi K. Cyclooxygenase-2/prostaglandin E2 accelerates the healing of gastric ulcers via EP4 receptors. Am J Physiol Gastrointest Liver Physiol. 2007;293:G788–G797. doi: 10.1152/ajpgi.00131.2007. [DOI] [PubMed] [Google Scholar]


