Abstract
Inteleurkin-22 (IL-22) is a IL-10 family cytokine member and is mainly produced by innate lymphoid cells (ILCs), Th17 cells, and Th22 cells. Previous studies have indicated that IL-23 and several transcription factors, including STAT3, RORγt, and the AhR are important stimulus. Recently, there is emerging evidence that Tregs can regulate IL-22 expression. In the review, we discuss the updated advancement on Tregs function and its regulatory role on IL-22 expression.
Keywords: Interleukin-22, regulatory (Treg) cells, T helper cells, innate immune cells
Introduction
IL-22 is a member of the IL-10 family, mainly produced by T cells and natural killer (NK) cells and represents an effector cytokine of the Th17 lineage [1-4]. The regulation of IL-22 expression consists of cytokine-mediated regulation such as IL-23 and transcriptional control including STAT3, RORγt, and the AhR [5]. Here, we focus on the regulatory role of T regulatory (Treg) cells in IL-22 expression.
IL-22
The IL-22-IL-22R pathway
IL-22 belongs to the IL-10 cytokine family and is produced by special immune cell populations, including Th22, Th1, and Th17 cells, classical and non-classical (NK-22) NK cells, NKT cells, and lymphoid tissue inducer cells [3]. IL-22 binds to a heterodimeric receptor consisting of IL-22R1 and IL-10R2. IL-22R1, the ligand binding subunit, is expressed by a variety of non-immune tissues: skin, lung, kidney, and pancreas [6,7]. Thus, IL-22 functions as a signaling mediator that can connect lymphocytes and epithelial cells. There is also a soluble IL-22R called IL-22-binding protein (IL-22BP) [6,7]. The binding of IL-22 to IL-22BP is of 20- to 1000-fold higher affinity compared to its binding to the membrane bound IL-22R1 [8].
IL-22 binding to IL-22R complex induces a cascade of downstream signaling pathways [5]. Initial studies utilizing a murine kidney cell line revealed that IL-22R ligation induced phosphorylation of STAT3, and to a lesser extent, STAT5 [4], while other studies observed phosphorylation of STAT1, STAT3, and STAT5 in a human kidney cell line [9]. Further analysis has concluded that IL-22 signaling utilizes Jak1 and Tyk2 to propagate downstream phosphorylation signals, including several MAPK pathways (ERK1/2, MEK1/2, JNK, and p38 kinase), and STAT1, STAT3, and STAT5 [10].
The regulation of IL-22 expression
IL-23 is an important stimulus for induction of IL-22 expression in both innate and adaptive immune cells. The importance of IL-23 in the induction of IL-22 in vivo is evident in several models [11-13]. IL-23 has also been found to be essential in the terminal differentiation of Th17 cells, aiding in their expansion and effector functions [14]. The ability of IL-23 to enhance Th17 cell expansion appears to be linked to IL-22 expression, as increased expansion was only observed in IL-22+Th17 cells but not in IL-17A+Th17 cells [2]. Several transcription factors, including STAT3, RORγt, and AhR have also been found to be essential in the regulation of IL-22 in multiple cell lineages [15-17]. In addition to expression, several functional properties of IL-22 can be regulated by different inflammatory cytokines such as IL-17A, 17-F and TNF-a in a synergistic or inhibitory manner [11,18,19].
The biological effect of IL-22
IL-22-IL-22R signaling in epithelial cells results in expression of genes involved in antimicrobial host defense including S100 proteins, defensins, Lipocalin 2, and RegIII-family proteins [20,21]. IL-22 also induces inflammatory molecules such as chemokines and cytokines including IL-6 [22,23]. In addition, IL-22 has an important function in tissue repair via induction of epithelial cell proliferation and survival [6,22,23]. By inducing such genes and by enhancing epithelial barrier function, IL-22 plays an important role in promoting resistance to extracellular pathogens [21-23].
During an inflammatory response, IL-22 can act to either promote or protect from inflammation. IL-22 prevents tissue destruction in several mouse models. In the intestine, IL-22 prevents tissue destruction in a murine model of inflammatory bowel diseases (IBD) [24,25] and in a mouse model of graft versus host disease [26]. In a Concanavalin A-induced hepatitis model, IL-22 protects from liver injury by enhancing the growth and survival of hepatocytes [27-29]. In contrast, IL-22 can promote pathological inflammatory responses in the skin and intestine in mouse models, and the concentration of IL-22 is increased in a variety of human diseases including psoriasis [21,22], rheumatoid arthritis and others [29,30]. Furthermore, excessive and aberrant IL-22 results in colon cancer development, as exemplified by mice lacking IL-22-binding protein (IL-22BP) [31].
Treg cells
Cells targeted by Tregs
Tregs, a subset of CD4+ T cells is characterized by the expression of the IL-2 receptor a-chain (CD25) [32-34]. CD25, however, is also expressed on activated T cells and therefore cannot serve as a Treg-specific marker molecule. Recently, the forkhead family transcription factor (Foxp3) has been described as a highly specific intracellular marker molecule for Treg [35,36]. Tregs regulate the activation and expansion of CD4+ T cells lineage, via expression of forkhead box P3 and/or their capacity to produce cytokines such as transforming growth factor (TGF)-β, IL-10, and IL-35 [37-40]. IL-10 antagonizes pro-inflammatory cytokines such as IL-6 and might also be an antagonist to the inflammatory IL-17. Additionally, IL-10 negatively regulates Th17 cell differentiation [41]. Moreover, besides CD4+ T and CD8+ T cells, also other cell types such as B-cells, dendritic cells (DCs), monocytes, mast cells, osteoblasts, NK cells and NK T cells were identified as target cell populations for Tregs [42-45].
Tregs function
Tregs can suppress the immune response of CD4+ and CD8+ T cells [46]. The suppressive functions are deficient or reduced in Foxp3 deficient mice and the human autoimmune diseases including autoimmune diabetes, autoimmune proliferative syndrome (APS) type II, multiple sclerosis (MS), graft versus host disease, autoimmune hepatitis and rheumatoid arthritis (RA) [47-49]. On the other hand, increased proportions of functionally active CD4+CD25+ Treg have been described in the synovial fluid of RA patients [47]. So far, conflicting data have been reported and no overall consensus has been reached. One of the major reasons for such controversial observations is the pleiotropic function of Tregs.
A recent focus may point to a new role for Tregs in the perpetuation of inflammatory processes, rather than in the suppression thereof [50-53]. A stable expression of Foxp3 is required for Treg differentiation and for their suppressor function, proliferative potential and metabolic fitness [36,54-56]. The transcriptional repressive effects of Foxp3 protein render Treg cells incapable of producing certain key cytokines such as interleukin-2 (IL-2). Therefore, Treg cells require an exogenous supply of these cytokines for their peripheral maintenance [57]. Suppression by Treg cells depends strongly on the local cytokine milieu and the proximity of Treg cells to effector cells during an immune response [34,58]. Tregs cells could lose their suppressive functions [59,60], because they may not effectively suppress by cytokine competition when cytokines are abundant during an infection. Moreover, during acute inflammation, Tregs cells promote Th17 cell differentiation leading to up-regulated the expression of pro-inflammatory IL-17A, IL-17F and IL-22 [61]. So Treg cells may have broader roles in immunity than just the previously recognized suppressor functions. Further studies on the function properties and the mechanism of action of these Treg are needed.
The regulation of Treg cells on IL-22 expression
IL-22 as a direct target gene of FOXP3
The IL-22 genomic locus is part of a small gene cluster comprising IFN-γ, IL-26, and IL-22. The IL-22 gene lies on the minus strand of the human genome and encodes for 5 exons [62]. Andreas et al has analyzed the Foxp3 transcription factor binding sites (TFBSs) in a human T-cell line by genomic tiling microarray (ChIP-on-chip), and observed that the down-regulation of IL-22 in stimulated Jurkat-Foxp3 (J-Foxp3) T cells was accompanied by Foxp3 binding only under stimulated conditions [63]. A reproducible Foxp3 binding site can be found in proximity (3646 bp) to the IL-22 TSS. In the middle of the ChIP region there was a perfectly matching Foxp3 consensus site with the following genomic coordinates: chr12 66937190 – 66937197 (hg18 reference assembly). Genomic site-specific real-time PCR of this binding region confirmed about 5-fold ChIP enrichment. A prerequisite for a direct Foxp3 target gene is the occurrence, suggesting IL-22 is a direct target gene of Foxp3 [63].
Treg cells regulate the expression of IL-22 on the transcriptional level
Consistent with the down-regulation of IL-22 in stimulated J-Foxp3 T cells, the decreased expression of IL-22 in Foxp3+ Treg cells has also been observed compared to conventional naïve Foxp3- T cells [63]. Recent studies reported a close relationship between CD4+Foxp3+ Tregs and proinflammatory IL-17-producing Th17 expressing the lineage-specific transcription factor RORγt [56,64]. It has been shown that IL-17 secreting Foxp3+ T cells that express RORγt share features of conventional RORγt+Th17 cells. However, RORγt+Foxp3+ Tregs mostly fail to secrete IL-22 after PMA/ionomycin stimulation [51]. Foxp3 TFBS in the IL-22 promoter restrains RORγt+Foxp3+ T cells to produce IL-22 on the transcriptional level [63].
Treg cells regulate the expression of IL-22 in CD4+ T cells
Despite the decreased expression of IL-22 in Foxp3+ Treg cells, it has been found that Treg cells can promote naïve T cell differentiation, to some extent, depending on inflammatory environment and the local cytokine milieu. In mouse mode of infection with an oral Candida albicans, Foxp3+ Treg cells can powerfully promote the transition of naïve CD4+ T cells to responding CD4+ cells (Tresp). Tresp cells markedly produce IL-22 [61]. Th17 cells are developed from naïve CD4+ T cells under the influence of a network of inflammatory cytokines, including IL-1, IL-6, IL-21 and TGF-β. Th17 cells produce pro-inflammatory cytokines IL-17A, IL-17F and IL-22 and the expression of the retinoic acid orphan receptor-related transcription factor (RORC). In the same study, the full differentiation program of Th17 cells promoted by the presence of Treg cells boosted the production of IL-22, in addition to IL-17A and IL-17F. Treg cells did not suppress, but actually promoted IL-17A, IL-22-dependent clearance of fungi during acute C.albicans infection [61]. These findings demonstrated plastic regulation of FOXP3+ Treg cells on IL-22 expression (Figure 1).
Figure 1.
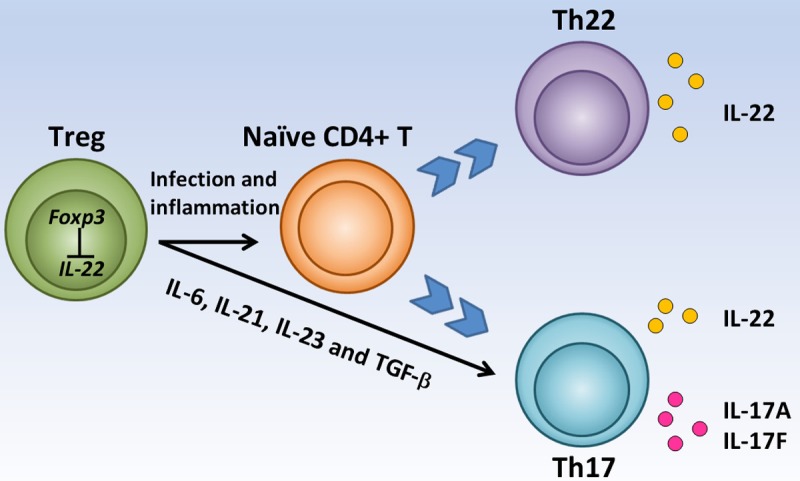
The regulation of Treg cells on IL-22 expression. Foxp3 suppresses IL-22 directly in Treg. Treg promotes naïve CD4+ cells to become Th22 and Th17 in the inflammatory conditions and then release IL-22, IL-17A and IL-17F. Treg also boosts the production of IL-22 in Th17.
Perspective
IL-22 is a critical cytokine in a number of immune processes and plays an important role in barrier surfaces as well as in the development and pathogenesis of autoimmunity. IL-22 is produced by T cells and innate immune cells including CD4+ T and CD8+ T cells, NK cells and NK T cells. Differentiation of these cells is, at least, partially regulated by Treg cells. Therefore, there is the presence of possibility that Treg cells might regulate the expression of IL-22. Despite the decreased expression of IL-22 in Foxp3+ Treg cells, Treg cells also induce the secretion of IL-22 from CD4+ T cells during acute inflammation. The evidence might be useful to further study on the exact association of Treg cells with IL-22 expression in immunity and infection. However, many questions remained to be answered. For example, Treg cells regulate the IL-22 expression on these cells: directly or indirectly? With a synergistic or inhibitory manner?
Acknowledgements
This work was partly supported by the NIH (AR-059103 and AI-084359), the Arthritis Foundation, the Rheumatology Research Foundation of the American College of Rheumatology (Within Our Reach program grant), and Science Foundation of Science and Technology Department of Zhejiang Province (No. 2007C3305).
Disclosure of conflict of interest
The authors declare no competing interests.
References
- 1.Kreymborg K, Etzensperger R, Dumoutier L, Haak S, Rebollo A, Buch T, Heppner FL, Renauld JC, Becher B. IL-22 is expressed by Th17 cells in an IL-23-depen, dent fashion, but not required for the development of autoimmune encephalomyelitis. J Immunol. 2007;179:8098–8104. doi: 10.4049/jimmunol.179.12.8098. [DOI] [PubMed] [Google Scholar]
- 2.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witte E, Witte K, Warszawska K, Sabat R, Wolk K. Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev. 2010;21:365–379. doi: 10.1016/j.cytogfr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–1819. doi: 10.4049/jimmunol.164.4.1814. [DOI] [PubMed] [Google Scholar]
- 5.Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol. 2010;107:1–29. doi: 10.1016/B978-0-12-381300-8.00001-0. [DOI] [PubMed] [Google Scholar]
- 6.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Wolk K, Sabat R. Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 2006;17:367–380. doi: 10.1016/j.cytogfr.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Wolk K, Witte E, Hoffmann U, Doecke WD, Endesfelder S, Asadullah K, Sterry W, Volk HD, Wittig BM, Sabat R. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn’s disease. J Immunol. 2007;178:5973–5981. doi: 10.4049/jimmunol.178.9.5973. [DOI] [PubMed] [Google Scholar]
- 9.Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–31339. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 10.Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277:33676–33682. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 11.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muñoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K, Loddenkemper C, Krell HW, Libert C, Lund LR, Frey O, Hölscher C, Iwakura Y, Ghilardi N, Ouyang W, Kamradt T, Sabat R, Liesenfeld O. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med. 2009;206:3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 14.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 16.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 17.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 18.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guilloteau K, Paris I, Pedretti N, Boniface K, Juchaux F, Huguier V, Guillet G, Bernard FX, Lecron JC, Morel F. Skin Inflammation Induced by the Synergistic Action of IL-17A, IL-22, Oncostatin M, IL-1{alpha}, and TNF-{alpha} Recapitulates Some Features of Psoriasis. J Immunol. 2010;184:5263–5270. doi: 10.4049/jimmunol.0902464. [DOI] [PubMed] [Google Scholar]
- 20.Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, Kasman I, Winer J, Modrusan Z, Danilenko DM, Ouyang W. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–2240. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 21.Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, Kasman I, Winer J, Modrusan Z, Danilenko DM, Ouyang W. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–23. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 22.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, More lF. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 23.Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A, Leclair S, Herrmann K, Seiderer J, Ochsenkühn T, Göke B, Auernhammer CJ, Dambacher J. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827–838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 24.Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Couturier M, Lamarthée B, Arbez J, Renauld JC, Bossard C, Malard F, Bonnefoy F, Mohty M, Perruche S, Tiberghien P, Saas P, Gaugler B. IL-22 deficiency in donor T cells attenuates murine acute graft-versus-host disease mortality while sparing the graft-versus-leukemia effect. Leukemia. 2013;27:1527–1537. doi: 10.1038/leu.2013.39. [DOI] [PubMed] [Google Scholar]
- 27.Pan H, Hong F, Radaeva S, Gao B. Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell Mol Immunol. 2004;1:43–49. [PubMed] [Google Scholar]
- 28.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 29.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, Ueki K, Nojima Y. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. 2005 Apr;52:1037–46. doi: 10.1002/art.20965. [DOI] [PubMed] [Google Scholar]
- 31.Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O’Connor W Jr, Murphy AJ, Valenzuela DM, Yancopoulos GD, Booth CJ, Cho JH, Ouyang W, Abraham C, Flavell RA. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2013;491:259–263. doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lan Q, Fan H, Quesniaux V, Ryffel B, Liu Z, Zheng SG. Induced Foxp3(+) regulatory T cells: a potential new weapon to treat autoimmune and inflammatory diseases? J Mol Cell Biol. 2012;4:22–28. doi: 10.1093/jmcb/mjr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran DQ. TGF-β: the sword, the wand, and the shield of FOXP3(+) regulatory T cells. J Mol Cell Biol. 2012;4:29–37. doi: 10.1093/jmcb/mjr033. [DOI] [PubMed] [Google Scholar]
- 34.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hori S, Takahashi T, Sakaguchi S. Control of autoimmunity by naturally arising regulatory CD4+ T cells. Adv Immunol. 2003;81:331–371. doi: 10.1016/s0065-2776(03)81008-8. [DOI] [PubMed] [Google Scholar]
- 36.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Z, Song X, Li B, Greene MI. FOXP3 and its partners: structural and biochemical insights into the regulation of FOXP3 activity. Immunol Res. 2008;42:19–28. doi: 10.1007/s12026-008-8029-x. [DOI] [PubMed] [Google Scholar]
- 38.Zhou X, Wang J, Shi W, Brand DD, Liu Z, Fan H, Zheng SG. Isolation of purified and live Foxp3+ regulatory T cells using FACS sorting on scatter plot. J Mol Cell Biol. 2010;2:164–169. doi: 10.1093/jmcb/mjq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 40.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+ CD25- precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 41.Ouyang X, Zhang R, Yang J, Li Q, Qin L, Zhu C, Liu J, Ning H, Shin MS, Gupta M, Qi CF, He JC, Lira SA, Morse HC 3rd, Ozato K, Mayer L, Xiong H. Transcription factor IRF8 directs a silencing programme for TH17 cell differentiation. Nat Commun. 2011;2:314. doi: 10.1038/ncomms1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–3932. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lan Q, Zhou X, Fan H, Chen M, Wang J, Ryffel B, Brand D, Ramalingam R, Kiela PR, Horwitz DA, Liu Z, Zheng SG. Polyclonal CD4+Foxp3+ Treg cells induce TGFβ-dependent tolerogenic dendritic cells that suppress the murine lupus-like syndrome. J Mol Cell Biol. 2012;4:409–419. doi: 10.1093/jmcb/mjs040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su W, Fan H, Chen M, Wang J, Brand D, He X, Quesniaux V, Ryffel B, Zhu L, Liang D, Zheng SG. Induced CD4+ forkhead box protein-positive T cells inhibit mast cell function and established contact hypersensitivity through TGF-β1. J Allergy Clin Immunol. 2012;130:444–452. e447. doi: 10.1016/j.jaci.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Zheng SG, Wang JH, Koss MN, Quismorio F, Gray JD, Horwitz DA. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J Immunol. 2004;172:1531–1539. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 47.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 48.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 51.Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, Valmori D. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci U S A. 2009;106:8635–8640. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, Hafler DA. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–4249. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, Zheng B, Littman DR, Liu YJ. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D, Brand D, Liu Z, Zheng SG. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J Immunol. 2010;185:2675–2679. doi: 10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng SG. Regulatory T cells vs Th17: differentiation of Th17 versus Treg, are the mutually exclusive? Am J Clin Exp Immunol. 2013;2:94–106. [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 57.Pandiyan PLM. The control of CD4+CD25+ Foxp3+ regulatory T cell survival. Biol Direct. 2008;3:6. doi: 10.1186/1745-6150-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Busse D, de la Rosa M, Hobiger K, Thurley K, Flossdorf M, Scheffold A, Höfer T. Competing feedback loops shape IL-2 signaling between helper and regulatory T lymphocytes in cellular microenvironments. Proc Natl Acad Sci U S A. 2010;107:3058–3063. doi: 10.1073/pnas.0812851107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, O’Brien S, Blank R, Lamb E, Natarajan S, Kastenmayer R, Hunter C, Grigg ME, Belkaid Y. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 61.Pandiyan P, Conti HR, Zheng L, Zheng L, Peterson AC, Mathern DR, Hernández-Santos N, Edgerton M, Gaffen SL, Lenardo MJ. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity. 2011;34:422–434. doi: 10.1016/j.immuni.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010;32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- 63.Jeron A, Hansen W, Ewert F, Buer J, Geffers R, Bruder D. ChIP-on-chip analysis identifies IL-22 as direct target gene of ectopically expressed FOXP3 transcription factor in human T cells. BMC Genomics. 2012;13:705. doi: 10.1186/1471-2164-13-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]


