Abstract
Objectives: Both Osteoradionecrosis (ORN) and Bisphosphonate associated osteonecrosis of the jaws (BRONJ) present clinically as regions of exposed necrotic bone. The study aimed to demonstrate the histopathological differences behind the observed clinical similarities. Study Design: Ten ORN specimens and ten BRONJ specimens were used, as well as ten samples of normal mandibular bone as control. Two bone-specific stainings were used, i.e. Sirius Red for the study of the relative presence of collagen types I and III and toluidine blue for the study the osteon density. Results: The Red Green Blue (RGB)-analysis of the specimens stained with Sirius Red identified significant differences between the chromatic patterns observed in bone preparations of patients suffering from ORN when compared to both BRONJ and control samples. Moreover, the osteon density of the BRONJ samples was significantly lower when compared to ORN and normal bone samples. Conclusions: The demonstrated differences in the bone architecture and in the bone collagen content between the two pathological conditions most likely reflect underlying pathophysiological differences.
Keywords: Osteoradionecrosis, bisphosphonate-associated osteonecrosis, bone structure, collagen, toluidine blue, Sirius red
Introduction
The clinicians that deal with the oral cavity often come across patients that present with exposed, apparently necrotic bone in the oral cavity. This exposed bone constitutes a significant health problem that needs to be dealt with, since it has significant implications for the quality of life and general health state of the patient. Frequently, the observed bone area is only the tip of the iceberg and might denote a significant range of necrosis of the mandible or the maxilla and radical therapeutic measures are deemed necessary to help the patient [1-4].
Various pathological conditions have been known to manifest in the form of exposed necrotic bone in the oral cavity, with osteoradionecrosis of the jaws (ORN - radiation related necrosis) and bisphosphonate-related osteonecrosis of the jaws (BRONJ - jaw necrosis attributed to the treatment of the patient with bisphosphonates) being among the commonest [1-8].
Radiation therapy is a frequently used treatment modality for head and neck cancer, either as a stand-alone option or in combination with surgery (adjuvant or neo-adjuvant radiotherapy) and/or chemotherapy. One of the most well documented complications of radiation therapy in the head and neck region is osteoradionecrosis (ORN) [3,9]. The first descriptions of this entity date back to 1922 [9] and 1926 [10]. The effect of ORN can be detrimental for the general well-being and the quality of life of affected patients. ORN typically represents a slow-healing radiation-induced ischemic necrosis of variable extent; tumour necrosis, recurrence or metastatic disease should have been excluded [3,9,11,12].
The clinical presentation of ORN is that of necrotic bone exposed to the oral cavity [3,8,9]. Marx defines ORN as “an area greater than 1 cm of exposed bone in a field of irradiation that had failed to show any evidence of healing for at least 6 months” [3,13-15]. However, the most commonly used definition of ORN limits the time of presence of the lesion to 3 months and reads as follows: ORN is a condition characterized by irradiated bone that becomes devitalized and exposed through the overlying skin or mucosa, without healing for 3 months and when tumour recurrence has been ruled out [3,9,16-19]. ORN is not necessarily associated with pain; however pain can occur when the surrounding soft tissues are inflamed. Cellulitis, fistulation, abscess formation or pathological fractures can complicate the clinical presentation [3,9,12,18-21].
Although a number of theories have been proposed to explain the pathogenesis of ORN, it seems that its pathophysiology can better be explained if seen in the context of radiation induced fibroatrophic process [22-25].
A relatively recently described clinical entity, Bisphosphonate-Related Osteonecrosis of the Jaws (BRONJ), has a clinical presentation quite similar to ORN. BRONJ also presents with an area of exposed bone in the maxillofacial region that persists for more than eight weeks. For establishment of a diagnosis of BRONJ two important factors must be present: current or recent exposure to bisphosphonates and no history of radiation therapy to the jaws. The above-mentioned complications can also superimpose the clinical presentation of BRONJ [4,7,26-35].
The fact that an increasing number of patients suffering with BRONJ are described every year can be attributed to the expansion of the use of bisphosphonates. Drugs of this class have been extensively used intravenously for the treatment of bone metastases of various cancer forms (lung, breast, prostate etc), the treatment of multiple myeloma, the management of tumour related hypercalcemia and severe osteoporosis among others [36,37]. The oral administration of bisphosphonates for the treatment of osteoporosis and the prevention of pathological fractures is also widespread [36,37]. The incidence of BRONJ is significantly higher in the group of patients that are receiving intravenous bisphosphonates [4,7,31].
The pathophysiology of this clinical entity is not clearly understood to this day [2,28,32,38-41]. A number of theories have been proposed, neither of which can provide an adequate explanation of the pathophysiological mechanism and the exclusivity of the presentation of this type of osteonecrosis to the jaws. Initially BRONJ was perceived as a type of avascular necrosis, something however that has not been confirmed by pathological findings [2,8]. Direct toxicity of the bisphosphonates to the bone [2], reduction of bone turnover [2] and more recently dissociation of the interaction circuits between osteoblasts, osteoclasts, fibroblasts, and keratinocytes during tissue remodelling [2,39,40] have been identified as possible explanations of the disease process.
Other authors favor the “outside to inside” hypothesis and highlight the role of mucosal injuries that allow infection of the bone with oral bacteria such as actinomyces. Inflammation and infection are seen as critical in this theory [8,32,42]. The bisphosphonate toxicity to the soft tissue should also be taken into account [2].
Recently, BRONJ was associated with impairment of the Msx-1-related osteoblast proliferation [40]. Msx-1 is a transcription factor that induces proliferation and inhibits terminal differentiation of osteoblasts [43] that plays an important role in alveolar bone regeneration [44,45]. It has been demonstrated that Msx-1 is expressed permanently in the jaws, whereas it is down-regulated in mesenchymal derived bones after maturation and is activated only during fracture healing [45-47].
It is often hypothesized that the similarities in the clinical presentation also extend to the microscopic level and denote similar pathophysiological mechanisms. By comparing the histopathological presentation of these two entities we aimed, to demonstrate differences on the microscopical level and thus potential differences in the pathophysiology underlying ORN and BRONJ, respectively.
Although such comparisons have also been performed in the past, the differences identified so far were more of a qualitative nature. In contrast, we focused on measurable parameters that can be independently reproduced. To do so we used two well-established staining techniques, i.e. Sirius Red stain and Toluidine Blue stain.
Materials and methods
The choice of the used bone stains - Sirius red
Sirius Red staining was used to demonstrate differences in collagen morphology; a new methodology to quantify the results obtained from this dying technique, based on the analysis of the obtained electronic images was used. At this point we would like to elaborate on the properties of this particular stain and the way that it was used in our study. The use of Sirius red stain in combination with polarization microscopy is a well-established method for the histochemical study of collagen that was first described by Junqueira in 1978 [48-59].
The term collagen is used to describe a family of glycoproteins that are contained in a variety of histological entities such as collagen fibres, reticulin fibres, basement membranes etc. [50,51,56,60,61]. The concept of the Sirius red staining technique in conjunction with polarization microscopy is based on the enhancement of the natural birefringence that collagen molecules display. This birefringence is explained by the fact that the collagen structures (at least the greatest amount of them) that can be observed under the optical microscope are composed of a number of collagen molecules that are orderly disposed in a parallel orientation [44-52].
By observing the different colors and intensities of birefringence with the existing knowledge for the biochemical distribution of collagens I, II and III Junqueira et al [48-50] proposed back in 1978 a scheme that different collagen types could be distinguished by using Sirius Red stain and polarization microscopy. According to their observations, collagen type I presents typically as thick, strongly birefringent, yellow or red fibres, while collagen III presents as thin, weakly birefringent, greenish fibres [48-56]. The difference in color and birefringence can be explained by the different patterns of physical aggregation of collagen fibres [48-56]. Collagen type I assembles in closely packed thick fibres [48-56,60,61], while the fibres of Collagen type III are made up of loosely packed thin fibrils. Collagen II, which does not form fibres, displays a weak birefringence of a varying color [48-56,60,61].
We used this method to detect the presence of Collagen type I and Collagen type III in the aforementioned specimens. Similar methods have been used in the literature in the study of the collagen content of the wall of cystic lesions, vesicular lesions and bone remodeling [57-59]. Although there have been several studies that used picrosirius red in the study of collagen the method was used mainly in a qualitative manner. The presence or prevalence of Collagen type I or of Collagen type III was determined by grossly identifying the predominant color or subjectively grading the specimen [57-59].
In our study we attempted to quantify this method so that we can obtain objective and reproducible results. To do so, pictures of our specimens under polarization microscopy were appropriately obtained and digitalized and finally analyzed with an appropriate software programme (the process is described in more details in the Methods section).
The sample analysis was based on the Red Green Blue (RGB) concept, on which the reproduction of color in digital monitors is based [62,63]. According to this concept, the apparent color of every pixel is created by the combination of three basic colors (red, green and blue) [58,59]. The intensity of each basic color in every pixel is expressed in a scale from 0 to 255. In this system the coordinates of black (absence of color) would be 0, 0, 0 while the coordinates of white would be 255, 255, 255 [62,63].
There are many ways to estimate the brightness of a pixel; the easiest way and most widely used way to do so is by calculating the value of the arithmetic mean of the intensity grade of the three basic colors (RGB=R+G+B, where RGB is the pixel RGB value, R the intensity of red, G the intensity of green and B the intensity of blue). This unique RGB value corresponds to the brightness [62,63] of the pixel. There is also the possibility to calculate a “weighted” RGB (RGB’) value that corresponds to the perceived luminance of the pixel using the formula RGB’=0.299*R+0.587*G+0.114*B [62,63]. In our study we used the concept of brightness by calculating the RGB value.
By using image analysis software, such as Image J (National Institutes of Health) it is possible to obtain an RGB analysis for a selected Region of Interest (ROI). The programme typically provides histograms for the three basic colors and a cumulative histogram for the RGB value. From the histograms the mean value of R, G, B and RGB for the ROI, as well as the statistical deviation of these values can be calculated.
The RGB values of a digitalized image are of course influenced from the light and contrast circumstances that characterize the actual photography. A more lucid image would have higher RGB values in comparison with the photography of the same image under darker circumstances. The absolute maintenance of lighting circumstances stability is not technically possible; moreover, such a prerequisite would make comparison of digital images obtained by different laboratories impossible.
To overcome this problem we propose to use for comparison not the mean R, G, B, values themselves, but the analogy of these values to the arithmetic mean RGB value (which is a measure of the image’s brightness). The mean RGB is a parameter that is sensitive to lighting conditions (for example a more lighted image would have a greater RGB value) and therefore can be used to alleviate the differences between various experimental settings. The chromatic pattern that will be documented in this way can be used to draw conclusions regarding the presence of Type I and Type III collagen fibres.
Toluidine blue
Toluidine blue (otherwise known as Tolonium Chloride) is a stain that is widely used in the study of bone biology [64-66]. It is one of the standard osseous stains that provide an adequate view of the bone architecture and its cellular composition. Toluidine Blue is a blue cationic stain. Its use is quite common for staining semi-thin (0.5 to 1 μm) sections of resin-embedded tissue [64]. An alkaline solution with a pH as high as 10 is used and this binds to the nucleic acids and all proteins of the specimen, enabling for the observation of its structural details [64].
Patients and tissue harvesting
Bone specimens of patients diagnosed with BRONJ and osteoradionecrosis were compared. A reference group of normal bone samples was used as control. A number of ten samples were studied in each category. Two separate analyses were performed. The specimens were coloured with toluidine blue to study the osteon density and with Sirius red to study the collagen morphology. The specimens stained with Sirius red were evaluated with polarization microscopy.
Bone specimens from 30 patients were included in this study. The ethical aspects of the study were approved by the ethical committee of the University of Erlangen-Nuremberg (Ref.-Nr. 4272) [39,40,42]. Ten specimens were obtained from 10 consecutive patients with clinically and histologically evident BRONJ that underwent sequestrotomy. Each specimen that was included was confirmed to exhibit histopathologic aspects of BRONJ. In addition to the histopathologic characteristics of BRONJ, the inclusion criteria for specimens were: patients that received intravenous application of either pamidronate or zoledronate for at least 12 months for treating carcinoma, and patients showed clinical evidence of an exposed jaw bone for at least 8 weeks. Specimens from patients who have formerly received radiotherapy were excluded.
All specimens were obtained during routine clinical procedures, where tissue was collected for standard diagnostics. Thus, no surgical procedure specific to this study was performed, and no additional material was collected from patients.
Five of the included patients were female and five male. The median age of the cohort was 64 years. Four of the male patients suffered from prostate cancer and one from multiple myeloma, while the underlying diagnosis in all the female patients was breast cancer. The controls comprised 10 bone specimens that were collected during intraoral surgery procedures in patients with no BP-history and no clinical signs of intraoral inflammation or periodontitis.
The 10 control specimens were harvested from the alveolar crest after a tooth extraction that required the removal of sharp bone ridges. No patient received any medication that could affect the histopathological appearance of the bone specimens. The gender and age of patients were matched in the BRONJ and control groups.
The osteoradionecrosis specimens (n=10) were from patients that had been treated with radiotherapy prior to surgery for oral squamous epithelial carcinoma. These patients received a mean total reference dose of 68 Gy in the lower jaw region. The specimens used in this study were collected after a mean interval of 36 months between radiotherapy and secondary surgery. Tissue samples were obtained from the soft tissue that surrounded the bone that was exposed during a sequestrectomy of osteoradionecrosis-affected mandibular bone. The osteoradionecrosis group consisted of 6 males and 4 females with a median age of 57 years.
Analysis of collagen using Sirius red staining and polarization microscopy
Our specimens were stained with Sirius red and then observed under polarization microscopy using a Zeiss light microscope (Axioskop, Zeiss, Jena, Germany). The obtained images were digitalized with the help of a video camera and stored in electronic form. For the analysis of the image the Image-J software was used and the observer was blinded to the specimen groups. One representative image of each specimen group is demonstrated in Figure 1.
Figure 1.
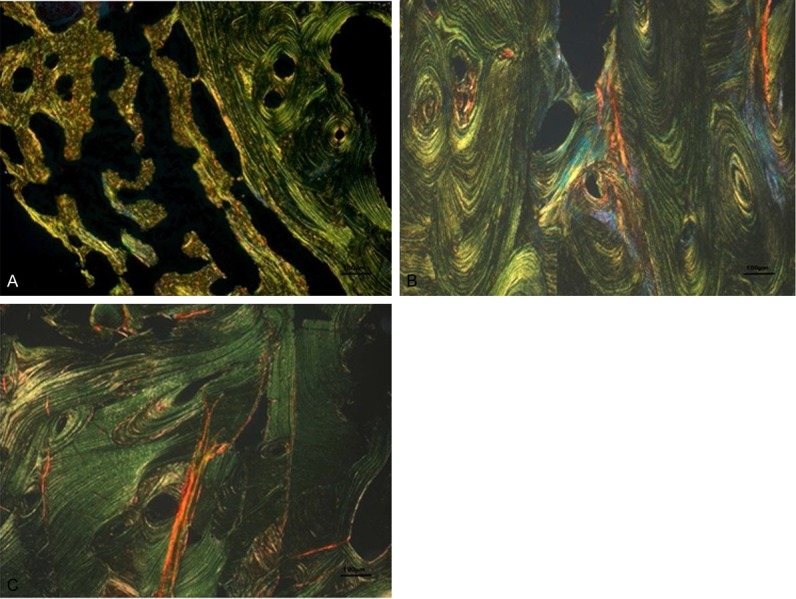
A-C: Representative images of the 3 groups using Sirius red under polarization microscopy with a magnification of 100x (A: BRONJ, B: ONJ, C: Normal bone). The ONJ specimens are characterized by a relatively higher representation of yellow and red colors.
Three ROIs were randomly selected for each specimen. By using the above-mentioned software we were able to evaluate the R, G, and B values, as well as the combined RGB value for our ROIs and consequently evaluate the R/RGB, G/RGB and B/RGB values of our specimens. These values were compared with the help of SPSS 21.0 software.
Study of the osseous histomorphometry using the toluidine blue stain
The toluidine blue stained sections were analyzed using a Zeiss light microscope (Axioskop, Zeiss, Jena, Germany) (Figure 2). The image was digitalized using a video camera and stored in an electronic form. For the analysis of the image the Image-J software and was implemented.
Figure 2.
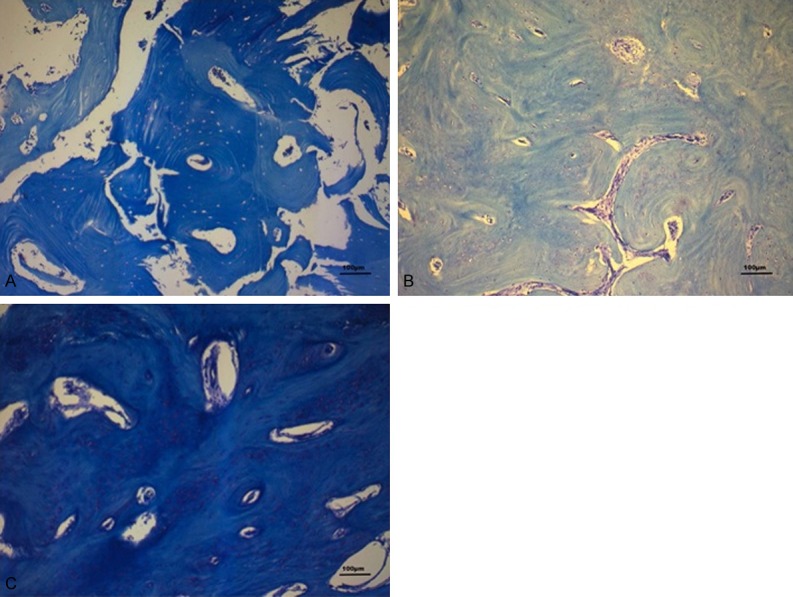
A-C: Representative images of the 3 groups using Toluidine Blue with a magnification of 100x (A: BRONJ, B: ONJ, C: Normal bone). BRONJ specimens are characterized by a lower osteon density. The thickness of the individual osteons is increased in the same specimens.
By using the above-mentioned software we were able to evaluate the surface area of randomly selected ROIs (3 for each specimen). In the same areas the number of osteons was counted. For an osteon to be counted, its Haversian canal should be fully included in the photographed area. Thus, we were able to calculate the osteon density, i.e. osteon number/surface area of our specimens. Finally, we compared the respective values of the two pathologic conditions to each other and to the values of the normal bone specimens. Statistical analysis was performed with the help of the SPSS 21.0 software.
Results
Results of the RGB-analysis
The respective mean values of R/RGB, G/RGB, and B/RGB in our specimens are shown in Table 1. One-Way ANOVA was used to compare the values among the three different specimen categories. Statistical analysis demonstrated that the values of R/RGB and G/RGB valued significantly (p<0.001) between our specimen categories.
Table 1.
Results of the RGB analysis for the three specimen categories (n=10 patients per group)
| Mean R/RGB | Mean G/RGB | Mean B/RGB | |
|---|---|---|---|
| BRONJ | 99.130 | 118.710 | 82.844 |
| ONJ | 153.489 | 161.121 | 85.390 |
| Normal bone | 93.394 | 119.781 | 86.825 |
After detection of this statistically significant variation post-hoc tests were performed to further elaborate the differences between the three categories [Tukey, Scheffe, Bonferroni, as well as Dunett 2-sided t-test (with normal bone used for the base of comparison for this test)]. All these tests revealed that the values R/RGB and G/RGB were significantly (p<0.001) higher in the ORN specimens in comparison to both normal bone and BRONJ specimens. The box plot analysis for the above-mentioned parameters (R/RGB, G/RGB and B/RGB) are shown in Figures 3, 4 and 5.
Figure 3.
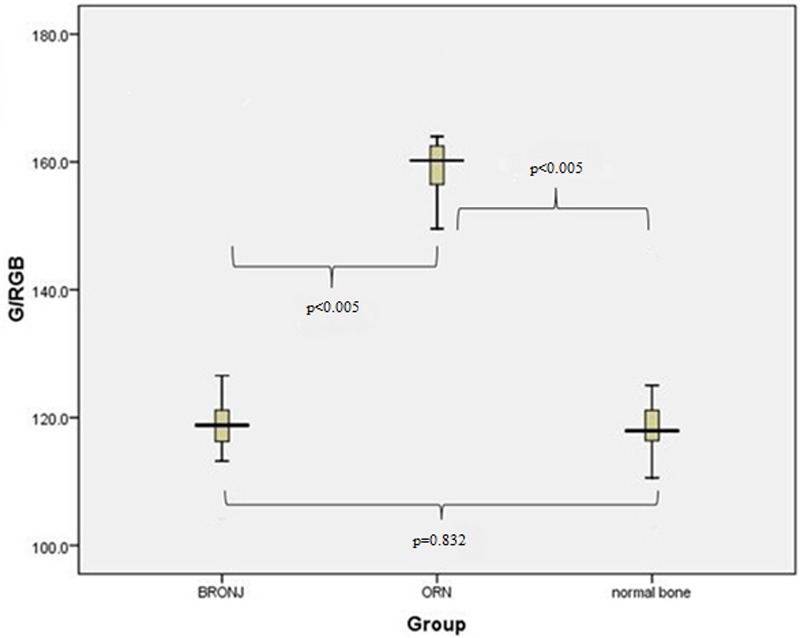
Boxplot representation of the parameter R/RGB for our three specimen groups. The value of the parameter varies significantly between BRONJ and ORN specimens, as well as between ORN and normal bone specimens (p<0.005).
Figure 4.
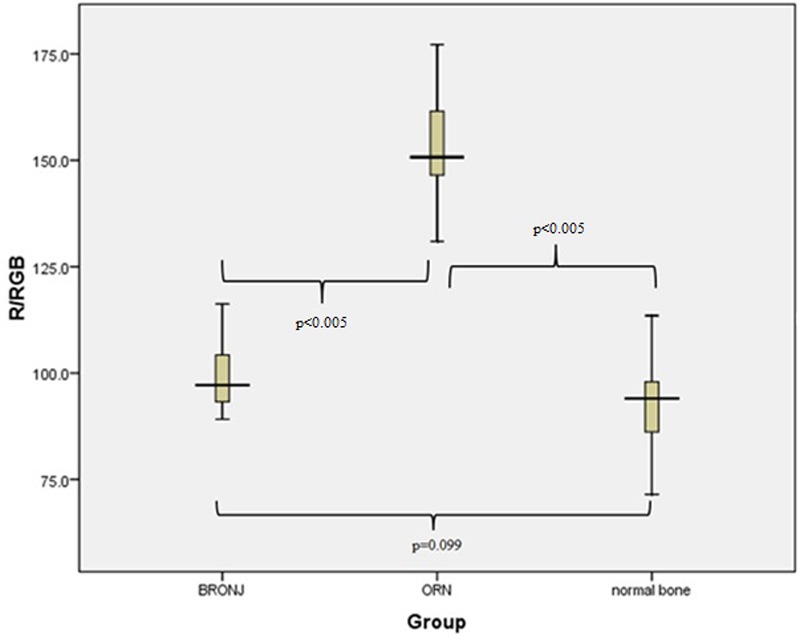
Boxplot representation of the parameter G/RGB for our three specimen groups. The value of the parameter varies significantly between BRONJ and ORN specimens, as well as between ORN and normal bone specimens (p<0.005).
Figure 5.
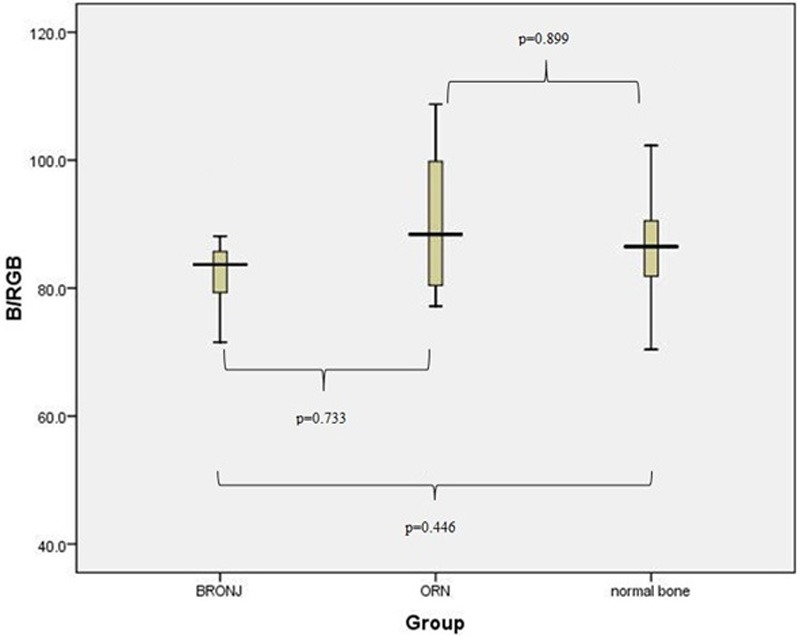
Boxplot representation of the parameter B/RGB for our three specimen groups. No significant variation between the two groups was identified for this parameter.
Results of the histomorphometrical analysis using toluidin blue
A One-Way ANOVA test was used to compare the osteon density among the three specimen categories. The mean values of osteon density for these categories can be seen in Table 2. There is a statistically significant (p<0.001) difference between the BRONJ specimens and the normal bone specimens. More specifically the analysis that was performed in the above-described fashion showed that the osteon density in the BRONJ specimens was significantly lower in comparison to the density measured in both the normal bone specimens (p<0.001) and ORN specimens (p=0.001). The osteon density in the ORN specimens was relatively lower in comparison to the density measured for normal bone, but the difference was not statistically significant. The box plot diagram that presents the results of measurement of the osteon density in the three groups is shown in Figure 6.
Table 2.
Mean osteon density for the three specimen categories (n=10 patients per group)
| Mean osteon density (osteons per mm2) | |
|---|---|
| BRONJ | 4.772 |
| ONJ | 9.034 |
| Normal bone | 9.035 |
Figure 6.
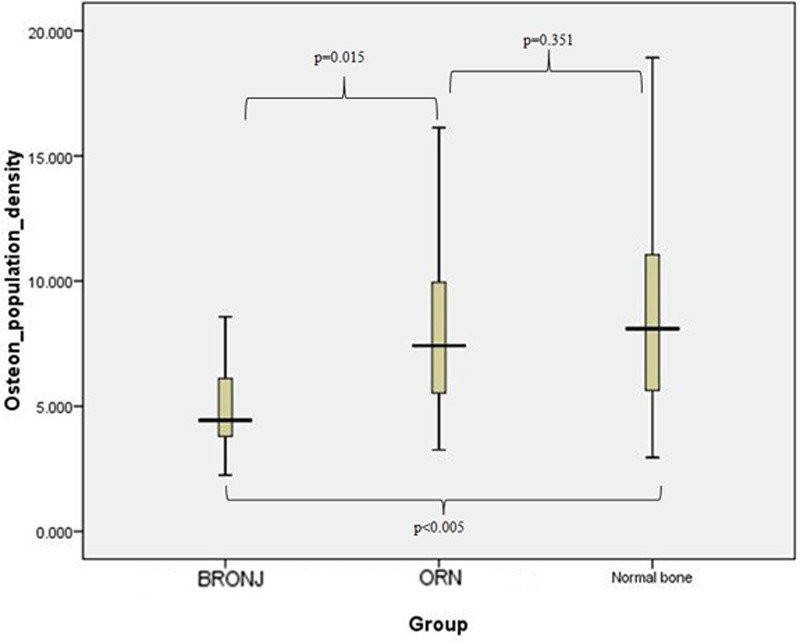
Boxplot representation of the osteon density for the three specimen groups. The value of the parameter varies significantly between BRONJ and normal bone specimens (p<0.005).
Discussion
ORN and BRONJ have a very similar clinical appearance [1,5,8,15,28], something that makes the differential diagnosis sometimes very difficult. In fact, the only significant difference that was observed in a study that compared their clinical presentation were more lesions per patient in patients suffering from BRONJ compared with ORN patients [5]. The authors also highlighted the fact that while BRONJ seems to affect both mandible and maxilla [5,8], ORN was almost exclusively developed in the mandible [17,19,67].
This similarity does not, however, extend to the histopathological presentation [8,15,28]. In a comparison between specimens of ORN and BRONJ conducted by Hansen et al [8] a number of differences were noted: ORN lesions were found to be more homogenous and the necrosis was more extensive. BRONJ specimens had a patchy appearance, where multiple, partially confluent areas of necrotic bone were mingled with vital bone residues. These and many other histopathological differences, such as the increased trabecular thickness that is observed in BRONJ [68,69] underline the fact that different pathophysiological mechanisms produce a different microscopic appearance, which however results in similar clinical manifestations.
Marx and Turson [15] also reported results of a comparison that was performed among histopathological specimens of BRONJ, ORN as well as osteomyelitis. The authors pointed out to a number of differences, i.e. an increased presence of collagen in ORN; BRONJ is described as a non-inflammatory necrosis, characterized by the suppression of bone renewal.
The results of our study allow two basic observations: (i). BRONJ is a disorder that is characterized by disruption of the normal bone architecture and organization and (ii) ORN is a condition characterized by increased fibrosis.
Architectural changes
The fact that the bone architecture of BRONJ specimens is altered has been described in other clinical studies. Paparella et al [69] compared BRONJ specimens to infectious osteomyelitis specimens and identified a characteristic Paget-like appearance of the BRONJ specimens, with increased trabecular thickness and decreased medullary spaces. The authors hypothesize that the structural alteration leads to formation of isolated bone sectors with no contact with the marrow and hence no source of nutrition. In other words they describe BRONJ as a form of avascular necrosis, where the lack of blood supply is attributed to extreme compartmentalization because of an abnormal process of bone remodelling.
Favia et al [68] also described a reduced Haversian canal density in BRONJ patients compared to normal bone, as well as morphological changes of the Haversian canal, such as maximal and minimal canal diameter. Our study confirmed this finding and also demonstrated that there is a significant difference in osteon density between ORN and BRONJ samples.
On the other hand no changes in the bone architecture per se are described in cases of ORN; the hallmark of the disease is the decrease in the numbers of osteoblasts, manifested as empty lacunae [3,13-15]. This was corroborated from our measurements, since no statistically significant difference in the osteon density was found between ORN specimens and normal bone specimens. There was a tendency to lower osteon density in the ORN specimens, but the difference was not statistically significant.
The collagen
The RGB-analysis of our specimens that were coloured with Sirius Red demonstrated that the chromatic patterns of the bone preparations from the patients suffering from ORN varied significantly from the respective bone preparations from patients suffering from BRONJ as, well as from our control samples. There was no significant difference noted between BRONJ and control samples. More specifically the mean value of R/RGB and G/RGB were considerably increased in the ORN specimens.
In a digitally stored image, areas that are perceived as red color are characterized by predominantly increased R/RGB value, while those perceived as yellow are typically characterized by an increase in both R/RGB and G/RGB values. Green coloured areas are typically characterized by a medium to high value of G/RGB with medium values of R/RGB. A statistically significant increased R/RGB value was found in the analysis of ORN specimens. Since a high R/RGB value is obtained in red as well as yellow coloured areas, it is safe to conclude that these areas are overrepresented in the ORN specimens. Overrepresentation of red and yellow areas is consisted with an increased presence of Type I collagen fibres.
The G/RGB value has also been found to be statistically significantly increased. An increased G/RGB value would be expected to be found in yellow as well as in green coloured areas. Since overrepresentation of yellow areas would be consisted with increased presence of collagen Type I and overrepresentation of green areas consisted with increased presence of collagen Type III, the increase in G/RGB value speaks for increased collagen presence; however, safe conclusions for the relative representation of Type I and Type III collagen cannot be extrapolated from only this value.
In other words we were able to demonstrate an overrepresentation of collagen type I and quite possibly an increase in collagen type III in ORN patients in comparison to BRONJ patients and our control group. The analogy of the increase of collagen I to collagen III could not be determined from the used method, since further standardization of this technique by performing RGB analysis in samples with known concentration of collagen I and III would be necessary for this purpose.
The fact that radiation-related damages are characterized by fibrosis (i.e. collagen overexpression) has been well documented in the literature [3,66-69]. In fact the etiopathogenic theories for ORN have gradually shifted to include this concept [3,9]. The initial perception for the etiology of ORN was proposed by Meyer [70], who explained the condition with the triad “radiation-trauma-infection”; more specifically Meyer believed that a trauma in the overlying mucosa provided the access to the compromised from radiation bone. Other authors like Titterington [71] also agreed with this concept and for a decade ORN was viewed as a special type of osteomyelitis [3,9,17].
That was until Marx [13,14] demonstrated that the isolated microorganisms in ORN did not represent bacterial invasion, but rather superinfection, a finding that challenged the central role in ORN pathophysiology. Marx proposed his own triad to replace Myer’s triad: Hypoxia-Hypocellularity-Hypovascularity were caused by the radiation and were viewed as the cornerstone of the disease [3,13,14,17]. This factor triad results according to Marx’s theory in tissue breakdown that causes the observed non-healing wounds. Based on this hypothesis Marx suggested Hyper-Baric Oxygen (HBO) therapy for the management of ORN [13]. The relatively poor results of HBO [72,73] in randomized control trials questioned the correctness of this etiopathogenic theory [3,17,73].
In 2004 Assael [3,74] challenged the above-mentioned widely accepted perception by pointing out that several of the observed radiogenic effects on the bone cells precede the vascular alterations. He considered the effect of radiation on osteoclasts to be the most important factor in ORN, since it interfered with normal bone turnover and compromised the bone healing capacity. Assael believed that ORN has a similar pathophysiologic mechanism to the one involved in BRONJ - since bisphosphonates mainly interfere with the function of osteoclasts and osteoclast dysfunction is one of the hallmarks of the disease [2,4,7]. The fact, however, that bisphosphonates have in fact been found to be advantageous in the management of ORN by some investigators [75] does not support this concept. What also argues against Assael’s theory is the number of histopathologic differences between BRONJ and ORN [3,8,64-66]; our observations regarding bone architecture and collagen also identified differences between BRONJ and ORN, thus making a common pathophysiological mechanism unlikely.
The cascade of Radiation Induced Fibroatrophic Process (RIF) is observed in tissues after radiation damage [22-25,75-77]. A number of cytokines is involved in this process, i.e. Interferon-ß (INF-ß), Platelet-derived Growth Factor (PDGF), Epidermal Growth Factor (EGF), Fibroblast Growth Factor (FGF), Connective tissue Growth Factor (CTGF), Interleukins, Prostaglandins and most importantly Transforming Growth Factor ß1 (TGF-ß1) [22-25,75-77].
TGF-ß1 is a known pro-fibrotic factor and its over-expression leads to increased collagen I and III expression and a reduced stimulation of ECM components [22-25,75-77]. Wehrhan et al [35] compared the expression of TGF-ß1 factor in soft tissue specimens of patients suffering from BRONJ and ORN and demonstrated that the expression of TGF-ß1 is diminished in the bisphosphonate specimens and increased in the ORN specimens.
Our study partially confirmed this finding. We found an increased representation of collagen I and possibly collagen III in ORN - patients consisted with an increased TGF-ß1 expression. On the other hand we failed to demonstrate any difference in the collagen expression between BRONJ patients and our control group.
Conclusion
In summary, the findings of our comparative study further highlighted the pathophysiological differences between these two common forms of jaw necrosis. ORN is a condition that is dominated by fibrosis and hyper-expression of Collagen I whereas this was not seen in the BRONJ specimens, which, on the other hand were characterized by deviation from the normal bone architecture. The fact that these two conditions have relatively similar clinical presentation underlines the fact that a resemblance in clinical presentation does not necessary denote a similar pathophysiology.
Acknowledgements
We acknowledge support by Deutsche Forschungsgemeinschaft and Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) within the funding programme Open Access Publishing.
Disclosure of conflict of interest
The authors disclose no conflict of interest.
References
- 1.Almazrooa SA, Woo SB. Bisphosphonate and nonbisphosphonate-associated osteonecrosis of the jaw: a review. J Am Dent Assoc. 2009;140:864–75. doi: 10.14219/jada.archive.2009.0280. [DOI] [PubMed] [Google Scholar]
- 2.Reid IR. Osteonecrosis of the jaw: who gets it, and why? Bone. 2009;44:4–10. doi: 10.1016/j.bone.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Chrcanovic BR, Reher P, Sousa AA, Harris M. Osteoradionecrosis of the jaws - a current overview - part 1: Physiopathology and risk and predisposing factors. Oral Maxillofac Surg. 2010;14:3–16. doi: 10.1007/s10006-009-0198-9. [DOI] [PubMed] [Google Scholar]
- 4.Ruggiero SL. Bisphosphonate-related osteonecrosis of the jaw: an overview. Ann N Y Acad Sci. 2011;1218:38–46. doi: 10.1111/j.1749-6632.2010.05768.x. [DOI] [PubMed] [Google Scholar]
- 5.Bagan JV, Jiménez Y, Hernández S, Murillo J, Díaz JM, Poveda R, Carbonell E, Sanchis JM, Gavaldá C, Scully C. Osteonecrosis of the jaws by intravenous bisphosphonates and osteoradionecrosis: a comparative study. Med Oral Patol Oral Cir Bucal. 2009;14:e616–9. doi: 10.4317/medoral.14.e616. [DOI] [PubMed] [Google Scholar]
- 6.Bagan J, Scully C, Sabater V, Jimenez Y. Osteonecrosis of the jaws in patients treated with intravenous bisphosphonates (BRONJ): A concise update. Oral Oncol. 2009 Jul;45:551–4. doi: 10.1016/j.oraloncology.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B Task Force on Bisphosphonate-Related Osteonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaw - 2009 update. Aust Endod J. 2009;35:119–30. doi: 10.1111/j.1747-4477.2009.00213.x. [DOI] [PubMed] [Google Scholar]
- 8.Hansen T, Kunkel M, Weber A, James Kirkpatrick C. Osteonecrosis of the jaws in patients treated with bisphosphonates - histomorphologic analysis in comparison with infected osteoradionecrosis. J Oral Pathol Med. 2006;35:155–60. doi: 10.1111/j.1600-0714.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 9.Lambade PN, Lambade D, Goel M. Osteoradionecrosis of the mandible: a review. Oral Maxillofac Surg. 2013 Dec;17:243–9. doi: 10.1007/s10006-012-0363-4. [DOI] [PubMed] [Google Scholar]
- 10.Regaud C. Sur la necrose des os attenté par un processus cancereux et traites par les radiaions. Compt Rend Soc Biol. 1922;87:427. [Google Scholar]
- 11.Ewing J. Radiation osteitis. Acta Radiol. 1926;6:399–412. [Google Scholar]
- 12.Jereczek-Fossa BA, Orecchia R. Radiotherapy-induced mandibular bone complications. Cancer Treat Rev. 2002;28:65–74. doi: 10.1053/ctrv.2002.0254. [DOI] [PubMed] [Google Scholar]
- 13.Marx RE. A new concept in the treatment of osteoradionecrosis. J Oral Maxillofac Surg. 1983;41:351–357. doi: 10.1016/s0278-2391(83)80005-6. [DOI] [PubMed] [Google Scholar]
- 14.Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41:283–8. doi: 10.1016/0278-2391(83)90294-x. [DOI] [PubMed] [Google Scholar]
- 15.Marx RE, Tursun R. Suppurative osteomyelitis, bisphosphonate induced osteonecrosis, osteoradionecrosis: a blinded histopathologic comparison and its implications for the mechanism of each disease. Int J Oral Maxillofac Surg. 2012;41:283–9. doi: 10.1016/j.ijom.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Harris M. The conservative management of osteoradionecrosis of the mandible with ultrasound therapy. Br J Oral Maxillofac Surg. 1992;30:313–8. doi: 10.1016/0266-4356(92)90181-h. [DOI] [PubMed] [Google Scholar]
- 17.Lyons A, Ghazali N. Osteoradionecrosis of the jaws: current understanding of its pathophysiology and treatment. Br J Oral Maxillofac Surg. 2008;46:653–60. doi: 10.1016/j.bjoms.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Teng MS, Futran ND. Osteoradionecrosis of the mandible. Curr Opin Otolaryngol Head Neck Surg. 2005;13:217–21. doi: 10.1097/01.moo.0000170527.59017.ff. [DOI] [PubMed] [Google Scholar]
- 19.Reuther T, Schuster T, Mende U, Kubler A. Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients—a report of a thirty year retrospective review. Int J Oral Maxillofac Surg. 2003;32:289–295. doi: 10.1054/ijom.2002.0332. [DOI] [PubMed] [Google Scholar]
- 20.Curi MM, Dib LL. Osteoradionecrosis of the jaws: a retrospective study of the background factors and treatment in 104 cases. J Oral Maxillofac Surg. 1997;55:540–4. doi: 10.1016/s0278-2391(97)90478-x. [DOI] [PubMed] [Google Scholar]
- 21.Oh HK, Chambers MS, Martin JW, Lim HJ, Park HJ. Osteoradionecrosis of the mandible: treatment outcomes and factors influencing the progress of osteoradionecrosis. J Oral Maxillofac Surg. 2009;67:1378–86. doi: 10.1016/j.joms.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Rustemeyer J, Bremerich A. Bisphosphonate-associated osteonecrosis of the jaw: what do we currently know? A survey of knowledge given in the recent literature. Clin Oral Investig. 2010;14:59–64. doi: 10.1007/s00784-009-0294-0. [DOI] [PubMed] [Google Scholar]
- 23.Hellstein JW, Marek CL. Bisphosphonate osteochemonecrosis (bis-phossy jaw): is this phossy jaw of the 21st century? J Oral Maxillofac Surg. 2005;63:682–9. doi: 10.1016/j.joms.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Gutta R, Louis PJ. Bisphosphonates and osteonecrosis of the jaws: science and rationale. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:186–93. doi: 10.1016/j.tripleo.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Wimalawansa SJ. Insight into bisphosphonate-associated osteomyelitis of the jaw: pathophysiology, mechanisms and clinical management. Expert Opin Drug Saf. 2008;7:491–512. doi: 10.1517/14740338.7.4.491. [DOI] [PubMed] [Google Scholar]
- 26.Landesberg R, Woo V, Cremers S, Cozin M, Marolt D, Vunjak-Novakovic G, Kousteni S, Raghavan S. Potential pathophysiological mechanisms in osteonecrosis of the jaw. Ann N Y Acad Sci. 2011;1218:62–79. doi: 10.1111/j.1749-6632.2010.05835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLeod NM, Brennan PA, Ruggiero SL. Bisphosphonate osteonecrosis of the jaw: a historical and contemporary review. Surgeon. 2012;10:36–42. doi: 10.1016/j.surge.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Lesclous P, Abi Najm S, Carrel JP, Baroukh B, Lombardi T, Willi JP, Rizzoli R, Saffar JL, Samson J. Bisphosphonate-associated osteonecrosis of the jaw: a key role of inflammation? Bone. 2009;45:843–52. doi: 10.1016/j.bone.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqi A, Payne AG, Zafar S. Bisphosphonate-induced osteonecrosis of the jaw: a medical enigma? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e1–8. doi: 10.1016/j.tripleo.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Allen MR, Burr DB. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. J Oral Maxillofac Surg. 2009;67:61–70. doi: 10.1016/j.joms.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Bagan J, Scully C, Sabater V, Jimenez Y. Osteonecrosis of the jaws in patients treated with intravenous bisphosphonates (BRONJ): A concise update. Oral Oncol. 2009;45:551–4. doi: 10.1016/j.oraloncology.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, Kavanagh KL, Triffitt JT, Lundy MW, Phipps RJ, Barnett BL, Coxon FP, Rogers MJ, Watts NB, Ebetino FH. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci. 2007;1117:209–57. doi: 10.1196/annals.1402.089. [DOI] [PubMed] [Google Scholar]
- 33.Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Silverman SL, Landesberg R. Osteonecrosis of the jaw and the role of bisphosphonates: a critical review. Am J Med. 2009;122:S33–45. doi: 10.1016/j.amjmed.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Wehrhan F, Hyckel P, Guentsch A, Nkenke E, Stockmann P, Schlegel KA, Neukam FW, Amann K. Bisphosphonate-associated osteonecrosis of the jaw is linked to suppressed TGFß1-signaling and increased Galectin-3 expression: a histological study on biopsies. J Transl Med. 2011;9:102. doi: 10.1186/1479-5876-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wehrhan F, Hyckel P, Amann K, Ries J, Stockmann P, Schlegel K, Neukam F, Nkenke E. Msx-1 is suppressed in bisphosphonate-exposed jaw bone analysis of bone turnover-related cell signalling after bisphosphonate treatment. Oral Dis. 2011;17:433–42. doi: 10.1111/j.1601-0825.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 37.Sarin J, DeRossi SS, Akintoye SO. Updates on bisphosphonates and potential pathobiology of bisphosphonate-induced jaw osteonecrosis. Oral Dis. 2008;14:277–85. doi: 10.1111/j.1601-0825.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- 38.Stockmann P, Vairaktaris E, Wehrhan F, Seiss M, Schwarz S, Spriewald B, Neukam FW, Nkenke E. Osteotomy and primary wound closure in bisphosphonate-associated osteonecrosis of the jaw: a prospective clinical study with 12 months follow-up. Support Care Cancer. 2010;18:449–60. doi: 10.1007/s00520-009-0688-1. [DOI] [PubMed] [Google Scholar]
- 39.Roybal PG, Wu NL, Sun J, Ting MC, Schafer CA, Maxson RE. Inactivation of Msx1 and Msx2 in neural crest reveals an unexpected role in suppressing heterotopic bone formation in the head. Dev Biol. 2010;343:28–39. doi: 10.1016/j.ydbio.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maxson R, Ishii M. The Bmp pathway in skull vault development. Front Oral Biol. 2008;12:197–208. doi: 10.1159/000115042. [DOI] [PubMed] [Google Scholar]
- 41.Babajko S, Petit S, Fernandes I, Méary F, LeBihan J, Pibouin L, Berdal A. Msx1 expression regulation by its own antisense RNA: consequence on tooth development and bone regeneration. Cells Tissues Organs. 2009;189:115–121. doi: 10.1159/000151748. [DOI] [PubMed] [Google Scholar]
- 42.Chung IH, Yamaza T, Zhao H, Choung PH, Shi S, Chai Y. Stem cell property of postmigratory cranial neural crest cells and their utility in alveolar bone regeneration and tooth development. Stem Cells. 2009;27:866–877. doi: 10.1002/stem.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orestes-Cardoso S, Nefussi JR, Lezot F, Oboeuf M, Pereira M, Mesbah M, Robert B, Berdal A. Msx1 is a regulator of bone formation during development and postnatal growth: in vivo investigations in a transgenic mouse model. Connect Tissue Res. 2002;43:153–160. doi: 10.1080/03008200290000547. [DOI] [PubMed] [Google Scholar]
- 44.Junqueira LCU, Cossermelli W, Berntani R. Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch Histol Jpn. 1978;41:267. doi: 10.1679/aohc1950.41.267. [DOI] [PubMed] [Google Scholar]
- 45.Junquiera LC, Junqueira LC, Brentani RR. A simple and sensitive method for the quantitative estimation of collagen. Anal Biochem. 1979;94:96–9. doi: 10.1016/0003-2697(79)90795-4. [DOI] [PubMed] [Google Scholar]
- 46.Montes GS, Junqueira LC. Biology of collagen. Rev Can Biol Exp. 1982;41:143–56. [PubMed] [Google Scholar]
- 47.Montes GS. Structural biology of the fibres of the collagenous and elastic systems. Cell Biol Int. 1996;20:15–27. doi: 10.1006/cbir.1996.0004. [DOI] [PubMed] [Google Scholar]
- 48.Junqueira LC, Montes GS, Toledo OM, Bexiga SR, Gordilho MA, Brentani RR. Evidence for collagen molecular orientation in basement membranes. Histochem J. 1983;15:785–94. doi: 10.1007/BF01003341. [DOI] [PubMed] [Google Scholar]
- 49.Junqueira LC, Roscoe JT. Reduced collagen content and fibre bundle disorganization in skin biopsies of patients with Ehlers-Danlos syndrome. Histochem J. 1985;17:1197–202. doi: 10.1007/BF01002502. [DOI] [PubMed] [Google Scholar]
- 50.Junqueira LC, Assis Figueiredo MT, Torloni H, Montes GS. Differential histologic diagnosis of osteoid. A study on human osteosarcoma collagen by the histochemical picrosirius-polarization method. J Pathol. 1986;148:189–96. doi: 10.1002/path.1711480210. [DOI] [PubMed] [Google Scholar]
- 51.López-De León A, Rojkind M. A simple micromethod for collagen and total protein determination in formalin-fixed Montes GS, Junqueira LC. The use of the picrosirius-polarization method for the study of the biopathology of collagen. J Histochem Cytochem. 1985;33:737–43. doi: 10.1177/33.8.2410480. [DOI] [PubMed] [Google Scholar]
- 52.Montes GS, Junqueira LCU. The use of the picrosirius-polarization method for the study of the biopathology of collagen. Mem Inst Oswaldo Cruz. 1991;86(Suppl 3):1–11. doi: 10.1590/s0074-02761991000700002. [DOI] [PubMed] [Google Scholar]
- 53.Borges LF, Gutierrez PS, Marana HR, Taboga SR. Picrosirius-polarization staining method as an efficient histopathological tool for collagenolysis detection in vesical prolapse lesions. Micron. 2007;38:580–3. doi: 10.1016/j.micron.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Hirshberg A, Lib M, Kozlovsky A, Kaplan I. The influence of inflammation on the polarization colors of collagen fibers in the wall of odontogenic keratocyst. Oral Oncol. 2007;43:278–82. doi: 10.1016/j.oraloncology.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 55.Vij R, Vij H, Rao NN. Evaluation of collagen in connective tissue walls of odontogenic cysts—a histochemical study. J Oral Pathol Med. 2011;40:257–62. doi: 10.1111/j.1600-0714.2010.00961.x. [DOI] [PubMed] [Google Scholar]
- 56.Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. J Cell Sci. 2007;120:1955–8. doi: 10.1242/jcs.03453. [DOI] [PubMed] [Google Scholar]
- 57.Okuyama K. Revisiting the molecular structure of collagen. Connect Tissue Res. 2008;49:299–310. doi: 10.1080/03008200802325110. [DOI] [PubMed] [Google Scholar]
- 58.Bezryadin S, Bourov P, Ilinikh D. International Symposium on Technologies for Digital Fulfillment Abstract Book and CD-ROM. Volume 1. Las Vegas, Nevada: 2007. Mar, Brightness Calculation in Digital Image Processing; pp. 10–15. [Google Scholar]
- 59.Wyszecki G, Stiles WS. Color Science: Concepts and Methods, Quantitative Data and Formulae. Second Edition. New York: John Wiley & Sons; 2000. [Google Scholar]
- 60.Carson FL. A Self-Instructional Text. 2nd edition. Chicago: American Society of Clinical Pathologists Press; 1997. Histotechnology; p. 188. [Google Scholar]
- 61.Bach-Gansmo FL, Irvine SC, Brüel A, Thomsen JS, Birkedal H. Calcified Cartilage Islands in Rat Cortical Bone. Calcif Tissue Int. 2013 Apr;92:330–8. doi: 10.1007/s00223-012-9682-6. [DOI] [PubMed] [Google Scholar]
- 62.Draenert FG, Kämmerer PW, Palarie V, Wagner W. Vertical bone augmentation with simultaneous dental implantation using crestal biomaterial rings: a rabbit animal study. Clin Implant Dent Relat Res. 2012;14:e169–74. doi: 10.1111/j.1708-8208.2011.00401.x. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz HC, Kagan AR. Osteoradionecrosis of the mandible: scientific basis for clinical staging. Am J Clin Oncol. 2002;25:168–71. doi: 10.1097/00000421-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 64.Favia G, Pilolli GP, Maiorano E. Histologic and histomorphometric features of bisphosphonate-related osteonecrosis of the jaws: an analysis of 31 cases with confocal laser scanning microscopy. Bone. 2009;45:406–13. doi: 10.1016/j.bone.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 65.Paparella ML, Brandizzi D, Santini-Araujo E, Cabrini RL. Histopathological features of osteonecrosis of the jaw associated with bisphosphonates. Histopathology. 2012;60:514–6. doi: 10.1111/j.1365-2559.2011.04061.x. [DOI] [PubMed] [Google Scholar]
- 66.Delanian S, Lefaix JL. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol. 2004;73:119–31. doi: 10.1016/j.radonc.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 67.Delanian S, Chatel C, Porcher R, Depondt J, Lefaix JL. Complete restoration of refractory mandibular osteoradionecrosis by prolonged treatment with a pentoxifylline-tocopherol-clodronate combination (PENTOCLO): a phase II trial. Int J Radiat Oncol Biol Phys. 2011;80:832–9. doi: 10.1016/j.ijrobp.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 68.Vozenin-Brotons MC, Milliat F, Sabourin JC, de Gouville AC, François A, Lasser P, Morice P, Haie-Meder C, Lusinchi A, Antoun S, Bourhis J, Mathé D, Girinsky T, Aigueperse J. Fibrogenic signals in patients with radiation enteritis are associated with increased connective tissue growth factor expression. Int J Radiat Oncol Biol Phys. 2003;56:561–72. doi: 10.1016/s0360-3016(02)04601-1. [DOI] [PubMed] [Google Scholar]
- 69.Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277–90. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 70.Meyer I. Infectious diseases of the jaws. J Oral Surg. 1970;28:17–26. [PubMed] [Google Scholar]
- 71.Titterington WP. Osteomyelitis and osteoradionecrosis of the jaws. J Oral Med. 1971 Jan-Mar;26:7–16. [PubMed] [Google Scholar]
- 72.Annane D, Depondt J, Aubert P, Villart M, Géhanno P, Gajdos P, Chevret S. Hyperbaric oxygen therapy for radionecrosis of the jaw: a randomized, placebo-controlled, double-blind trial from the ORN96 study group. J. Clin. Oncol. 2004;22:4893–900. doi: 10.1200/JCO.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 73.Bennett MH, Feldmeier J, Hampson N, Smee R, Milross C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2012 May 16;5:CD005005. doi: 10.1002/14651858.CD005005.pub2. [DOI] [PubMed] [Google Scholar]
- 74.Assael LA. New foundations in understanding osteonecrosis of the jaws. J Oral Maxillofac Surg. 2004;62:125–126. doi: 10.1016/j.joms.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 75.Delanian S, Depondt J, Lefaix JL. Major healing of refractory mandible osteoradionecrosis after treatment combining pentoxifylline and tocopherol: a phase II trial. Head Neck. 2005;27:114–23. doi: 10.1002/hed.20121. [DOI] [PubMed] [Google Scholar]
- 76.Schultze-Mosgau S, Blaese MA, Grabenbauer G, Wehrhan F, Kopp J, Amann K, Rodemann HP, Rödel F. Smad-3 and Smad-7 expression following anti-transforming growth factor beta 1 (TGFbeta1)-treatment in irradiated rat tissue. Radiother Oncol. 2004;70:249–59. doi: 10.1016/j.radonc.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 77.Wehrhan F, Rödel F, Grabenbauer GG, Amann K, Brückl W, Schultze-Mosgau S. Transforming growth factor beta 1 dependent regulation of Tenascin-C in radiation impaired wound healing. Radiother Oncol. 2004;72:297–303. doi: 10.1016/j.radonc.2004.07.011. [DOI] [PubMed] [Google Scholar]


