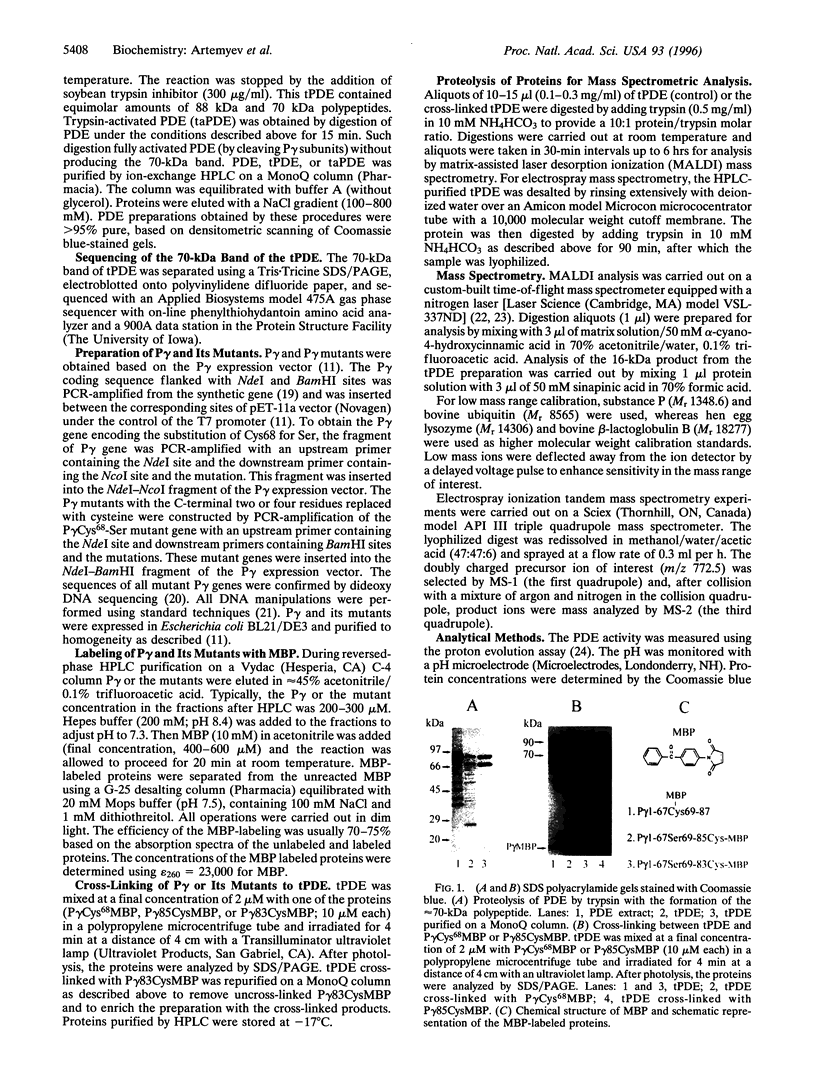
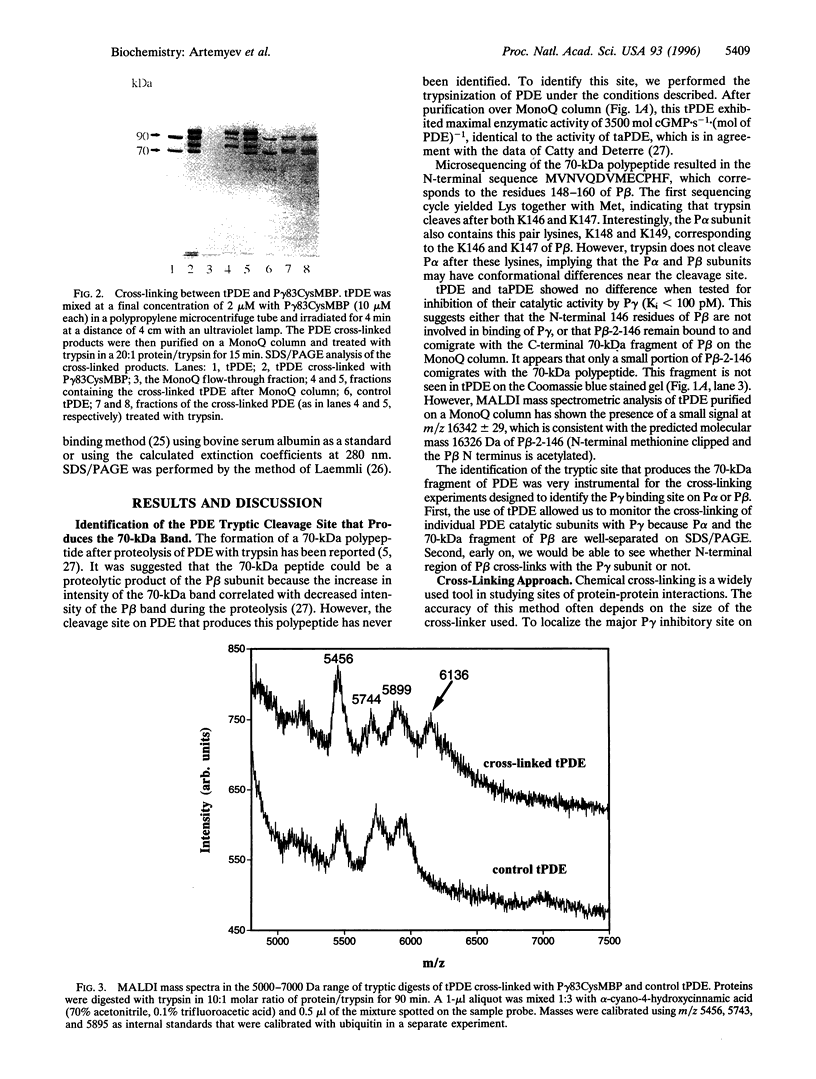
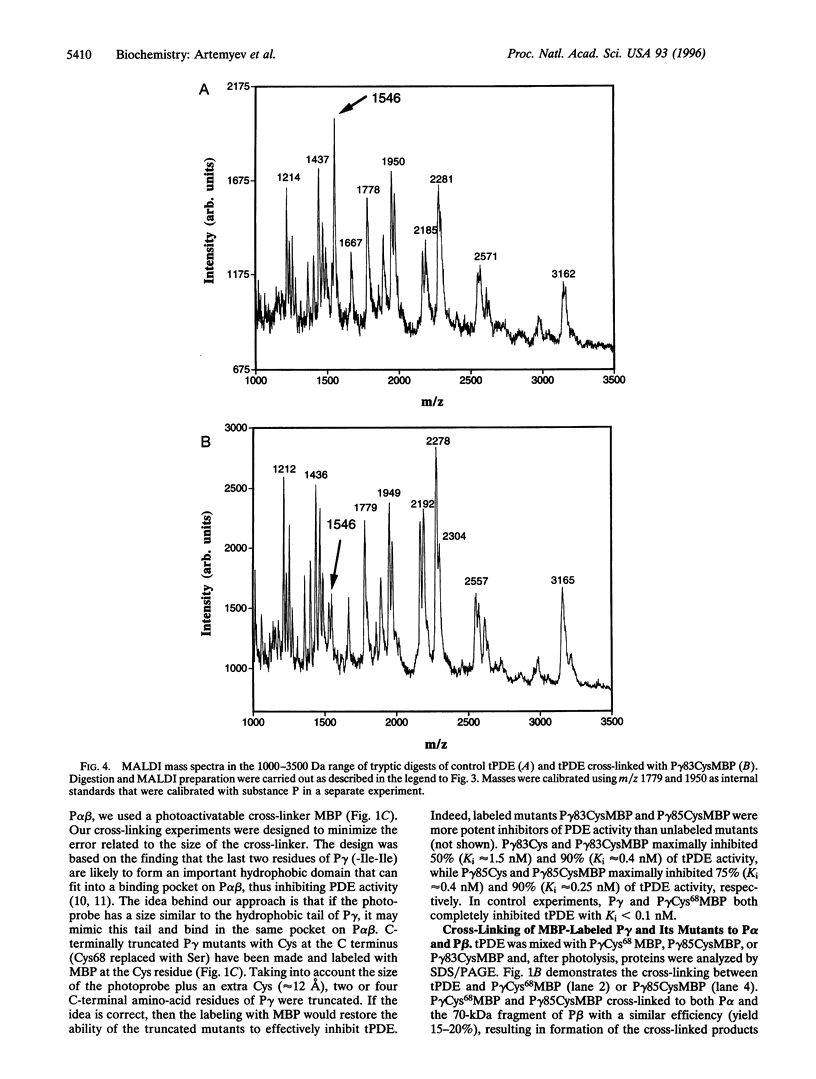
Abstract
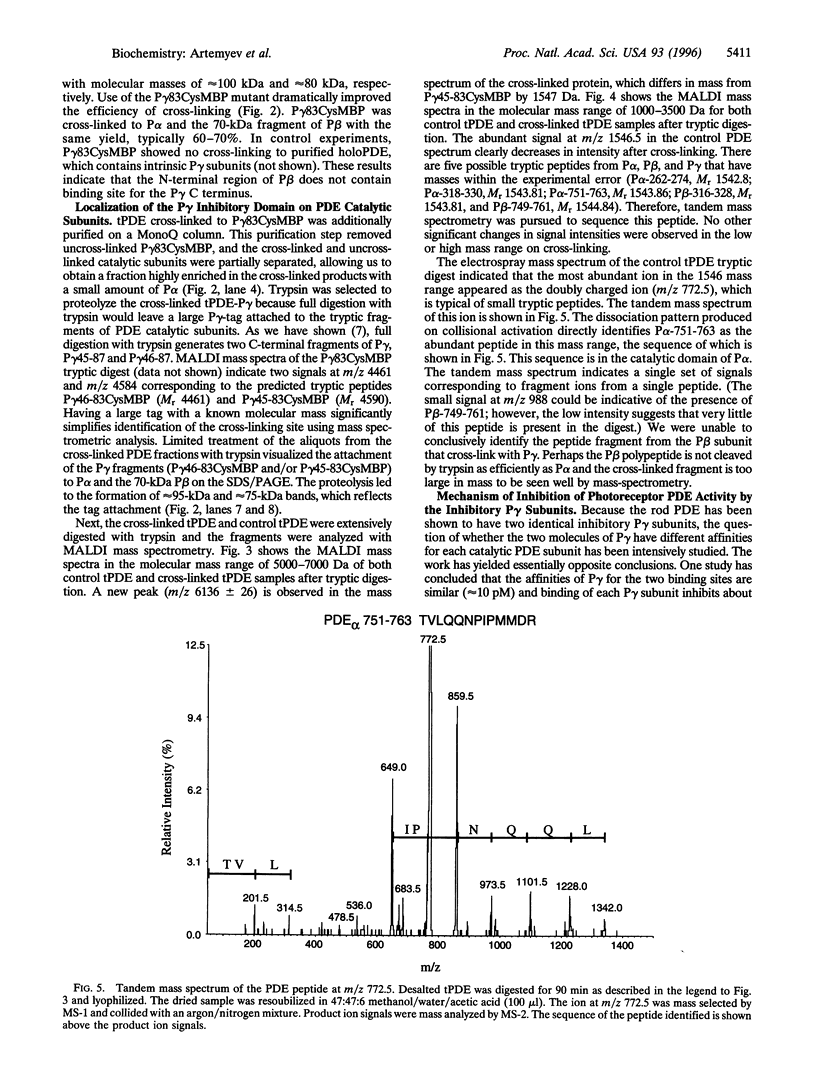
cGMP phosphodiesterase (PDE) is the key effector enzyme of vertebrate photoreceptor cells that regulates the level of the second messenger, cGMP. PDE consists of catalytic alpha and beta subunits (Palpha and Pbeta) and two inhibitory gamma subunits (Pgamma) that block PDE activity in the dark. The major inhibitory region has been localized to the C terminus of Pgamma. The last C-terminal residues -IleIle form an important hydrophobic domain critical for the inhibition of PDE activity. In this study, mutants of Pgamma were designed for cross-linking experiments to identify regions on Palpha and Pbeta subunits that bind to the Pgamma C terminus. In one of the mutants, the cysteine at position 68 was substituted with serine, and the last four C-terminal residues of Pgamma were replaced with a single cysteine. This mutant, Pgamma83Cys, was labeled with photoprobe 4-(N-maleimido) benzophenone (MBP) at the cysteine residue. The labeled Pgamma83CysMBP mutant was a more potent inhibitor of PDE activity than the unlabeled mutant, indicating that the hydrophobic MBP probe mimics the Pgamma hydrophobic C terminus. A specific, high-yield cross-linking of up to 70% was achieved between the Pgamma83CysMBP and PDE catalytic subunits. Palpha and the N-terminally truncated Pbeta (lacking 147 aa residues) cross-linked to Pgamma83CysMBP with the same efficiency. Using mass spectrometric analysis of tryptic fragments from the cross-linked PDE, we identified the site of cross-linking to aa residues 751-763 of Palpha. The corresponding region of Pbeta, Pbeta-749-761, also may bind to the Pgamma C terminus. Our data suggest that Pgamma blocks PDE activity through the binding to the catalytic site of PDE, near the NKXD motif, a consensus sequence for interaction with the guanine ring of cGMP.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artemyev N. O., Hamm H. E. Two-site high-affinity interaction between inhibitory and catalytic subunits of rod cyclic GMP phosphodiesterase. Biochem J. 1992 Apr 1;283(Pt 1):273–279. doi: 10.1042/bj2830273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr W., Devlin M. J., Applebury M. L. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979 Nov 25;254(22):11669–11677. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown R. L. Functional regions of the inhibitory subunit of retinal rod cGMP phosphodiesterase identified by site-specific mutagenesis and fluorescence spectroscopy. Biochemistry. 1992 Jun 30;31(25):5918–5925. doi: 10.1021/bi00140a031. [DOI] [PubMed] [Google Scholar]
- Brown R. L., Stryer L. Expression in bacteria of functional inhibitory subunit of retinal rod cGMP phosphodiesterase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4922–4926. doi: 10.1073/pnas.86.13.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catty P., Deterre P. Activation and solubilization of the retinal cGMP-specific phosphodiesterase by limited proteolysis. Role of the C-terminal domain of the beta-subunit. Eur J Biochem. 1991 Jul 15;199(2):263–269. doi: 10.1111/j.1432-1033.1991.tb16119.x. [DOI] [PubMed] [Google Scholar]
- Chabre M., Deterre P. Molecular mechanism of visual transduction. Eur J Biochem. 1989 Feb 1;179(2):255–266. doi: 10.1111/j.1432-1033.1989.tb14549.x. [DOI] [PubMed] [Google Scholar]
- Deterre P., Bigay J., Forquet F., Robert M., Chabre M. cGMP phosphodiesterase of retinal rods is regulated by two inhibitory subunits. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2424–2428. doi: 10.1073/pnas.85.8.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung B. K., Young J. H., Yamane H. K., Griswold-Prenner I. Subunit stoichiometry of retinal rod cGMP phosphodiesterase. Biochemistry. 1990 Mar 20;29(11):2657–2664. doi: 10.1021/bi00463a006. [DOI] [PubMed] [Google Scholar]
- Hamilton S. E., Prusti R. K., Bentley J. K., Beavo J. A., Hurley J. B. Affinities of bovine photoreceptor cGMP phosphodiesterases for rod and cone inhibitory subunits. FEBS Lett. 1993 Mar 1;318(2):157–161. doi: 10.1016/0014-5793(93)80012-j. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Stryer L. Purification and characterization of the gamma regulatory subunit of the cyclic GMP phosphodiesterase from retinal rod outer segments. J Biol Chem. 1982 Sep 25;257(18):11094–11099. [PubMed] [Google Scholar]
- Jurnak F. The three-dimensional structure of c-H-ras p21: implications for oncogene and G protein studies. Trends Biochem Sci. 1988 Jun;13(6):195–198. doi: 10.1016/0968-0004(88)90080-1. [DOI] [PubMed] [Google Scholar]
- Karas M., Hillenkamp F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal Chem. 1988 Oct 15;60(20):2299–2301. doi: 10.1021/ac00171a028. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li T. S., Volpp K., Applebury M. L. Bovine cone photoreceptor cGMP phosphodiesterase structure deduced from a cDNA clone. Proc Natl Acad Sci U S A. 1990 Jan;87(1):293–297. doi: 10.1073/pnas.87.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Evanczuk A. T. Real time assay of rod disk membrane cGMP phosphodiesterase and its controller enzymes. Methods Enzymol. 1982;81:532–542. doi: 10.1016/s0076-6879(82)81074-4. [DOI] [PubMed] [Google Scholar]
- Lipkin V. M., Dumler I. L., Muradov K. G., Artemyev N. O., Etingof R. N. Active sites of the cyclic GMP phosphodiesterase gamma-subunit of retinal rod outer segments. FEBS Lett. 1988 Jul 18;234(2):287–290. doi: 10.1016/0014-5793(88)80100-5. [DOI] [PubMed] [Google Scholar]
- Lipkin V. M., Khramtsov N. V., Vasilevskaya I. A., Atabekova N. V., Muradov K. G., Gubanov V. V., Li T., Johnston J. P., Volpp K. J., Applebury M. L. Beta-subunit of bovine rod photoreceptor cGMP phosphodiesterase. Comparison with the phosphodiesterase family. J Biol Chem. 1990 Aug 5;265(22):12955–12959. [PubMed] [Google Scholar]
- McAllister-Lucas L. M., Sonnenburg W. K., Kadlecek A., Seger D., Trong H. L., Colbran J. L., Thomas M. K., Walsh K. A., Francis S. H., Corbin J. D. The structure of a bovine lung cGMP-binding, cGMP-specific phosphodiesterase deduced from a cDNA clone. J Biol Chem. 1993 Oct 25;268(30):22863–22873. [PubMed] [Google Scholar]
- Miki N., Baraban J. M., Keirns J. J., Boyce J. J., Bitensky M. W. Purification and properties of the light-activated cyclic nucleotide phosphodiesterase of rod outer segments. J Biol Chem. 1975 Aug 25;250(16):6320–6327. [PubMed] [Google Scholar]
- Noel J. P., Hamm H. E., Sigler P. B. The 2.2 A crystal structure of transducin-alpha complexed with GTP gamma S. Nature. 1993 Dec 16;366(6456):654–663. doi: 10.1038/366654a0. [DOI] [PubMed] [Google Scholar]
- Oppert B., Cunnick J. M., Hurt D., Takemoto D. J. Identification of the retinal cyclic GMP phosphodiesterase inhibitory gamma-subunit interaction sites on the catalytic alpha-subunit. J Biol Chem. 1991 Sep 5;266(25):16607–16613. [PubMed] [Google Scholar]
- Oppert B., Takemoto D. J. Identification of the gamma-subunit interaction sites in the retinal cyclic-GMP phosphodiesterase beta-subunit. Biochem Biophys Res Commun. 1991 Jul 31;178(2):474–479. doi: 10.1016/0006-291x(91)90131-p. [DOI] [PubMed] [Google Scholar]
- Papermaster D. S., Dreyer W. J. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974 May 21;13(11):2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiba N. P., Artemyev N. O., Hamm H. E. The carboxyl terminus of the gamma-subunit of rod cGMP phosphodiesterase contains distinct sites of interaction with the enzyme catalytic subunits and the alpha-subunit of transducin. J Biol Chem. 1995 Jun 2;270(22):13210–13215. doi: 10.1074/jbc.270.22.13210. [DOI] [PubMed] [Google Scholar]
- Stryer L. Visual excitation and recovery. J Biol Chem. 1991 Jun 15;266(17):10711–10714. [PubMed] [Google Scholar]
- Takemoto D. J., Hurt D., Oppert B., Cunnick J. Domain mapping of the retinal cyclic GMP phosphodiesterase gamma-subunit. Function of the domains encoded by the three exons of the gamma-subunit gene. Biochem J. 1992 Feb 1;281(Pt 3):637–643. doi: 10.1042/bj2810637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trong H. L., Beier N., Sonnenburg W. K., Stroop S. D., Walsh K. A., Beavo J. A., Charbonneau H. Amino acid sequence of the cyclic GMP stimulated cyclic nucleotide phosphodiesterase from bovine heart. Biochemistry. 1990 Nov 6;29(44):10280–10288. doi: 10.1021/bi00496a018. [DOI] [PubMed] [Google Scholar]
- Wensel T. G., Stryer L. Activation mechanism of retinal rod cyclic GMP phosphodiesterase probed by fluorescein-labeled inhibitory subunit. Biochemistry. 1990 Feb 27;29(8):2155–2161. doi: 10.1021/bi00460a028. [DOI] [PubMed] [Google Scholar]
- Whalen M. M., Bitensky M. W. Comparison of the phosphodiesterase inhibitory subunit interactions of frog and bovine rod outer segments. Biochem J. 1989 Apr 1;259(1):13–19. doi: 10.1042/bj2590013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarfitz S., Hurley J. B. Transduction mechanisms of vertebrate and invertebrate photoreceptors. J Biol Chem. 1994 May 20;269(20):14329–14332. [PubMed] [Google Scholar]