Abstract
The morbidity and mortality of heart failure with preserved left ventricular ejection fraction (HFpEF) were similar to those of systolic heart failure, but the pathogenesis of HFpEF remains poorly understood. It was demonstrated that, in systolic heart failure, microRNA-21 (miR-21) could inhibit the apoptosis of cardiac fibroblasts, leading to cardiac hypertrophy and myocardial fibrosis, but the role of miR-21 in HFpEF remains unknown. By employing cell culture technique, rat myocardiocytes and cardiac fibroblasts were obtained. The expression of miR-21 in the two cell types under different conditions was compared and we found that the miR-21 expression was significantly higher in cardiac fibroblasts than in myocardiocytes. We established a rat HFpEF model and harvested the tissues of cardiac apex for pathological examination, Northern blotting and so forth. We found that miR-21 expression was significantly higher in model rats than in sham-operated rats, and the model rats developed the cardiac atrophy and cardiac fibrosis. After injection of miR-21 antagonist, the the cardiac atrophy and cardiac fibrosis were conspicuously ameliorated. Both in vivo and in vitro, inhibition of miR-21 expression resulted in reduced Bcl-2 expression while over-expression of miR-21 led to elevation of Bcl-2 expression. Our study suggested that miR-21 promoted the development of HFpEF by up-regulating the expression of anti-apoptotic gene Bcl-2 and thereby suppressing the apoptosis of cardiac fibrosis.
Keywords: Cardiac fibrosis, miR-21, Bcl-2, HFpEF
Introduction
In patients with hypertension, coronary disease and diabetics, the elasticity of their hearts tend to be abnormal and, as a result, the ventricular walls become increasingly stiff and cardiac muscles suffer from fibrosis, manifested as heart failure with persevered left ventricular ejection fraction (HFpEF). In 2007, European Society of Cardiology (ESC) and the Cardiovascular Imaging Association (EAVCI) of the ESC defined this condition as heart failure with normal ejection fraction [1], which is also known as heart failure with persevered left ventricular ejection fraction (HFpEF). It was estimated that, among patients with heart failure, about a half (range: 40%-71%, average: 56%) had preserved systolic function and its morbidity and mortality were similar to those of systolic heart failure [1]. It was believed that HFpEF patients usually had hypertrophy of left ventricle, with activated renin-angiotensin-aldosterone system (RAAS), and angiotensin receptor blocker could improve ventricular remodeling. However, in 2008, i-PRESERVE, the first largest-scale international clinical study, failed to confirm that Irbesartan, an angiotensin receptor blocker, could improve the prognosis as compared with placebo [2]. A study [3] revealed that, in models of heart failure caused by cardiac hypertrophy and cardiac fibrosis due to aortic stenosis, the expression of miR-21 showed no evident increase, and elevated or reduced miR-21 expression in myocardiocytes didn’t affect the size, shape, number and function of the cardiac muscle cells. On the other hand, the cardiac fibroblasts of rats with heart failure can highly express miR-21 and miR-21 can promote the development of cardiac hypertrophy and cardiac fibrosis by increasing the secretion of fibroblast growth factor-2 (FGF-2) and inhibiting the apoptosis of cardiac fibroblasts (CFs). These studies suggested that miR-21 might be a vital regulator in HFpEF and it play its roles by working on CFs.
Apoptosis is an active process of cell death in the individual development and is subjected to the regulation of a wide array of genes. In the proto-oncogene Bcl-2 protein family, Bcl-2 is believed to be the most important gene [4-6]. Mayorga et al. [7] by culturing fibroblasts in vitro, found that Bcl-2 gene was an important anti-apoptotic factor, and inhibition of Bcl-2 expression could promote the apoptosis of cardiac fibroblasts. Ji et al. [8] observed that, in the model of vascular damage, miR-21 worked on its target gene Bcl-2, which is responsible for the proliferation/apoptosis of vascular endothelia, and increased miR-21 expression directly promoted the expression of Bcl-2 gene, thereby further inhibiting apoptosis. Si et al. [9] demonstrated that miR-21 mediated tumor growth also by up-regulating Bcl-2 expression. Since miR-21 is highly expressed in the fibroblasts of rats with cardiac failure and Bcl-2 is an anti-apoptotic gene, in this study, we explored if miR-21 inhibits the apoptosis of cardiac fibroblasts and further triggers the development of HFpEF also by regulating Bcl-2 in HFpEF rats.
Materials and methods
Reagents and antibodies
All reagents and antibodies were bought online.
Animals
Adult male SD rats were purchased from the Animal Center, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China and were fed and maintained in the center before and after the surgery. All experiments were carried out in compliance with the Guidelines for the care and use of Laboratory Animals (NIH Publication NO. 23-85, revised 1996, Science and Technology Department of Hubei Province).
Experimental design
To study the mechanism of the development of HFpHF, we made a rat model of HFpHF [10-12]. The rats, weighing 150 g-170 g, aged 5-6 weeks, were divided into two groups at random: a transverse aortic constriction group (TAC group) and a sham-operation group (sham group). The rats of operation group received transverse aortic constriction (TAC) in order to reduce the internal diameter by 40% and then the abdominal cavity was closed. The animals were raised for another 8 weeks. Serving as controls, the rats in the sham-operation group were subjected to all surgical steps except partial ligation of abdominal aorta.
To examine the changes in myocardiocytes after miR-21 was inhibited, we further divided HFpEF rats into anti-miR-21 group and PBS group. In anti-miR-21 group, miR-21 antagonist was given via right jugular vein at 5 OD/rat each day for three days in a row. In PBS group, PBS was injected instead of miR-21 antagonist. The animals were raised for another three days for later detection.
The assessment of HFpEF model
After TAC, the rats from TAC group and sham group were examined echocardiography and hemodynamics. The ultrasound examination measured diastolic function (LWEDD, VST, PWT, IVRT), systolic function (EF), left ventricular systolic pressure (LVSP), end-diastolic pressure (LVEDV) and left ventricular pressure maximum rise and fall rate (± LVdp/dtmax). The rats were anesthetized with ketamine and xylazine, to reduce the heart rate to 180-250 beats/minute, which would facilitate the echocardiographic measurement (such as determination of IVRT). Moreover, compared with chloral hydrate and pentobarbital, ketamine works fast, exerts less respiration-inhibitory effect. For quantitative assessment of heart size, we measured the ratio of ventricular myocardium weight and body weight (VW/BW).
Harvesting of cardiac fibroblasts and cardiomyocytes
The myocardiocytes and cardiac fibroblasts were harvested by using cell culture technique. Briefly, hearts were removed from newborn rats (aged 0-3 days), digested with a mixture of type II collagenase and trypsin enzyme. The two kinds of cells were separated by employing differential adhesion. The supernatant (containing cardiomyocytes) and adherent cells (cardiac fibroblasts) were collected. The cells were identified by staining with different antibodies. The method for harvesting cardiomyocytes and cardiac fibroblasts from adult rats were identical to that for neonatal rats, except that the concentration of digestive enzyme was different.
In vitro induction of fibrosis
Fibroblasts were harvested from SD neonate rats and cultured. Cells of the third generation were collected and serum was removed within 24 hours for synchronization. Angiotensin at 10-6-10-10 mol/L was added and the cells were cultured for another 24 hours. Then, these cells were divided into mock group, NC group, and Anti-miR-21 group.
Pathological examination of murine cardiac tissues
MUSSON staining was employed to observe the fibrosis of cardiac muscles in the groups.
In situ hybridization
In situ hybridization was carried out to observe the expression of miR-21 and their locations in rat cardiac tissues. The digoxin-labeled probes for in situ hybridization were from JRDUN Biotechnology Co. Ltd., Shanghai, China. The tissues at the cardiac apex (less than 0.5 cm) were taken, paraffin-embedded, HE-stained and then subjected to in situ hybridization. The results were analyzed by using an image analyzing software package.
Northern blotting
Classic Northern blotting was performed to detect miR-21 expression by using γ-32P (a synthesized isotope probe). Total RNA was isolated from the tissues of left ventricle.
Real-time PCR
RT-PCR was performed to detect the miR-21 and Bcl-2 expression in the myocardiocytes and/or cardiac fibroblasts from the rats of sham and TAC groups. miR-21 and Bcl-2 primers were designed and synthesized by Guangzhou RiboBio Company, China. (www.sirma.cn).
Western blotting
After in vivo and in vitro inhibition of miR-21 expression, we examined the Bcl-2 expression at protein level by employing Western blotting. For in vivo study, miR-21 antagonist was injected via jugular vein. With in vitro observation, AngII was used to stimulate fibroblasts to induce fibrosis and miR-21 antagonist was added.
Bcl-2 expression after induction of miR-21 over-expression
We also examined bcl-2 expression after miR-21 over-expression induced by Pre-miR-21 (Genepharma, Shanghai. 1 × 109 U/ml). Briefly, the viral stock solution and DMEM containing 10% FBS were mixed at 1:10 to prepare a working solution. The working solution was added to the culture of cardiac fibroblasts for 24 h and then medium was replaced. The cells were cultured and collected for real-time PCR.
Statistical analysis
The data were analyzed with SPSS software 13.0. ANOVA with Newman-Keuls test was used to compare the data among different groups. The significance level was set at p<0.05.
Results
Evaluation of HFpEF model
The HFpEF model was assessed by echocardiography and hemodynamics. Before TAC, we detected LWEDD, VST, PWT, IVRT and EF and statistical analyses exhibited that, before the operation, there were no significant differences in the parameters between the two groups.
Eight weeks after the operation, we detected the above-mentioned parameters (Figure 1A) and statistical analyses showed that there existed statistically significant differences in the parameters between the two groups, suggesting that the diastolic functions of TAC group were impaired, as indicated by inverted E and A peaks. Moreover, IVRT was significantly protracted in TAC group (Figure 1B). Hemodynamic examination revealed that there were significant differences in the parameters reflecting diastolic functions between the two groups (P<0.05) (Figure 1C), indicating that the diastolic functions in TAC group was impaired. What is more, compared with rats in TAC group, rats in sham group were conspicuously more active and ate more. On the basis of these findings, we were led to conclude that the model was successfully established.
Figure 1.

Evaluation of HFpEF model. Eight weeks after TAC, assess the HFPEF model by echocardiography. The ultrasound examination measured diastolic function (LWEDD, VST, PWT, IVRT) and systolic function (EF), which reflected that diastolic functions of TAC group were impaired (A), as indicated by inverted E and A peaks and significant protracted IVRT (B). Meanwhile, the parameters of hemodynamic examination (LVEDV, ± LVdp/dtmax) were decreased absolutely in TAC group, which indicating the diastolic functions in TAC group were impaired (C).
High miR-21 expression in fibroblasts of HFpEF rats
As seen from the in situ hybridization figures, miR-21 expression was higher in TAC group than in sham group and the finding was more obvious in cardiac intestinal tissues (Figure 2A). There was a significant difference in the positive area between the two groups (P<0.05) (Figure 2B). Northern blotting also exhibited that miR-21 was highly expressed in TAC rats (Figure 2C, 2D).
Figure 2.

High miR-21 expression in fibroblasts of HFpEF rats. In situ hybridization, miR-21 expression was higher in TAC group than in sham group, and it was more obvious in cardiac interstitial tissues (A). While, the positive area reflected the same finding as mentioned above (B) (*P<0.05). Northern-blot also exhibited that miR-21 was highly expressed in TAC rats (C, D). Detecting miR-21 by RT-PCR in cardiac fibroblasts, compared with sham group, miR-21 expression significantly higher in TAC group (E). In TAC group, the expression of miR-21 is more higher in cardiac fibroblasts than in myocardiocytes (F). miR-21 expression significantly higher in cardiac fibroblasts than in myocardiocytes.
High miR-21 expression in cardiac fibroblasts of HFpEF rats
By cell culture, we obtained myocardiocytes and cardiac fibroblasts from HFpEF rats, with the purity reaching as high as 90%. By using RT-PCR, we compared the miR-21 expression in the two cells. We found that miR-21 expression significantly higher in cardiac fibroblasts than in myocardiocytes (P<0.01) (Figure 2F).
The association between miR-21 expression and cardiac hypertrophy and fibrosis
We examined the relationship between miR-21 expression and pathological changes of HFpEF by assessing the cardiac index and histological samples of HFpEF rats before and after injection of miR-21 antagonist via jugular vein.
Grossly, the rat hearts of TAC group were evidently larger than those of sham group. Moreover, there was significant difference in cardiac index between the two group, indicating that cardiac muscles in TAC group were significantly more hypertrophic (P<0.05) (Figure 3C). Three weeks after the injection of miR-21 antagonist, compared with rats given PBS, the hearts of antagonist-treated rats became significantly diminished in size (P<0.05) and cardiac index was lower (Figure 4C).
Figure 3.

The association between mir-21 expression and cardiac hypertrophy and fibrosis. The cardiomyocytes was irregularly arranged in TAC group (A) by HE staining. MUSSON staining showed that it had an obvious cardiac fibrosis in HFpEF rats (B). The cardiac index was increased in TAC group than in sham group (C), **P<0.01.
Figure 4.
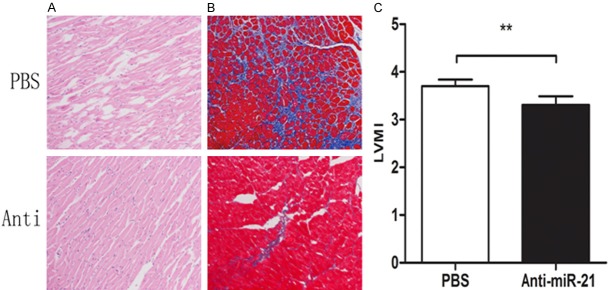
The association between mir-21 expression and cardiac hypertrophy and fibrosis. Three weeks after the injection of miR-21 antagonist via jugular vein, HE staining showed that the arrangement of cardiomyocytes of TAC group was improved (A). The fibrosis of TAC group was ameliorated (B). And, cardiac index of antagonist-treated rats was lower (C).
Pathological staining revealed the similar changes. Eight weeks after the operation, HE-staining showed that the myocardiocytes in TAC group were large and irregularly arranged (Figure 3A). After administration of miR-21 antagonist, the cardiac hypertrophy was ameliorated (Figure 4A). MASSON staining showed obvious fibrosis in TAC group (Figure 3B). After treatment with miR-21 antagonist, the fibrosis was improved. In PBS group, fibrosis was markedly aggravated and (Figure 4B) and it worsened over time.
Regulation of Bcl-2 by miR-21
Both in vivo and in vitro tests demonstrated miR-21 could regulate the expression of Bcl-2. Inhibition of miR-21 led to the reduced Bcl-2 expression while over-expression of miR-21 resulted in elevated expression of Bcl-2.
Three weeks after jugular injection of miR-21 antagonist into HFpEF rat model (5 OD/rat, for three consecutive days), RT-PCR showed that Bcl-2 expression in rat cardiac tissues was decreased (Figure 5D) and Western blotting revealed that Bcl-2 expression was lower at protein level (Figure 5B). It was also found that, after induction of fibrosis in vitro, Bcl-2 expression in the fibroblasts transfected with anti-miR-21 was lowered at both gene (Figure 5E) and protein level (Figure 5C). On the other hand, 48 hours (Figure 5F) and 72 hours (Figure 5G) after in vitro transfection with pre-miR21, Bcl-2 expression was increased at RNA level.
Figure 5.

Regulation of Bcl-2 by miR-21. Cell fractions from rat hearts stained with antibodies against prolyl 4-hydroxylase (P4HB) and a2-actinin (ACTN2). The cardiac fibroblasts were full of the vision and the purity is up to 98% (A). After jugular injection of miR-21 antagonist into HFpEF rat model, western blotting revealed that Bcl-2 expression was lower at protein level (B), and RT-PCR showed that Bcl-2 expression in rat cardiac tissues was decreased (D). After induction of fibrosis by AngII, Bcl-2 expression in the fibroblasts transfected with anti-miR-21 was lowered at both gene (E) and protein level (C). RT-PCR showed that Bcl-2 expression was decreased 48 hours (F) and 72 hours (G) after transfection with pre-miR21, **P<0.01.
Discussion
HFpEF refers to cardiac insufficiency caused by elevated left ventricular pressure due to impaired relaxing capability of the left ventricle and resultant reduced ventricular compliance. With this condition, the cardiac contractility is at or close to normal level (LVEF>50 or 45) [1]. HFpEF tends to affect elderly, female, obese or anemic people. Compared with HFrEF, HFpEF victims usually had higher incidence of hypertension or ventricular fibrillation [5]. HFpEF has been on rise steadily and is expected to become a major factor causing heart failure in the coming 10 years. HFpEF is characterized by diastolic heart insufficiency, with or without normal or slightly abnormal cardiac contractility. Nevertheless, its pathogenesis has been poorly understood. Some researchers [13] believed that diastolic heart insufficiency is associated with the cardiac stiffness due to fibrosis. The development and progression of HFpEF are associated with the activation of cytokines, mainly released by macrophages. The macrophages-secreted MMP promotes the decomposition of matrix collagen, which causes collagen re-modeling and thereby aggravates cardiac stiffness. Therefore, cardiac fibrosis may play a vital role in the development of HFpEF.
A previous study [3] demonstrated that, in the model of TAC, the cardiac fibroblasts highly expressed miR-21 and elevated miR-21 expression could promote the cardiac hypertrophy and cause fibrosis by increasing the secretion of fibroblast growth factor-2 (FGF-2) and inhibiting the apoptosis of cardiac fibroblasts (CFs). HFpEF model can be made by carefully controlling the degree of aortic stenosis [4-6]. In this study, we ligated the aortic artery to decrease its blood flow by 40% and evaluated the model 8 weeks later. Echocardiography showed that the systolic functions of the model rats were normal (EF greater than 70%) but diastolic functions were obviously impaired. Hemodynamic study revealed the similar results. Moreover, rats in the sham group were more active than those in TAC group and they ate more. We were led to conclude that the rat HFpEF model was successfully established.
In 2001, Lagos-Quintana et al. [14] confirmed that miR-21 gene is found in both vertebrates and invertebrates. Since then, miR-21 gene has been increasingly drawing the interest of researchers and has been a subject of active investigations. These studies have been focusing on gastric, pulmonary, hepatic cancer and chronic lymphatic leukemia [15-18], and some researches also examined the relationship between miR-21 and the cardiovascular diseases in humans [19-22].
Some studies verified that miR-21 gene was highly expressed in vascular smooth muscles [23], endothelia [24], myocardiocytes [19] and cardiac fibroblasts [25]. However, researchers did not agree on if miR-21 was expressed in cardiac cells. Thum et al. [3] believed that mir-21 was highly expressed not in myocardiocytes but in cardiac fibroblasts, and the miR-21 expression was stronger with the severity of heart insufficiency. On the other hand, Cheng and other researchers [19,26,27] found that mir-21 expression was high in myocardiocytes. This study exhibited that, in HFpEF rats, miR-21 expression was high in cardiac fibroblasts not in myocardiocytes, which was consistent with the finding made by Thum et al. [3]. The discrepancies among different studies might be ascribed to developmental status of the experimental animals since miR-21 expression was evidently higher in neonate rats than their adult counterparts.
Cardiac fibrosis is an important pathological factor implicated in the development of heart insufficiency and miR-21 is highly expressed in fibrotic cardiac muscles [3]. Thum et al [3] demonstrated that up-regulated miR-21 expression could enhance the signal transduction of EPK-MAP pathway, thereby leading to the proliferation of fibroblasts and cardiac fibrosis. Silencing of miR-21 gene could inhibit the transduction of EPK-MAP signals and thus inhibiting the fibrosis and improving cardiac functions. Roy et al substantiated that miR-21 gene played a pivotal role in the cardiac fibrosis and remodeling in a rat model of ischemia-reperfusion. Their study identified PTEN as a target gene of miR-21 and found that PTEN led to cardiac fibrosis by working on MMP-2. Our study showed that elevated miR-21 expression could bring about cardiac hypertrophy and fibrosis. In this study, both Northern blotting and in situ hybridization revealed that miR-21 expression was significantly higher in rats of TAC group than in those of sham group. Moreover, pathological examination exhibited that rats in TAC group were suffered from cardiac hypertrophy and fibrosis. After inhibition of miR-21 gene, both cardiac hypertrophy and fibrosis were ameliorated. These findings suggested that can lead to cardiac fibrosis and the results were coincident with those reported by Thum and Roy [21,25].
Oncogene Bcl-2 is thought to be one of the ultimate pathways of apoptosis regulation and it is an important apoptosis suppressor in cardiac fibroblasts, vascular endothelia and even tumor cells. Our study found that miR-21 could regulate the expression of Bcl-2. RT-PCR and Western blotting revealed that, after inhibition of miR-21 expression with anti-miR-21, Bcl-2 expression, at both gene and protein levels, was down-regulated. After transfection of anti-miR-21 into fibroblasts, we observed the similar results as we did in HFpEF model. On the contrary, 48 and 72 hours later, up-regulation of miR-21 expression, Bcl-2 expression level was increased at RNA level. Therefore, we were led to believe that Bcl-2 expression was regulated by miR-21 gene. So far, the following common target genes of miR-21 have been identified: SPRY1, SFRS8, PPARA, TIMP3, NFIB, SPRY2, PDCD4, ARID1A, Bcl-2 and so forth. Among them, PDCD4, PTEN, spry1 and spry2 are associated with cardiovascular system [3,19,28,29]. It’s generally believed that a single gene can regulated a variety of target genes and different genes can regulate one target gene simultaneously. Therefore, miR-21 regulating mechanism is complicated. By studying the rat HFpEF model and in vitro fibrosis, we demonstrated that miR-21 regulates the expression of Bcl-2 and further causes cardiac hypertrophy and fibrosis. Nevertheless, if other genes, such as spry1, are involved in the process warrants further study.
So far, the treatment of HFpEF principally consists of controlling inducing factors, band relieving symptoms and improving survival. Nevertheless, the medications available don’t work as effectively on HFpEF as they do on SHF. i-PRESERVE research program failed to confirm that irbesartan could significantly improve the prognosis of HFpEF as compared to placebo [2]. β-receptor blockers were found to be able to improve heart functions in patients with reduced EF of left ventricle but it did not improve the survival and re-hospitalization rates in HFpEF patients [30]. Though Ca2+ blockers can improve the diastolic heart functions and reverse the hypertrophy of left ventricle, they are contraindicated in many cases, such as bradycardia, conduction block, and severe cardiac insufficiency [31]. Reports on the use of diuretics and aldosterones for the treatment of HFpEF are scanty. It was reported [32] that injection of adenoviral RNAi into rats with heart failure could restore the size of cardiac cavities and significantly improve cardiac hypertrophy and fibrosis. Moreover, no aberrant activities of miRNA were observed and hepatic functions were not conspicuously impaired. Our study exhibited that, after inhibition of miR-21 expression, the pathological changes, such as cardiac hypertrophy and fibrosis, were obviously ameliorated and their eating and mental status was evidently improved. These findings provided some insight into the treatment of HFpEF at gene level. However, these results were obtained in animal models and further human studies are still needed.
To sum up, our study suggested that miR-21 promotes the development of HFpEF by up-regulating anti-apoptosis gene Bcl-2 and thereby inhibiting the apoptosis of fibroblasts.
Acknowledgements
We are particularly grateful to Chaohong Yu and Pengfei Zhu for their expert technical assistance. This study was funded by grant from National Natural Science Foundation of China (NO. 30971103).
Disclosure of conflict of interest
None.
Abbreviations
- CF
Cardiac Fibroblast
- FGF-2
Fibroblast Growth Factor 2
- TAC
Transverse Aortaventralis Constriction
- Bcl-2
B-cell lymphoma-2
- miR-21
MicroRNA-21
- HFpEF
Heart Failure with Preserved Left Ventricular Ejection Fraction
References
- 1.Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the heart failure and echocardiography associations of the European society of cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 2.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 3.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signaling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 4.Zavadil J, Narasimhan M, Blumenberg M, Schneider RJ. Transforming growth factor-β and microRNA: mRNA regulatory networks in epithelial plasticity. Cells Tissues Organs. 2007;185:157–161. doi: 10.1159/000101316. [DOI] [PubMed] [Google Scholar]
- 5.Prabhu SD, Wang G, Luo J, Gu Y, Ping P, Chandrasekar B. Beta-adrenergic receptor blockade modulates Bcl-X (S) expression and reduces apoptosis in failing myocardium. J Mol Cell Cardiol. 2003;35:483. doi: 10.1016/s0022-2828(03)00052-x. [DOI] [PubMed] [Google Scholar]
- 6.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O’Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumors. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 7.Mayorga M, Bahi N, Ballester M, Comella JX, Sanchis D. Bcl-2 is a key factor for cardiac fiborblast resistance to programmed cell death. J Biol Chem. 2004;279:34882–34889. doi: 10.1074/jbc.M404616200. [DOI] [PubMed] [Google Scholar]
- 8.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of microRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 9.Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21 mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 10.Dubi S, Arbel Y. Large animal models for diastolic dysfunction and diastolic heart failure-a review of the literature. Cardiovasc Pathol. 2009;19:147–152. doi: 10.1016/j.carpath.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Tang HF, Wu SL, Deng CY, Zhang WC, Kuang SJ. Bisoprolol inhibits sodium current in ventricular myocytes of rats with diastolic heart failure. Clin Exp Pharmacol Physiol. 2007;34:714–719. doi: 10.1111/j.1440-1681.2007.04628.x. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch JC, Borton AR, Albayya FP, Russell MW, Ohye RG, Metzger JM. Comparative analysis of parvalbumin and SERCA2a cardiac myocyte gene transfer in a large animal model of diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2004;286:H2314–2321. doi: 10.1152/ajpheart.01137.2003. [DOI] [PubMed] [Google Scholar]
- 13.van Heerebeek L, Franssen CP, Hamdani N, Verheugt FW, Somsen GA, Paulus WJ. Molecular and cellular basis for diastolic dysfunction. Curr Heart Fail Rep. 2012;9:293–302. doi: 10.1007/s11897-012-0109-5. [DOI] [PubMed] [Google Scholar]
- 14.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 15.Guo J, Miao Y, Xiao B, Huan R, Jiang Z, Meng D, Wang Y. Differential expression of micro RNA species in human gastric cancer versus non tumorous tissues. J Gastroenterol Hepatol. 2009;24:652–657. doi: 10.1111/j.1440-1746.2008.05666.x. [DOI] [PubMed] [Google Scholar]
- 16.Xie Y, Todd NW, Liu Z, Zhan M, Fang H, Peng H, Alattar M, Deepak J, Stass SA, Jiang F. Altered miRNA expression in sputum for diagnosis of non small cell lung cancer. Lung Cancer. 2010;67:170–176. doi: 10.1016/j.lungcan.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fulci V, Chiaretti S, Goldoni M, Azzalin G, Carucci N, Tavolaro S, Castellano L, Magrelli A, Citarella F, Messina M, Maggio R, Peragine N, Santangelo S, Mauro FR, Landgraf P, Tuschl T, Weir DB, Chien M, Russo JJ, Ju J, Sheridan R, Sander C, Zavolan M, Guarini A, Foà R, Macino G. Quantitative technologies establish a novel microRNA profile of chronic lymphocytic leukemia. Blood. 2007;109:4944–4951. doi: 10.1182/blood-2006-12-062398. [DOI] [PubMed] [Google Scholar]
- 19.Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. Micro RNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol. 2007;170:1831–1840. doi: 10.2353/ajpath.2007.061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, Gross C, Engelhardt S, Ertl G, Bauersachs J. MicroRNA in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda S, Kong SW, Lu J, Bisping E, Zhang H, Allen PD, Golub TR, Pieske B, Pu WT. Altered microRNA expression in human heart disease. Physiol Genomics. 2007;31:367–373. doi: 10.1152/physiolgenomics.00144.2007. [DOI] [PubMed] [Google Scholar]
- 23.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 24.Suárez Y, Fernández-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 25.Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ, Sen CK. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C. MicroRNA-21 protects against the H2O2-induced injury on cardiac myocytes via its target gene PDCD 4. J Mol Cell Cardiol. 2009;47:5–14. doi: 10.1016/j.yjmcc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284:29514–29525. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 29.Sayed D, Rane S, Lypowy J, He M, Chen IY, Vashistha H, Yan L, Malhotra A, Vatner D, Abdellatif M. MicroRNA-21 targets Sprouty2 and promotes cellular outgrowths. Mol Biol Cell. 2008;19:3272–3282. doi: 10.1091/mbc.E08-02-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen-Solal A, Kotecha D, van Veldhuisen DJ, Babalis D, Böhm M, Coats AJ, Roughton M, Poole-Wilson P, Tavazzi L, Flather M SENIORS Investigators. Efficacy and safety of nebivolol in elderly heart failure patients with impaired renal function: insights from the SENIORS trial. Eur J Heart Fail. 2009;11:872–880. doi: 10.1093/eurjhf/hfp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westermann D, Knollmann BC, Steendijk P, Rutschow S, Riad A, Pauschinger M, Potter JD, Schultheiss HP, Tschöpe C. Diltiazem treatment prevents diastolic heart failure in mice with familial hypertrophic cardiomyopathy. Eur J Heart Fail. 2006;8:115–121. doi: 10.1016/j.ejheart.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Suckau L, Fechner H, Chemaly E, Krohn S, Hadri L, Kockskämper J, Westermann D, Bisping E, Ly H, Wang X, Kawase Y, Chen J, Liang L, Sipo I, Vetter R, Weger S, Kurreck J, Erdmann V, Tschope C, Pieske B, Lebeche D, Schultheiss HP, Hajjar RJ, Poller WC. Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation. 2009;119:1241–1252. doi: 10.1161/CIRCULATIONAHA.108.783852. [DOI] [PMC free article] [PubMed] [Google Scholar]


