Abstract
To explore whether hypoxia and interleukin 8 (IL-8) regulate the viability and apoptosis of cervical carcinomas cells and the possible mechanism. We evaluated the expression of hypoxia inducible factor-1α (HIF-1α), IL-8 and its receptors (CXCR1 and CXCR2) in cervical cancer and cervicitis tissues by immunohistochemistry. Then the effects of hypoxia and IL-8 on the viability and apoptosis of HeLa and SiHa cells were detected by the SRB and apoptosis assays. Here we observed that the expression of HIF-1α, IL-8 and CXCR1 in cervical cancer tissues was significantly higher than that in cervicitis tissues. Hypoxic condition stimulated the secretion of IL-8 and the expression of CXCR1 and CXCR2 on HeLa and SiHa cells. Recombinant human IL-8 enhanced the viability and reduced the apoptosis in HeLa and SiHa cells. HeLa and SiHa cells cultured in 1% oxygen showed the increased viability and apoptosis, and the former effect could be partly reversed by anti-human IL-8 neutralizing antibody. This data suggested that IL-8 secreted by cervical carcinomas cells induced by hypoxia can stimulate the viability of cervical carcinomas cells in an autocrine dependent manner, and contribute to the pathogenesis of cervical cancer.
Keywords: Hypoxia, IL-8, cervical carcer cells, viabilty, apoptosis
Introduction
Cervical cancer is the third most commonly diagnosed cancer in women worldwide, and its global incidence increased at an annual rate of 0.6% between 1980 and 2010 [1]. There are more than 273,000 deaths worldwide from cervical cancer each year accounting for 9% of total cancer deaths in women [2].
Oxygen is a molecule that is central to cellular respiration and viability, yet there are multiple physiologic and pathological contexts in which cells experience conditions of insufficient oxygen availability, a state known as hypoxia. Tumor hypoxia can drive the tumor toward a more aggressive malignant phenotype through clonal selection and genomic and proteomic changes [3]. The role of hypoxia in the growth of cervical cancer and the mechanism, however, has not been investigated.
Hypoxia-inducible factor-1α (HIF-1α) has been extensively studied as an endogenous hypoxia marker [4,5]. High level of HIF-1α has predictive and prognostic significance in cervical cancer [6]. In addition, HIF-1α is over-expressed in preinvasive and precancerous lesions of the cervix [7], endometrium [8], breast [9,10], and prostate [9,11].
Chemokine CXCL8 (also named IL-8) is produced by many cell types, such as peripheral blood monocytes [12], mesothelial cells [13], endometrial cells [14,15], cervical cancer cells [16]. This chemokine activates multiple intracellular signaling pathways downstream of two cell-surface G protein-coupled receptors (CXCR1 and CXCR2) [17,18]. It has been found that IL-8 promoted migration and metastasis of cancer cells by regulating the secretion of matrix metalloproteinases MMP2 and MMP9 [19], played an important role in angiogenesis and participated in tumor vasifaction, which correlated with the infiltration and metastasis of cancers [20,21]. Although hypoxia and IL-8 had been linked with various cancers, little research has been done on the relationship and effect of hypoxia and IL-8 in cervical cancer.
Therefore, the present study is undertaken to investigate whether hypoxia modulates the growth of cervical carcinomas cells by IL-8. Our current findings show that hypoxia up-regulates the secretion of IL-8 and the expression of it receptors, and further stimulates the viability of cervical carcinoma HeLa and SiHa cells that thereby maybe involved in the pathogenesis of cervical cancer.
Materials and methods
Tissue collection
All tissue samples were obtained with informed consent in accordance with the requirements of the Research Ethics Committee in the Obstetrics and Gynecology, Fudan University Shanghai Medical College. Samples from 18 patients in International Federaton of Gynecology and Obstetrics (FIGO) stages of cervical cancer were obtained from women age 29-56 years. Among them, 2 (11.1%) patients had FIGO Stage Ib2 disease, 4 (22.2%) had Stage IIa, 6 (33.3%) had Stage IIb, 5 (27.8%) had Stage IIIb, and 1 (5.6%) had Stage IVa disease. Moreover, certivical tissues from cervicitis (n=10) (age 24-44 years) were collected as control. All the samples were confirmed histologically according to established criteria. In cancer group, squamous cell carcinoma was found in 17 (94.4%) patients and 1 with adenocarcinoma.
Immunohistochemistry
Paraffin sections (5 um) of the certivical tissues from cervical cancer (n=18) or cervicitis (n=10) were dehydrated in graded ethanol, and incubated with hydrogen peroxide in 1% bovine serum albumin (BSA)/TBS to block endogenous peroxidase. The samples were then incubated with mouse anti-human HIF-1α antibody (8 ug/ml, R&D Systems, USA), IL-8 antibody (8 ug/ml, R&D Systems), CXCR1 antibody (15 ug/ml, R&D Systems), CXCR2 antibody (15 ug/ml, R&D Systems) or mouse IgG isotype antibody overnight at 4°C in a humid chamber. After washing three times with TBS, the sections were overlaid with peroxidase-conjugated anti-mouse IgG antibody (SP-9002; Golden Bridge International, Inc., Beijing, China), and the reaction was developed with 3, 3-diaminobenzidine (DAB), and counterstained with hematoxylin. The experiments were repeated five times.
Cell culture
Cervical epidermoid carcinoma HeLa and SiHa cells were grown in 1640 (Gibco, USA) supplemented with 1% antibiotic-antimycotic and 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA).
These cells were incubated either under the normoxic condition (21% O2, 5% CO2, 74% N2 at 37°C) in a humidified incubator (Heal Force, HF 100, Shanghai, China) or under the hypoxic condition (1% O2, 5% CO2, 94% N2 at 37°C) in a humidified incubator (Heal Force, HF 100, Shanghai, China).
Enzyme-linked immunosorbent assay for IL-8 determination
HeLa and SiHa cells (3×105 cells/well) were seeded in 24-well plates, and cultured either under the normoxic condition or under the hypoxic condition for 24h. Then the secretion of IL-8 by the supernatant of HeLa and SiHa cells was detected by ELISA (Shanghai ExCell Biology, Inc, Shanghai, China), according to the manufacturer’s instruction.
The expression of CXCR1 and CXCR2 on HeLa and SiHa cells by flow cytometry
HeLa and SiHa cells (1×105 cells/well) were seeded in 24-well plates, and cultured either under the normoxic condition or under the hypoxic condition for 8h. Then we trypsinized and collected these cells, and evaluated the expression of CXCR1 and CXCR2 by flow cytometry. Antibodies against human antigens were: FITC-conjugated anti-human CXCR1 (R&D Systems) and PE-conjugated anti-human CXCR2 (R&D Systems). Samples were analyzed in a FACS Calibur flow cytometer (Becton Dickinson, USA) by using Cellquest software (Becton Dickinson). Statistical analysis was conducted by using isotype matched controls.
IL-8 and anti-IL-8 neutralizing antibody treatments
HeLa and SiHa cells were treated with recombinant human IL-8 (rhIL-8) at different concentration (1, 10, 100 ng/ml) (R&D Systems) for 48h, with vehicle as control. In addition, we treated HeLa and SiHa cells under the normoxic condition or under the hypoxic condition for 8h with anti-human IL-8 neutralizing antibody (α-IL-8) (0.04, 2, 10 ug/ml) (R&D Systems), and then cultured these cells under the normoxic condition for 48h, with mouse isotype (Sino-America Co. Ltd) as control. Then Sulforhodamine B (SRB) proliferation assay and apoptosis assay (the concentration of rhIL-8 was 10 ng/ml and α-IL-8 was 2 ug/ml for the latter group) were performed to detect respectively the effect of rhIL-8 on the proliferation and apoptosis of HeLa and SiHa cells.
Cell proliferation and apoptosis assay
For SRB proliferation assay, 50 μl of 30% trichloroacetic acid was added for 60 min at 4°C. After washing and drying the plate, 100 μl of 0.4% SRB was added for 30 min. Next, the plate was rinsed with 0.1% acetic acid and air dried, and 100 μl of Tris base (10 mmol/L) was added before shaking the plate for 10 min. The SRB value was measured at a wavelength of 570 nm. The experiment was performed in sextuplicates and repeated five times.
Phosphatidylserine externalization was quantified by flow cytometry with a commercially available annexin V-FITC apoptosis detection kit (Invitrogen, USA) according to the manufacturer’s guideline. The cells were resuspended in PBS, and washed twice and resuspended in the kit binding buffer (100 ul/pellet) containing annexin V solution (2.5 ul/pellet) and propidium iodide (1 mg/ml). Samples were incubated in dark for 15 min, and the percent of annexin V-positive cells in HeLa and SiHa cells was determined by FACS Calibur flow cytometry. The experiments were performed in sextuplicates, and repeated three times.
Statistics
All values are shown as the mean±SD. Data were analyzed by using t test or one-way analysis of variance with Statistical Package for the Social Sciences software version 11.5. Differences were considered as statistically significant at P<0.05.
Results
Cervical cancer cells highly express IL-8 and it receptors in cervical cancer tissues
To investigate the possible regulation of hypoxia and IL-8 on the biological behavior of cervical cancer cells, we first compared the expression level of HIF-1α, IL-8 and it receptors in cervical cancer and cervicitis tissues by immunohistochemistry. As shown in Figure 1, in contrast to tissues from cervicitis, HIF-1α, IL-8 and it receptor CXCR1 were highly staining in cervical cancer cells from all cervical cancer samples. However, the expression of CXCR2 in these two groups had no significantly difference. Our observations indicated that the difference of hypoxic condition and IL-8 signals in the cervical cancer and cervicitis tissues might be associated with the unique biological behavior of cervical cancer cells.
Figure 1.
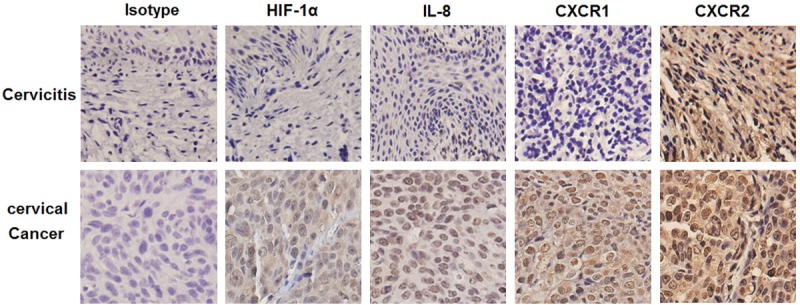
Cervical cancer cells highly express IL-8 and it receptors in cervical cancer tissues. The expression of HIF-1α, IL-8, CXCR1 and CXCR2 in the cervical tissues from cervical cancer (n=18) and cervicitis patients (n=10) was analyzed respectively by immunohistochemistry. Original magnification: ×400.
Hypoxia induces the secretion of IL-8 and the expression of it receptors on HeLa and SiHa cells
To clarify the relationship between hypoxia and IL-8 signals, we first investigated the effect of hypoxic conditions on the IL-8 and it receptors (CXCR1 and CXCR2) of HeLa and SiHa cells. As depicted in Figure 2, hypoxic conditions stimulated the secretion of IL-8 in both HeLa and SiHa cells (P<0.05) (Figure 2A). Subsequently, flow cytometry was used to measure the expression of CXCR1 or CXCR2 on HeLa and SiHa cells. We found that the percentage of either CXCR1 or CXCR2 positive cells in HeLa and SiHa cells was increased after hypoxia (P<0.05, P<0.01, or P<0.001) (Figure 2B-D). In view of these results above, we proposed that IL-8 and it receptors might be the down-stream molecules of hypoxic condition, and involved in the regulatory effect on cervical cancer cells mediated by hypoxia.
Figure 2.

Hypoxia induces the secretion of IL-8 and the expression of it receptors on HeLa and SiHa cells. We cultured HeLa and SiHa cells under hypoxic condition (1% oxygen) or normoxic condition (21% oxygen) for 24h (for ELISA assay) or 8h (for flow cytometry assay), and then detected the secretion level of IL-8 in the supernatant by ELISA (A) and the expression of CXCR1 and CXCR2 by flow cytometry (B-D). Normal HeLa/SiHa: HeLa/SiHa cells cultured under normoxic condition; Hypoxic HeLa/SiHa: HeLa/SiHa cells cultured under hypoxic condition. Results are highly reproducible in three independent experiments. Data are mean±SD. *P<0.05, **P<0.01 or ***P<0.001 compared to the normoxic condition control.
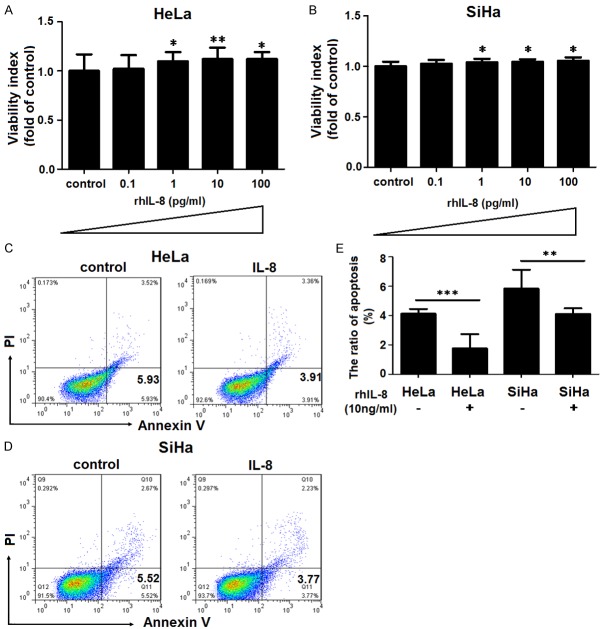
Recombinant human IL-8 promotes the proliferation and reduces the apoptosis of HeLa and SiHa cells
To analyze the possible role of IL-8 in the proliferation and apoptosis of cervical cancer cells, we treated HeLa and SiHa cells with recombinant human IL-8 (rhIL-8) at different concentration (0, 0.1, 1, 10, 100 pg/ml) for 48 hours. Data presented in Figure 3A and 3B showed that rhIL-8 stimulated proliferation of HeLa and SiHa cells at the concentration of 1-100 ng/ml (P<0.05 or P<0.01) (Figure 3A and 3B). On the contrary, 10 ng/ml rhIL-8 markedly inhibited the apoptosis of HeLa and SiHa cells (P<0.01 or P<0.001) (Figure 3C and 3D). Our results indicated that IL-8 derived from cervical carcinoma cells might enhance proliferation and anti-apoptosis of cervical cancer cells in an autocrine manner via binding CXCR1 and or CXCR2.
Figure 3.
Recombinant human IL-8 promotes the proliferation and reduces the apoptosis of HeLa and SiHa cells. HeLa and SiHa cells (7×103 cell/well for proliferation assay; 1×105 cell/well for apoptosis assay) were treated respectively with recombinant human IL-8 (rhIL-8) (1, 10, 100 ng/ml for proliferation assay; only 10 ng/ml for apoptosis assay) for 48h, with vehicle or mouse isotype as controls. Then Sulforhodamine B (SRB) proliferation assay (A, B) and annexin V-FITC apoptosis detection assay (C-E) were conducted to analyze proliferation and apoptosis of HeLa and SiHa cells, respectively. *P<0.05, **P<0.01 or ***P<0.001 compared to the vehicle control.
The stimulatory effect of hypoxia on the proliferation not apoptosis of cervical cancer cells is dependent on IL-8 signals
In order to probe into whether hypoxia regulates cervical cancer cells through IL-8 signals, we pre-treated HeLa and SiHa cells with anti-human IL-8 neutralizing antibody (0, 0.04, 2, 10 ug/ml) for 8h, cultured these cells under hypoxic condition or normoxic condition for another 48h, and then detected the proliferation and apoptosis of HeLa and SiHa cells by SRB assay and apoptosis assay. Hypoxic condition notably increased proliferation of HeLa and SiHa cells (P<0.01 or P<0.001) (Figure 4A and 4B). However, it could be reversed by anti-human IL-8 neutralizing antibody, especially at the concentration of 2 ug/ml (P<0.01) (Figure 4A and 4B). The results of apoptosis assay showed that hypoxia led to increased apoptosis of HeLa and SiHa cells (P<0.05 or P<0.001) (Figure 4C and 4D), but anti-human IL-8 neutralizing antibody did not influence these effects.
Figure 4.
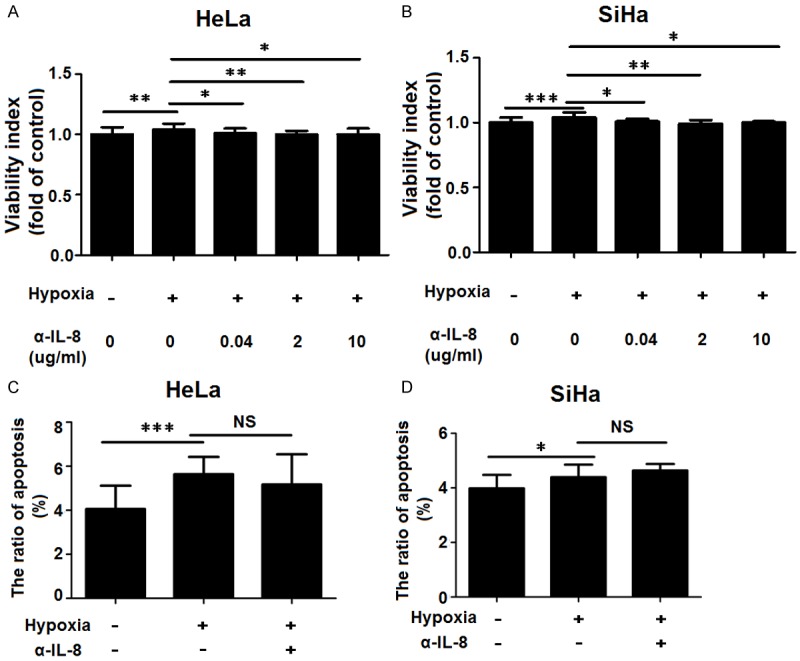
The stimulatory effect of hypoxia on the proliferation not apoptosis of cervical cancer cells is dependent on IL-8 signals. We treated HeLa and SiHa cells under the normoxic condition or under the hypoxic condition for 8h with anti-human IL-8 neutralizing antibody (α-IL-8) (0.04, 2, 10 ug/ml for proliferation assay; only 2 ug/ml for apoptosis assay), and then cultured these cells under the normoxic condition for 48h, with mouse isotype (Sino-America Co. Ltd) as control. Then SRB proliferation assay (A, B) and apoptosis assay (C, D) were performed to analyze the proliferation and apoptosis of HeLa and SiHa cells. Data are mean±SD. NS: no statistically difference.
Discussion
Cancer cells are characterized by rapid proliferation and require adaptive metabolic responses to allow continued biosynthesis and cell growth in the setting of decreased oxygen (O2) and nutrient availability [22,23]. The hypoxia-inducible factors (HIFs) are a common link between adaptation to low O2, changes in cancer metabolism, and malignant progression. In our current work, contrast to cervicitis tissues, cervical cancer cells in cervical cancer highly expressed HIF-1α, IL-8 and it receptor CXCR1, suggesting that IL-8 signals maybe associated with hypoxic status and the biological behavior in cervical cancer cells.
The key findings of the present study indicate that hypoxic condition stimulates the secretion of IL-8 and the expression of CXCR1 and CXCR2 on cervical cancer cells HeLa and SiHa cells, and further promotes the proliferation of HeLa and SiHa cells through up-regulating IL-8/it receptors signals. Moreover, hypoxia promoted the apoptosis; on the contrary, recombinant human IL-8 repressed the apoptosis of HeLa and SiHa cells. These results suggest that hypoxia and it’s downstream IL-8 signals have important roles in promoting the growth of cervical cancer cells, and further stimulating the development and progression of cervical cancer. These findings further add to our understanding about the biological function and role manner of hypoxia in the cervical cancer progression.
Tumors grow in immunocompetent hosts via autocrine/paracrine mechanisms that maintain proliferation using escape mechanisms, and lead to the ineffectiveness of host elimination by producing cytokines such as IL-8 and the chemokine CC chemokine ligand 28 (CCL28) that facilitate invasion and metastasis [24,25]. As a potent pro-angiogenic factor, IL-8 is also involved in tumor progression and cell invasion; thereby stimulating cancer cell growth and contributing to metastasis and recurrence [26,27]. It has been reported that many types of human carcinomas, including breast, colon, gastric, lung, and ovarian cancers, express high levels of IL-8 relative to normal tissues [28]. Over-expression of IL-8 has been reported in multiple malignancies and is frequently associated with poor clinical outcome [29,30]. In this study, we demonstrated that IL-8 and its receptors were the downstream molecules, and involved in the modulating the proliferation not apoptosis of cervical cancer cells mediated by hypoxia. However, the exact mechanisms still need further research.
Hypoxia, a condition that is known to drive angiogenesis in tumors, results in the release of damage-associated pattern molecules, which can trigger the rejection of tumors by the immune system [31]. Facciabene et al had found that tumour hypoxia promotes the recruitment of regulatory T (Treg) cells through induction of expression of CCL28, which, in turn, promotes tumour tolerance and angiogenesis [25]. Therefore, our current results offered a new possibility that the stimulatory effect of hypoxia on tumor angiogenesis was partly achieved by increasing IL-8. However, this speculation still requires detailed research.
Collectively, as shown in Figure 5, based on previous reports and our finding, it has been demonstrated that cervical carcinoma cells express high level of IL-8, CXCR1 and CXCR2 under hypoxic conditions. The increased IL-8/CXCR1/CXCR2 signals induced by hypoxia, on the one hand, may stimulate the proliferation, restrict the apoptosis and promote the migration and metastasis of cervical cancer cells in an autocrine-dependent manner; On the other hand, hypoxia may strengthen the dialogue between cervical cancer cells and vascular endothelial cells through stimulating the production of pro-angiogenic factor IL-8. In addition, hypoxia promotes the apoptosis of cervical cancer cells in an IL-8-independent manner. These effects finally contribute to growth and development of cervical cancer.
Figure 5.
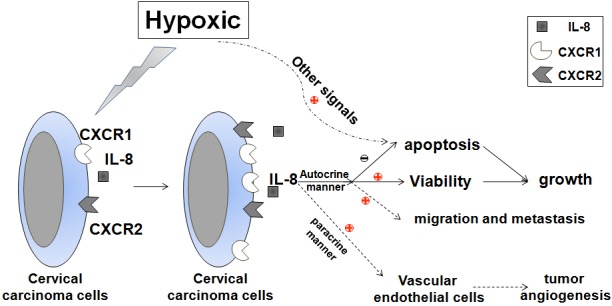
Schematic roles of hypoxia in regulating biological behavior of cervical cancer cells. Under hypoxia conditions, cervical carcinoma cells secrete high level of IL-8 and express more CXCR1 and CXCR2. The increased IL-8/CXCR1/CXCR2 signals induced by hypoxia, on the one hand, may stimulate the proliferation, restrict the apoptosis and promote the migration and metastasis of cervical cancer cells in an autocrine-dependent manner; On the other hand, enhance the angiogenesis of vascular endothelial cells (VEC) in a paracrine-dependent manner. In addition, hypoxia promotes the apoptosis of cervical cancer cells through other signals. These effects finally contribute to growth and development of cervical cancer.
Acknowledgements
This study was supported by National and Shanghai Leading Academic Discipline Project (211XK22) to Da-Jin Li; Program for Outstanding Medical Academic Leader of Shanghai to Da-Jin Li; National Natural Science Foundation of China (NSFC) 31101064 to Ming-Qing Li, Program for ZhuoXue of Fudan University to Ming-Qing Li; Program for Wuxi Science and Technology Bureau CSE01N1113 to Jin-Jin Yu; NSFC 81302260 to Feng Xie.
Disclosure of conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJ, Naghavi M. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011;378:1461–1484. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- 2.Arbyn M, Castellsagué X, de Sanjosé S, Bruni L, Saraiya M, Bray F, Ferlay J. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 3.Dachs GU, Chaplin DJ. Microenvironmental control of gene expression: implications for tumor angiogenesis, progression, and metastasis. Semin Radiat Oncol. 1998;8:208–216. doi: 10.1016/s1053-4296(98)80046-5. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 5.Lee WY, Huang SC, Hsu KF, Tzeng CC, Shen WL. Roles for hypoxia-regulated genes during cervical carcinogenesis: somatic evolution during the hypoxia-glycolysis-acidosis sequence. Gynecol Oncol. 2008;108:377–384. doi: 10.1016/j.ygyno.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Bachtiary B, Schindl M, Pötter R, Dreier B, Knocke TH, Hainfellner JA, Horvat R, Birner P. Overexpression of hypoxia-inducible factor 1alpha indicates diminished response to radiotherapy and unfavorable prognosis in patients receiving radical radiotherapy for cervical cancer. Clin Cancer Res. 2003;9:2234–2240. [PubMed] [Google Scholar]
- 7.Acs G, Zhang PJ, McGrath CM, Acs P, McBroom J, Mohyeldin A, Liu S, Lu H, Verma A. Hypoxia-inducible erythropoietin signaling in squamous dysplasia and squamous cell carcinoma of the uterine cervix and its potential role in cervical carcinogenesis and tumor progression. Am J Pathol. 2003;162:1789–1806. doi: 10.1016/S0002-9440(10)64314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horree N, van Diest PJ, van der Groep P, Sie-Go DM, Heintz AP. Hypoxia and angiogenesis in endometrioid endometrial carcinogenesis. Cell Oncol. 2007;29:19–27. doi: 10.1155/2007/434731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 10.Bos R, Zhong H, Hanrahan CF, Mommers EC, Semenza GL, Pinedo HM, Abeloff MD, Simons JW, van Diest PJ, van der Wall E. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J Natl Cancer Inst. 2001;93:309–314. doi: 10.1093/jnci/93.4.309. [DOI] [PubMed] [Google Scholar]
- 11.Zhong H, Semenza GL, Simons JW, De Marzo AM. Up-regulation of hypoxia-inducible factor 1 alpha is an early event in prostate carcinogenesis. Cancer Detect Prev. 2004;28:88–93. doi: 10.1016/j.cdp.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimura TK, Matsushima K, Oppenheim JJ, Leonard EJ. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin-1 (IL-1) J Immunol. 1987;139:788–793. [PubMed] [Google Scholar]
- 13.Arici A, Tazuke SI, Attar E, Kliman HJ, Olive DL. Interleukin-8 concentration in peritoneal fluid of patients with endometriosis and modulation of interleukin-8 expression in human mesothelial cells. Mol Hum Reprod. 1996;2:40–45. doi: 10.1093/molehr/2.1.40. [DOI] [PubMed] [Google Scholar]
- 14.Arici A, Head JR, MacDonald PC, Casey ML. Regulation of interleukin-8 gene expression in human endometrial cells in culture. Mol Cell Endocrinol. 1993;94:195–204. doi: 10.1016/0303-7207(93)90168-j. [DOI] [PubMed] [Google Scholar]
- 15.Li MQ, Luo XZ, Meng YH, Mei J, Zhu XY, Jin LP, Li DJ. CXCL8 enhances proliferation and growth and reduces apoptosis in endometrial stromal cells in an autocrine manner via a CXCR1-triggered PTEN/AKT signal pathway. Hum Reprod. 2012;27:2107–2116. doi: 10.1093/humrep/des132. [DOI] [PubMed] [Google Scholar]
- 16.Xie F, Meng YH, Liu LB, Chang KK, Li H, Li MQ, Li DJ. Cervical carcinoma cells stimulate the angiogenesis through TSLP promoting growth and activation of vascular endothelial cells. Am J Reprod Immunol. 2013;70:69–79. doi: 10.1111/aji.12104. [DOI] [PubMed] [Google Scholar]
- 17.Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–1280. [PubMed] [Google Scholar]
- 18.Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. J Immunol. 2009;183:2895–2897. [PubMed] [Google Scholar]
- 19.Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, Bar-Eli M. Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol. 1997;151:1105–1113. [PMC free article] [PubMed] [Google Scholar]
- 20.Kitadai Y, Takahashi Y, Haruma K, Naka K, Sumii K, Yokozaki H, Yasui W, Mukaida N, Ohmoto Y, Kajiyama G, Fidler IJ, Tahara E. Transfection of interleukin-8 increases angiogenesis and tumorigenesis of human gastric carcinoma cells in nude mice. Br J Cancer. 1999;81:647–653. doi: 10.1038/sj.bjc.6690742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strieter RM, Polverini PJ, Arenberg DA, Walz A, Opdenakker G, Van Damme J, Kunkel SL. Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. J Leukoc Biol. 1995;57:752–762. doi: 10.1002/jlb.57.5.752. [DOI] [PubMed] [Google Scholar]
- 22.Mucaj V, Shay JE, Simon MC. Effects of hypoxia and HIFs on cancer metabolism. Int J Hematol. 2012;95:464–470. doi: 10.1007/s12185-012-1070-5. [DOI] [PubMed] [Google Scholar]
- 23.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu S, Shang H, Cui L, Zhang Z, Zhang Y, Li Y, Wu J, Li RK, Xie J. Targeted blockade of interleukin-8 abrogates its promotion of cervical cancer growth and metastasis. Mol Cell Biochem. 2013;375:69–79. doi: 10.1007/s11010-012-1529-y. [DOI] [PubMed] [Google Scholar]
- 25.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 26.Ju D, Sun D, Xiu L, Meng X, Zhang C, Wei P. Interleukin-8 is associated with adhesion, migration and invasion in human gastric cancer SCG-7901 cells. Med Oncol. 2012;29:91–99. doi: 10.1007/s12032-010-9780-0. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Chen L, Li JY, Mukaida N, Wang Q, Yang C, Yin WJ, Zeng XH, Jin W, Shao ZM. ERbeta and PEA3 co-activate IL-8 expression and promote the invasion of breast cancer cells. Cancer Biol Ther. 2011;11:497–511. doi: 10.4161/cbt.11.5.14667. [DOI] [PubMed] [Google Scholar]
- 28.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 29.Fujimoto J, Sakaguchi H, Aoki I, Tamaya T. Clinical implications of expression of interleukin 8 related to angiogenesis in uterine cervical cancers. Cancer Res. 2000;60:2632–2635. [PubMed] [Google Scholar]
- 30.Lokshin AE, Winans M, Landsittel D, Marrangoni AM, Velikokhatnaya L, Modugno F, Nolen BM, Gorelik E. Circulating IL-8 and anti-IL-8 autoantibody in patients with ovarian cancer. Gynecol Oncol. 2006;102:244–251. doi: 10.1016/j.ygyno.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Lotfi R, Lee JJ, Lotze MT. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J Immunother. 2007;30:16–28. doi: 10.1097/01.cji.0000211324.53396.f6. [DOI] [PubMed] [Google Scholar]