Abstract
Recent studies have found that periostin (PN), as a kind of secreted glycoprotein, is closely related to the metastatic potential and prognosis of many kinds of tumors. This study aimed to examine the expression of PN in patients with esophageal squamous cell carcinoma (ESCC) and explore the relationship of PN expression with clinicopathologic factors, tumor angiogenesis and prognosis. The results showed that increased PN protein expression was prevalent in ESCC and was significantly associated with lymphatic metastasis (P=0.008), tumor differentiation (P=0.04), venous invasion (P=0.014) and TNM stage (P=0.001). Additionally, expression of PN was found to be an independent prognostic factor in ESCC patients. High expression of PN protein is closely correlated to the tumor progression and angiogenesis and poor survival of ESCC. Taken together, PN is a promising biomarker to identify individuals with poor prognostic potential and concludes the possibility of its use as a prognostic marker in patients with ESCC.
Keywords: Periostin, esophageal squamous cell carcinoma, angiogenesis, prognosis
Introduction
Esophageal squamous cell carcinoma (ESCC) is one of the most common cancers in incidence and sixth in lethal worldwide, but it is still insufficiently studied [1-3]. Recently, albeit treatment strategies for ESCC have made great progress, a majority of the patients died of local relapse, advanced disease, distant metastasis and resistance to adjuvant therapy [4,5]. Due to lack of specific early symptoms or effective tumor biomarkers, most patients with ESCC are diagnosed at the advanced stages and their prognosis still remains poor. Thus, molecular biomarkers for recurrence and progression of ESCC are the effective ways for the choice of therapy for ESCC patients.
Periostin (PN), also named osteoblast-specific factor 2 (OSF-2), was originally identified in 1993 as an 90 kDa protein acids, which was secreted from the mouse osteoblastic cell line MC3T3-E1 [6]. It shows homologous sequence to an insect cell adhesion protein named fasciclin I (FAS I) and is involved in many biologic processes, such as cell adhesion, motility, angiogenesis and metastatic growth [7-9]. Accumulative findings showed that PN was overexpressed in various human cancers of head and neck, oral, nasopharyngeal, thyroid, breast, colon, pancreatic, and ovary [8-16], etc. In spite of some studies have demonstrated PN as a promising marker for tumor aggression in different types of human cancers, few were reported about the expression of PN in ESCC and its potential clinical significance.
In the present study, the expression levels of PN, vascular endothelial growth factor (VEGF) and microvessel density (MVD) in ESCC or corresponding paracarcinomatous normal tissues were examined using by immunohistochemistry. Further, the survival analysis was observed by Kaplan-Meier method. A multivariate survival analysis was performed for all parameters that were significant in the univariate analysis using the Cox regression model. All the above mentioned aimed to elucidate the expression of PN in ESCC and its correlation with clinicopathological characteristics, tumor angiogenesis, and prognosis.
Materials and methods
Patients and specimens
Sixty-eight patients with ESCC were selected who underwent curative esophagectomy at the Department of Thoracic Surgery, between 2006 and 2008. All cases were diagnosed on a clinical basis with pathological confirmation and no patients received additional treatment prior to the operation. All patients’ tissue specimens were formalin-fixed and paraffin-embedded for immunohistochemistry staining. Clinical and pathological data of all patients were obtained from medical records and follow-ups, with a follow-up deadline of June 13, 2013. The clinical and pathological data collected included gender, age, tumor size, degree of tumor differentiation, lymphatic metastasis, venous invasion, tumor location and TNM. This study was approved by the Institutional Review Board of the Anhui Provincial Hospital affiliated to Anhui Medical University and written consent was obtained from all participants.
Immunohistochemistry
The expressions of PN, VEGF and CD31 were detected by immunohistochemistry using a two-step method. In brief, specimen slices were dewaxed, rinsed in phosphate-buffered saline (PBS). After incubation in 10 mmol/L pH 6.0 citrate buffer, antigen was retrieved heating in a microwave oven. 3% hydrogen peroxide was used to block the endogenous peroxidase activity. Then sections were then incubated with primary polyclonal rabbit anti-PN antibody or anti-VEGF antibody or anti-CD31 antibody (all 1:100, Zhongshan Jinqiao Co., Beijing, China) at 4°C overnight. After incubating with labeled secondary antibody (mouse antirabbit IgG; Zhongshan Jinqiao Co., Beijing, China) at room temperature for 30 min, sections were colored through reacts with 3, 3-diaminobenzidine (DAB; Zhongshan Jinqiao Co., Beijing, China). Hematoxylin was used for counterstaining. Negative control slides were processed with PBS and a known positive tissue slice was used as a positive control.
The percentage of staining cells was used to express the results of immunohistochemistry. Results were evaluated based on the average percentage of positive cells per 100 cells in 10 high-power fields, as follows: 0-10%, negative (-); 10-30%, weak positive (+); >30%, strong positive (++). MVD evaluation acted in accordance with following principles. It was quantified in 5 fields which high expression using high-power lens (400 ×) and values were expressed by averages measurements. The slices were assessed by two pathologists who were blind to diagnosis independently.
Western blot analysis
Fresh frozen tumor tissues and corresponding paracarcinomatous normal tissues of two ESCC patients were lysed with cell lysis buffer as previously described [17]. About 50 μg of protein extract was resolved in 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA) in an ice-water environment. The membranes were blocked with 5% non-fat milk solution for 2 h at room temperature and then incubated with primary antibodies of rabbit polyclonal anti-PN (1:200, Beijing Biosynthesis Biothchnology Co., Ltd., China), VEGF (1:200, Beijing Biosynthesis Biothchnology Co., Ltd., China) and mouse monoclonal anti β-actin (1:400, Beijing Zhongshan Jinqiao Co., Ltd., China) at 4°C overnight. After washing with Tris-buffered saline Tween-20 (TBST), membranes were incubated with anti-rabbit or anti-mouse secondary antibodies conjugated with horseradish peroxidase (HRP) (1:20,000, Beijing Zhongshan Jinqiao Co., Ltd., China) for 1 h at 37°C. The membrane was then developed using the enhanced chemiluminescence systems. β-actin was used to confirm that an equal amount of protein was loaded in each lane. Band intensities were quantified with a computerized densitometer (Image J Launcher, Broken Symmetry Software).
Statistical analysis
Statistical analyses were completed with SPSS 13.0 (SPSS, Inc., Chicago, IL) and P<0.05 was considered statistically. The Mann-Whitney U test, χ2 tests and Spearman’s rank correlation test were used as appropriate for the comparison of variables. Survival rates were calculated using the Kaplan-Meier method. Univariate survival analyses were performed using the log-rank test, and multivariate survival analyses were performed using the Cox regression model.
Results
Expression of PN in ESCC tissues and its correlations with clinicopathological characteristics
In the present study, the expression of PN in a total of 68 primary ESCC tumor samples and their corresponding paracarcinomatous normal tissues were interpretable by immunohistochemical method. PN staining mainly located at cytoplasm whereas a very little positive expression was observed in extracellular matrix around tumor cell. As shown in Table 1, PN immunostaining in the tumor tissues was observed in 50/68 (73.5%), whereas expression was negative in 18/68 (26.5%) (Figure 1A-D). PN expression in corresponding paracarcinomatous normal tissues was significantly lower than that in ESCC tissues (12/68, P<0.05). Based on immunostaining evaluation and the statistical analysis, we also assessed the correlation between PN expression and clinicopathological parameters available for the patients (Table 3). Positive expression of PN in ESCC tissues were significantly correlated with lymphatic metastasis (P=0.008), tumor differentiation (P=0.04), venous invasion (P=0.014) and TNM stage (P=0.001). However, PN expression was not correlated with age, gender, and tumor size or tumor location.
Table 1.
Differential expression of PN between ESCC and corresponding paracarcinomatous normal tissues (cases)
| Tissues | Case number | Periostin expression | Positive rate (%) | |
|---|---|---|---|---|
|
|
||||
| +~++ | - | |||
| ESCC tissues | 68 | 50 | 18 | 73.5 |
| Paracarcinomatous tissues | 68 | 12 | 56 | 17.6 |
Figure 1.
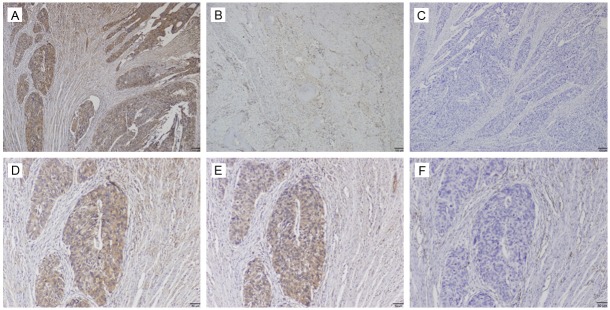
Immunohistochemical staining of periostin, VEGF and CD31 in esophageal squamous cell carcinoma (ESCC) tissues. Periostin (PN) mainly expressed in the cytoplasm of ESCC tissues. A: ++ PN staining; B: + PN staining; C: Negative for PN staining; D: ++ PN staining; E: Positive for VEGF staining; F: Positive for CD31 staining. A-C: Bar=100 μm; D-F: Bar=50 μm, examined by using serial sections.
Table 3.
PN expression status in relation to selected clinicopathologic features in 68 ESCC patients (cases)
| Clinicopathologic data | Case number | Periostin | χ2 | P value | |
|---|---|---|---|---|---|
|
| |||||
| +~++ | - | ||||
| Age | |||||
| ≤60 | 45 | 31 | 14 | 1.472 | 0.225 |
| >60 | 23 | 19 | 4 | ||
| Gender | |||||
| Male | 36 | 24 | 12 | 1.851 | 0.174 |
| Female | 32 | 26 | 6 | ||
| Tumor size (cm) | |||||
| ≤5 | 28 | 19 | 9 | 0.787 | 0.375 |
| >5 | 40 | 31 | 9 | ||
| Lymphatic metastasis | |||||
| No | 43 | 27 | 16 | 6.930 | 0.008 |
| Yes | 25 | 23 | 2 | ||
| Tumor differentiation | |||||
| High, Moderate | 35 | 22 | 13 | 4.220 | 0.04 |
| Low | 33 | 28 | 5 | ||
| Venous invasion | |||||
| No | 40 | 25 | 15 | 6.071 | 0.014 |
| Yes | 28 | 25 | 3 | ||
| Tumor location | |||||
| Upper | 20 | 16 | 4 | 0.609 | 0.435 |
| Middle/Low | 48 | 34 | 14 | ||
| TNM | |||||
| I/II | 42 | 25 | 17 | 11.070 | 0.001 |
| III/IV | 26 | 25 | 1 | ||
Expression of VEGF and MVD in ESCC tissues and its correlation with PN
The relationships between expression of PN and VEGF were calculated and have been outlined in Table 2. The expression of VEGF in all 68 ESCC tumor samples was mainly localized in the cytoplasm (Figure 1E) and positive rates in ESCC tissues was 67.6 % (46/68). The result also showed that high expression of PN correlated with VEGF expression (r=0.369; P=0.002; Table 2).
Table 2.
The expression correlation between PN and VEGF (cases)
| Stain | Periostin | r | P value | |
|---|---|---|---|---|
|
|
||||
| +~++ | - | |||
| VEGF | ||||
| +~++ | 39 | 7 | 0.369 | 0.002 |
| - | 11 | 11 | ||
To further investigate the relationship between PN and angiogenesis, we detected the expression of MVD in the ESCC by using an antibody against CD31 (Figure 1F). Our study showed that tumors with PN-positive expression significantly had higher MVD (46.7 ± 15.6 vs. 24.3 ± 8.2; P=0.000) compared to those in PN-negative tissues (Figure 2).
Figure 2.
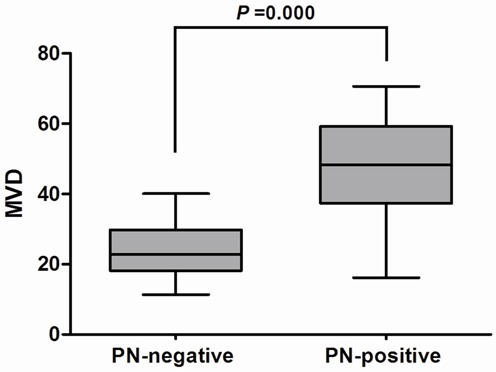
Intratumoral microvessel density (MVD) in relation to periostin (PN) protein immunoreactivity. Mann-Whitney U test demonstrated that tumors with PN-positive expression showed significantly higher intratumoral MVD than that with PN-negative expression (P=0.000).
Additionally, to further verify the difference of expression, we detected the expression levels of PN and VEGF proteins in two fresh frozen ESCC and corresponding paracarcinomatous normal specimens by western blot analysis. As shown in Figure 3, similar to the results of immunohistochemical analysis, the expression levels of PN and VEGF in ESCC were higher than that in cancer-surrounding tissues.
Figure 3.
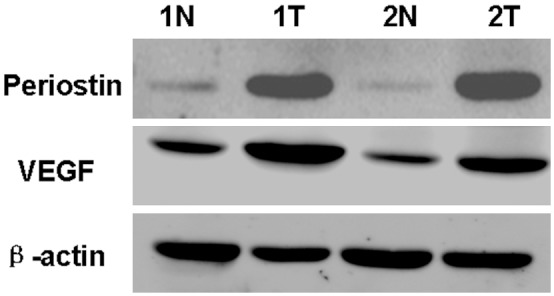
Western blot analysis of periostin (PN, 90 kDa) and VEGF proteins in two fresh frozen esophageal squamous cell carcinoma (ESCC) and corresponding paracarcinomatous normal tissues. T: ESCC tissues; N: corresponding paracarcinomatous normal tissues.
Relationship between PN expression and prognosis
Patients with PN-positive expression showed a poorer prognosis than those with PN-negative expression by the Kaplan-Meier analysis. The log-rank test revealed that the overall survival time of ESCC patients with PN-positive expression (34.44 ± 3.05 months) was markedly shorter than that with PN-negative expression (50.27 ± 5.53 months; P=0.027; Figure 4A; Table 4). Furthermore, similar results were also observed in the disease free survival analysis (32.46 ± 3.20 months vs. 49.05 ± 5.93 months; P=0.026; Figure 4B). To understand deeply about the relationship between the PN positive expression and prognosis, two different degrees of positive expression were analyzed by using the Kaplan-Meier method (Figure 5). Moreover, as seen in Table 4, Multivariate Cox analysis indicated PN expression was one of the independent prognostic factors, along with tumor differentiation, venous invasion and TNM stage (Table 5).
Figure 4.
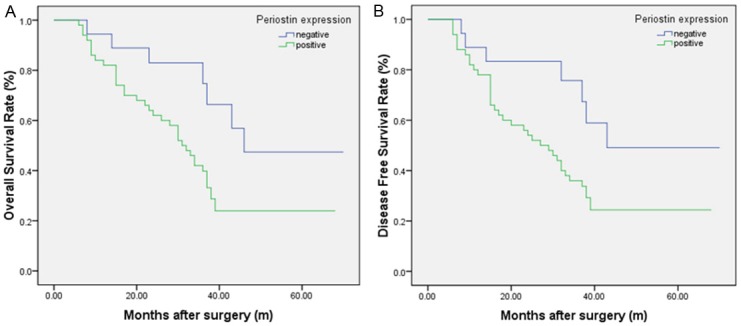
Kaplan-Meier analysis of overall survival (OS) and disease-free survival (DFS) curves of patients with esophageal squamous cell carcinoma (ESCC) based on periostin expression as positive or negative. A: OS curve of patients with ESCC based on periostin expression; B: DFS curve of patients with ESCC based on periostin expression. The ESCC patients with periostin positive showed significantly poorer OS and DFS rates than those with periostin negative.
Table 4.
Univariate analysis of factors associated with OS and DFS
| Variable | OS | DFS | ||
|---|---|---|---|---|
|
|
|
|||
| 95% CI | P value | 95% CI | P value | |
| Periostin | ||||
| Negative | 39.420-61.111 | 0.027 | 37.440-60.667 | 0.026 |
| Positive | 28.469-40.414 | 26.181-38.736 | ||
| Age | ||||
| ≤60 | 30.380-43.991 | 0.618 | 28.969-43.090 | 0.647 |
| >60 | 31.655-50.077 | 28.260-48.548 | ||
| Gender | ||||
| Male | 33.580-48.243 | 0.570 | 31.141-46.965 | 0.628 |
| Female | 27.508-44.423 | 25.894-43.407 | ||
| Tumor size (cm) | ||||
| ≤5 | 35.173-52.197 | 0.144 | 32.461-50.951 | 0.175 |
| >5 | 27.930-42.312 | 26.165-41.140 | ||
| Lymphatic metastasis | ||||
| No | 35.710-49.906 | 0.087 | 33.764-48.910 | 0.084 |
| Yes | 23.238-39.882 | 20.938-38.102 | ||
| Tumor differentiation | ||||
| High, Moderate | 39.954-55.609 | 0.002 | 38.833-55.196 | 0.001 |
| Low | 22.472-34.740 | 19.465-32.232 | ||
| Venous invasion | ||||
| No | 37.739-52.335 | 0.004 | 36.590-51.802 | 0.004 |
| Yes | 22.228-36.908 | 18.976-34.572 | ||
| Tumor location | ||||
| Upper | 20.603-37.328 | 0.745 | 18.323-30.950 | 0.783 |
| Middle/Low | 25.542-39.390 | 23.315-37.979 | ||
| TNM | ||||
| I/II | 31.333-48.183 | 0.000 | 29.442-46.806 | 0.000 |
| III/IV | 14.903-25.174 | 12.138-21.093 | ||
Figure 5.
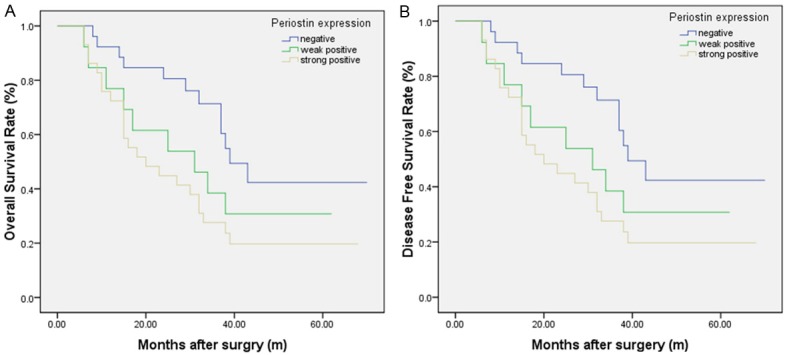
Kaplan-Meier analysis of overall survival (OS) and disease-free survival (DFS) curves of patients with esophageal squamous cell carcinoma (ESCC) based on periostin expression as strongly positive, weakly positive or negative. A: OS curve of patients with ESCC based on periostin expression; B: DFS curve of patients with ESCC based on periostin expression. The ESCC patients with periostin positive showed significantly poorer OS and DFS rates than those with periostin negative. The survival of patients in the strongly positive periostin expression was poorest.
Table 5.
Multivariate analysis of factors associated with OS and DFS
| Characteristics | OS | DFS | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |
| Periostin (negative vs. positive) | 2.393 | 1.065-5.377 | 0.035 | 2.395 | 1.066-5.381 | 0.034 |
| Age (≤60 vs. >60) | 1.184 | 0.654-2.143 | 0.577 | 0.864 | 0.458-1.632 | 0.653 |
| Gender (male vs. female) | 0.853 | 0.452-1.610 | 0.624 | 1.155 | 0.638-2.091 | 0.634 |
| Tumor size, cm (≤5 vs. >5) | 1.567 | 0.846-2.902 | 0.153 | 1.519 | 0.818-2.818 | 0.185 |
| Lymphatic metastasis (no vs. yes) | 1.658 | 0.915-3.004 | 0.096 | 1.665 | 0.918-3.018 | 0.093 |
| Tumor differentiation (high, moderate vs. low) | 0.392 | 0.211-0.726 | 0.003 | 0.363 | 0.196-0.673 | 0.001 |
| Venous invasion (no vs. yes) | 2.292 | 1.262-4.162 | 0.006 | 2.313 | 1.275-4.194 | 0.006 |
| Tumor location (upper vs. middle, low) | 0.899 | 0.470-1.722 | 0.749 | 0.918 | 0.491-1.718 | 0.789 |
| TNM (no vs. yes) | 2.934 | 1.635-5.266 | 0.000 | 3.047 | 1.714-5.417 | 0.000 |
Discussion
Recent studies have demonstrated that PN, as a extracellular matrix protein, was upregulated in a variety of cancers encompassing head and neck [11,13], colon [8], pancreatic [15], oral [14], breast [9], lung [18], ovarian [10] and liver cancer [19,20], etc. It functioned as a cell adhesion molecule and participated in many biological processes, including cell adhesion, invasion, metastasis and tumor angiogenesis [7,8,13,14]. As for ESCC, there were only several experiments focusing on the correlation between PN and that. Available cumulative findings provided mounting evidence for the importance of PN in tumor invasion, tumor cell dissemination as well as creating a supportive environment for metastatic colonization of ESCC [21-23]. However, most of previous studies did not clarify the relationship between PN expression and tumor angiogenesis and/or clinicopathological parameters of ESCC.
In the present study, we first describe the prognostic relevance of PN expression in patients with ESCC. Overexpression of PN was detectable in 50 of 68 (73.5%) tumor tissues. Furthermore, we observed that ESCC tumors with PN-positive expression were more frequently lymphatic metastasis, tumor differentiation, venous invasion and TNM stage III-IV than ESCC tumors with PN-negative expression. Further assessment demonstrated that OS and DFS were better in patients without PN expression than those in patients with PN-positive expression. Both Kaplan-Meier and multivariate analysis showed that the expression of PN was independent predictors of poor prognosis for both OS and DFS. The relationship between PN staining intensity and the patients’ survival was also analyzed, and a general inverse trend between a descending in patient survival and ascending PN staining intensity was observed. Therefore, these results indicated that PN expression had an adverse influence on the ESCC patient’s outcome. PN may be used as a marker to predict ESCC patient’s prognosis.
Angiogenesis plays a vital role in tumor initiation, progression and metastasis, and it has been considered one of the hallmarks of cancer [24,25]. There is a plethora of evidence supporting significance of angiogenesis in development and aggressiveness of esophageal cancer. For instance, Kitadai et al. [26] found that esophageal cancer cell lines (both ESCC and esophageal adenocarcinoma) express high levels of VEGF and MVD. Upregulation of VEGF in tissue is one of the potential drivers of progression of metaplasia to dysplasia and adenocarcinoma in esophagus [27]. Oshima et al. [28] demonstrated that increased serum level of VEGF is shown to be a marker of higher stage and is associated with poor response to chemoradiotherapy. Moreover, higher levels of serum VEGF -C & D, two of the VEGF subtypes, are also shown to be predictive of patient survival and tumor progression in esophageal cancer [29].
Thus, in order to verify whether there is a relationship between PN protein and tumor angiogenesis, we quantified the levels of VEGF and MVD, which were the most widely accepted markers of tumor angiogenesis. Our results showed that tumors with PN-positive group expressed higher VEGF and had higher MVD than those in PN-negative group. We analyzed the relationship between VEGF and PN by spearman’s rank correlation test and found there were a significant positive correlation between PN and VEGF. Taken together, these findings suggested that PN plays a crucial role in ESCC tumorigenesis by the induction and/or promotion of tumor angiogenesis. Recently, some studies revealed that PN binding to the integrins activates the FAK- and Akt/PKB-mediated signaling pathways which promote tumor angiogenesis, invasion and metastasis [30,31]. To demonstrate these hypotheses, further investigations will still be required to explore its possible molecular mechanisms.
In conclusion, our study found that PN is overexpressed in ESCC tissues compared with their adjacent tissues. It is possible that PN expression has a close correlation with tumor angiogenesis and metastasis, and its overexpression is of predictive value on ESCC development and progression. In future, if this activity could be blocked by some specific inhibitors, we may provide a new target for the anti-angiogenic therapy of ESCC.
Acknowledgements
We sincerely appreciate Dr. Hang-Cheng Zhou and Dr. Wen Zhong (two pathologists, Department of Pathology, Anhui Provincial Hospital, Hefei, China) for their kind assistance in pathology. This work was partly supported by the National Natural Science Foundation of China (No. 81201906).
Disclosure of conflict of interest
The authors have no conflicts of interest.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.Sano A, Kato H, Sakurai S, Sakai M, Tanaka N, Inose T, Saito K, Sohda M, Nakajima M, Nakajima T, Kuwano H. CD24 expression is a novel prognostic factor in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16:506–514. doi: 10.1245/s10434-008-0252-0. [DOI] [PubMed] [Google Scholar]
- 4.Mariette C, Balon JM, Piessen G, Fabre S, Van Seuningen I, Triboulet JP. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer. 2003;97:1616–1623. doi: 10.1002/cncr.11228. [DOI] [PubMed] [Google Scholar]
- 5.Ren Y, Cao B, Law S, Xie Y, Lee PY, Cheung L, Chen Y, Huang X, Chan HM, Zhao P, Luk J, Vande Woude G, Wong J. Hepatocyte growth factor promotes cancer cell migration and angiogenic factors expression: a prognostic marker of human esophageal squamous cell carcinomas. Clin Cancer Res. 2005;11:6190–6197. doi: 10.1158/1078-0432.CCR-04-2553. [DOI] [PubMed] [Google Scholar]
- 6.Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993;294:271–278. doi: 10.1042/bj2940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002;62:5358–5364. [PubMed] [Google Scholar]
- 8.Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson RM, Rich JN, Wang XF. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5:329–339. doi: 10.1016/s1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 9.Shao R, Bao S, Bai X, Blanchette C, Anderson RM, Dang T, Gishizky ML, Marks JR, Wang XF. Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol. 2004;24:3992–4003. doi: 10.1128/MCB.24.9.3992-4003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ismail RS, Baldwin RL, Fang J, Browning D, Karlan BY, Gasson JC, Chang DD. Differential gene expression between normal and tumor-derived ovarian epithelial cells. Cancer Res. 2000;60:6744–6749. [PubMed] [Google Scholar]
- 11.Chang Y, Lee TC, Li JC, Lai TL, Chua HH, Chen CL, Doong SL, Chou CK, Sheen TS, Tsai CH. Differential expression of osteoblast-specific factor 2 and polymeric immunoglobulin receptor genes in nasopharyngeal carcinoma. Head Neck. 2005;27:873–882. doi: 10.1002/hed.20253. [DOI] [PubMed] [Google Scholar]
- 12.Fluge O, Bruland O, Akslen LA, Lillehaug JR, Varhaug JE. Gene expression in poorly differentiated papillary thyroid carcinomas. Thyroid. 2006;16:161–175. doi: 10.1089/thy.2006.16.161. [DOI] [PubMed] [Google Scholar]
- 13.Kudo Y, Ogawa I, Kitajima S, Kitagawa M, Kawai H, Gaffney PM, Miyauchi M, Takata T. Periostin promotes invasion and anchorage-independent growth in the metastatic process of head and neck cancer. Cancer Res. 2006;66:6928–6935. doi: 10.1158/0008-5472.CAN-05-4540. [DOI] [PubMed] [Google Scholar]
- 14.Siriwardena BS, Kudo Y, Ogawa I, Kitagawa M, Kitajima S, Hatano H, Tilakaratne WM, Miyauchi M, Takata T. Periostin is frequently overexpressed and enhances invasion and angiogenesis in oral cancer. Br J Cancer. 2006;95:1396–1403. doi: 10.1038/sj.bjc.6603431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baril P, Gangeswaran R, Mahon PC, Caulee K, Kocher HM, Harada T, Zhu M, Kalthoff H, Crnogorac-Jurcevic T, Lemoine NR. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: role of the beta4 integrin and the PI3k pathway. Oncogene. 2007;26:2082–2094. doi: 10.1038/sj.onc.1210009. [DOI] [PubMed] [Google Scholar]
- 16.Erkan M, Kleeff J, Gorbachevski A, Reiser C, Mitkus T, Esposito I, Giese T, Buchler MW, Giese NA, Friess H. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132:1447–1464. doi: 10.1053/j.gastro.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 17.Kwon YJ, Lee SJ, Koh JS, Kim SH, Kim YJ, Park JH. Expression patterns of aurora kinase B, heat shock protein 47, and periostin in esophageal squamous cell carcinoma. Oncol Res. 2009;18:141–151. doi: 10.3727/096504009790217407. [DOI] [PubMed] [Google Scholar]
- 18.Ouyang G, Liu M, Ruan K, Song G, Mao Y, Bao S. Upregulated expression of periostin by hypoxia in non-small-cell lung cancer cells promotes cell survival via the Akt/PKB pathway. Cancer Lett. 2009;281:213–219. doi: 10.1016/j.canlet.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 19.Lv Y, Wang W, Jia WD, Sun QK, Huang M, Zhou HC, Xia HH, Liu WB, Chen H, Sun SN, Xu GL. High preoparative levels of serum periostin are associated with poor prognosis in patients with hepatocellular carcinoma after hepatectomy. Eur J Surg Oncol. 2013;39:1129–1135. doi: 10.1016/j.ejso.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Lv Y, Wang W, Jia WD, Sun QK, Li JS, Ma JL, Liu WB, Zhou HC, Ge YS, Yu JH, Xia HH, Xu GL. High-level expression of periostin is closely related to metastatic potential and poor prognosis of hepatocellular carcinoma. Med Oncol. 2013;30:385. doi: 10.1007/s12032-012-0385-7. [DOI] [PubMed] [Google Scholar]
- 21.Michaylira CZ, Wong GS, Miller CG, Gutierrez CM, Nakagawa H, Hammond R, Klein-Szanto AJ, Lee JS, Kim SB, Herlyn M, Diehl JA, Gimotty P, Rustgi AK. Periostin, a cell adhesion molecule, facilitates invasion in the tumor microenvironment and annotates a novel tumor-invasive signature in esophageal cancer. Cancer Res. 2010;70:5281–5292. doi: 10.1158/0008-5472.CAN-10-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong GS, Lee JS, Park YY, Klein-Szanto AJ, Waldron TJ, Cukierman E, Herlyn M, Gimotty P, Nakagawa H, Rustgi AK. Periostin cooperates with mutant p53 to mediate invasion through the induction of STAT1 signaling in the esophageal tumor microenvironment. Oncogenesis. 2013;2:e59. doi: 10.1038/oncsis.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo JH, Zhou J, Gao Y. Correlation between periostin and SNCG and esophageal cancer invasion, infiltration and apoptosis. Asian Pac J Trop Med. 2013;6:516–519. doi: 10.1016/S1995-7645(13)60088-7. [DOI] [PubMed] [Google Scholar]
- 24.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Kitadai Y, Haruma K, Tokutomi T, Tanaka S, Sumii K, Carvalho M, Kuwabara M, Yoshida K, Hirai T, Kajiyama G, Tahara E. Significance of vessel count and vascular endothelial growth factor in human esophageal carcinomas. Clin Cancer Res. 1998;4:2195–2200. [PubMed] [Google Scholar]
- 27.Vallbohmer D, Peters JH, Kuramochi H, Oh D, Yang D, Shimizu D, DeMeester SR, Hagen JA, Chandrasoma PT, Danenberg KD, Danenberg PV, DeMeester TR. Molecular determinants in targeted therapy for esophageal adenocarcinoma. Arch Surg. 2006;141:476–481. doi: 10.1001/archsurg.141.5.476. discussion 481-472. [DOI] [PubMed] [Google Scholar]
- 28.Oshima Y, Yajima S, Yamazaki K, Matsushita K, Tagawa M, Shimada H. Angiogenesis-related factors are molecular targets for diagnosis and treatment of patients with esophageal carcinoma. Ann Thorac Cardiovasc Surg. 2010;16:389–393. [PubMed] [Google Scholar]
- 29.Kozlowski M, Kowalczuk O, Milewski R, Chyczewski L, Niklinski J, Laudanski J. Serum vascular endothelial growth factors C and D in patients with oesophageal cancer. Eur J Cardiothorac Surg. 2010;38:260–267. doi: 10.1016/j.ejcts.2010.01.061. [DOI] [PubMed] [Google Scholar]
- 30.Li G, Jin R, Norris RA, Zhang L, Yu S, Wu F, Markwald RR, Nanda A, Conway SJ, Smyth SS, Granger DN. Periostin mediates vascular smooth muscle cell migration through the integrins alphavbeta3 and alphavbeta5 and focal adhesion kinase (FAK) pathway. Atherosclerosis. 2010;208:358–365. doi: 10.1016/j.atherosclerosis.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Utispan K, Sonongbua J, Thuwajit P, Chau-In S, Pairojkul C, Wongkham S, Thuwajit C. Periostin activates integrin alpha5beta1 through a PI3K/AKTdependent pathway in invasion of cholangiocarcinoma. Int J Oncol. 2012;41:1110–1118. doi: 10.3892/ijo.2012.1530. [DOI] [PubMed] [Google Scholar]


