Abstract
MicroRNA-155 (miR-155) is overexpressed in many human cancers; however, the function of miR-155 is largely unknown in esophageal squamous cell carcinoma (ESCC). In the present study, we found that miR-155 is dramatically increased in ESCC tissues compared with the paired adjacent normal tissues, which suggested that miR-155 acts as an oncogene in ESCC. We predicted that tumor protein p53-induced nuclear protein 1 (TP53INP1) is a candidate target gene of miR-155 given that miR-155 expression decreased mRNA and protein levels of TP53INP1 as determined by RT-PCR and Western blot analysis. In addition, miR-155 and TP53INP1 showed a negative relation in ESCC tissues. Dual luciferase-based reporter assay indicated direct regulation of TP53INP1 by miR-155. Furthermore, we demonstrated that RNA interference of TP53INP1 increased the proliferation and colonies formation of EC-1 cells. Up-regulation of TP53INP1 abrogated miR-155 induced growth in EC-1 cells and mutation of TP53INP1 in 3’-UTR restored the effects when co-transfected with miR-155. We also indicated that overexpression of miR-155 significantly promoted the proliferation of EC-1 cells in vitro and the development of tumors in nude mice. Taken together, our study reveals that miR-155 acts as an oncogene by targeting TP53INP1 in ESCC.
Keywords: ESCC, TP53INP1, miR-155
Introduction
Esophageal squamous cell carcinoma (ESCC) is the major histologic subtype of esophageal cancer. ESCC is about 2% of total human malignant tumors and is the sixth leading cause of death from cancer [1]. Its morbidity shows a significant geographic difference and appears to be high in china. ESCC occurs in familial aggregates. Cohort studies in high-risk areas like Shanxi, Shandong province of China reported that 25%-50% ESCC patients had the family history of esophageal cancer [2]. It is well-known that Nitrite chronic stimulation, inflammation and trauma, genetic factor and microelement in food are risk factors of esophageal cancer. Statistics reveal that mutation of tumor suppressor gene p53 is associated with the development of ESCC in the cohort [3]. The most frequent symptom of ESCC is progressive dysphagia. Generally, patients apply to a physician at a stage when they cannot swallow solid foods anymore, which mean an advanced stage for ESCC, and in this stage patients lose their chances of curable surgical treatment. Therefore, developing effective early diagnosis method is particularly important for detecting and treating ESCC.
MicroRNAs (miRNAs) are a group of small non-coding RNAs with 18-25 nucleotides in length that negatively regulate gene expression by imprecisely binding to complementary sequence in the 3’-UTR of their target mRNAs [4]. MiRNAs play an important role in biological and pathologic processed including cell differentiation, proliferation, apoptosis and metabolism. They can function as oncogenes or tumor suppressors [5]. Aberrant miRNA expression may contribute to many types of human disease and they have been associated with every aspect of tumorigenesis. A recent study has shown that differential expression of miRNA was correlated with esophageal carcinoma survival [6].
In this study, we discovered that the expression of miR-155 in ESCC tissues was higher than that of adjacent tissues, which was consistent with Ran Liu [7]. Furthermore, TP53INP1 (tumor protein 53-induced nuclear protein 1) is a target gene of miR-155. Meanwhile, TP53INP1 is a proapoptotic stress-induced p53 target gene [8]. Regulation of miR-155 expression affected the expression of TP53INP1 in EC-1 cell lines. Finally, we validated that TP53INP1 is a direct target of miR-155 in the context of human ESCC.
Materials and methods
Tissue specimens and cell lines
30 pairs of fresh frozen ESCC and their adjacent non-tumor tissue specimens were obtained from surgical specimens from Anyang Tumor Hospital (Anyang, Henan, China) with approval of the Ethics Committee of Anyang Tumor Hospital. All samples used in this study were approved by the committee for ethical review of research. The whole procedure of consent was approved and documented by the Ethics Committee of Anyang Tumor Hospital. The ESCC cell lines EC-1 (provided by professor Kui-sheng Chen, Department of Pathology. The University of Zhengzhou, Henan, China) were stored in our own laboratory. Cells were maintained in 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Hy-Clone, Logan, UT, USA) and cultivated at 37°C in 5% CO2.
Quantitative real-time PCR analysis
Total RNA was extracted from isolated from tissues/cells by Trizol method (Invitrogen, Carlsbad, CA, USA). The first-strand of cDNA was synthesized with M-MLV Reverse Transcriptase (Promega, Madison, WI, USA). Quantitative real-time PCR (qRT-PCR) was performed as follows: 20 μl PCR mix was initial incubated at 95°C for 45 s, followed by 40 cycles of 95°C for 15 s and 60°C for 30 s. The primers sequences are as follows: mir-155 RT: 5’-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG ACCCCTAT-3’; mir-155 F: 5’-ACACTCCAGCTGGGTTAATGCTAATCGTGAT-3’, R: 5’-TGGTGTCGTGGAGT CG-3’. U6: F: 5’-CTCGCTTCGGCAGCACA-3’, R: 5’-AACGCTTCACGAATTTGCGT-3’. TP53INP1F: 5’-CCA CGTACAATGACTCTTCT-3’, R: 5’-TTCTTGGTTGGAGGAAGAAC-3’.
MTT assay
Two groups including the experimental group and the control group were involved in this study. Cells were dispensed in a 96-well plate with 1500 cells per well. Each group consisted of three wells. The cells were incubated for 24 h, 48 h, 72 h, 96 h and 120 h after transfection respectively. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed by adding 20 μl of MTT (5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) for 4 h. When MTT incubation was completed, the supernatants were removed. Then, 150 μl of dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA) was added to each well. After 15 min, the absorbance (OD) of each well was measured and the value was recorded using a microplate reader set at a wavelength of 490 nm. The experiments were performed in triplicate.
BrdU staining assay
For analysis of EC-1 proliferation, 5-bromo-2- deoxyuridine (BrdU) (10 μmol/L) was used to label the cells transfected with miRNA-155. Cells were then visualized with the fluorescence microscope.
Plate clone formation assay
200 cells were added to each well of a 6-well culture plate and incubated for 14 days, the cells were fixed by 70% ethanol and stained with 0.5% crystal violet. The number of colonies was analyzed using Image J software. These experiments were performed in triplicate.
Immunohistochemical staining (IHC)
We use IHC to study the expression and localization of the TP53INP1 proteins on paraffin tissue sections (4 μm). The tissue sections were from ESCC and their adjacent non-tumor tissues. The antibody was bought from Abcam (Inc. Cambridge, MA). We can see the localization of the TP53INP1 proteins, and the staining intensity was examined and classified into: absent and positive.
Tumorigenicity assay
A lentiviral based system of miR-155 was constructed and used to infect EC-1 cells. Cells (5×106) were suspended in 100 μl PBS and then injected into nude mouse (Bikai, Shanghai, China) at 5 to 6 weeks of age. According to the recommendations guidelines of the Animal Care and Use Committee of The Tenth People’s Hospital of Shanghai, the studies were performed strictly with the Permit number: 2011-RES1. The protocol was approved by Science and Technology Commission of Shanghai Municipality (ID: SYXK 2007-0006). Each group consisted of 3 mice. Tumor growth was examined every week for 6 weeks. After 6 weeks, mice were killed and tumors were collected to measure the volume and weight.
Luciferase reporter assays
TP53INP1-3’-UTR were constructed into psiCHECK-2 report plasmid (Progma, Madison, WI, USA). Plasmids and miR-155 were co-transfected into EC-1 cells using LipofectamineTM 2000 (Invitrogen, USA). The luciferase activity was measured 24 h post transfection using the Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA). Fold-activation values were measured relative to the levels of Renilla Luciferase activity in cells transfected with negative control and normalized by luciferase activities.
Western blot analysis
EC-1 cells were seeded into 6-well plates (1×105 cells per well) and transfected when cells outgrowth was confluent. Cells were collected and lysed 48 h post transfection. Equal amounts of proteins were separated by 10% gradient SDS-polyacrylamide gels. The proteins were transferred onto a NC membrane, blocked with 5% fat-free milk powder for 1 h. Blots were then incubated with rabbit monoclonal TP53INP1 antibody (Abcam Inc. Cambridge, MA), followed by incubation with HRP-conjugated secondary antibody. β-actin was used as control to verify equal amounts of protein.
Statistical analysis
The SPSS 18.0 version (SPSS Inc. Chicago, IL, USA) was used for conducting the statistical analyses. Data was tested using Student’s t-test, One-way ANOVA and Chi-square test. In all samples, P≤0.05 (*) and P≤0.01 (**) was considered to be statistically significant.
Results
MiR-155 is upregulated in ESCC tissues and promotes the proliferation of EC-1 cells
MiR-155 expression level between ESCC tissues and paired adjacent non-tumor tissues from 30 individual patients were measured using quantitative real-time PCR. miRNA-155 was markedly upregulated (>3 times) respectively in ESCC samples compared with normal samples (Figure 1A). Among all the samples, miR-155 was expressed more than two-fold higher in 60% of ESCC tissues (Figure 1B). To confirm the role of miR-155 in ESCC cells proliferation, miR-155 was over-expressed in EC-1 cell lines in vitro and then detected cell viability by MTT assay. MTT assay results indicated that cells over-expressed miR-155 showed stronger proliferation ability than control (Figure 1C).
Figure 1.
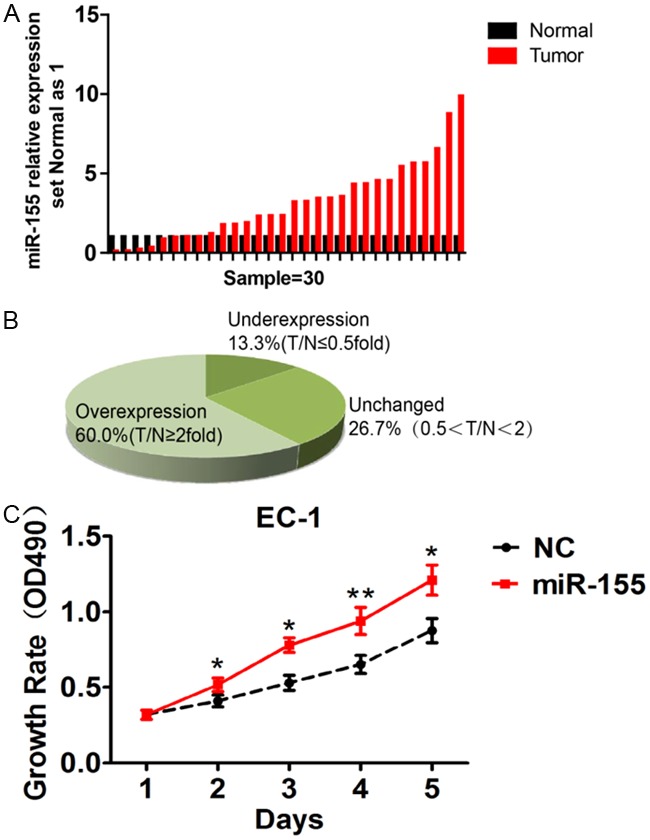
miR-155 was upregulated in ESCC tissues and promoted the growth of EC-1 cells. A: Quantitative real-time PCR analysis showed upregulation of miRNA-155 in ESCC tissues compared to paired adjacent normal tissues. B: 60% of ESCC samples showed twofold higher expression of miRNA-155. C: MTT assay showed that miR-155promoted proliferation in EC-1 cells. (*, P<0.05; **, P<0.01).
TP53INP1 is the putative candidate target gene of miR-155
Putative miR-155 targets were predicted using target prediction programs TargetScan. Our analysis revealed that TP53INP1 was a potential target of miR-155. The 3’-UTR of TP53INP1 contained a binding site for the seed region of miR-155 (Figure 2A).
Figure 2.

TP53INP1 is a direct target of miR-155. (A) putative miR-155 binding sequence in the 3’-UTR of TP53INP1 mRNA. (B) quantitative real-time PCR and (C) western blot were used to monitor the expression level of TP53INP1 after transfection with miR-155. β-actin was used as an internal control (**, P<0.01). (D) Inverse Correlation between miR-155 and TP53INP1 mRNA Levels. (E) dual luciferase reporter assay showed that wtTP53INP1 (wild-type TP53INP1 3’-UTR) co-transfected with miR-155 was significantly increased (**, P<0.01).
The effect of miR-155 on the expression of TP53INP1 was further examined by quantitative real-time PCR and western blot. We found that over-expression miR-155 caused a significant decrease in mRNA (Figure 2B) and protein level (Figure 2C). Moreover, we tested mRNA level of TP53INP1 and miR-155 in 30 ESCC tissues using quantitative real-time PCR, and the data showed obvious negative relation between expression of TP53INP1 and miR-155 (r=-0.7, P=0.001) (Figure 2D).
To validate whether TP53INP1 is a direct target of miR-155, a human TP53INP1
3’-UTR was cloned into the reporter vector and the dual luciferase reporter assay was performed. The relative luciferase activity of the reporter that contains wild-type 3’-UTR was significantly increased when miR-155 was co-transfected (Figure 2E). In contrast, the activity of mutant 3’-UTR was unaffected by simultaneous transfection of miR-155 compared with NC. The results indicated that miR-155 may suppress TP53INP1 expression through binding the 3’-UTR of TP53INP1.
The low expression of TP53INP1 is associated with tumor differentiation, stage and size
Next, we examined the expression of TP53INP1 in ESCC tumor and normal tissue sections with IHC. Compared to the normal tissue, it’s obvious that the positive staining intensity of ESCC is much stronger (Figure 3). Moreover, we found that the positive staining of TP53INP1 was localized within the nucleus.
Figure 3.
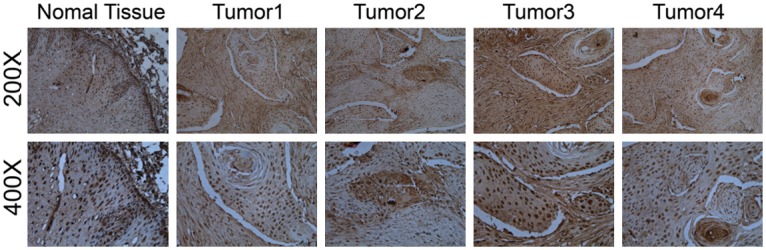
Representative IHC photos of TP53INP1 expression in normal tissue (200× and 400×) and ESCC tumors. TP53INP1 staining was mainly localized within the nucleus of cells in the form of yellow brown granules. It’s obvious that the positive staining intensity of ESCC is much more stronger than that of normal tissue.
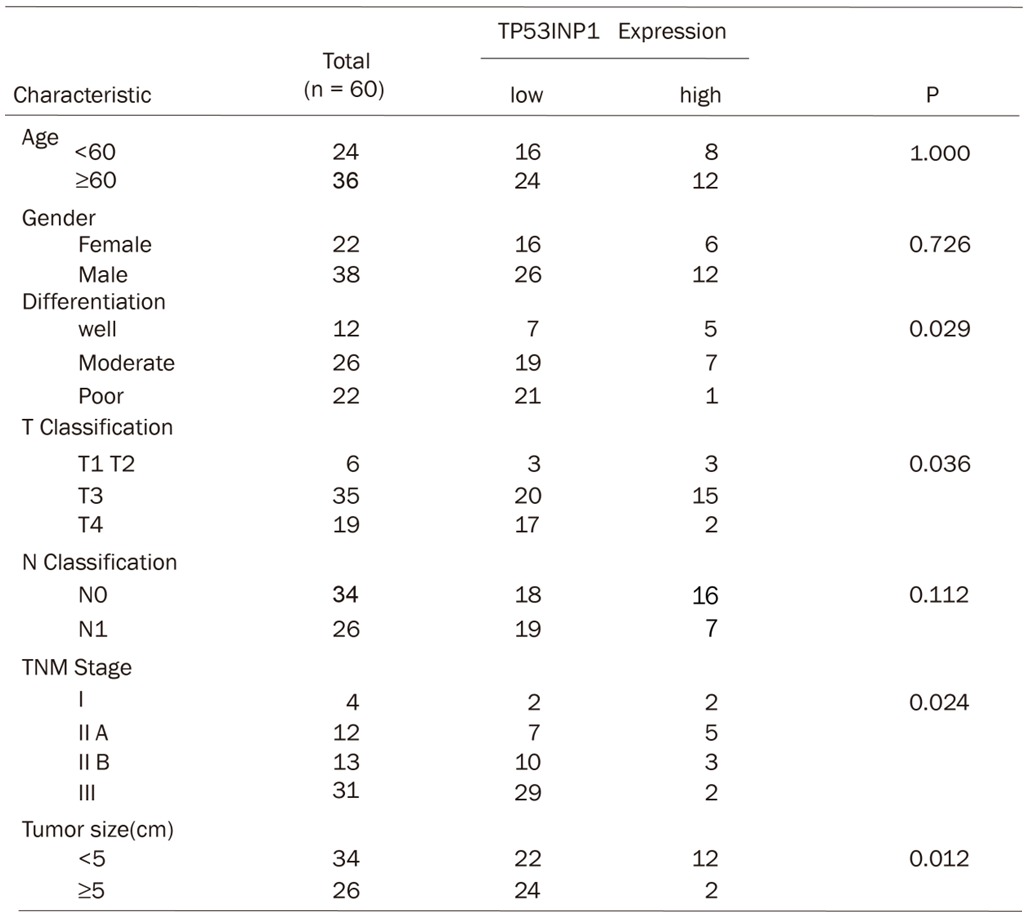
After that, we investigated the relevance of TP53INP1 expression and clinical pathological features. Data analysis showed that TP53INP1 mRNA low expression correlates with tumor grade (P=0.024), tumor differentiation (P=0.029) and tumor size (P=0.012) in ESCC (Table 1). Moreover, the TP53INP1 expression in advanced TNM stage ESCC (III)is lower than in early TNM stage tumor (I, type IIA and IIB) (Table 1). However, TP53INP1 mRNA is not related to the patients’ age and gender.
Table 1.
The correlation of TP53INP1 mRNA level with clinicopathologic features like age, gender, differentiation, tumor size and TNM stage
|
Targeting TP53INP1 is a mechanism of miR-155 on oncomiR in EC-1 cells
To investigate whether miR-155 exerts its oncogenic function by targeting TP53INP1, we examined whether RNA interference (RNAi) knockdown of TP53INP1 expression could recapitulate the oncogenic effects of miR-155 in EC-1 cells. We found that siRNA knockdown of TP53INP1 in EC-1 cells significantly promoted cell proliferation (Figure 4A, 4B) and anchorage-independent growth (Figure 4C). These results indicate that a reduction of TP53INP1 expression can mimic miR-155 in promoting EC-1 cells to proliferate. We then performed rescue experiments to further validate that TP53INP1 targeting is involved in miR-155-mediated oncogenesis in ESCC. MTT assays and BrdU staining assay were used to evaluate the proliferation level of EC-1 cells. Two expression vectors, wtTP53INP1 and mutTP53INP1 (whole TP53INP1 sequence without 3’-UTR) were constructed. Overexpression of TP53INP1 protein greatly suppressed the proliferation of EC-1 cells overexpressing miR-155 (Figure 4D, 4E). This growth inhibition was significantly rescued when mutTP53INP1 was co-transfection with miR-155. As expected, miR-155 acts as an oncomiR in EC-1 cells by down-regulating TP53INP1 expression.
Figure 4.
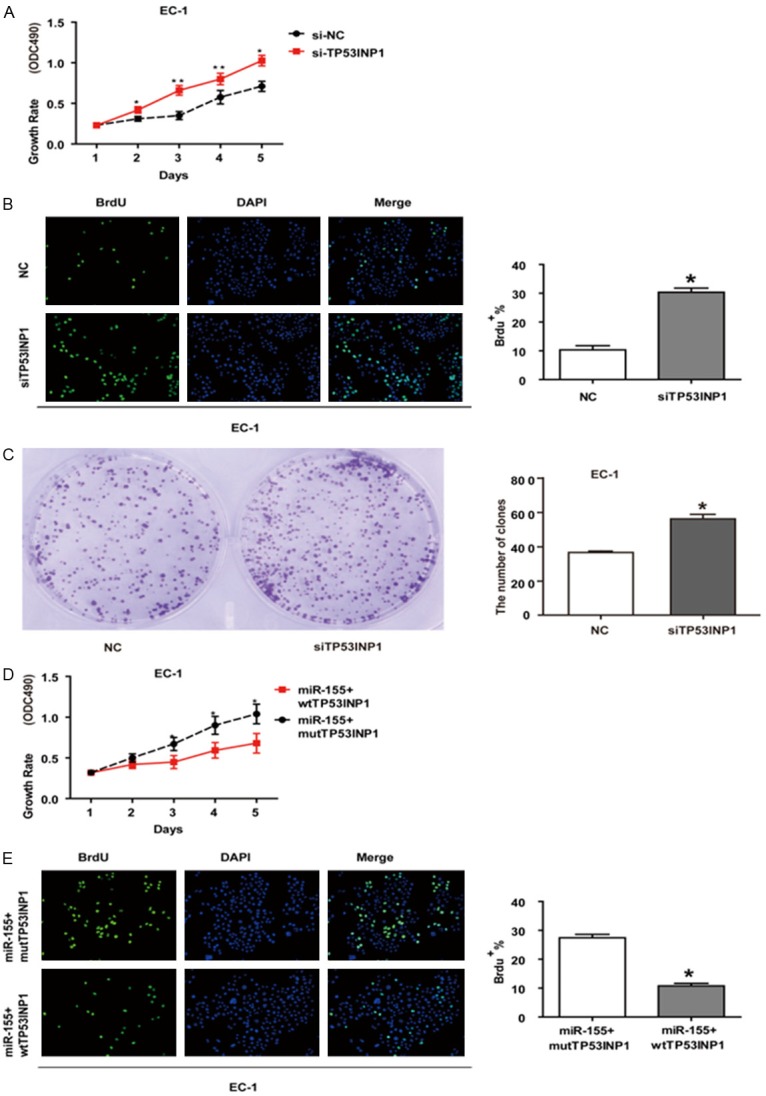
Targeting TP53INP1 is involved in the oncogenic function of miR-155 in EC-1 cells. (A) MTT assay and (B) BrdU staining assay RNAi knockdown of TP53INP1 in EC-1 cells significantly promoted cell proliferation. (C) RNAi knockdown of TP53IINP1 stimulated colonies growth in EC-1 cells. (D) MTT assay, (E) BrdU staining assay showed that TP53INP1 overexprssion inhibits the oncogenic function of miR-155. (*, P<0.05; **, P<0.01).
MiR-155 promotes cell proliferation in vitro and in vivo by targeting TP53INP1
The significant increased expression of miR-155 in ESCC tissues and EC-1 cells prompted us to explore the possible biological significance of miR-155 in tumorigenesis. BrdU staining assay found that 35.9% cells transfected with miR-155 were labeled by BrdU while control group was 20.3%, which verified that miR-155 could promote the proliferation ability of EC-1 cells (Figure 5A).The capacity of colony formation was evaluated on EC-1 cell lines transfected with miR-155. The data indicated that miR-155 significantly stimulated EC-1 cells to grow more and larger colonies on soft agar (Figure 5B).
Figure 5.
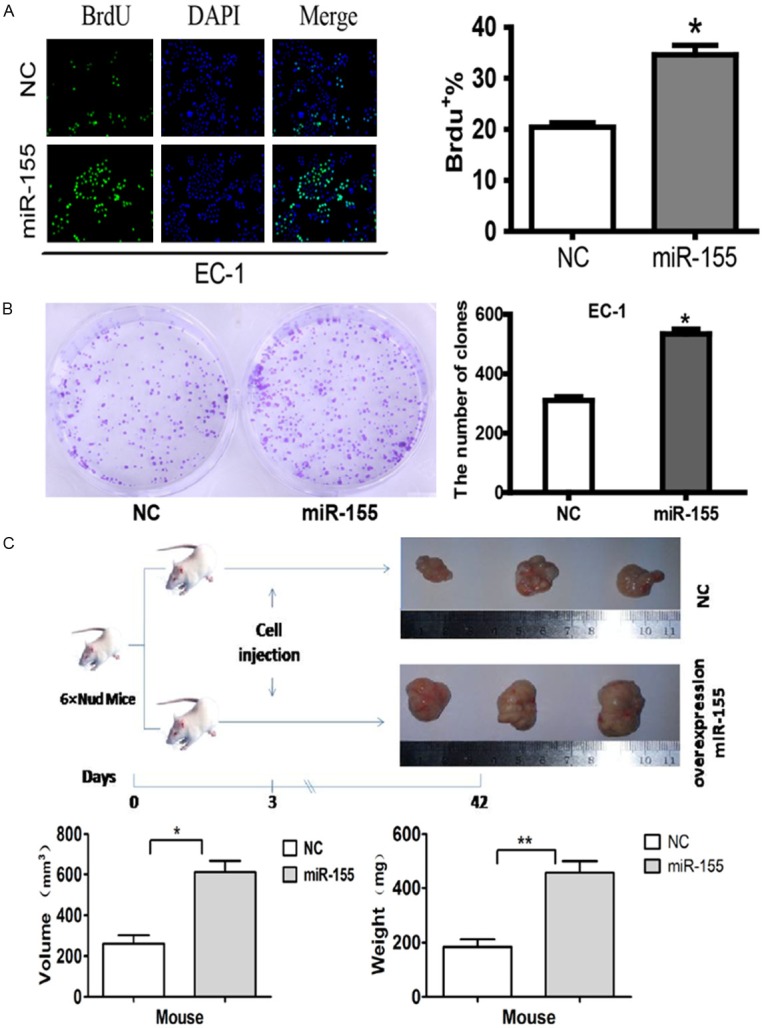
miR-155 promotes colony formation in vitro and tumorigenicity in vivo. A: BrdU staining assay showed that miR-155 promoted proliferation in EC-1 cells. B: miR-155 promotes colonies formation of EC-1 cells. C: miR-155 stimulates tumor growth of EC-1 cells in nude mouse. (*, P<0.05; **, P<0.01).
To further confirm the biological function of miR-155, an in vivo model was used
MiR-155-transfected EC-1 cells were injected into nude mice and produced tumors with mean size of 611.3 ± 100.2 mm3, mean weight of 456.0 ± 77.5 mg, whereas control group produced tumors in the size of 277.0 ± 77.0 mm3 and weight of 184.0 ± 47.8 mg. The size and weight of the former showed significantly difference than the latter with P<0.05 and P<0.01 respectively (Figure 5C).
Discussion
MiRNAs are a class of small non-coding RNAs which inhibit gene expression by either blocking translation or inducing degradation of mRNA. MiR-155 is a classic immuno-related miRNA. In the immune system, miR-155 is unique due to its ability to shape the transcriptome of activated myeloid and lymphoid cells controlling multiple physiological processes ranging from inflammation to immunological memory [9]. Accumulating evidence shows that miR-155 is an oncogenic miRNA. Studies indicate frequent increase of miR-155 in various types of human malignancy, including breast cancer [10], squamous cell lung cancer [11], thyroid tumor [12] and pancreatic tumor [13]. The results demonstrate that miR-155 plays an important role in carcinogenesis.
In this study, we reveal the molecular mechanism for the first time that TP53INP1 decreased as a direct target gene of miR-155 in ESCC. We show that miR-155 directly interacts with 3’-UTR of TP53INP1 and blocks TP53INP1 translation. Then we want to know how does miR-155 function on cells? Decreased TP53INP1 expression has been found in 45 cases of breast carcinoma [14]. The TP53INP1 expression level is inversely related to tumor size, positive lymph node metastasis, high histological grade and aberrant p53 expression. The study has verified that TP53INP1 is a tumor suppressor gene in carcinogenesis. A previous study reports that TP53INP1 expression is repressed by miR-155 in pancreatic ductal adenocarcinoma (PDAC) [13]. Therefore, we focus on the mechanism of TP53INP1 in the p53 pathway. Our study shows that miR-155 acts as an oncogene in ESCC, its expression level is associated with the tumor malignance and overexpression of miR-155 promoted tumor development in vivo. These findings are consistent with previous reports.
MiR-155 has to modulate the expression of target transcript to realize its biological function. Therefore, we predicted the candidate target genes of miR-155 and TP53INP1 gene turned out to be one of the candidates. Data above have confirmed that overexpression of miR-155 led to lower TP53INP1 mRNA level and promoted the EC-1 cells proliferation. In addition, the expression of miR-155 and TP53INP1 showed negative relation in ESCC tissues. Accumulating evidences show that TP53INP1 are downregulated expression in many malignancy including breast cancer, lung cancer and pancreatic cancer [13-15], which is also in agreement with our data. Thus, our studies suggest that miR-155 interact along with TP53INP1 in a regulatory pathway and play a crucial role in tumor development.
TP53INP1 is a proapoptotic stress-induced p53 target gene [8,16]. TP53INP1 acts as an anti-tumoral gene and allows regulation of cell cycle progression and apoptosis, dependently or independently from p53 in the p53-induced apoptotic pathway [17]. P53 pathway is a crucial tumor suppressor pathway, mutations of p53 gene increase with tumor progression in many tumors. P53 is a conserved nuclear transcription factor that regulates the transcription of numerous target genes. P53 protein can inhibit cell cycle, induce apoptosis, and hinder angiogenesis in response to stress or DNA damage. Here we show that TP53INP1 inhibit the proliferation of EC-1 cells. Since TP53INP1 is a direct target gene of miR-155, we propose that miR-155/TP53INP1 might affect cell growth by p53 pathway.
Normal cell cycle is necessary for the cell growth and the disorder cell cycle control may induce unlimited cell proliferation [18,19]. Numerous studies have shown that microRNAs can regulate the expression of protein-coding genes through imperfect base pairing with the 3’-UTR of target miRNAs. The abnormal expression of these proteins causes disordered cell cycle and cell proliferation. Oncogenic miRNAs may facilitate cell cycle entry and progression by targeting related proteins. Our studies reveal that miR-155 acts as an oncogene in ESCC and has important roles in the growth of EC-1 cells by directly targeting TP53INP1. TP53INP1 is involved in p53 pathway. So we assume that miR-155 leads to the dysfunction of p53 pathway through decreasing expression of TP53INP1. The alteration of p53 pathway may disrupt the cell cycle control and drive the cell cycle to G1 progression and S-phase entry.
Acknowledgements
We thank Professor Kui-sheng Chen (Department of Pathology, The University of Zhengzhou, Henan, China) for providing ESCC tissues and cell lines. This research was supported by the Key program for the fundamental research of the science and technology commission of Shanghai under Grant No. 10JC1412800.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Gao Y, Hu N, Han XY, Ding T, Giffen C, Goldstein AM, Taylor PR. Risk factors for esophageal and gastric cancers in Shanxi Province, China: a case–control study. Cancer Epidemiol. 2011;35:e91–e99. doi: 10.1016/j.canep.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong Y, Miao X, Zhang X, Ding F, Luo A, Guo Y, Tan W, Liu Z, Lin D. The role of P53 and MDM2 polymorphisms in the risk of esophageal squamous cell carcinoma. Cancer Res. 2005;65:9582–9587. doi: 10.1158/0008-5472.CAN-05-1460. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nature Reviews Genetics. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo Y, Chen Z, Zhang L, Zhou F, Shi S, Feng X, Li B, Meng X, Ma X, Luo M. Distinctive microRNA profiles relating to patient survival in esophageal squamous cell carcinoma. Cancer Res. 2008;68:26–33. doi: 10.1158/0008-5472.CAN-06-4418. [DOI] [PubMed] [Google Scholar]
- 7.Liu R, Liao J, Yang M, Shi Y, Peng Y, Wang Y, Pan E, Guo W, Pu Y, Yin L. Circulating miR-155 expression in plasma: a potential biomarker for early diagnosis of esophageal cancer in humans. J Toxicol Environ Health A. 2012;75:1154–1162. doi: 10.1080/15287394.2012.699856. [DOI] [PubMed] [Google Scholar]
- 8.Tomasini R, Azizi Samir A, Pebusque MJ, Calvo EL, Totaro S, Dagorn JC, Dusetti NJ, Iovanna JL. P53-dependent expression of the stress-induced protein (SIP) Eur J Cell Biol. 2002;81:294–301. doi: 10.1078/0171-9335-00248. [DOI] [PubMed] [Google Scholar]
- 9.Vigorito E, Kohlhaas S, Lu D, Leyland R. miR-155: an ancient regulator of the immune system. Immunol Rev. 2013;253:146–157. doi: 10.1111/imr.12057. [DOI] [PubMed] [Google Scholar]
- 10.Kong W, He L, Coppola M, Guo J, Esposito NN, Coppola D, Cheng JQ. MicroRNA-155 regulates cell survival, growth, and chemosensitivity by targeting FOXO3a in breast cancer. J Biol Chem. 2010;285:17869–17879. doi: 10.1074/jbc.M110.101055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Raponi M, Dossey L, Jatkoe T, Wu X, Chen G, Fan H, Beer DG. MicroRNA classifiers for predicting prognosis of squamous cell lung cancer. Cancer Res. 2009;69:5776–5783. doi: 10.1158/0008-5472.CAN-09-0587. [DOI] [PubMed] [Google Scholar]
- 12.Nikiforova MN, Tseng GC, Steward D, Diorio D, Nikiforov YE. MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. J Clin Endocrinol Metab. 2008;93:1600–1608. doi: 10.1210/jc.2007-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gironella M, Seux M, Xie MJ, Cano C, Tomasini R, Gommeaux J, Garcia S, Nowak J, Yeung ML, Jeang KT, Chaix A, Fazli L, Motoo Y, Wang Q, Rocchi P, Russo A, Gleave M, Dagorn JC, Iovanna JL, Carrier A, Pébusque MJ, Dusetti NJ. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc Natl Acad Sci U S A. 2007;104:16170–16175. doi: 10.1073/pnas.0703942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito Y, Motoo Y, Yoshida H, Iovanna JL, Takamura Y, Miya A, Kuma K, Miyauchi A. Decreased expression of tumor protein p53-induced nuclear protein 1 (TP53INP1) in breast carcinoma. Anticancer Res. 2006;26:4391–4395. [PubMed] [Google Scholar]
- 15.Jiang PH, Motoo Y, Garcia S, Iovanna JL, Pébusque MJ, Sawabu N. Down-expression of tumor protein p53-induced nuclear protein 1 in human gastric cancer. World J Gastroenterol. 2006;12:691–696. doi: 10.3748/wjg.v12.i5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomasini R, Samir AA, Vaccaro MI, Pebusque MJ, Dagorn JC, Iovanna JL, Dusetti NJ. Molecular and functional characterization of the stress-induced protein (SIP) gene and its two transcripts generated by alternative splicing. SIP induced by stress and promotes cell death. J Biol Chem. 2001;276:44185–44192. doi: 10.1074/jbc.M105647200. [DOI] [PubMed] [Google Scholar]
- 17.Okamura S, Arakawa H, Tanaka T, Nakanishi H, Ng CC, Taya Y, Monden M, Nakamura Y. p53DINP1, a p53-Inducible Gene, Regulates p53-Dependent Apoptosis. Mol Cell. 2001;8:85–94. doi: 10.1016/s1097-2765(01)00284-2. [DOI] [PubMed] [Google Scholar]
- 18.Song L, Wang L, Li Y, Xiong H, Wu J, Li J, Li M. Sam68 up-regulation correlates with, and its down-regulation inhibits, proliferation and tumourigenicity of breast cancer cells. J Pathol. 2010;222:227–237. doi: 10.1002/path.2751. [DOI] [PubMed] [Google Scholar]
- 19.Xia J, Wu Z, Yu C, He W, Zheng H, He Y, Jian W, Chen L, Zhang L, Li W. miR-124 inhibits cell proliferation in gastric cancer through down-regulation of SPHK1. J Pathol. 2012;227:470–480. doi: 10.1002/path.4030. [DOI] [PubMed] [Google Scholar]


