Abstract
Objective: Physiological hypertrophy is featured by the hypertrophy of pre-existing cardiomyocytes and the formation of new cardiomyocytes. C-kit positive cardiac progenitor cells increased their numbers in exercise-induced physiological hypertrophy. However, the participation of Sca-1 positive cells in the physiological adaptation of the heart to exercise training is unclear. Methods: Physiological hypertrophy was induced by swimming and the mRNA levels of GATA binding protein 4 (GATA4), atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), endogenous hepatocyte growth factor (HGF), and insulin like growth factor-1 (IGF-1) from the whole heart were determined by real-time polymerase chain reactions (RT-PCRs) analysis. Immunofluorescent staining was used to compare the number of C-kit and Sca-1 positive cardiac progenitor cells. In addition, mRNA levels of C-kit and Sca-1 in left ventricle (LV), right ventricle (RV), and outflow tract (OFT) were determined in mice swimming for 7, 14, and 21 days by RT-PCRs. Results: The ratio of heart weight (HW) to body weight and HW to tibia length and the mRNA level of GATA4 were increased while mRNA levels of ANP and BNP remained unchanged. C-kit and Sca-1 positive cardiac progenitor cells were activated by swimming training. An increased endogenous production of HGF and IGF was observed at least at the mRNA level. Swimming induced a significant up-regulation of C-kit in LV of mice swimming for 1, 2 and 3 weeks and in RV of mice swimming for 3 weeks. Sca-1 positive cardiac progenitor cells were increased in LV and OFT in mice swimming for 3 weeks. Conclusion: This study presents that swimming-induced physiological hypertrophy initiates activation of cardiac progenitor cells.
Keywords: Exercise, hypertrophy, physiological, cardiac progenitor cells
Introduction
Traditionally accepted paradigm has been that the heart is a post-mitotic organ with no regenerative capacity to compensate for cardiomyocytes losses occurring in heart failure and many other cardiac diseases [1]. However, burgeoning evidence has indicated that the adult heart maintains endogenous regenerative capacity to some extent, producing new cardiomyocytes either from progenitor/stem cells or pre-existing cardiomyocytes [2-4]. By utilizing carbon-14 dating, it is reported that cardiomyocytes were renewed at a rate of 1% and 0.45% per year at the age of 25 and 75, respectively, in human [2]. In other words, half of cardiomyocytes are replaced during a normal human lifespan [2].
The heart responses to the physiological stimuli, such as exercise in a form called physiological hypertrophy [5,6]. Unlike pathological hypertrophy that ultimately decompensate to cardiovascular diseases such as heart failure and arrhythmia [7-9], physiological hypertrophy is an adaptive beneficial response [10]. Physiological hypertrophy is featured by the hypertrophy of pre-existing cardiomyocytes and the formation of new cardiomyocytes [10]. The source of newly formed cardiomyocyte might be pre-existing cardiomyocytes and cardiac progenitor cells, requiring using the myocyte-restricted lineage tracing studies to unravel this question [5,10-12].
Cardiac ischemia-reperfusion injury and pregnancy have been reported to initiate time-dependent and robust signs of up-regulation of cardiac progenitor cells [13]. In addition, a recent study has indicated that C-kit positive cardiac stem cells increased their numbers in exercise-induced physiological hypertrophy [12], however, the participation of other cardiac stem cell-like population especially Sca-1 positive cells in the physiological adaptation of the heart to exercise training is unclear [14]. Moreover, whether the activation of cardiac progenitor cells is focal or global throughout the myocardium is also unknown.
Materials and methods
This study was approved by the local ethical committees and all animal experiments were conducted under the guidelines on humane use and care of laboratory animals for biomedical research published by National Institutes of Health (No. 85-23, revised 1996).
Swimming-induced physiological hypertrophy model
C57/BL6 male mice aged 10-12 weeks, purchased from Shanghai SLAC Laboratory Animal CO. LTD were used in this study. Mice swam in a ramp protocol starting from 10 min twice a day, with an increment of 10 min per day until 90 min twice per day was reached. The mice were checked all the time to avoid submerging under the water surface. After 21 days, the swimming mice were sacked and the body weight, heart weight and tibia length were determined and compared with age-matched sedentary controls. The whole heart tissues except the apex were snap-frozen in liquid nitrogen and kept in minus 80°C until RNA isolation. The apex of the heart was frozen in OCT in liquid nitrogen and sectioned at 10 μm.
To explore the time and local dependent effects, the hearts from the swimming and sedentary controls from another cohort were harvested at day 7, 14, and 21. The hearts were kept cold on dry-ice and divided into three different regions: left ventricle (LV), right ventricle (RV), and outflow tract (OFT, defined as the outflow tract of the right ventricle).
Quantitative real-time polymerase chain reactions (RT-PCRs) analysis
Total RNA were isolated from cardiac tissues as indicated using RNeasy Mini Kit (QIAGEN) according to the manufacturer’s instruction. Real-time PCRs with SYBR Green I, which was validated with respect to reproducibility and linearity within the measuring range, was performed in quadruplicate using the 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA), and SYBR Green PCR Master Mix (Bio-Rad, Hercules, CA, USA) as reagent. The cycling parameters were as follows: denaturation at 94°C for 1 min; annealing at 55-60°C for 1 min (depending on the primer); and elongation at 72°C for 1 min (40 cycles). β-actin was used for normalization. Primer sequences (forward and reverse) are as follows: atrial natriuretic peptide (ANP), AGGCAGTCGATTCTGCTTGA and CGTGATAGATGAAGGCAGGAAG; brain natriuretic peptide (BNP), TAGCCAGTCTCCA GAGCAATTC and TTGGTCCTTCAAGAGCTGTCTC; GATA binding protein 4 (GATA4), CCCTACCCAGCCTACATGG and ACATATCGAGATTGGGGTGTCT; endogenous hepatocyte growth factor (HGF), ATGTGGGGG ACCAAACTTCTG and GGATGGCGA CATGAAGCAG; insulin like growth factor-1 (IGF-1), GTGGGGGCTCGT GTTTCTC and GATCACCGTGCAGTT TTCCA; and C-kit, GAATCTCCGAAGAGGCCAGAA and GC TGCAACAGGGGGTAACAT; Sca-1, ATGTC GGATTTTGACAGCAACC and GTCCCG GTGGCACATTTCT; and β-actin, ATCGCTGACAGGATGCAGAA and CAGGAGGAG CAATGATCTTGA.
Immunofluorescent staining
Frozen sections (10 μm thick) were mounted on Superfrost Plus slides (Shitai, China) and were postfi xed with 4% paraformaldehyde dissolved in 0.1 M phosphate buffer (pH=7.4) for at least 15 minutes. Sections were immersed in 10% goat serum for 1 hour after washed with phosphate buffer saline (PBS) for three times. After that, sections were incubated overnight at 4°C with rabbit polyclonal anti-c-kit (Abcam, ab5506), which was diluted by 1:100 in 1 × PBS with 0.25% Triton X-100. After that, sections were incubated to goat anti-rabbit labeled with rhodamine secondary antibodies (Santa Cruz, sc-362262) diluted by 1:200 in phosphate buffer for 1 hour. Finally, the sections were stained with DAPI (ProLong® Gold, Life technology). The same protocol was used in Rat monoclonal to Sca-1 (Abcam, ab51317, 1:100) and goat anti-rat labeled with FITC (Santa Cruz, sc-2011). Slides (n=4) from each heart, 6 hearts from each group were counted at 40 × magnification using confocal laser scanning microscope (LSM 710, Carl Zeiss MicroImaging GmbH, Germany). All imaging was performed in a blinded way whereby at least 20 random images were obtained from each slide and expressed as number/106 myocytes.
Statistical analysis
For the analysis of qRT-PCR data, the relative expression level for each mRNA was calculated using the 2-ΔΔCt method. The data were presented as the mean ± SE. An independent-samples t-test was conducted to evaluate the one-way layout data. Non-parametric (Mann-Whitney U) test was employed to calculate the difference between two independent groups when appropriate. All analyses were performed using SPSS 17.0, and all statistical tests were two-sided. P values less than 0.05 were considered to be statistically significant.
Results
Swimming induces physiological hypertrophy in mice
Figure 1A showed that the ratio of heart weight (HW)/body weight (BW) was significantly increased by swimming. To exclude the effects of weight loss by exercise, the ratio of HW to tibia length (TL) was also determined and was found to be increased in swimming mice (Figure 1B), indicating that cardiac hypertrophy was induced in this ramp swimming exercise mouse model as expected. In addition, the mRNA level of a common hypertrophy marker GATA-4 was also found to be elevated in swimming mice (Figure 1C), further confirming that hypertrophy occurred in these mice. To exclude the occurrence of pathological hypertrophy, mRNA levels of ANP and BNP were determined. Both genes were not changed in swimming mice (Figure 1D and 1E), indicating that healthy physiological hypertrophy other than pathological one was induced in these swimming mice.
Figure 1.
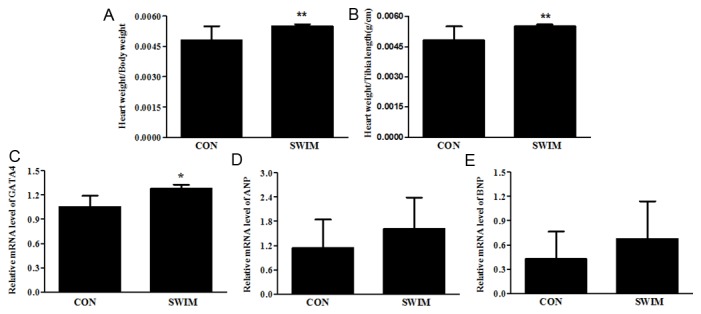
Physiological hypertrophy is induced by swimming. (A) Heart weight/body weight (B) Heart weight/tibia length (C) GATA binding protein 4 (GATA4) mRNA level (D) Atrial natriuretic peptide (ANP) mRNA level (E) Brain natriuretic peptide (BNP) mRNA level. *, Compared to the control group, P less than 0.05. **, Compared to the control group, P less than 0.01. n=6 per group. CON, control; SWIM, swimming.
Swimming-induced physiological hypertrophy initiates activation of cardiac progenitor cells
As C-kit positive and Sca-1 positive cells are two major types of cardiac progenitor cells [5,15,16], double-blinded semi-quantitative immunofluorescent staining analyses of C-kit and Sca-1 positive cells were performed. As shown in Figure 2, swimming activates C-kit and Sca-1 positive cardiac progenitor cells, which might partly contributes to the newly formed cardiomyocytes and cardiac protective effects during exercise.
Figure 2.
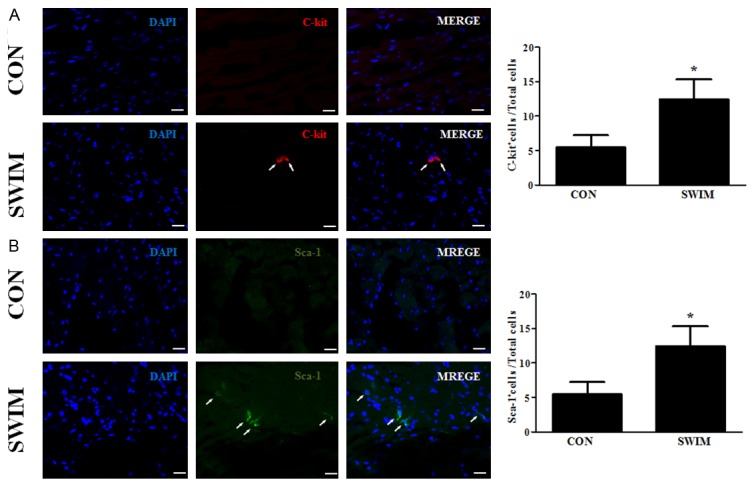
Swimming initiates activation of cardiac progenitor cells. Swimming activates C-kit (A) and Sca-1 (B) positive cardiac progenitor cells. *, Compared to the control group, P less than 0.05. **, Compared to the control group, P less than 0.01. n=6 per group. CON, control; SWIM, swimming.
HGF and IGF-1 are up-regulated in swimming-induced physiological hypertrophy
As increased endogenous production of HGF and IGF have been reported to be involved in the activation of progenitor cells during cardiac ischemia-reperfusion [13], we sought to determine whether these two factors might also contribute to the activation of cardiac progenitor cells as observed in this study. We found that at least at mRNA levels, HGF and IGF were significant increased (Figure 3), indicating that they might also play a similar role for the activation of progenitor cells as in cardiac ischemia-reperfusion.
Figure 3.
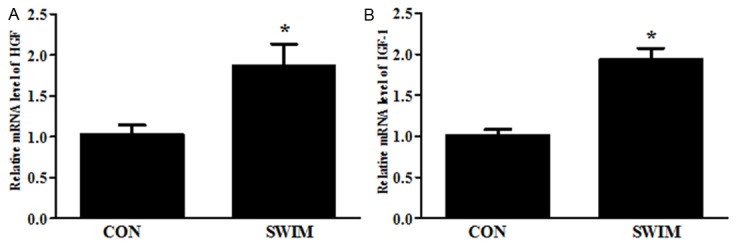
Swimming up-regulates mRNA levels of endogenous hepatocyte growth factor (HGF) and insulin like growth factor-1 (IGF-1). HGF (A) and IGF-1 (B) mRNA levels. *, Compared to the control group, P less than 0.05. n=6 per group. CON, control; SWIM, swimming.
A time-dependent activation of cardiac progenitor cells is induced by swimming
Swimming induced a significant up-regulation of C-kit in LV of mice swimming for 1, 2 and 3 weeks (Figure 4A). In addition, the increase of C-kit was also observed in RV of mice swimming for 3 weeks (Figure 4A). However, no significant changes occurred in OFT (Figure 4A). As for Sca-1, it was increased in LV and OFT in mice swimming for 3 weeks (Figure 4B). These data indicate that a time-dependent activation of cardiac progenitor cells is induced by swimming.
Figure 4.
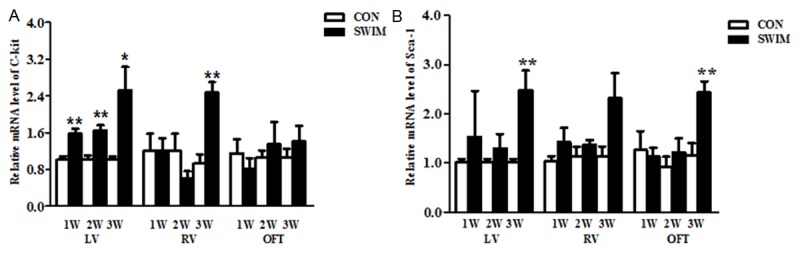
Swimming induces a time-dependent activation of cardiac progenitor cells. C-kit (A) and Sca-1 (B) mRNA levels in left ventricle (LV), right ventricle (RV), and outflow tract (OFT) of mice swimming for 7, 14, and 21 days. *, Compared to the control group, P less than 0.05. **, Compared to the control group, P less than 0.01. n=6 per group. CON, control; SWIM, swimming.
Discussion
Adult mammalian heart maintains a considerable capacity for cardiomyocyte renewal though this limited regenerative capacity is not sufficient to restore contractile function after substantial cardiac injury [1,11,17]. Myocardial regeneration by means of promoting the endogenous regenerative processes in situ is an attractive approach as it avoids the issues with engraftment and immune rejection in stem cell implantation [1,11]. Besides that, it can also potentially provide a noninvasive therapy [1,11]. Thereafter, the development of strategies to stimulate the endogenous regenerative capacity of the heart might be a rational alternative to cell transplantation for cardiomyocyte replacement after cardiac injury [1,11,18].
The heart can undergo an adaptive form of cardiac hypertrophy called physiological hypertrophy, which is fundamentally different from pathological hypertrophy [6,19,20]. Unlike pathological hypertrophy, physiological hypertrophy induced by exercise does not develop disease and is usually beneficial in healthy individuals [19]. Exercise-induced physiological hypertrophy has been consistently proven providing protection against myocardial infarction and myocardial ischemia-reperfusion injury in animal models [10,21-23]. Thus, physiological hypertrophy is generally accepted to be protective for pathological cardiac remodeling [10]. Besides the hypertrophy of pre-existing cardiomyocytes, physiological hypertrophy is also accompanied by new cardiomyocytes formation in vivo though to what extent they contribute to the formation of physiological hypertrophy and the corresponding cardiac protective effects is largely unknown [10]. C-kit and Sca-1 positive cardiac stem cells are two major types of cardiac stem cells within the heart [12,14,18]. Adult C-kit positive cardiac stem cells have been shown to be necessary and sufficient for functional cardiac regeneration and repair [18]. In addition, Sca-1 positive cardiac stem cells have also been indicated as a source of myocardial renewal in the murine adult heart [14]. C-kit positive cardiac stem cells have been reported to be activated in response to treadmill exercise training [12]. The present study adds the evidence that C-kit positive cardiac stem cells are activated by swimming exercise training. Besides that, to the best of knowledge, here we firstly show that Sca-1 positive cardiac stem cells are activated by swimming exercise training. Collectively, the present study shows that exercise-induced physiological hypertrophy initiates activation of cardiac progenitor cells.
However, how cardiac progenitor cells get activated during exercise is nuclear, though it might be related to the increased endogenous production of HGF and IGF as observed in the present study. Dissecting the molecular mechanisms for the activation of cardiac progenitor cells during exercise, particularly development of means to enhance the endogenous cardiac progenitor cell activity, will pave the way for establishing novel effective therapies, encompassing heart regeneration for a large spectrum of cardiovascular diseases [1,11].
In conclusion, this study presents that swimming-induced physiological hypertrophy initiates activation of cardiac progenitor cells. Better understanding of the mechanisms controlling the activation of cardiac progenitor cells could be of significant clinical value in treating cardiac pathological remodeling.
Acknowledgements
This work was supported by the grants from National Natural Science Foundation of China (81200169 to J. Xiao; 81270314 to J. Xu), Innovation Program of Shanghai Municipal Education Commission (13YZ014 to J. Xiao), Foundation for University Young Teachers by Shanghai Municipal Education Commission (year 2012, to J. Xiao), Innovation Foundation of Shanghai University (sdcx2012038, to J. Xiao), and partially by Leading Academic Discipline Project of Shanghai Municipal Education Commission “Molecular Physiology” and Shanghai Municipal Science and Technology Committee (13DZ2272100).
Disclosure of conflict of interest
None declared.
References
- 1.Rosenzweig A. Medicine. Cardiac regeneration. Science. 2012;338:1549–1550. doi: 10.1126/science.1228951. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellison GM, Waring CD, Vicinanza C, Torella D. Physiological cardiac remodelling in response to endurance exercise training: cellular and molecular mechanisms. Heart. 2012;98:5–10. doi: 10.1136/heartjnl-2011-300639. [DOI] [PubMed] [Google Scholar]
- 6.Fu S, Zhuo R, Yao M, Zhang J, Zhou H, Xiao J. MicroRNA basis of physiological hypertrophy. Front Genet. 2013;4:253. doi: 10.3389/fgene.2013.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubois-Deruy E, Belliard A, Mulder P, Chwastyniak M, Beseme O, Henry JP, Thuillez C, Amouyel P, Richard V, Pinet F. Circulating plasma serine(208) -phosphorylated troponin T levels are indicator of cardiac dysfunction. J Cell Mol Med. 2013;17:1335–1344. doi: 10.1111/jcmm.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosello-Lleti E, Alonso J, Cortes R, Almenar L, Martinez-Dolz L, Sanchez-Lazaro I, Lago F, Azorin I, Juanatey JR, Portoles M, Rivera M. Cardiac protein changes in ischaemic and dilated cardiomyopathy: a proteomic study of human left ventricular tissue. J Cell Mol Med. 2012;16:2471–2486. doi: 10.1111/j.1582-4934.2012.01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu X, Wang H, Liu F, Chen L, Luo W, Su P, Li W, Yu L, Yang X, Cai J. Identification of micro-RNA networks in end-stage heart failure because of dilated cardiomyopathy. J Cell Mol Med. 2013;17:1173–1187. doi: 10.1111/jcmm.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bostrom P, Mann N, Wu J, Quintero PA, Plovie ER, Panakova D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, Spiegelman BM. C/EBPbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mann N, Rosenzweig A. Can exercise teach us how to treat heart disease? Circulation. 2012;126:2625–2635. doi: 10.1161/CIRCULATIONAHA.111.060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waring CD, Vicinanza C, Papalamprou A, Smith AJ, Purushothaman S, Goldspink DF, Nadal-Ginard B, Torella D, Ellison GM. The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur Heart J. 2013 doi: 10.1093/eurheartj/ehs338. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genead R, Fischer H, Hussain A, Jaksch M, Andersson AB, Ljung K, Bulatovic I, Franco-Cereceda A, Elsheikh E, Corbascio M, Smith CI, Sylven C, Grinnemo KH. Ischemia-reperfusion injury and pregnancy initiate time-dependent and robust signs of up-regulation of cardiac progenitor cells. PLoS One. 2012;7:e36804. doi: 10.1371/journal.pone.0036804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida S, De Gaspari P, Kostin S, Jenniches K, Kilic A, Izumiya Y, Shiojima I, Grosse Kreymborg K, Renz H, Walsh K, Braun T. Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem Cell Reports. 2013;1:397–410. doi: 10.1016/j.stemcr.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song D, Li Y, Cao J, Han Z, Gao L, Xu Z, Yin Z, Wang G, Fan Y, Wang C. Effect of iron deficiency on c-kit(+) cardiac stem cells in vitro. PLoS One. 2013;8:e65721. doi: 10.1371/journal.pone.0065721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen Z, Mai Z, Zhang H, Chen Y, Geng D, Zhou S, Wang J. Local activation of cardiac stem cells for post-myocardial infarction cardiac repair. J Cell Mol Med. 2012;16:2549–2563. doi: 10.1111/j.1582-4934.2012.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagai T, Matsuura K, Komuro I. Cardiac side population cells and Sca-1-positive cells. Methods Mol Biol. 2013;1036:63–74. doi: 10.1007/978-1-62703-511-8_5. [DOI] [PubMed] [Google Scholar]
- 18.Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, Henning BJ, Stirparo GG, Papait R, Scarfo M, Agosti V, Viglietto G, Condorelli G, Indolfi C, Ottolenghi S, Torella D, Nadal-Ginard B. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827–842. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 19.Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol. 2013;14:38–48. doi: 10.1038/nrm3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das S, Aiba T, Rosenberg M, Hessler K, Xiao C, Quintero PA, Ottaviano FG, Knight AC, Graham EL, Bostrom P, Morissette MR, del Monte F, Begley MJ, Cantley LC, Ellinor PT, Tomaselli GF, Rosenzweig A. Pathological role of serum- and glucocorticoid-regulated kinase 1 in adverse ventricular remodeling. Circulation. 2012;126:2208–2219. doi: 10.1161/CIRCULATIONAHA.112.115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farah C, Kleindienst A, Bolea G, Meyer G, Gayrard S, Geny B, Obert P, Cazorla O, Tanguy S, Reboul C. Exercise-induced cardioprotection: a role for eNOS uncoupling and NO metabolites. Basic Res Cardiol. 2013;108:389. doi: 10.1007/s00395-013-0389-2. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson CK, Lambert JP, Chow CW, Lefer DJ, Calvert JW. Chronic exercise downregulates myocardial myoglobin and attenuates nitrite reductase capacity during ischemia-reperfusion. J Mol Cell Cardiol. 2013;64:1–10. doi: 10.1016/j.yjmcc.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvert JW, Condit ME, Aragon JP, Nicholson CK, Moody BF, Hood RL, Sindler AL, Gundewar S, Seals DR, Barouch LA, Lefer DJ. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of beta(3)-adrenergic receptors and increased nitric oxide signaling: role of nitrite and nitrosothiols. Circ Res. 2011;108:1448–1458. doi: 10.1161/CIRCRESAHA.111.241117. [DOI] [PMC free article] [PubMed] [Google Scholar]


