Abstract
Upper urinary tract urothelial carcinomas (UUTUC) are infrequent and show an occurrence of about 5-10% of all urothelial carcinomas. In this study, we investigated the HER2 status of 171 UUTUC patients with nephroureterectomy. The number of patients is the largest of any HER2 study. All 171 cases were analyzed for both HER2 overexpression using immunohistochemistry and HER2 gene amplification using dual-color in situ hybridization. The scoring system proposed by the ASCO/CAP and ToGA trials was used. Out of 171 patients, 140 patients had a HER2 score-0 or score-1 (81.9%), 17 a score-2 (9.9%), and 14 a score-3 (8.2%) with immunohistochemistry. HER2 gene amplification was observed in 31 out of 171 cases (18.1%). A good correlation was observed between protein overexpression and gene amplification (p<0.0001). Twenty-three UUTUC (13.5%) were determined as HER2-positive cancer according to ASCO/CAP and ToGA criteria. HER2 positivity in patients over 70 years old was higher than that of patients under 70 years old (p=0.0132). HER2 expression correlated to a high histological grade (p=0.0003) and the coexistence of a high grade carcinoma in situ (p=0.0089). No HER2-positive cancer was observed in patients with renal pelvic UUTUC (0 out of 76, p<0.0001). HER2-positive UUTUC showed a shorter recurrence time in the residual urinary bladder after nephroureterectomy with Kaplan-Meier analysis (p=0.0284) and multivariate analysis (p=0.0034). The results suggest that HER2 positivity in UUTUC is an independent predictive marker for early recurrence of urothelial carcinoma in the residual urinary bladder after surgery.
Keywords: HER2, immunohistochemistry, DISH, urothelial carcinoma, upper urinary tract, tissue microarray
Introduction
Upper urinary tract urothelial carcinomas (UUTUC) are infrequent and have an occurrence of about 5-10% of all urothelial carcinomas [1-5]. Compared with bladder carcinomas, UUTUC show a less favorable prognosis because of late presentation and/or diagnosis [6]. Chemotherapy has been considered effective for UUTUC [7,8], but overall survival of patients with advanced UUTUC has not significantly improved despite advancements in chemotherapy regimens in recent decades [9,10]. Combination chemotherapy consisting of weekly paclitaxel and gemcitabine plus cisplatin resulted in higher response rates to treatment, but did not provide significant overall survival rates because of increased hematological toxicity [11]. The identification of molecular targeted therapy is an important step to provide treatments that are better than systemic toxic chemotherapy.
Human epidermal growth factor receptor type 2 (HER2) is located on chromosome 17q21 and is a transmembrane tyrosine kinase growth factor receptor [12]. It consists of a growth factor binding ectodomain, a single transmembrane segment, an intracellular protein-tyrosine kinase catalytic domain, and a tyrosine-containing cytoplasmic tail. The genes for the four members of this family, HER1-HER4, are found on different human chromosomes [13]. HER1-HER4 are associated with cell development, proliferation and differentiation, protein overexpression, and/or gene amplification associated with a poor prognosis, rapid progression and metastasis in several types of carcinomas [14]. HER2 protein overexpression and gene amplification are observed in various malignancies, such as breast, ovary, stomach, colon, small intestine, and lung cancers [15-19].
HER2 protein overexpression and/or gene amplification are investigated routinely for almost all breast carcinomas and for chemotherapy-resistant advanced gastric cancers. The molecular targeted therapies for HER2 are performed in neoadjuvant, adjuvant and/or metastatic settings in both breast and gastric cancers, and the effects have been reported to be favorable [18,20,21].
There are many previous studies examining HER2 overexpression and/or gene amplification in urothelial carcinomas of the urinary bladder [22-29], but only four using systematic analysis have investigated UUTUC [30-33]. Several reports on urothelial carcinomas have yielded conflicting results, with extensive variability in the incidence rates of HER2 overexpression, ranging between 0 and 89%, and HER2 gene amplification, ranging between 0 and 59% [22-48]. One paper reported no strong association between HER2 protein overexpression and gene amplification [34]; however, HER2 protein overexpression and gene amplification has been reported to correlate well in both breast and gastric cancers [18,49].
With variability in the information available, the aim of this study was to clarify the HER2 status of UUTUC, such as protein overexpression and gene amplification, and examine for any correlations and relationships with clinical outcomes using 171 UUTUC patients.
Materials and methods
Study population
One hundred and seventy-one patients with UUTUC (76 of pelvis, 49 of ureter and 46 of both ureter and pelvis) (119 men and 52 women) who underwent nephroureterectomy at The University of Tokyo Hospital between 1996 and 2012 were included in the study (Table 1). No patient received neoadjuvant chemotherapy. In 31 cases, bladder cancer was found and treated before or at the time of nephroureterectomy.
Table 1.
Correlations between HER2 positivity and clinicopathological features in UUTUC patients who underwent nephroureterectomy (n=171)
| HER2 status | p-value | |||
|---|---|---|---|---|
|
|
||||
| N | Positive | Negative | ||
| Total | 171 | 23 (13.5%) | 148 (86.5%) | |
| Gender | ||||
| Male | 119 | 14 (11.8%) | 105 (8.2%) | p=0.3284 |
| Female | 52 | 9 (17.3%) | 43 (82.7%) | |
| Age | ||||
| Under 70 | 93 | 7 (7.5%) | 86 (92.5%) | p=0.0132 |
| Over 70 | 78 | 16 (20.5%) | 62 (79.5%) | |
| Side | ||||
| Right | 85 | 13 (15.3%) | 72 (84.7%) | p=0.4823 |
| Left | 86 | 10 (11.6%) | 76 (88.4%.) | |
| Previous bladder tumor | ||||
| Present | 31 | 7 (22.6%) | 24 (77.4%) | p=0.0996 |
| Absent | 140 | 16 (11.4%) | 124 (88.6%) | |
| Tumor Stage | ||||
| pTa, pTis, pT1 | 75 | 7 (9.3%) | 68 (90.7%) | p=0.1630 |
| pT2-pT4 | 96 | 16 (16.7%) | 80 (83.3%) | |
| Location of tumor | ||||
| Ureter | 49 | 9 (18.4%) | 40 (81.6%) | p<0.0001 |
| Pelvis | 76 | 0 (0%) | 76 (100%) | |
| Both | 46 | 14 (30.4%) | 32 (69.6%) | |
| Tumor growth | ||||
| Papillary | 126 | 15 (11.9%) | 111 (88.1%) | p=0.3216 |
| Sessile | 45 | 8 (17.8%) | 37(82.2%) | |
| Histological grade | ||||
| Grade 3 | 97 | 21 (21.6%) | 76 (78.4%) | p=0.0003 |
| Non-Grade 3 | 74 | 2 (2.7%) | 72 (97.3%) | |
| Lymphovascular invasion | ||||
| present | 74 | 13 (17.6%) | 61 (82.4%) | p=0.1681 |
| absent | 97 | 10 (10.3%) | 87 (89.7%) | |
| Co-existence of HGCIS | ||||
| Present | 83 | 17 (20.5%) | 66 (79.5%) | p=0.0089 |
| Absent | 88 | 6 (6.8%) | 17 (93.2%) | |
| Lymph node metastasis | ||||
| Present | 19 | 3 (15.8%) | 16 (94.2%) | p=0.7513 |
| Absent | 152 | 20 (13.2%) | 132 (86.8%) | |
All research protocols were approved by the institutional review board at The University of Tokyo Hospital.
Histopathological evaluation
Each operative specimen was fixed in 10% formalin and embedded in paraffin. All cases with hematoxylin and eosin-stained sections were examined by a certified surgical pathologist (T.M.) unaware of clinical outcome data. Tumor histology and grade were defined by the World Health Organization/International Society of Urologic Pathology consensus classification [50,51]. Staging of the tumors was performed according to the TNM classification [50]. All cases were urothelial carcinomas. Lymphovascular invasion was examined by hematoxylin and eosin staining and Elastica van Gieson staining.
Immunohistochemical analysis and dual-color in situ hybridization analysis
Specimens were processed using a manual tissue microarray (TMA; Beecher Instruments, Silver Spring, MD, USA). Two pieces of 2-mm tissue core, larger than that widely used (0.6-mm), were selected from representative tumor areas and transferred to each TMA block to assess for protein and gene expression [51,52]. Eight TMA blocks were constructed in total. Immunohistochemistry for HER2 were performed using PATHWAY HER-2/neu (clone 4B5) primary antibody (Roche Ventana Medical Systems Inc., Tokyo, Japan) as per the standardized Ventana PATHWAY HER2/neu protocol using a BenchMark Autoimmune Stainer (Roche Ventana Medical Systems Inc., Tokyo, Japan). Staining was performed on 4-μm tissue microarray sections, and membrane staining was evaluated. Protein expression was scored according to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines [49] and the results of the Trastuzumab for Gastric Cancer (ToGA) trial [18] of HER2 staining, defined as score-0 (no staining), score-1 (incomplete membrane staining), score-2 (complete but weak membrane staining in >10% of tumor cells) and score-3 (intense membrane staining in >30% of tumor cells) [49]. Immunoreactivity was evaluated by a certified pathologist (T.S.) blind to other data.
HER2 gene amplification was examined using dual-color in situ hybridization (DISH). Dual-color means black and red colors, that is, silver precipitation is deposited in the nuclei, and single copies of the HER2 gene are visualized as single black dots (metallic silver signals) and single copies of chromosome 17 (CEP17) as red dots (alkaline-phosphatase) on the same slide. All DISH steps were performed using an automatic Ventana BenchMark XT (Roche Ventana Medical Systems Inc.). Slides were then counterstained with hematoxylin. The numbers of CEP17 and HER2 signals were counted in 20 or 40 non-overlapping nuclei per core by conventional bright-field microscopy (Olympus 51BX, Olympus Japan, Tokyo, Japan). Only cells on which at least two CEP17 reference probe signals could be identified on a section were included for review. HER2 gene amplification was examined in all 171 cases by DISH. We defined a HER2/CEP17 ratio >2.2 as HER2 gene amplified UUTUC according to the ASCO/CAP recommendation for evaluation of gene amplification of breast cancer [18]. A single certified pathologist (T.S.) evaluated each microarray specimen to determine HER2 gene amplification.
Statistical analysis
All statistical analyses were performed using SAS software (version 9.3, SAS Institute, Cary, NC, USA). All p-values were two-sided. Differences were considered significant at p<0.05. For categorical data, the chi-square test was performed. Kaplan-Meier and log-rank tests were used for survival analysis. To control for confounding variables, multivariate Cox proportional hazards regression models were used. The multivariate models included gender, age at diagnosis, tumor side, tumor location, tumor architecture, lymphovascular invasion, concomitant high grade carcinoma in situ (HGCIS), tumor stage (pTa-pT2 vs. pT3-pT4), and lymph node metastasis.
Results
HER2 protein overexpression and gene amplification in UUTUC
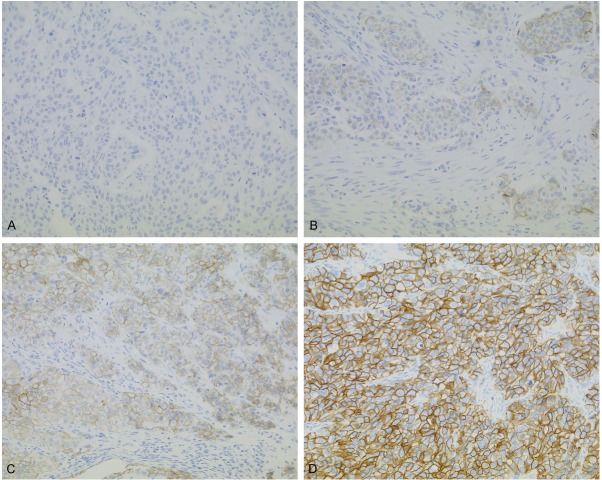
Immunohistochemical staining patterns of HER2 are shown in Figure 1. Out of 171 patients, 140 showed score-0 or score-1 (81.9%), 17 showed score-2 (9.9%), and 14 showed score 3 (8.2%) with immunohistochemistry. Gene amplification was observed in 31 (18.1%) out of 171 cases (Figure 2). A good correlation between HER2 protein overexpression and gene amplification was observed (p<0.0001, Table 2). Twenty-three patients were determined to be HER2-positive UUTUC (Table 2) according to ASCO/CAP and ToGA recommendations [18,49].
Figure 1.
HER2 expression using immunohistochemistry in UUTUC. A: Negative for HER2 was score-0. B: Weakly positive for HER2 was score-1. Weak staining was categorized as negative. C: Moderately positive for HER2 was score-2. Gene examination using DISH or FISH is recommended for score-2 in UUTUC, and group was divided into HER2-positive or negative urothelial carcinomas. D: Strongly positive for HER2 was score-3. A-D: Magnification ×200.
Figure 2.
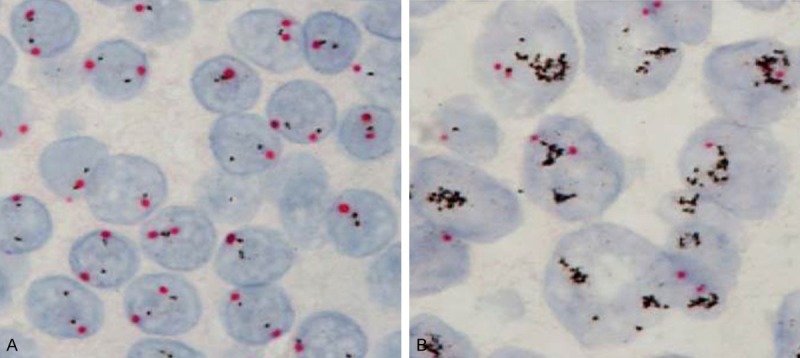
HER2 amplification by DISH in UUTUC. Red dots indicate centromere (CEP17) and black dots represent HER2. A: HER2/CEP17=0.97 mean negative for amplification of HER2 gene. B: HER2/CEP17=6.72 mean amplification of HER2 gene. Magnification ×1,000 (oil emersion lens).
Table 2.
HER2 analysis using immunohistochemistry and dual-color in situ hybridization (p<0.0001)
| HER2 gene amplification | ||||
|---|---|---|---|---|
|
|
||||
| (-) | (+) | Total | ||
| HER2 protein overexpression | 0, 1+ | 128 | 12 | 140 |
| 2+ | 8 | 9* | 17 | |
| 3+ | 4* | 10* | 14 | |
| Total | 140 | 31 | 171 | |
HER2-positive cancer.
Clinicopathological analysis of HER2-positive UUTUC
The relationship between HER2 positivity and clinicopathological factors are shown in Table 1. A statistically significant difference was observed for HER2 positivity between patients aged over 70 years (16 positive cases in 78 patients, 20.5%) and patients aged under 70 years (7 positive cases in 93 patients, 7.5%) (p=0.0132). For tumor location (localized to the ureter, to the renal pelvis, or to both the ureter and renal pelvis), a significant difference was observed for HER2 positivity. No HER2-positive patient showed tumors localized to only the renal pelvis (0 out of 76 renal pelvic urothelial carcinomas, p<0.0001). For histological grade, HER2-positive UUTUC was determined as Grade 3 for 21 (21.7%) out of 97 patients, but non-Grade 3 cases were determined in only two (2.7%) out of 74 patients, there was a significant statistical difference (p=0.0003). For concomitance of HGCIS, a significant difference was observed for HER2 positivity between patients with and without HGCIS; 17 (20.5%) patients in 83 cases with HGCIS vs. six (6.6%) patients in 88 cases without HGCIS (p=0.0089). No statistical differences were observed between the other clinicopathological factors and HER2 positivity (Table 1).
HER2 expression and clinical outcome of UUTUC
One hundred and forty patients without previous bladder cancers before nephroureterectomy were analyzed, and Kaplan-Meier analysis revealed that HER2-positive patients showed a significant association with shorter time to recurrence in the residual urinary bladder after nephroureterectomy (log-rank p=0.0284, Figure 3). This shorter time to recurrence in HER2-positive patients showed a statistically significant difference not only in univariate but also in multivariate Cox models. The multivariate hazard ratio was 3.70 (95% confidence interval, 1.54-8.87) for time to recurrence in the residual urinary bladder (p=0.0034, Table 3).
Figure 3.
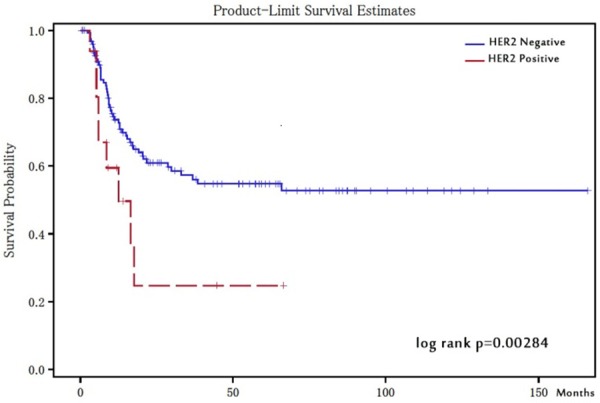
Time to recurrence in urinary bladder-comparison with HER2 positive and negative patients. Kaplan-Meier analysis revealed that HER2-positive patients showed significant association with a shorter bladder recurrence after nephroureterectomy (log-rank p=0.0284).
Table 3.
Univariate and multivariate analyses of HER2 positivity and patient outcome (time to recurrence in the urinary bladder)
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR | (95% CI) | p value | HR | (95% CI) | p value | ||
| HER2 positivity | 2.19 | (1.07-4.51) | 0.0325 | 3.70 | (1.54-8.87) | 0.0034 | |
| Sex (female vs. male) | 0.76 | (0.41-1.38) | 0.3625 | 0.75 | (0.39-1.46) | 0.4003 | |
| Age (≥70 vs. <70) | 0.79 | (0.47-1.35) | 0.3959 | 0.60 | (0.33-1.10) | 0.0994 | |
| Side (right vs. left) | 1.09 | (0.65-1.82) | 0.7563 | 1.02 | (0.59-1.79) | 0.9395 | |
| Tumor location (ureter vs. renal pelvis) | 1.36 | (0.81-2.29) | 0.2513 | 1.45 | (0.79-2.67) | 0.2347 | |
| Tumor architecture (sessile vs. papillary) | 0.82 | (0.44-1.51) | 0.517 | 0.78 | (0.36-1.69) | 0.5212 | |
| Histological grade (Grade 1, 2 vs. Grade 3) | 0.70 | (0.42-1.18) | 0.1818 | 0.34 | (0.14-0.83) | 0.0173 | |
| Concomitant carcinoma in situ (present vs. absent) | 0.99 | (0.58-1.67) | 0.9637 | 1.53 | (0.78-3.02) | 0.2187 | |
| Tumor stage (pT3-pT4 vs. pTa-pT2) | 0.92 | (0.54-1.55) | 0.7433 | 1.00 | (0.34-2.94) | 0.3399 | |
| Lymphovascular invasion (present vs. absent) | 1.24 | (0.73-2.09) | 0.4317 | 1.65 | (0.68-4.03) | 0.2719 | |
| Lymph node metastasis (present vs. absent) | 1.30 | (0.62-2.76) | 0.4874 | 2.24 | (0.85-5.90) | 0.1040 | |
CI, confidence interval; HR, hazard ratio.
Discussion
HER2 positivity in UUTUC was significantly associated with age, a high histological grade, tumor location, and concomitant HGCIS. HER2-positive patients with UUTUC showed a shorter time to recurrence in the urinary bladder after nephroureterectomy using Kaplan-Meier analysis. These results suggest that HER2 positivity in UUTUC is an independent prognostic factor of shorter time to recurrence in the urinary bladder after nephroureterectomy as shown by multivariate analysis.
Many studies have reported on HER2 status in a multitude of cancer types. Targeted therapy using trastuzumab is commonly applied worldwide for gastric and mammary carcinoma in preoperative (neoadjuvant), postoperative (adjuvant) and metastatic settings.
For urothelial carcinomas, there are many papers reporting on the status of HER2 protein overexpression and gene amplification, but several studies have reported conflicting results. For example, the reported incidence of HER2 overexpression varies between 0 and 89%, and gene amplification between 0 and 59% [22-48]. One of the reasons for this variation may be because of the characterization of special membrane staining, such as U-shaped and/or basolateral staining with immunohistochemistry. In the present study, several cases showed such immunostaining patterns, and these particular patterns are similar to those of gastric cancer [18], and are determined as being negative for HER2 because of the incomplete staining pattern under the previous ASCO/CAP criteria. This difficulty can be overcome by applying the same ToGA recommendations adopted for gastric cancer [48] (that is, positive staining for HER2 as U-shaped and basolateral) for the estimation of HER2 status with immunohistochemistry in UUTUC.
Another point to note in the literature are reports that scored cases as score-2 and score-3 using only immunohistochemistry and treated them as HER2-positive cancers, and then investigated correlations with clinicopathological outcomes [36]. HER2-positive cancers should be determined using a combination of immunohistochemistry and fluorescence in situ hybridization or DISH if the HER2 status is score-2 with immunohistochemistry for both breast and gastric cancers according to ASCO/CAP and ToGA recommendations [18,49]. Therefore, in the present study, 12 HER2 amplification cases were determined as being HER2-negative cancers because they showed score-0 or score-1 with immunohistochemistry. This group (HER2 gene amplification but negative for HER2 protein overexpression) showed a poor response to trastuzumab in subgroup analysis in the ToGA trial [18].
In the present study, HER2-positive cancers showed a higher occurrence in patients aged over 70 years (p=0.0132). Previous reports found no statistically significant correlation between HER2 status and age in urothelial carcinomas [21,54], and the incidence rate of HER2-positive breast cancer tended to higher in patients less than 40 years old [55]. With increasing age comes a higher chance of a cancer being inoperable because of increased complications. Such cases may be better suited to oncological therapy using anti-HER2 targeted drugs, such as trastuzumab, lapatinib and pertuzumab.
With regard to tumor location, no HER2-positive patients were observed to have renal pelvic urothelial carcinomas (0 out of 76 patients). A few studies have examined tumor location in UUTUC. Langner C et al. [32] reported that HER2 overexpression and gene amplification were infrequent in UUTUC, and only a small number of patients might benefit from HER2-targeted cancer therapy. In their report, no strong overexpression (score-3) of HER2 with immunohistochemistry was observed in 53 patients with UUTUC. The researchers examined 48 cases of urothelial carcinoma in the renal pelvis and only five in the ureter. The rate of pelvic urothelial carcinoma in their study was very high, so it is possible that cases of strong overexpression (score-3) of HER2 were not observed.
For HER2 positivity and histological grade, Grade 3 UUTUC showed a statistically higher rate compared with Grade 2 or Grade 1 (p=0.0003) in the present study. This finding is consistent with other studies [30,51]. A more frequent coexistence with HGCIS in HER2-positive UUTUC was observed compared with HER2-negative UUTUC also.
For urothelial carcinomas of the urinary bladder, urothelial carcinoma with HGCIS were aggressive and multifocal, so cystectomy was adopted even for non-muscle invasive urothelial carcinomas, as recommended by the European Association of Urology [56]. It is suggested that the careful examination of another foci of urothelial carcinoma may be needed in HER2-positive UUTUC.
HER2 positivity was significantly associated with shorter recurrence of urothelial carcinomas in the residual urinary bladder after nephroureterectomy (p=0.0284, Figure 3). Recurrence in the urinary bladder after nephroureterectomy occurs in 30-51% of UUTUC patients [57,58]. Tsai et al. [33] reported that HER2 expression of UUTUC was significantly associated with tumor recurrence. In their study, they investigated HER2 status in 94 patients with UUTUC using immunohistochemistry, and found that the incidence of subsequent tumor recurrence in the urinary bladder significantly correlated to ureteral tumor involvement and HER2 expression. They also reported that tumor staging and HER2 expression were independent predictors of disease progression, disease-free survival and overall survival using univariate and multivariate analyses. In the current study, both HER2 positivity and high grade (Grade 3) UUTUC recurred in the urinary bladder early after surgery, but we found no statistically significant relationship between HER2 status and disease-free survival and overall survival. HER2 positivity and a high histological grade of UUTUC were also associated with a shorter recurrence time in the bladder using multivariate analysis. This suggests that HER2 positivity in UUTUC is an independent predictive marker for early recurrence of urothelial carcinoma in the residual urinary bladder after nephroureterectomy, and that the residual urinary bladder should be examined after a short period following operation.
In conclusion, since HER2-positive UUTUC have a high histological grade and recur in the residual urinary bladder early after surgery, the HER2 status with UUTUC should be examined to provide information to help manage aggressive UUTUC.
Acknowledgements
The authors thank Kei Sakuma, Aiko Nishimoto and Harumi Yamamura for their excellent technical support. This work was supported by Kei Sato of Roche in Japan for DISH examination.
Disclosure of conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Izquierdo L, Mengual L, Gazquez C, Ingelmo-Torres M, Alcaraz A. Molecular characterization of upper urinary tract tumours. BJU Int. 2010;106:868–72. doi: 10.1111/j.1464-410X.2009.09135.x. [DOI] [PubMed] [Google Scholar]
- 2.Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000;164:1523–5. [PubMed] [Google Scholar]
- 3.Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52:594–601. doi: 10.1016/s0090-4295(98)00295-7. [DOI] [PubMed] [Google Scholar]
- 4.Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, Lotan Y, Weizer A, Raman JD, Wood CG Upper Tract Urothelial Carcinoma Collaboration. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115:1224–33. doi: 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]
- 5.Olgac S, Mazumdar M, Dalbagni G, Reuter VE. Urothelial carcinoma of the renal pelvis: a clinicopathologic study of 130 cases. Am J Surg Pathol. 2004;28:1545–52. doi: 10.1097/00000478-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000;164:1523–5. [PubMed] [Google Scholar]
- 7.Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester R, Burger M, Cowan N, Böhle A, Van Rhijn BW, Kaasinen E, Palou J, Shariat SF. Euro-pean guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. 2013;63:1059–71. doi: 10.1016/j.eururo.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Ito A, Shintaku I, Satoh M, Ioritani N, Aizawa M, Tochigi T, Kawamura S, Aoki H, Numata I, Takeda A, Namiki S, Namima T, Ikeda Y, Kambe K, Kyan A, Ueno S, Orikasa K, Katoh S, Adachi H, Tokuyama S, Ishidoya S, Yamaguchi T, Arai Y. Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: the THP Monotherapy Study Group Trial. J. Clin. Oncol. 2013;31:1422–7. doi: 10.1200/JCO.2012.45.2128. [DOI] [PubMed] [Google Scholar]
- 9.Shelley MD, Cleves A, Wilt TJ, Mason MD. Gemcitabine chemotherapy for the treatment of metastatic bladder carcinoma. BJU Int. 2011;108:168–79. doi: 10.1111/j.1464-410X.2011.10341.x. [DOI] [PubMed] [Google Scholar]
- 10.Vassilakopoulou M, de la Motte Rouge T, Colin P, Ouzzane A, Khayat D, Dimopoulos MA, Papadimitriou CA, Bamias A, Pignot G, Nouhaud FX, Hurel S, Guy L, Bigot P, Roumiguié M, Rouprêt M French Collaborative National Database on UUT-UCC. Outcomes after adjuvant che-motherapy in the treatment of high-risk urothelial carcinoma of the upper urinary tract (UUT-UC): results from a large multicenter collaborative study. Cancer. 2011;117:5500–8. doi: 10.1002/cncr.26172. [DOI] [PubMed] [Google Scholar]
- 11.Sella A, Kovel S. Combination of gemcitabine and carboplatin in urothelial cancer patients unfit for cisplatin due to impaired renal or cardiac function. Int Braz J Urol. 2012;38:49–56. doi: 10.1590/s1677-55382012000100007. [DOI] [PubMed] [Google Scholar]
- 12.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roskoski R Jr. The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem Biophys Res Commun. 2004;319:1–11. doi: 10.1016/j.bbrc.2004.04.150. [DOI] [PubMed] [Google Scholar]
- 14.Ménard S, Fortis S, Castiglioni F, Agresti R, Balsari A. HER2 as a prognostic factor in breast cancer. Oncology. 2001;61:67–72. doi: 10.1159/000055404. [DOI] [PubMed] [Google Scholar]
- 15.Shepard HM, Lewis GD, Sarup JC, Fendly BM, Maneval D, Mordenti J, Figari I, Kotts CE, Palladino MA Jr, Ullrich A, et al. Monoclonal antibody therapy of human cancer: taking the HER2 protooncogene to the clinic. J Clin Immunol. 1991;11:117–27. doi: 10.1007/BF00918679. [DOI] [PubMed] [Google Scholar]
- 16.Kern JA, Schwartz DA, Nordberg JE, Weiner DB, Greene MI, Torney L, Robinson RA. p185neu expression in human lung adenocarcinomas predicts shortened survival. Cancer Res. 1990;50:5184–7. [PubMed] [Google Scholar]
- 17.Ochs AM, Wong L, Kakani V, Neerukonda S, Gorske J, Rao A, Riggs M, Ward H, Keville L. Expression of vascular endothelial growth factor and HER2/neu in stage II colon cancer and correlation with survival. Clin Colorectal Cancer. 2004;4:262–7. doi: 10.3816/ccc.2004.n.025. [DOI] [PubMed] [Google Scholar]
- 18.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 19.Gu MJ, Hong SM, Jung SJ. HER2 protein expression and HER2 gene amplification are infrequent in small intestinal carcinomas. Virchows Arch. 2013;462:603–7. doi: 10.1007/s00428-013-1425-1. [DOI] [PubMed] [Google Scholar]
- 20.Dent S, Oyan B, Honig A, Mano M, Howell S. HER2-targeted therapy in breast cancer: a systematic review of neoadjuvanttrials. Cancer Treat Rev. 2013;39:622–31. doi: 10.1016/j.ctrv.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Jelovac D, Wolff AC. The adjuvant treatment of HER2-positive breast cancer. Curr Treat Options Oncol. 2012;13:230–9. doi: 10.1007/s11864-012-0186-4. [DOI] [PubMed] [Google Scholar]
- 22.Verdoorn BP, Kessler ER, Flaig TW. Targeted therapy in advanced urothelial carcinoma. Oncology. 2013;27:219–26. [PubMed] [Google Scholar]
- 23.Olsson H, Fyhr IM, Hultman P, Jahnson S. HER2 status in primary stage T1 urothelial cell carcinoma of the urinary bladder. Scand J Urol Nephrol. 2012;46:102–7. doi: 10.3109/00365599.2011.637955. [DOI] [PubMed] [Google Scholar]
- 24.Simonetti S, Russo R, Ciancia G, Altieri V, De Rosa G, Insabato L. Role of polysomy 17 in transitional cell carcinoma of the bladder: immunohis-tochemical study of HER2/neu expression and fish analysis of c-erbB-2 gene and chromosome 17. Int J Surg Pathol. 2009;17:198–205. doi: 10.1177/1066896909333415. [DOI] [PubMed] [Google Scholar]
- 25.Kolla SB, Seth A, Singh MK, Gupta NP, Hemal AK, Dogra PN, Kumar R. Prognostic significance of Her2/neu overexpression in patients with muscle invasive urinary bladder cancer treated with radical cystectomy. Int Urol Nephrol. 2008;40:321–7. doi: 10.1007/s11255-007-9283-x. [DOI] [PubMed] [Google Scholar]
- 26.Hauser-Kronberger C, Peham K, Grall J, Rausch W, Hutarew G, Dietze O. Novel approach of human epidermal growth factor receptor 2 detection in noninvasive and invasive transitional cell carcinoma of the bladder. J Urol. 2006;175:875–80. doi: 10.1016/S0022-5347(05)00411-8. [DOI] [PubMed] [Google Scholar]
- 27.Eissa S, Ali HS, Al Tonsi AH, Zaglol A, El Ahmady O. HER2/neu expression in bladder cancer: relationship to cell cycle kinetics. Clin Biochem. 2005;38:142–8. doi: 10.1016/j.clinbiochem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Chen PC, Yu HJ, Chang YH, Pan CC. Her2 amplification distinguishes a subset of non-muscle-invasive bladder cancers with a high risk of pro-gression. J Clin Pathol. 2013;66:113–9. doi: 10.1136/jclinpath-2012-200944. [DOI] [PubMed] [Google Scholar]
- 29.Hansel DE, Swain E, Dreicer R, Tubbs RR. HER2 overexpression and amplification in urothelial carcinoma of the bladder is associated with MYC coamplification in a subset of cases. Am J Clin Pathol. 2008;130:274–81. doi: 10.1309/41VLTFX3YPP1HF6F. [DOI] [PubMed] [Google Scholar]
- 30.Vershasselt-Crinquette M, Colin P, Ouzzane A, Gnemmi V, Robin YM, Aubert S, Villers A, Leroy X. Assessment of human epidermal growth factor receptor 2 status in urothelial carcinoma of the upper urinary tract: a study using dual-color in situ hybridization and immunohistochemistry. Appl Immunohistochem Mol Morphol. 2012;20:363–6. doi: 10.1097/PAI.0b013e318241cab9. [DOI] [PubMed] [Google Scholar]
- 31.Alexa A, Baderca F, Zăhoi DE, Lighezan R, Izvernariu D, Raica M. Clinical significance of Her2/neu overexpression in urothelial carcinomas. Rom J Morphol Embryol. 2010;51:277–82. [PubMed] [Google Scholar]
- 32.Langner C, Gross C, Rehak P, Ratschek M, Rüschoff J, Zigeuner R. HER2 protein overexpression and gene amplification in upper urinary tract transitional cell carcinoma: systematic analysis applying tissue microarray technique. Urology. 2005;65:176–80. doi: 10.1016/j.urology.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Tsai YS, Tzai TS, Chow NH, Wu CL. Frequency and clinicopathologic correlates of ErbB1, ErbB2, and ErbB3 immunoreactivity in urothelial tumors of upper urinary tract. Urology. 2005;66:1197–202. doi: 10.1016/j.urology.2005.06.117. [DOI] [PubMed] [Google Scholar]
- 34.Caner V, Turk NS, Duzcan F, Tufan NL, Kelten EC, Zencir S, Dodurga Y, Bagci H, Duzcan SE. No strong association between HER-2/neu protein overexpression and gene amplification in high-grade invasive urothelial carcinomas. Pathol Oncol Res. 2008;14:261–6. doi: 10.1007/s12253-008-9027-y. [DOI] [PubMed] [Google Scholar]
- 35.Enache M, Simionescu CE, Stepan A. Rom EGFR and Her2/neuimmunoexpression in papillary urothelial bladder carcinomas. J Morphol Embryol. 2013;54:137–41. [PubMed] [Google Scholar]
- 36.Naik DS, Sharma S, Ray A, Hedau S. Epidermal growth factor receptor expression in urinary bladder cancer. Indian J Urol. 2011;27:208–14. doi: 10.4103/0970-1591.82839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ching CB, Amin MB, Tubbs RR, Elson P, Platt E, Dreicer R, Fergany A, Hansel DE. HER2 gene amplification occurs frequently in the micropapillary variant of urothelial carcinoma: analysis by dual-color in situ hybridization. Mod Pathol. 2011;24:1111–9. doi: 10.1038/modpathol.2011.69. [DOI] [PubMed] [Google Scholar]
- 38.Laé M, Couturier J, Oudard S, Radvanyi F, Beuzeboc P, Vieillefond A. Assessing HER2 gene amplification as a potential target for therapy in invasive urothelial bladder cancer with a standardized methodology: results in 1005 patients. Ann Oncol. 2010;21:815–9. doi: 10.1093/annonc/mdp488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skagias L, Politi E, Karameris A, Sambaziotis D, Archondakis A, Vasou O, Ntinis A, Michalopoulou F, Moreas I, Koutselini H, Patsouris E. Prog-nostic impact of HER2/neu protein in urothelial bladder cancer. Survival analysis of 80 cases and an overview of almost 20 years’ re-search. J BUON. 2009;14:457–62. [PubMed] [Google Scholar]
- 40.Tsai YS, Tzai TS, Chow NH. Does HER2 immunoreactivity provide prognostic information in locally advanced urothelial carcinoma patients re-ceiving adjuvant M-VEC chemotherapy? Urol Int. 2007;79:210–6. doi: 10.1159/000107952. [DOI] [PubMed] [Google Scholar]
- 41.Gårdmark T, Wester K, De la Torre M, Carlsson J, Malmström PU. Analysis of HER2 expression in primary urinary bladder carcinoma and cor-responding metastases. BJU Int. 2005;95:982–6. doi: 10.1111/j.1464-410X.2005.05452.x. [DOI] [PubMed] [Google Scholar]
- 42.Latif Z, Watters AD, Dunn I, Grigor KM, Underwood MA, Bartlett JM. HER2/neu overexpression in the development of muscle-invasive transi-tional cell carcinoma of the bladder. Br J Cancer. 2003;89:1305–1309. doi: 10.1038/sj.bjc.6601245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Latif Z, Watters AD, Dunn I, Grigor K, Underwood MA, Bartlett JM. HER2/neu gene amplification and protein overexpression in G3 pT2 transi-tional cell carcinoma of the bladder: a role for anti-HER2 therapy? Eur J Cancer. 2004;40:56–63. doi: 10.1016/j.ejca.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 44.Coogan CL, Estrada CR, Kapur S, Bloom KJ. HER-2/neu protein overexpression and gene amplification in human transitional cell carcinoma of the bladder. Urology. 2004;63:786–790. doi: 10.1016/j.urology.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 45.Matsubara H, Yamada Y, Naruse K, Nakamura K, Aoki S, Taki T, Tobiume M, Zennami K, Katsuda R, Honda N. Potential for HER-2/neu molecular targeted therapy for invasive bladder carcinoma: comparative study of immunohistochemistry and fluorescent in situ hybridization. Oncol Rep. 2008;19:57–63. [PubMed] [Google Scholar]
- 46.Miyamoto H, Kubota Y, Noguchi S, Takase K, Matsuzaki J, Moriyama M, Takebayashi S, Kitamura H, Hosaka M. C-ERBB-2 gene amplification as a prognostic marker in human bladder cancer. Urology. 2000;55:679–683. doi: 10.1016/s0090-4295(99)00604-4. [DOI] [PubMed] [Google Scholar]
- 47.Krüger S, Weitsch G, Büttner H, Matthiensen A, Böhmer T, Marquardt T, Sayk F, Feller AC, Böhle A. Overexpression of c-erbB-2 oncoprotein in muscle-invasive bladder carcinoma: relationship with gene amplification, clinicopathological parameters and prognostic outcome. Int J Oncol. 2002;21:981–987. [PubMed] [Google Scholar]
- 48.de Pinieux G, Colin D, Vincent-Salomon A, Couturier J, Amsellem-Ouazana D, Beuzeboc P, Vieillefond A. Confrontation of immunohistochemistry and fluorescent in situ hybridization for the assessment of HER-2/neu (c-erbb-2) status in urothelial carcinoma. Virchows Arch. 2004;444:415–419. doi: 10.1007/s00428-004-0986-4. [DOI] [PubMed] [Google Scholar]
- 49.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF American Society of Clinical Oncology; College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 50.Cheng L, Montironi R, Davidson DD, Lopez-Beltran A. Staging and reporting of urothelial carcinoma of the urinary bladder. Mod Pathol. 2009;22:S70–95. doi: 10.1038/modpathol.2009.1. [DOI] [PubMed] [Google Scholar]
- 51.Grignon DJ. The current classification of urothelial neoplasms. Mod Pathol. 2009;22:S60–9. doi: 10.1038/modpathol.2008.235. [DOI] [PubMed] [Google Scholar]
- 52.Morikawa T, Maeda D, Kume H, Homma Y, Fukayama M. Ribonucleotide reductase M2 subunit is a novel diagnostic marker and a potential therapeutic target in bladder cancer. Histopathology. 2010;57:885–92. doi: 10.1111/j.1365-2559.2010.03725.x. [DOI] [PubMed] [Google Scholar]
- 53.Morikawa T, Kawai T, Abe H, Kume H, Homma Y, Fukayama M. UBE2C is a marker of unfavorable prognosis in bladder cancer after radical cystectomy. Int J Clin Exp Pathol. 2013;15:1367–74. [PMC free article] [PubMed] [Google Scholar]
- 54.Krüger S, Mahnken A, Kausch I, Feller AC. Value of clusterinimmunoreactivity as a predictive factor in muscle-invasive urothelial bladder car-cinoma. Urology. 2006;67:105–9. doi: 10.1016/j.urology.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 55.Swede H, Moysich KB, Freudenheim JL, Quirk JT, Muti PC, Hurd TC, Edge SB, Winston JS, Michalek AM. Breast cancer risk factors and HER2 over-expression in tumors. Cancer Detect Prev. 2001;25:511–9. [PubMed] [Google Scholar]
- 56.Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou-Redorta J European Association of Urology (EAU) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2008;54:303–14. doi: 10.1016/j.eururo.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 57.Azémar MD, Comperat E, Richard F, Cussenot O, Rouprêt M. Bladder recurrence after surgery for upper urinary tract urothelial cell carci-noma: frequency, risk factors, and surveillance. Urol Oncol. 2011;29:130–6. doi: 10.1016/j.urolonc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Raman JD, Ng CK, Scherr DS, Margulis V, Lotan Y, Bensalah K, Patard JJ, Kikuchi E, Montorsi F, Zigeuner R, Weizer A, Bolenz C, Koppie TM, Isbarn H, Jeldres C, Kabbani W, Remzi M, Waldert M, Wood CG, Roscigno M, Oya M, Langner C, Wolf JS, Ströbel P, Fernández M, Karakiewcz P, Shariat SF. Impact of Tumor Location on Prognosis for Patients with Upper Tract Urothelial Carcinoma Managed by Radical Nephroureterectomy. Eur Urol. 2010;57:1072–9. doi: 10.1016/j.eururo.2009.07.002. [DOI] [PubMed] [Google Scholar]