Abstract
The identification of molecular prognostic markers for pancreatic cancer patients could provide insightful information for their management in the clinic. The aim of the study is to investigate whether or not the expressions of c-Myc and Fas (CD95/APO1) have prognostic relevance for overall survival (OS) in patients with pancreatic cancer. we used immunohistochemistry on tissue microarrays containing 162 pancreatic cancer specimens to assess the protein expression levels of c-Myc and Fas. Kaplan-Meier survival analysis demonstrated that high level of c-Myc cytoplasmic expression was significantly correlated with worse survival in patients with pancreatic cancer (P = 0.012), while high level of Fas cytoplasmic expression was significantly associated with better outcome of pancreatic cancer (P = 0.046). However, multivariate Cox model analysis showed that tumor differentiation, lymph node status and c-Myc cytoplasmic expression were significant independent prognostic factors for OS (P < 0.001, P = 0.023, P = 0.001, respectively). On the contrary, Fas cytoplasmic expression did not independently influence patient’s prognosis (P = 0.249). Our data suggested that high level of c-Myc cytoplasmic expression may be considered as a valuable marker for prognosis of pancreatic cancer.
Keywords: Pancreatic cancer, c-Myc, Fas (CD95/APO1), immunohistochemistry, survival analysis
Introduction
Pancreatic cancer is a fourth or fifth leading cause of cancer-related death for both men and women in the western world [1,2]. For all stages combined, the 5-year survival rates is < 5% if left untreated [3]. Complete surgical resection is the only chance for long-term survival, improving five-year relative survival rates to 25-35% after surgery [4,5]. Unfortunately, because pancreatic cancer is often advanced at the time of diagnosis, only 15 to 20 out of every 100 diagnosed cases can be considered candidates for potentially curative resection [6]. Although there has been a remarkable improvement in the treatment of the disease, the prognosis in patients with pancreatic cancer remains extremely poor.
Identification of prognostic factors may provide useful information for clinical management. The current prognosis prediction for pancreatic cancer very greatly depend on the TNM staging system (Tumor, Node, Metastasis), However, prognosis varies among patients with a similar tumor stage, therefore the TNM classification alone can not accurately predict the outcome for individual patients. Over recent years, researchers have extensively investigated the potential predictive molecular markers, and also have identified some molecular markers are significantly correlated with clinicopathological variables and survival rates of pancreatic cancer [7-9]. So far, however, no prognostic and predictive molecular marker in pancreatic cancer is recommended in clinical practice.
Previous studies have demonstrated that aberrant expressions of c-Myc and Fas (CD95/APO1) have been described in multiple malignancies and implicated in the pathogenesis of various malignancies, which include epithelial malignancies as well as pancreatic cancer [10-12]. Many studies have shown a significant association between the over-expression of c-Myc and poor prognosis in numerous tumor types [13,14], whereas some other reports do not find such a relationship [15,16]. Moreover, a recent study has shown that higher levels of c-Myc mRNA in breast cancer are correlated with better survival [17]. Currently, the final prognostic value of the c-Myc expression with respect to overall survival in pancreatic cancer remains uncertain. In addition, only a few studies regarding the expression of Fas in human pancreatic cancer tissues have been reported, and the available data for prognostic value of Fas in pancreatic cancer is very limited. Recently, a previous study showed that the over-expression of c-Myc and low-expression of Fas were significantly correlated with the PNI of pancreatic cancer [18]. Consequently, we speculated that the expressions of c-Myc and Fas may have potential prognostic value in patients with pancreatic cancer. Therefore, in order to clarify our thought, we performed an immunohistochemical study on 162 pancreatic cancer using tissue microarrays technology. In addition, we also examined the association of c-Myc and Fas expression with each other and their association with patient clinicopathological characteristics.
Materials and methods
Patients and tissue samples
The paraffin-embedded samples from patients investigated in this study were collected retrospectively from archival material stored in the biobank center in National Engineering Center for Biochip at Shanghai. Samples included a total of 162 patients who had undergone a resection surgery between 1995 and 2009. Written informed consent for the tissue specimens was received from all participants, and the study was approved by the ethical committee of biobank center related hospitals.
The following clinicopathological data was obtained from original pathology reports, including age, gender, tumor size, location, and invasion, LN metastases, grade of differentiation, and tumor stage. Staging of pancreatic cancer were assessed according to the American Joint Committee on Cancer (AJCC) criteria. A detailed description of clinical and pathological data of these 162 patients is provided in Table 1.
Table 1.
Characteristics features of the 162 patients with pancreatic cancer
| Clinicopathologic features | Number | Percentage (%) |
|---|---|---|
| Age (years) | ||
| < 60 | 89 | 54.9 |
| ≥ 60 | 73 | 45.1 |
| Gender | ||
| female | 60 | 37 |
| male | 102 | 63 |
| Size (cm) | ||
| < 4 | 74 | 45.7 |
| ≥ 4 | 88 | 54.3 |
| Location | ||
| head | 117 | 72.2 |
| body and rear | 45 | 27.8 |
| Differentiation | ||
| well | 117 | 72.2 |
| moderate | 14 | 8.7 |
| poor | 31 | 19.1 |
| Nodal status | ||
| Negative | 97 | 59.9 |
| Positive | 65 | 40.1 |
| Perineural Invasion status | ||
| Negative | 79 | 48.8 |
| Positive | 83 | 51.2 |
| Stage | ||
| stage I | 80 | 49.4 |
| stage II | 80 | 49.4 |
| stage III | 0 | 0 |
| stage IV | 2 | 1.2 |
All follow-up times was measured from the date of surgery to patient’s death from pancreatic cancer. Unfortunately, at the time of last follow-up ended in December 2011, 92 patients were lost to follow-up and excluded from the 5-year survival analysis, only 70 patient samples were available for the survival analysis. Among remaining 70 patients, 46 patients died during the follow-up period.
Tissue microarray construction
Tissue microarrays (TMAs) were constructed using appropriate tissue cores from formalin-fixed and paraffin-embedded (FFPE) samples as previously described [19]. Briefly, appropriate tumor areas and its corresponding non-tumor pancreatic samples were selected by the pathologists, and then a single core with a diameter of 0.6 mm was taken from each case. TMA blocks were constructed using an automated tissue arrayer (Beecher Instruments, Sun Prarie, WI). The array blocks were cut into five-micron sections, and then Sections were stained with hematoxylin-eosin to verify the presence of tumor cells. In all cases, tissue cores obtained from normal adjacent pancreas were severed as internal controls.
Immunohistochemistry and scoring
The TMA sections were deparaffinized in xylene, rehydrated with graded ethanol, washed in Tris-buffered saline. Antigen retrieval was conducted at high temperature under high pressure in sodium citrate buffer (pH 6) for 10 minutes. After quenching of endogenous peroxidase activity, c-Myc polyclone antibody (Santa company, expression in cytoplasm and cytomembrane) and Fas monoclonal antibody (Abcam company, expression in cytoplasm) was used at 1:300 dilution, respectively, and then specimens were incubated with the antibodies overnight at 4°C. Slides were then incubated with an appropriate dilution of the corresponding secondary biotinylated rabbit antibody for 30 minutes at room temperature. And then slides were washed 3 times in Tris-buffered saline and incubated in streptavidin-horseradish peroxidase (1:100, Dako) at room temperature for 30 minutes. Chromogenic immunolocalization was conducted using 3,3-diaminobenzidine (Dako, inc, CA, USA). Slides were then counterstained with hematoxylin. After washing, slides were dehydrated, and then mounted with cover-slips. Other tissue cores containing pancreatic cancer tissues used as positive controls. The negative control consisted of normal serum substituted for primary antibody.
Each slide was scored semi-quantitatively on the basis of percentage and intensity of stained normal or neoplastic epithelial cells as described previously [20]. The percentages of stained cells were scored as following: 0 points for no staining; 1 point for < 20%; 2 points for 20-75%; 3 points for > 75% of cells stained. The intensity of staining was graded on the following scale: 0, negative; 1, low; 2, moderate; and 3, strong intensity. The total score was the product of the scores for the intensity and positive rate of staining. In this study, a final total score of 0-4 and 5-9 in c-Myc expression was considered to be low or high expression, while a total score > 2 in Fas expression was defined as high-expression. The stained TMA slides were scored independently by two experienced pathologists in a blinded manner.
Statistical analysis
Associations between clinicopathological parameters and expressions of c-Myc and Fas were compared using the χ2-test. Relationship between c-Myc and Fas protein expressions were compared using the Spearman correlation coefficient analysis. Overall survival was calculated and survival curves were plotted using Kaplan-Meier method, and the differences between groups were compared using log-rank test. The significant variables in univariate models were further analyzed by the multivariate Cox proportional hazards regression models for independent prognostic value. All analyses were performed using the SPSS software package (SPSS Inc, Chicago, IL, USA, version 17.0). All tests were two-sided and P values < 0.05 were considered statistically significant.
Results
Expression of c-Myc and Fas in pancreatic cancer tissues and paracancerous tissues
Immunohistochemistry staining showed that c-Myc protein was mainly located in the cytoplasm of the cell, but also a small fraction (11.7%) was detected in the cell membrane. While, staining of Fas was detected only in the cytoplasm of the cell. The representative photographs were shown in Figure 1. In the present study, we found a significant increase of c-Myc expression in pancreatic cancer tissues compared with paracancerous tissues (P < 0.001). On the contrary, the expression level of Fas was significantly decreased in cancer tissues compared with paracancerous tissues (P < 0.001).
Figure 1.
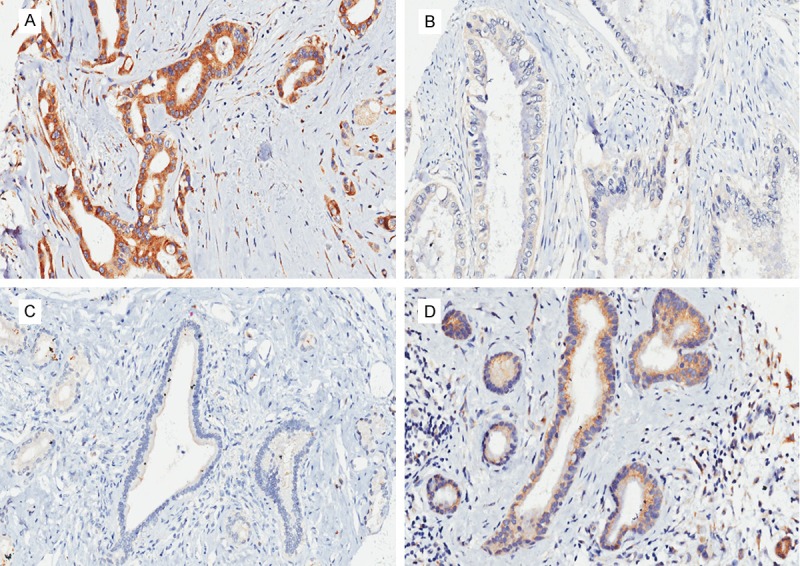
Immunohistochemical staining of c-Myc and Fas in human pancreatic cancer tissues and paracancerous tissues. A: Showing the positive expression of c-Myc in cancer tissues. B: Showing the negative expression of Fas in cancer tissues. C: Showing the negative expression of c-Myc in paracancerous tissues. D: Showing the positive expression of Fas in paracancerous tissues. All images were taken at 100× magnification.
Relationships between expression of c-Myc and Fas and clinicopathological features in pancreatic cancer
The cytoplasmic expression of c-Myc was significantly increased in pancreatic cancers with high tumor stage and with perineural invasion (PNI) (P = 0.019, P = 0.048, respectively), while no significant difference was observed between the cell membrane expression of c-Myc and all the clinicopathological features (all P > 0.05). In addition, the cytoplasmic expression level of Fas tended to be lower in pancreatic cancers with PNI than in those without (P = 0.022). However, the results showed that there was no significant correlation between the cytoplasmic expressions of c-Myc and Fas and the other variables, including age, gender, tumor size, location, grade of differentiation and lymph node status (all P > 0.05; Table 2).
Table 2.
Associations between the various clinicopathological factors and the expression of c-Myc and Fas
| c-Myc cytoplasmic expression | Fas cytoplasmic expression | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Features | NO. of cases | High | Low | p-value | High | Low | p-value | |
| Age (years) | < 60 | 89 | 50 | 39 | 0.296 | 54 | 35 | 0.686 |
| ≥ 60 | 73 | 35 | 38 | 42 | 31 | |||
| Gender | female | 60 | 30 | 30 | 0.629 | 31 | 29 | 0.131 |
| male | 102 | 55 | 47 | 65 | 37 | |||
| Location | head | 117 | 59 | 58 | 0.401 | 68 | 49 | 0.634 |
| body/rear | 45 | 26 | 19 | 28 | 17 | |||
| Size (cm) | < 4 | 74 | 39 | 35 | 0.956 | 46 | 28 | 0.490 |
| ≥ 4 | 88 | 46 | 42 | 50 | 38 | |||
| Differentiation | poor | 31 | 13 | 18 | 0.192 | 22 | 9 | 0.140 |
| moderate/well | 131 | 72 | 59 | 74 | 57 | |||
| LNM | positive | 65 | 32 | 33 | 0.499 | 37 | 28 | 0.620 |
| negative | 97 | 53 | 44 | 59 | 38 | |||
| PNI | positive | 83 | 51 | 32 | 0.019* | 42 | 41 | 0.022* |
| negative | 79 | 34 | 45 | 54 | 25 | |||
| Stage | stage I | 80 | 35 | 45 | 0.048* | 48 | 32 | 0.952 |
| stage II | 80 | 48 | 32 | 47 | 33 | |||
| stage IV | 2 | 2 | 0 | 1 | 1 | |||
Note: LNM: Lymph node metastasis; PNI: perineural invasion.
C-Myc had significantly higher cytoplasmic expression level in patients with PNI (P = 0.019) and high stage (P = 0.048) than those in patients with low stage and without PNI.
Fas had significantly lower cytoplasmic expression level in patients with PNI (P = 0.022) than those in patients without PNI.
Relationship between c-Myc and Fas expression in pancreatic cancer
The results showed that both the cytoplasmic expression and cell membrane expression of c-Myc were not significantly correlated with the cytoplasmic expression of Fas in pancreatic cancer tissues (r = 0.091, r = 0.037, respectively; P = 0.248, P = 0.641, respectively; Table 3). In addition, no significant correlation was found between the c-Myc expression in cytoplasm and in cell membrane (r = 0.107, P = 0.174; Table 4).
Table 3.
Relationship between c-Myc and Fas protein expression
| c-Myc cytoplasmic expression | c-Myc cell membrane expression | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| High | Low | p-value | High | Low | p-value | ||
| Fas cytoplasmic expression | High | 54 | 42 | 0.248 | 3 | 93 | 0.641 |
| Low | 31 | 35 | 3 | 63 | |||
Table 4.
Relationship between cytoplasmic and cell membrane expression of c-Myc
| c-Myc cytoplasmic expression | ||||
|---|---|---|---|---|
|
|
||||
| High | Low | p-value | ||
| c-Myc cell membrane expression | High | 5 | 1 | 0.174 |
| Low | 80 | 76 | ||
Survival analysis
The median overall survival (OS) in the study cohort was 14 months. Kaplan-Meier analysis demonstrated that LNM, grade of differentiation and AJCC stage were negatively significant prognostic predictors for overall survival of pancreatic cancer (P = 0.01, P = 0.003, P = 0.036, respectively), whereas, the other clinicopathological characteristics including age, gender, tumor size and location were not significantly associated with prognosis (data not shown).
In addition, the prognosis of pancreatic cancer patients with high c-Myc cytoplasmic expression was significantly worse than that of pancreatic cancer patients with low c-Myc cytoplasmic expression (P = 0.012; Figure 2A). Median survival times were 10.0 months for high cytoplasmic c-Myc expression and 37.0 months for low cytoplasmic c-Myc expression. After stratification of patients according to AJCC stage, high c-Myc cytoplasmic expression remained a significant predictor of poor survival in stage I (P = 0.005). In contrast, patients with high Fas cytoplasmic expression had significantly better outcomes than those with low Fas cytoplasmic expression ones (high expression: median 33.0 months, low expression: median 10.0 months, P = 0.046; Figure 2B). However, there was no significant correlation in prognosis of pancreatic cancer between the patients with high c-Myc cell membrane expression than those with low c-Myc cell membrane expression (P = 0.183).
Figure 2.
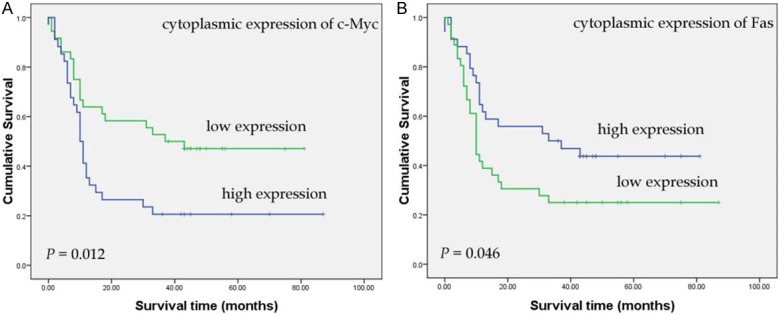
Kaplan-Meier curves show that high cytoplasmic expression level of c-Myc (A) was significantly correlated with worse survival of patients with pancreatic cancer, while high cytoplasmic expression level of Fas (B) was significantly associated with better survival (n = 70; P = 0.012, P = 0.046, respectively; log rank test).
Furthermore, variables that were significantly associated with OS in univariate analysis were included in Cox proportional hazards multivariate regression analysis. The analysis demonstrated that poor differentiation [hazard ratio (HR), 3.98; 95% CI, 1.92-8.23; P < 0.001, LNM (HR, 3.30; 95% CI, 1.17-9.26; P = 0.023) and high c-Myc cytoplasmic expression (HR, 3.36; 95% CI, 1.69-6.66; P = 0.001) were independently correlated with increased risk of death, whereas Fas cytoplasmic expression (HR, 1.43; 95% CI, 0.77-2.63; P = 0.249) and tumor stage (HR, 1.05; 95% CI, 0.39-2.82; P = 0.123) were not statistically significant (Table 5).
Table 5.
Multivariate Cox regression analysis of potential prognostic factors for survival of pancreatic cancer
| variables | HR | 95% CI | P-value |
|---|---|---|---|
| Differentiation, poor v moderate/well | 3.98 | 1.92-8.23 | < 0.001* |
| Stage, II v I | 1.05 | 0.39-2.82 | 0.922 |
| LNM, yes v no | 3.3 | 1.17-9.26 | 0.023* |
| c-Myc cytoplasmic expression, high v low | 3.36 | 1.69-6.66 | 0.001* |
| Fas cytoplasmic expression, low v high | 1.43 | 0.77-2.63 | 0.249 |
Note: HR: hazard ratio; CI: confidence interval; LNM: Lymph node metastasis.
P < 0.05, statistically significant.
Discussion
Finding molecular biomarkers useful for predicting outcomes of pancreatic cancer patients could provide important information for management in the clinic. The present study demonstrated that both the aberrant cytoplasmic expressions of c-Myc and Fas were significantly correlated with OS in patients with resected pancreatic cancer. Multivariate survival analysis showed that high cytoplasmic expression level of c-Myc protein was the independent factor predicting decreased overall survival. However, Fas protein expression level lost its independent prognostic power on multivariate analysis for OS.
The c-Myc is a member of the MYC family of transcription factors, which also includes N-Myc and L-Myc. Mounting evidence suggests that c-Myc participates in most aspects of cellular function, including metabolism, growth, differentiation, apoptosis, adhesion, and migration [21]. Previous studies have demonstrated that the amplification and/or over-expression of c-Myc are frequently detected in various cancer cells including pancreatic cancer [22-24]. In the present study, we found that the expression level of c-Myc was higher in pancreatic cancer tissues than that in paracancerous tissues. Our results were in agreement with previous reports that c-Myc was a very strong proto-oncogene and involved in the genesis of pancreatic cancer [10]. Fas belongs to a member of the death receptor subfamily of the tumor necrosis factor (TNFR) superfamily, and it is involved in apoptotic cell death. Previous studied have showed that down-regulation or loss of Fas expression has been also observed in many types of human cancer. Our data demonstrated that the expression level of Fas was lower in pancreatic cancer tissues than that in paracancerous tissues. The data presented here confirmed previous reports that absence or low-expression of Fas contributed to the pathogenesis of pancreatic cancer, and supported the idea that cancer cells might be resistant to apoptosis induced by the loss or low-expression of Fas protein. Moreover, our present results showed that high level of c-Myc cytoplasmic expression and low level of Fas cytoplasmic expression were significantly correlated with PNI. These findings suggested that the aberrant expression of c-Myc and Fas played important roles not only in the pancreatic tumorigenesis, but also in the progression of pancreatic cancer, and thereby contributed to the poor clinical outcome.
With respect to prognosis, our results showed that high level of c-Myc expression was a highly significant independent predictor of reduced overall survival (HR, 3.36, 95% CI, 1.69 to 6.66, P = 0.001). Our data suggested that high cytoplasmic expression of c-Myc might be considered as a valuable marker for prognosis of pancreatic cancer. This result was consistent with the data of some previous studies. For example, a report by Naidu et al [25] suggested that elevated c-Myc expression played an important role in breast cancer progression and might act as a potential prognostic marker for predicting the prognosis in patients with breast cancer. Schrader et al [26] also found a significant correlation between c-Myc over-expression and poor prognosis in diffuse large B cell lymphoma (DLBCL). The date revealed that c-Myc was an independent negative prognostic factor in a subgroup of patients with DLBCL. However, Some studies do not find any association between c-Myc expression and prognosis [27]. Moreover, a recent study [17] showed that over-expression of c-Myc was significantly correlated with a low incidence of LNM (P = 0.006) and increased DFS (P = 0.04). The results of this study suggested that tumors with higher expression level of c-myc were correlated with better survival. It is difficult to explain these inconsistent results. However, some researchers have suggested that c-Myc protein may induce cells to differentiation and apoptosis, and may suppress expression of vascular endothelial growth factor in tumor cells. This may in part explain why over-expression of c-myc is correlated with a better outcome.
Similarly, some researchers have also found that Fas may be an independent prognostic factor in cancers. For instance, Macher et al. [28] constructed a tissue microarray containing 617 patients with renal cell carcinoma (RCC) to investigate the relationship between the expression of Fas and prognosis of RCC. Authors found high expression of Fas was correlated with LNM and negatively associated with disease-specific survival. In multivariate analysis, the result identified high expression of Fas was a negative independent prognostic factor in RCC. However, some studies do not find any association between Fas expression and prognosis [29,30]. Moreover, some other reports have demonstrated that high level of Fas expression was correlated with a worse prognosis. For instance, Sejima et al [31] conducted a study using mRNA quantification and immunohistochemistry to investigate the expressions of Fas, Fas ligand (FasL) and Bcl-2 in surgically resected tumors from 82 patients with renal cell carcinoma (RCC). Multivariate analysis showed that high level of Fas mRNA was significantly associated with poorer outcome in patients with RCC (P = 0.0002). In this study, our data showed low cytoplasmic expression of Fas had significant impact on overall survival of pancreatic cancer, but no statistically significant association was found between Fas expression and OS in multivariate analysis. Moreover, there was no association between Fas cytoplasmic expression and tumor stage. Therefore, we agree with Markovic et al. [32] who demonstrated that Fas negativity was an unfavorable factor for therapy response and worse survival in univariate analysis in patients with diffuse large B-cell lymphoma (DLBCL), but Fas does not affect overall survival independently.
In conclusion, the results of this study suggested that the abnormal expression of c-Myc and Fas were two of the most crucial factors in the process of pancreatic tumorigenesis and progression, and both aberrant cytoplasmic expression of c-Myc and Fas were significantly correlated with OS of pancreatic cancer in univariate analysis. Furthermore, multivariate analysis demonstrated that only cytoplasmic c-Myc expression was an independent prognostic biomarker in patients with pancreatic cancer. These data contribute to further improving prognostic stratification of patients with pancreatic cancer and provide us with a potential molecular target for anticancer therapy. Currently, only few data are available regarding the prognostic impact of c-Myc and Fas expression in patients with pancreatic cancer after surgery. Therefore, more large population-based studies with long-term follow-up are required to support our findings.
Acknowledgements
This work was supported partly by Key Discipline Construction Project of Pudong Health Bureau of Shanghai, China (Grant No: PWZxkq2010-05) to HJ, and partly by China National 863 Project Foundation for Cancer Genomics (Pancreas Genomics) (Grant No: 1006AA02A302) to HG.
Disclosure of conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Jemal A, Siegel R, Xu JQ, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Bond-Smith G, Banga N, Hammond TM, Imber CJ. Pancreatic adenocarcinoma. BMJ. 2012;344:e2476. doi: 10.1136/bmj.e2476. [DOI] [PubMed] [Google Scholar]
- 3.Gong ZH, Holly EA, Bracci PM. Survival in Population-based Pancreatic Cancer Patients: San Francisco Bay Area, 1995-1999. Am J Epidemiol. 2011;174:1373–1381. doi: 10.1093/aje/kwr267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchem JB, Hamilton N, Gao F, Hawkins WG, Linehan DC, Strasberg SM. Long-Term Results of Resection of Adenocarcinoma of the Body and Tail of the Pancreas Using Radical Antegrade Modular Pancreatosplenectomy Procedure. J Am Coll Surg. 2012;214:46–52. doi: 10.1016/j.jamcollsurg.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PWT, Vauthey JN, Wang H, Cleary KR, Staerkel GA, Charnsangavej C, Lano EA, Ho L, Lenzi R, Abbruzzese JL, Wolff RA. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J. Clin. Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 6.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammad N, Heilbrun LK, Philip PA, Shields AF, Zalupski MM, Venkatramanamoorthy R, El-Rayes BF. CA19-9 as a predictor of tumor response and survival in patients with advanced pancreatic cancer treated with gemcitabine based chemotherapy. Asia Pac J Clin Oncol. 2010;6:98–105. doi: 10.1111/j.1743-7563.2010.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dabritz J, Preston R, Hanfler J, Oettle H. Follow-Up Study of K-ras Mutations in the Plasma of Patients With Pancreatic Cancer Correlation With Clinical Features and Carbohydrate Antigen 19-9. Pancreas. 2009;38:534–541. doi: 10.1097/MPA.0b013e31819f6376. [DOI] [PubMed] [Google Scholar]
- 9.Blackford A, Serrano OK, Wolfgang CL, Parmigiani G, Jones S, Zhang XS, Parsons DW, Lin JCH, Leary RJ, Eshleman JR, Goggins M, Jaffee EM, Iacobuzio-Donahue CA, Maitra A, Cameron JL, Olino K, Schulick R, Winter J, Herman JM, Laheru D, Klein AP, Vogelstein B, Kinzler KW, Velculescu VE, Hruban RH. SMAD4 Gene Mutations Are Associated with Poor Prognosis in Pancreatic Cancer. Clin Cancer Res. 2009;15:4674–4679. doi: 10.1158/1078-0432.CCR-09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skoudy A, Hernandez-Munoz I, Navarro P. Pancreatic ductal adenocarcinoma and transcription factors: role of c-Myc. J Gastrointest Cancer. 2011;42:76–84. doi: 10.1007/s12029-011-9258-0. [DOI] [PubMed] [Google Scholar]
- 11.Grippo PJ, Sandgren EP. Acinar-to-ductal metaplasia accompanies c-myc-induced exocrine pancreatic cancer progression in transgenic rodents. Int J Cancer. 2012;131:1243–1248. doi: 10.1002/ijc.27322. [DOI] [PubMed] [Google Scholar]
- 12.Bernstorff WV, Glickman JN, Odze RD, Farraye FA, Joo HG, Goedegebuure PS, Eberlein TJ. Fas (CD95/APO-1) and Fas ligand expression in normal pancreas and pancreatic tumors - Implications for immune privilege and immune escape. Cancer. 2002;94:2552–2560. doi: 10.1002/cncr.10549. [DOI] [PubMed] [Google Scholar]
- 13.O’Toole SA, McNeil CM, Morey AL, Millar EK, Elena-Lopez-Knowles , Musgrove EA, Sutherland RL. 29. C-Myc Gene Amplification Is Associated With A Poor Prognosis in Invasive Ductal Carcinoma. Pathology - Journal of the RCPA. 2010;42:S89–S90. [Google Scholar]
- 14.Wu X, Cai ZD, Lou LM, Zhu YB. Expressions of p53, c-MYC, BCL-2 and apoptotic index in human osteosarcoma and their correlations with prognosis of patients. Cancer Epidemiol. 2012;36:212–216. doi: 10.1016/j.canep.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Li YJ, Wei ZM, Meng YX, Ji XR. beta-catenin up-regulates the expression of cyclinD1, c-myc and MMP-7 in human pancreatic cancer: Relationships with carcinogenesis and metastasis. World J Gastroenterol. 2005;11:2117–2123. doi: 10.3748/wjg.v11.i14.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawksworth D, Ravindranath L, Chen Y, Furusato B, Sesterhenn IA, McLeod DG, Srivastava S, Petrovics G. Overexpression of C-MYC oncogene in prostate cancer predicts biochemical recurrence. Prostate Cancer Prostatic Dis. 2010;13:311–315. doi: 10.1038/pcan.2010.31. [DOI] [PubMed] [Google Scholar]
- 17.Kanthan R, Fried I, Rueckl T, Senger JL, Kanthan SC. Expression of cell cycle proteins in male breast carcinoma. World J Surg Oncol. 2010;8:10. doi: 10.1186/1477-7819-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He C, Jiang H, Geng S, Sheng H, Shen X, Zhang X, Zhu S, Chen X, Yang C, Gao H. Expression of c-Myc and Fas correlates with perineural invasion of pancreatic cancer. Int J Clin Exp Pathol. 2012;5:339–346. [PMC free article] [PubMed] [Google Scholar]
- 19.Lin MS, Chen WC, Huang JX, Gao HJ, Zhang BF, Fang J, Zhou Q, Hu Y. Tissue Microarrays in Chinese Human Rectal Cancer: Study of Expressions of the Tumor-Associated Genes. Hepatogastroenterology. 2011;58:1937–1942. doi: 10.5754/hge11262. [DOI] [PubMed] [Google Scholar]
- 20.Ohuchida K, Mizumoto K, Ishikawa N, Fujii K, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M, Tanaka M. The role of S100A6 in pancreatic cancer development and its clinical implication as a diagnostic marker and therapeutic target. Clin Cancer Res. 2005;11:7785–7793. doi: 10.1158/1078-0432.CCR-05-0714. [DOI] [PubMed] [Google Scholar]
- 21.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nature reviews. Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 22.Joensuu K, Hagström J, Leidenius M, Haglund C, Andersson L, Sariola H, Heikkilä P. Bmi-1, c-myc, and Snail expression in primary breast cancers and their metastases—elevated Bmi-1 expression in late breast cancer relapses. Virchows Archiv. 2011;459:31–39. doi: 10.1007/s00428-011-1096-8. [DOI] [PubMed] [Google Scholar]
- 23.Schleger C, Verbeke C, Hildenbrand R, Zentgraf H, Bleyl U. c-MYC activation in primary and metastatic ductal adenocarcinoma of the pancreas: incidence, mechanisms, and clinical significance. Mod Pathol. 2002;15:462–469. doi: 10.1038/modpathol.3880547. [DOI] [PubMed] [Google Scholar]
- 24.Li YJ, Wei ZM, Meng YX, Ji XR. Beta-catenin up-regulates the expression of cyclinD1, c-myc and MMP-7 in human pancreatic cancer: relationships with carcinogenesis and metastasis. World J Gastroenterol. 2005;11:2117–2123. doi: 10.3748/wjg.v11.i14.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naidu R, Wahab NA, Yadav M, Kutty MK. Protein expression and molecular analysis of c-myc gene in primary breast carcinomas using immunohistochemistry and differential polymerase chain reaction. Int J Mol Med. 2002;9:189–196. [PubMed] [Google Scholar]
- 26.Schrader A, Bentink S, Spang R, Lenze D, Hummel M, Kuo M, Arrand JR, Murray PG, Trumper L, Kube D, Vockerodt M. High myc activity is an independent negative prognostic factor for diffuse large B cell lymphomas. Int J Cancer. 2012;131:E348–E361. doi: 10.1002/ijc.26423. [DOI] [PubMed] [Google Scholar]
- 27.Hayry V, Makinen LK, Atula T, Sariola H, Makitie A, Leivo I, Keski-Santti H, Lundin J, Haglund C, Hagstrom J. Bmi-1 expression predicts prognosis in squamous cell carcinoma of the tongue. Br J Cancer. 2010;102:892–897. doi: 10.1038/sj.bjc.6605544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macher-Goeppinger S, Bermejo JL, Wagener N, Hohenfellner M, Haferkamp A, Schirmacher P, Roth W. Expression and prognostic relevance of the death receptor CD95 (Fas/APO1) in renal cell carcinomas. Cancer Lett. 2011;301:203–211. doi: 10.1016/j.canlet.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Gryko M, Guzinska-Ustymowicz K, Pryczynicz A, Cepowicz D, Kuklinski A, Czyzewska J, Kemona A, Kedra B. Correlation between Fas and FasL proteins expression in normal gastric mucosa and gastric cancer. Folia Histochem Cytobiol. 2011;49:142–7. doi: 10.5603/fhc.2011.0020. [DOI] [PubMed] [Google Scholar]
- 30.Takikita M, Hu N, Shou JZ, Wang QH, Giffen C, Taylor PR, Hewitt SM. Biomarkers of apoptosis and survival in esophageal squamous cell carcinoma. BMC Cancer. 2009;9:310. doi: 10.1186/1471-2407-9-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sejima T, Morizane S, Hinata N, Yao A, Isoyama T, Saito M, Takenaka A. Fas Expression in Renal Cell Carcinoma Accurately Predicts Patient Survival after Radical Nephrectomy. Urol Int. 2012;88:263–270. doi: 10.1159/000334453. [DOI] [PubMed] [Google Scholar]
- 32.Markovic O, Marisavljevic D, Cemerikic V, Perunicic M, Savic S, Filipovic B, Mihaljevic B. Clinical and prognostic significance of apoptotic profile in patients with newly diagnosed nodal diffuse large B-cell lymphoma (DLBCL) Eur J Haematol. 2011;86:246–255. doi: 10.1111/j.1600-0609.2010.01567.x. [DOI] [PubMed] [Google Scholar]


