Abstract
Although recent progress has been made in the diagnosis and treatment of cancer, the prognosis of esophageal squamous cell carcinoma (ESCC) remains poor. The identification of biomarkers for ESCC prognosis is important for treatment decisions. The aim of this study was to evaluate the relationship between the expressions of Annexin A1 (ANXA1), three prime repair exonuclease 1 (TREX1) and apurinic/apyrimidinic endonuclease-1 (APE1) and clinical outcome of patients with ESCC. The expressions of ANXA1, TREX1 and APE1 in 93 pairs of ESCC and paracancerous tissues were tested using immunohistochemistry. ANX1, TREX1 and APE1 were dysregulated in ESCC. Nuclear expressions of ANXA1 and APE1 were significantly associated with pathologic type (P = 0.004 and 0.040, respectively). Patients with low expression of nuclear ANXA1 had a better prognosis than those with high expression of nuclear ANXA1 (HR = 0. 448, 95% CI 0.236-0.849, P = 0.014), especially for those with histologic grade 1 and 2 (HR = 0.303, 95% CI: 0.155-0.593, P < 0.001). In conclusion, nuclear ANXA1 may be potentially used as a prognostic biomarker for ESCC.
Keywords: Annexin A1, three prime repair exonuclease 1, apurinic/apyrimidinic endonuclease-1, esophageal squamous cell carcinoma, prognosis
Introduction
Esophageal cancer is the fifth most common cancer and the fourth most cause of cancer-related death in China [1]. Esophageal squamous cell carcinoma (ESCC) is the most common histological type, accounting for > 90% of cases [2]. Despite the improvement achieved in the diagnosis and treatment, the prognosis of ESCC remains poor, with the five-year survival rate of ~30% [3]. Most ESCC patients who undergo curative resection will eventually relapse and die of treatment-resistant disease, with local and regional recurrence being the most prevalent pattern of failure. Treatment for ESCC remains one of the most challenging tasks for cancer clinicians. Therefore, there is an urgent need for safer and more effectively therapies to improve the prognosis of ESCC patients.
Annexins are a family of Ca2+-regulated phospholipid-binding proteins, with 12 members in mammals, which have been implicated in the regulation of several biological processes, including cell differentiation, proliferation and apoptosis [4]. There is increasing evidence that annexins play important roles in cancer incidence and progression [5-11]. Annexin A1 (ANXA1), the first characterized member of the annexin superfamily, is an intracellular protein which is aberrantly expressed in many types of cancer, such as gastric [6,9,12], breast [10,13] and esophageal cancer [14,15]. Dysregulation of ANXA1 is related to the incidence, invasion, metastasis and drug resistance of cancers [16].
DNA repair enzymes are vitally important for protecting cells against damage caused by endogenous and exogenous agents. Apurinic/apyrimidinic endonuclease-1 (APE1) is the major AP endonuclease, which is important for the base excision repair pathway. In addition to DNA repair activity, APE1 has important roles in protection against oxidative stress- and hypoxia-induced apoptosis and necrosis [17]. Overexpression of APE1 in cancer cells is closely linked to poor prognosis and chemo- and radio-resistance [18]. Three prime repair exonuclease 1 (TREX1), the major 3’ to 5’ exonuclease in mammalian cells, excises bases from 3’ end of single- and double-stranded DNA templates with a preference for mismatched nucleotides [19]. In human fibroblasts, TREX1 was predominantly localized in the cytoplasm and translocated into the nucleus upon ultraviolet light exposure [20]. However, TREX1 is overexpressed in the nucleus of cancer cells [20].
In the present study, we investigated the expression levels of ANXA1, TREX1 and APE1 in 93 patients with ESCC and examined their associations with clinicopathologic factors and overall survival.
Materials and methods
Patients and tissue samples
A total of 93 patients were recruited who were underwent surgery between August 2008 and February 2010. All patients had histologically confirmed primary ESCC and had no history of other malignancy. ESCC and matched adjacent normal tissues were collected before patients were treated with any anti-cancer therapy, including surgery, chemotherapy and radiotherapy. This study was approved by the ethical committees of Taizhou People’s Hospital and National Engineering Center for Biochip at Shanghai. Written informed consent was obtained from each patient before enrolling in the study. The clinicopathologic features of ESCC patients were summarized in Table 1.
Table 1.
Clinicopathological characteristics of patients with ESCC
| Characteristics | No. of Patients | % |
|---|---|---|
| Age (years) | ||
| median | 63 | |
| range | 49-85 | |
| Gender | ||
| female | 22 | 23.7 |
| male | 71 | 76.3 |
| Pathologic type | ||
| medullary | 23 | 24.7 |
| ulcerative | 55 | 59.1 |
| others | 13 | 14.0 |
| unknown | 2 | 3.2 |
| AJCC stage | ||
| stage I | 7 | 7.5 |
| stage II | 52 | 55.9 |
| stage III | 29 | 31.2 |
| stage IV | 1 | 1.1 |
| unknown | 4 | 4.3 |
| LNM | ||
| negative | 55 | 59.1 |
| positive | 37 | 39.8 |
| unknown | 3 | 3.2 |
| Tumor size (cm) | ||
| median | 4.0 | |
| range | 1.7-10 | |
| Histologic grade | ||
| well | 15 | 16.1 |
| moderate | 63 | 67.7 |
| poor | 15 | 16.1 |
Tumor tissue microarray (TMA) and immunohistochemistry (IHC)
ESCC TMA was constructed as previously described [21]. Hematoxylin- and eosin-stained slides were reviewed by a pathologist, and representative areas that contained invasive tumor cells were marked on both the slides and corresponding paraffin block for TMA construction. TMA was constructed using an automated tissue arrayer (Beecher Instruments, Sun Prarie, WI). A single 600-μm tissue core was taken from each donor block and assembled into a recipient block. Subsequently, sections (4 μm thick) were cut from the array blocks and prepared for IHC analysis.
Sections were deparaffinized with xylene and rehydrated through graded alcohol. Antigen retrieval was performed using microwave treatment for 10 min in 10 mM sodium citrate buffer (pH 6.0). Endogenous peroxidase activity was quenched by incubating the sections in methanol with 6% hydrogen peroxide. Slides were then incubated with antibodies for 30 min (anti-ANXA1 antibody, 1:3000; anti-TREX1 antibody, 1:250; anti-APE1 antibody, 1:200). Staining was completed after a 10-min incubation with a freshly prepared substrate–chromogen solution (20 μL DAB chromogen per 1 mL of PBS), which results in a brown-colored precipitate at the antigen site. Slides were subsequently counterstained with hematoxylin, dehydrated, and mounted.
The slides stained by IHC were assessed by two pathologists who were blinded to clinical information. The staining intensity of cancer cells was scored as 0, negative; 1, weak; 2, moderate; 3, strong staining. For statistical evaluation, tumors were scored as 0, non-staining; 1, 1-10%; 2, 11-50%; 3, 51-80%; 4, 81-100% positive cells. The total histological score, which was the result of multiplication of intensity and percentage scores, was utilized to determine the result. The total histological score < 4 indicated as a low level of expression, whereas a total histological score ≥ 4 denotes a high level of expression [21].
Statistical analysis
The expression levels of ANXA1, TREX1 and APE1 between ESCC and paracancerous tissues were compared using the non-parametric Mann-Whitney U-test. The relationship between ANXA1, TREX1 and APE1, and clinicopathologic characteristics were tested by Chi-square test or Fisher’s exact test. The Kaplan-Meier method and the long-rank test were performed to compare the survival rates. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were calculated using Cox regression models for overall survival in uni- and multivariate analysis. A P-value < 0.05 was deemed statistically significant. All analyses were performed using the SPSS 19.0 software package (Chicago, IL, USA).
Results
The expression levels of ANXA1, TREX1 and APE1 in ESCC and paracancerous tissues
The expression levels of ANXA1, TREX1 and APE1 in 93 ESCC tissues were evaluated by IHC. The ANXA1-positive site was located in the cytoplasm and nucleus (Figure 1). The majority of ESCC showed negative or low expression of cytoplasmic and nuclear ANXA1 (68/93, 73.1%; 58/93, 62.4%, respectively), whereas a subset (25/93, 26.9%; 35/93, 37.6%) of ESCC showed high expression of cytoplasmic and nuclear ANXA1. The levels of cytoplasmic ANXA1 in cancer tissues were significantly higher than those in paracancerous tissues (P < 0.001), but nuclear ANXA1 showed a lower expression in ESCC tissues (P < 0.001). TREX1 was mainly localized in nucleus of both ESCC and adjacent non-cancerous cells. A weak positive cytoplasmic staining for TREX1 was observed in 26% (29/93) of cancer samples, whereas positive nuclear staining was found in 93.5% (87/93) of cancer samples. There were significant difference in cytoplasmic and nuclear expression of TREX1 between cancer and paracancerous tissues (P = 0.038 and < 0.001, respectively). APE1 was mainly localized in nucleus, whereas cytoplasmic APE1 expression was relatively rare. Nuclear expression of APE1 was observed in 100% (93/93) of cancer samples. The levels of nuclear APE1 in cancer tissues were also significantly higher than those in paracancerous tissues (P < 0.001).
Figure 1.
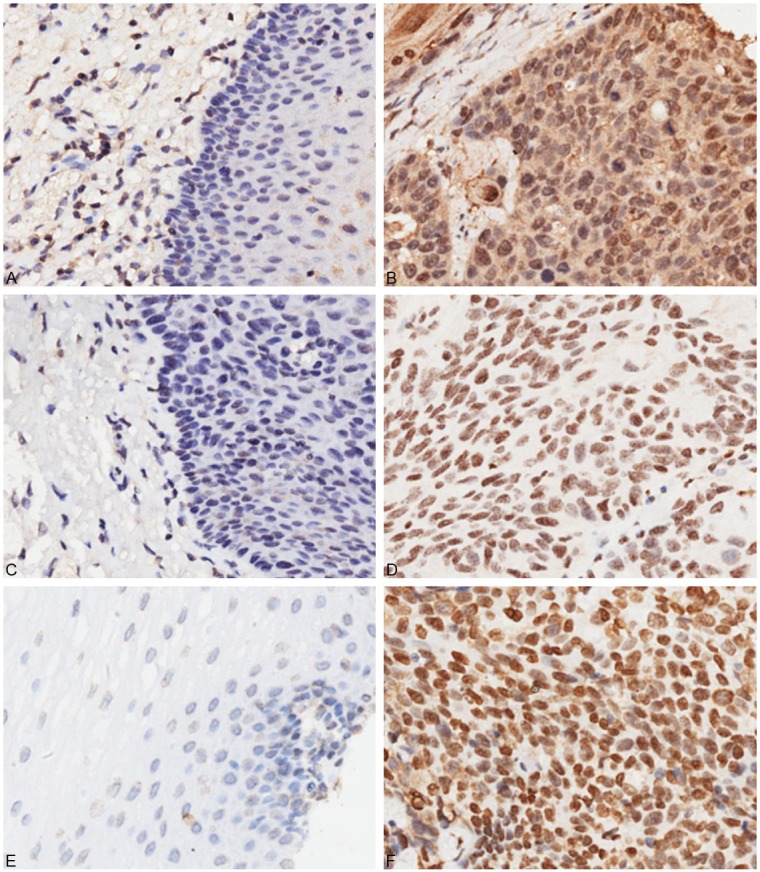
Immunohistochemical analysis of ANAX1, TREX1 and APE1 in ESCC and paracancerous tissues. A: Negative ANXA1 expression in adjacent non-cancerous tissue. B: Positive ANXA1 expression in ESCC tissue (cytoplasmic and nuclear staining). C: Negative TREX1 expression in adjacent non-cancerous tissue. D: Positive nuclear expression of TREX1 in ESCC tissue. E: Negative APE1 expression in adjacent non-cancerous tissue. F: Positive nuclear expression of APE1 in ESCC tissue.
The association of ANXA1, TREX1 and APE1 with clinicopathologic characteristics of ESCC patients
Since the expressions of cytoplasmic TREX1 and APE1 were low and rare in ESCC, respectively, cytoplasmic expressions of TREX1 and APE1 were excluded from further analysis. Nuclear expressions of ANXA1 and APE1 were significantly associated with pathologic type (P = 0.004 and 0.040, respectively) (Table 2). No other difference between ANXA1, TREX1 and APE1 expression and clinicopathologic characteristics was found.
Table 2.
Associations between the expressions of ANXA1, TREX1 and APE1 and the clinicopathological features
| Variables | Nuclear ANXA1 | Cytoplasmic ANXA1 | Nuclear TREX1 | Nuclear APE1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Low | High | P value | Low | High | P value | Low | High | P value | Low | High | P value | |
| Age (years) | 0.271 | 0.334 | 0.809 | 0.469 | ||||||||
| ≤ 60 | 18 | 15 | 22 | 11 | 9 | 24 | 10 | 23 | ||||
| > 61 | 40 | 20 | 46 | 14 | 15 | 45 | 14 | 46 | ||||
| Gender | 0.803 | 1 | 0.578 | 0.578 | ||||||||
| male | 45 | 26 | 52 | 19 | 17 | 54 | 17 | 54 | ||||
| female | 13 | 9 | 16 | 6 | 7 | 15 | 7 | 15 | ||||
| Size (cm) | 0.83 | 0.636 | 0.472 | 0.811 | ||||||||
| > 4 | 25 | 14 | 30 | 9 | 12 | 27 | 11 | 28 | ||||
| ≤ 4 | 33 | 21 | 38 | 16 | 12 | 42 | 13 | 41 | ||||
| Pathologic type | 0.004 | 0.376 | 0.765 | 0.040 | ||||||||
| medullary | 19 | 4 | 19 | 4 | 5 | 18 | 8 | 15 | ||||
| ulcerative | 27 | 28 | 40 | 15 | 16 | 39 | 9 | 46 | ||||
| others | 11 | 2 | 8 | 5 | 3 | 10 | 6 | 7 | ||||
| Histologic grade | 0.923 | 0.626 | 0.349 | 0.677 | ||||||||
| well | 9 | 6 | 10 | 5 | 3 | 12 | 3 | 12 | ||||
| moderate | 39 | 24 | 48 | 15 | 19 | 44 | 18 | 45 | ||||
| poor | 10 | 5 | 10 | 5 | 2 | 13 | 3 | 12 | ||||
| LNM | 0.263 | 0.458 | 0.627 | 0.617 | ||||||||
| positive | 25 | 10 | 28 | 7 | 10 | 25 | 7 | 28 | ||||
| negative | 32 | 23 | 39 | 16 | 13 | 42 | 14 | 41 | ||||
| AJCC stage | 0.764 | 0.606 | 0.937 | |||||||||
| I | 4 | 3 | 4 | 3 | 3 | 4 | 0.293 | 2 | 5 | |||
| II | 32 | 20 | 38 | 14 | 10 | 42 | 12 | 40 | ||||
| III | 20 | 9 | 23 | 6 | 10 | 19 | 7 | 22 | ||||
| IV | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | ||||
Survival analyses
Overall survival was calculated as the time from the date of ESCC diagnosis to death. Follow-up period ranged from 0.13 month to 52 months (median, 35 months). Among 93 patients with ESCC, 42 (45.2%) patients died as a result of disease progression during the follow-up. The overall survival rates at 1 and 3 years were 65.6% and 52.0%, respectively. Twenty patients of the 35 who had high expression of nuclear ANXA1 died from ESCC (20/35, 57.1%). In the group of patients with low expression of nuclear ANXA1, 22 died from the disease (22/58, 37.9%). ESCC patients with low expression of nuclear ANXA1 had a significantly longer survival time compared with those with high expression of nuclear ANXA1 (HR = 0.464, 95% CI 0.252-0.855, P = 0.014) (Table 3, Figure 2). Furthermore, TNM and LNM were significantly associated with shorter survival time in univariate analyses (Table 3). Upon multivariate analysis, only low expression of nuclear ANXA1 was significantly associated with better survival rate (HR = 0. 448, 95% CI 0.236-0.849, P = 0.014). Stratified analysis revealed that low expression of nuclear ANXA1 was significantly associated with better prognosis in ESCC patients with TNM stages III and IV (HR = 0.212, 95% CI: 0.050-0.899, P = 0.035) or histologic grade 1 and 2 (HR = 0.371, 95% CI: 0.193-0.713, P = 0.003). After adjustment for LNM and TNM, the association between nuclear ANXA1 and overall survival in patients with histologic grade 1 and 2 remained significant (HR = 0.303, 95% CI: 0.155-0.593, P < 0.001).
Table 3.
Univariate and multivariable Cox regression analysis of overall survival (n = 93)
| Features | Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years), > 60 vs ≤ 60 | 1.016 (0.540-1.914) | 0.961 | ||
| Gender, female vs male | 0.782 (0.374-1.635) | 0.513 | ||
| Pathologic type, medullary vs others | 0.932 (0.564-1.540) | 0.784 | ||
| Histologic grade, 1+2 vs 3 | 0.648 (0.255-1.649) | 0.362 | ||
| Tumor size (cm), > 4 vs ≤ 4 | 1.313 (0.703-2.449) | 0.393 | ||
| LNM, positive vs negative | 2.181 (1.092-4.357) | 0.027 | 1.425 (0.443-4.591) | 0.552 |
| TNM, III+IV vs I+II | 2.677 (1.235-5.802) | 0.013 | 3.515 (0.973-12.700) | 0.055 |
| Nuclear ANXA1 (low vs high) | 0.464 (0.252-0.855) | 0.014 | 0.448 (0.236-0.849) | 0.014 |
| Cytoplasm ANXA1 (low vs high) | 1.129 (0.554-2.301) | 0.738 | ||
| Nuclear TREX1 (low vs high) | 1.138 (0.560-2.316) | 0.721 | ||
| Nuclear APE1 (low vs high) | 1.328 (0.635-2.777) | 0.451 | ||
Figure 2.
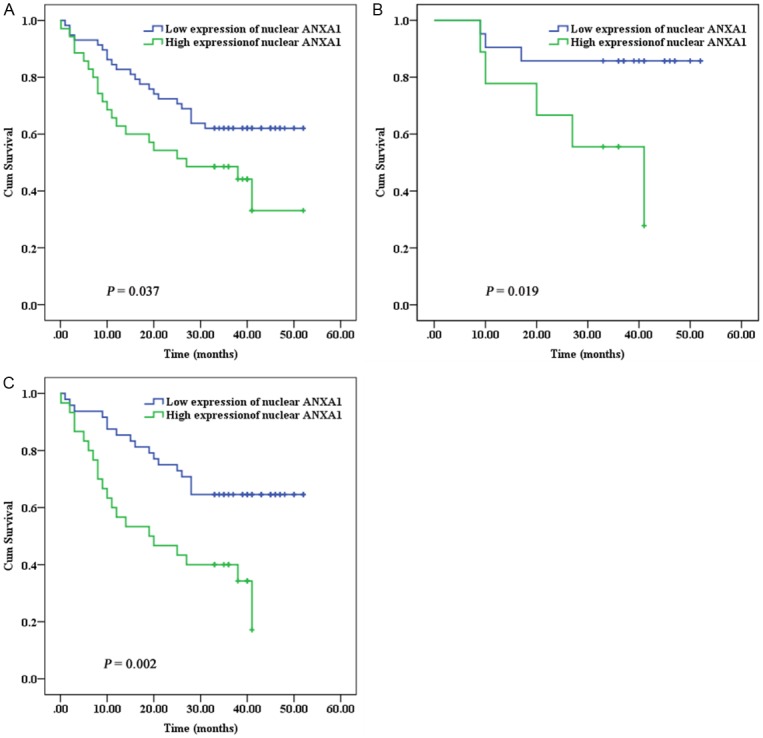
Kaplan-Meier survival curves of ESCC patients according to nuclear expression of ANXA1. A: All ESCC patients. B: Patients with TNM stages III and IV. C: Patients with histologic grade 1 and 2.
Discussion
In the present study, we investigated the relationship between the expressions of ANXA1, TREX1 and APE1 and clinical outcome of patients with ESCC. ANXA1, TREX1 and APE1 were dysregulated in ESCC. In addition, decreased expression of nuclear ANXA1 in ESCC was correlated with a favorable prognosis. These findings indicate that nuclear ANXA1 may have an influence on the progression of ESCC.
The role of ANXA1 in cancer is complicated by the fact that ANXA1 is downregulated in some cancers, including gastric [6,12], breast [10,13], prostate [22], cervical [23] and thyroid cancer [24], but upregulated in other types of cancer, such as pancreatic cancer [25]. A controversy exists regarding the expression of ANXA1 in gastric cancer. Cheng et al. [6] and Yu et al. [12] reported that ANXA1 was downregulated in gastric cancer, but Jorg et al. [9] showed overexpression of ANXA1 in gastric cancer. TNM stage may be the one of the main reasons for the inconsistent and contradictory findings. ANXA1 appears to play multifaceted roles in cancer, and acts as context-depending tumor suppressor or oncogene. Inhibition of ANXA1 facilitates the growth of prostate cancer cells [5], whereas overexpression of ANXA1 facilitates the migration and invasion of gastric cancer cells [6]. Previous studies revealed that ANXA1 was downregulated in both ESCC and esophageal adenocarcinoma [14,15]. In the current study, the majority of ESCC showed negative or low expression of ANXA1. However, there was no association between both cytoplasmic and nuclear expression of ANXA1 and histologic grade, which was inconsistent with previous study that ANXA1 was predominantly in well-differentiated ESCC [15]. In this study, most cases were TNM stages I and II, while cases were almost TNM stage III in previous study [15,26], which may partly explain the discrepant results. Furthermore, recent studies have demonstrated that the levels of ANXA1 may influence the survival in cancer patients [6,27-30]. Although no association between cytoplasmic expression of ANXA1 and overall survival of ESCC patients was observed, patients with low expression of nuclear ANXA1 had longer survival time, which was in agreement with previous study [27]. Further studies are required to fully understand roles of ANXA1 in ESCC.
Although TREX1 knockout mice does not show an increase in cancer incidence [31], inhibition of TREX1 promotes cell death in malignant glioma and melanoma cells treated with anti-cancer agents [20]. Furthermore, Dong et al. reported that polymorphism in TREX1 [32] was associated with survival in patients with pancreatic cancer. APE1 are frequently overexpressed in some types of cancer, such as gastric cancer [33,34]. The levels of APE1 have previously been shown to correlate with survival in cancer patients [33]. In the present study, TREX1 and APE1 were upregulated in ESCC tissues, which were consistent with previous studies [20,34]. However, the level of cytoplasmic TREX1 was significantly lower than those of nuclear TREX1 in paracancerous tissues, which differ from fibroblasts [20]. Tomicic et al. [20] found that nuclear TREX1 seems to be associated with replication. The details of the mechanism of nuclear TREX1 in esophageal cell and ESCC need further studies. However, nuclear expression of TREX1 and APE1 did not correlate with survival. It was amazing that nuclear expressions of both ANXA1 and APE1 correlated with pathologic type. Are there different mechanisms in carcinogenesis between pathologic types? Further studies are warranted to verify our findings and to determine molecular mechanisms underlying the pathogenesis of ESCC.
In summary, high expression of nuclear ANXA1 was found to be a strong risk factor for the overall survival of ESCC. Even though further studies are required to precisely elucidate the role and significance of nuclear ANXA1 in the setting of ESCC, it may be a valuable biomarker for the prediction of ESCC prognosis.
Acknowledgements
This work was supported by the Jiangsu Province Ministry of Health, China (grant No. H201260), the 12th Five-Year Plan Key Project of Science and Technology, China (grant No. 2013ZX10002007), the Shanghai Committee of Science and Technology, China (grant No. 13440701500), and the Jiangsu Province Science and Technology Support Program, China (grant No. BE2012729).
Disclosure of conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.He J, Chen W. 2012 Chinese Cancer Registry Annual Report. Military Medical Science Press; 2012. pp. 27–31. [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Yan W, Wistuba II, Emmert-Buck MR, Erickson HS. Squamous Cell Carcinoma - Similarities and Differences among Anatomical Sites. Am J Cancer Res. 2011;1:275–300. [PMC free article] [PubMed] [Google Scholar]
- 4.Calmon MF, Mota MT, Babeto E, Candido NM, Girol AP, Mendiburu CF, Bonilha JL, Silvestre RV, Rosa BM, Thome JA, Medeiros GH, Soares FA, Guimaraes GC, de Arruda JG, Oliani SM, Villa LL, Vassallo J, Rahal P. Overexpression of ANXA1 in penile carcinomas positive for high-risk HPVs. PLoS One. 2013;8:e53260. doi: 10.1371/journal.pone.0053260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mu D, Gao Z, Guo H, Zhou G, Sun B. Sodium Butyrate Induces Growth Inhibition and Apoptosis in Human Prostate Cancer DU145 Cells by Up-Regulation of the Expression of Annexin A1. PLoS One. 2013;8:e74922. doi: 10.1371/journal.pone.0074922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng TY, Wu MS, Lin JT, Lin MT, Shun CT, Huang HY, Hua KT, Kuo ML. Annexin A1 is associated with gastric cancer survival and promotes gastric cancer cell invasiveness through the formyl peptide receptor/extracellular signal-regulated kinase/integrin beta-1-binding protein 1 pathway. Cancer. 2012;118:5757–5767. doi: 10.1002/cncr.27565. [DOI] [PubMed] [Google Scholar]
- 7.Kang H, Ko J, Jang SW. The role of annexin A1 in expression of matrix metalloproteinase-9 and invasion of breast cancer cells. Biochem Biophys Res Commun. 2012;423:188–194. doi: 10.1016/j.bbrc.2012.05.114. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu T, Kasamatsu A, Yamamoto A, Koike K, Ishige S, Takatori H, Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H, Uzawa K. Annexin A10 in human oral cancer: biomarker for tumoral growth via G1/S transition by targeting MAPK signaling pathways. PLoS One. 2012;7:e45510. doi: 10.1371/journal.pone.0045510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jorge YC, Mataruco MM, Araujo LP, Rossi AF, de Oliveira JG, Valsechi MC, Caetano A, Miyazaki K, Fazzio CS, Thome JA, Rahal P, Oliani SM, Silva AE. Expression of annexin-A1 and galectin-1 anti-inflammatory proteins and mRNA in chronic gastritis and gastric cancer. Mediators Inflamm. 2013;2013:152860. doi: 10.1155/2013/152860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yom CK, Han W, Kim SW, Kim HS, Shin HC, Chang JN, Koo M, Noh DY, Moon BI. Clinical significance of annexin A1 expression in breast cancer. J Breast Cancer. 2011;14:262–268. doi: 10.4048/jbc.2011.14.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu DW, Liu Y, Yang X, Yang CZ, Ma J, Yang X, Qiao JK, Wang LZ, Li J, Zhang CP, Zhang ZY, Zhong LP. Low Annexin A1 expression predicts benefit from induction chemotherapy in oral cancer patients with moderate or poor pathologic differentiation grade. BMC Cancer. 2013;13:301. doi: 10.1186/1471-2407-13-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu G, Wang J, Chen Y, Wang X, Pan J, Li Q, Xie K. Tissue microarray analysis reveals strong clinical evidence for a close association between loss of annexin A1 expression and nodal metastasis in gastric cancer. Clin Exp Metastasis. 2008;25:695–702. doi: 10.1007/s10585-008-9178-y. [DOI] [PubMed] [Google Scholar]
- 13.Shen D, Nooraie F, Elshimali Y, Lonsberry V, He J, Bose S, Chia D, Seligson D, Chang HR, Goodglick L. Decreased expression of annexin A1 is correlated with breast cancer development and progression as determined by a tissue microarray analysis. Hum Pathol. 2006;37:1583–1591. doi: 10.1016/j.humpath.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Wang KL, Wu TT, Resetkova E, Wang H, Correa AM, Hofstetter WL, Swisher SG, Ajani JA, Rashid A, Hamilton SR, Albarracin CT. Expression of annexin A1 in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Clin Cancer Res. 2006;12:4598–4604. doi: 10.1158/1078-0432.CCR-06-0483. [DOI] [PubMed] [Google Scholar]
- 15.Hu N, Flaig MJ, Su H, Shou JZ, Roth MJ, Li WJ, Wang C, Goldstein AM, Li G, Emmert-Buck MR, Taylor PR. Comprehensive characterization of annexin I alterations in esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10:6013–6022. doi: 10.1158/1078-0432.CCR-04-0317. [DOI] [PubMed] [Google Scholar]
- 16.Guo C, Liu S, Sun MZ. Potential role of Anxa1 in cancer. Future Oncol. 2013;9:1773–1793. doi: 10.2217/fon.13.114. [DOI] [PubMed] [Google Scholar]
- 17.Xia L, Guan W, Wang D, Zhang YS, Zeng LL, Li ZP, Wang G, Yang ZZ. Killing effect of Ad5/F35-APE1 siRNA recombinant adenovirus in combination with hematoporphrphyrin derivative-mediated photodynamic therapy on human nonsmall cell lung cancer. Biomed Res Int. 2013;2013:957913. doi: 10.1155/2013/957913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai N, Cao XJ, Li MX, Qing Y, Liao L, Lu XF, Zhang SH, Li Z, Yang YX, Wang D. Serum APE1 autoantibodies: a novel potential tumor marker and predictor of chemotherapeutic efficacy in non-small cell lung cancer. PLoS One. 2013;8:e58001. doi: 10.1371/journal.pone.0058001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang CJ, Lam W, Bussom S, Chang HM, Cheng YC. TREX1 acts in degrading damaged DNA from drug-treated tumor cells. DNA Repair (Amst) 2009;8:1179–1189. doi: 10.1016/j.dnarep.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomicic MT, Aasland D, Nikolova T, Kaina B, Christmann M. Human three prime exonuclease TREX1 is induced by genotoxic stress and involved in protection of glioma and melanoma cells to anticancer drugs. Biochim Biophys Acta. 2013;1833:1832–1843. doi: 10.1016/j.bbamcr.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, He C, He C, Chen B, Liu Y, Kong M, Wang C, Lin L, Dong Y, Sheng H. Nuclear PKM2 expression predicts poor prognosis in patients with esophageal squamous cell carcinoma. Pathol Res Pract. 2013;209:510–515. doi: 10.1016/j.prp.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Patton KT, Chen HM, Joseph L, Yang XJ. Decreased annexin I expression in prostatic adenocarcinoma and in high-grade prostatic intraepithelial neoplasia. Histopathology. 2005;47:597–601. doi: 10.1111/j.1365-2559.2005.02300.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang LD, Yang YH, Liu Y, Song HT, Zhang LY, Li PL. Decreased expression of annexin A1 during the progression of cervical neoplasia. J Int Med Res. 2008;36:665–672. doi: 10.1177/147323000803600407. [DOI] [PubMed] [Google Scholar]
- 24.Petrella A, Festa M, Ercolino SF, Zerilli M, Stassi G, Solito E, Parente L. Annexin-1 downregulation in thyroid cancer correlates to the degree of tumor differentiation. Cancer Biol Ther. 2006;5:643–647. doi: 10.4161/cbt.5.6.2700. [DOI] [PubMed] [Google Scholar]
- 25.Bai XF, Ni XG, Zhao P, Liu SM, Wang HX, Guo B, Zhou LP, Liu F, Zhang JS, Wang K, Xie YQ, Shao YF, Zhao XH. Overexpression of annexin 1 in pancreatic cancer and its clinical significance. World J Gastroenterol. 2004;10:1466–1470. doi: 10.3748/wjg.v10.i10.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang J, Hu N, Goldstein AM, Emmert-Buck MR, Tang ZZ, Roth MJ, Wang QH, Dawsey SM, Han XY, Ding T, Li G, Giffen C, Taylor PR. High frequency allelic loss on chromosome 17p13.3-p11.1 in esophageal squamous cell carcinomas from a high incidence area in northern China. Carcinogenesis. 2000;21:2019–2026. doi: 10.1093/carcin/21.11.2019. [DOI] [PubMed] [Google Scholar]
- 27.Lin CY, Jeng YM, Chou HY, Hsu HC, Yuan RH, Chiang CP, Kuo MY. Nuclear localization of annexin A1 is a prognostic factor in oral squamous cell carcinoma. J Surg Oncol. 2008;97:544–550. doi: 10.1002/jso.20992. [DOI] [PubMed] [Google Scholar]
- 28.Wang D, Zhang H, Fang Z, Yu G. Annexin-1 downregulation is associated with clinical outcome in Chinese patients with hilar cholangiocarcinoma. Eur Surg Res. 2010;45:151–157. doi: 10.1159/000320237. [DOI] [PubMed] [Google Scholar]
- 29.Wang LP, Bi J, Yao C, Xu XD, Li XX, Wang SM, Li ZL, Zhang DY, Wang M, Chang GQ. Annexin A1 expression and its prognostic significance in human breast cancer. Neoplasma. 2010;57:253–259. doi: 10.4149/neo_2010_03_253. [DOI] [PubMed] [Google Scholar]
- 30.Li CF, Shen KH, Huang LC, Huang HY, Wang YH, Wu TF. Annexin-I overexpression is associated with tumour progression and independently predicts inferior disease-specific and metastasis-free survival in urinary bladder urothelial carcinoma. Pathology. 2010;42:43–49. doi: 10.3109/00313020903434405. [DOI] [PubMed] [Google Scholar]
- 31.Morita M, Stamp G, Robins P, Dulic A, Rosewell I, Hrivnak G, Daly G, Lindahl T, Barnes DE. Gene-targeted mice lacking the Trex1 (DNase III) 3’-->5’ DNA exonuclease develop inflammatory myocarditis. Mol Cell Biol. 2004;24:6719–6727. doi: 10.1128/MCB.24.15.6719-6727.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong X, Li Y, Hess KR, Abbruzzese JL, Li D. DNA mismatch repair gene polymorphisms affect survival in pancreatic cancer. Oncologist. 2011;16:61–70. doi: 10.1634/theoncologist.2010-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Attar A, Gossage L, Fareed KR, Shehata M, Mohammed M, Zaitoun AM, Soomro I, Lobo DN, Abbotts R, Chan S, Madhusudan S. Human apurinic/apyrimidinic endonuclease (APE1) is a prognostic factor in ovarian, gastro-oesophageal and pancreatico-biliary cancers. Br J Cancer. 2010;102:704–709. doi: 10.1038/sj.bjc.6605541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fareed KR, Al-Attar A, Soomro IN, Kaye PV, Patel J, Lobo DN, Parsons SL, Madhusudan S. Tumour regression and ERCC1 nuclear protein expression predict clinical outcome in patients with gastro-oesophageal cancer treated with neoadjuvant chemotherapy. Br J Cancer. 2010;102:1600–1607. doi: 10.1038/sj.bjc.6605686. [DOI] [PMC free article] [PubMed] [Google Scholar]


