Abstract
Purpose: To investigate the correlation among p300, CBP and MLL expression and the clinicopathological characteristics in resected SCLC patients. Methods: Two hundred and twenty-two resected SCLC patients were included in this study. We evaluated p300, CBP and MLL expression by immunohistochemistry. Results: Patients with high p300 expression had shorter OS and DFS than those with low p300 expression (p = 0.01; p = 0.009, respectively). The patients with CBP-positive tumors had significantly lower OS and DFS than those with CBP-negative tumors (p = 0.005 and p = 0.007, respectively). Moreover, the p300- and CBP-positive (+) group had a significantly poor OS and DFS. The multivariate Cox regression analysis showed that high p300 and CBP expression are independent markers of poor overall survival (p = 0.006; p = 0.017, respectively) in operable SCLC patients. Conclusions: High p300 and CBP expression are independent prognostic markers of poor overall survival for resected SCLC patients. The combination of p300 and CBP expression may be useful in identifying patients with increased risks of cancer recurrence of SCLC.
Keywords: p300, CBP, MLL, survival, prognosis, lung cancer
Introduction
The incidence and mortality of small-cell lung cancer (SCLC), known as an aggressive lung tumor subtype with poor prognosis, make this disease a notable health-care issue worldwide [1]. However, although platinum-based chemotherapy and radiotherapy have all exerted beneficial effects, the increase in survival has been slight since the mid-1980s [2]. Median survival for patients with limited-stage disease is currently 15-20 months, with 20-40% surviving to 2 years, and for those with extensive-stage disease the values are 8-13 months and 5% respectively [3]. Therefore, it is pivotal to clarify the mechanism of carcinogenesis, and to distinguish the population with high-risk recurrent and metastatic disease for better management of lung cancer patients by specific biomarkers. Histone modifications include acetylation, phosphorylation, methylation, ubiquitination and ADP-ribosylation [4]. Abnormalities in the epigenetic regulation of chromatin structure and function can lead to aberrant gene expression and cancer development [5]. p300, which was originally identified using protein-interaction assays with the adenoviral E1Aoncoprotein [6], is highly homologous to the cyclic AMP response element-binding (CREB) protein (CBP), with 63% homology at the amino-acid level [7]. p300 and CBP are members of the histone acetyltransferase (HAT) family of transcriptional coactivators [8,9] of various sequence-specific DNA-binding transcription factors and are involved in a wide range of cellular activities, such as DNA repair, cell growth, differentiation, and apoptosis [10]. Somatic mutations in the p300 gene are found in gastric cancer, colon cancer, glioblastoma, acute myeloid leukemia and small cell lung cancer [11-14]. In addition, the oncogenic effect of P300 has been reported in lung, colorectal, breast and prostate cancers, and its overexpression is indicative of a poor prognosis [15-17]. Moreover, germline mutations in the CBP gene have been reported in Rubinstein-Taybi disease, while somatic alterations are detected in hepatocellular carcinoma and acute myeloid leukemia [18,19]. Thus, dysfunction of CBP and/or p300 is considered to contribute to tumorigenesis in several human malignancies.
Mixed lineage leukemia (MLL) with histone methyltransferase activity is involved in chromosome translocations resulting in acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), or leukemia of mixed immunophenotype [20]. According to a recent study, genomic alterations affect the histone-modifying enzymes p300, CBP and MLL and become the second most frequently mutated class of genes in SCLC [14]. Nevertheless, the clinical significance of p300, CBP and MLL expression in SCLC is still unclear.
Our study retrospectively investigates the correlations among the expression of p300, CBP and MLL and the clinicopathological features in surgically resected SCLC patients for the purpose of identifying patients with increased risks of cancer recurrence and providing a theoretical basis for further clinical prevention of SCLC.
Materials and methods
Patients and sample collection
In this retrospective study, we analyzed specimens from 222 patients with SCLC who had undergone surgical resection for lung cancer at the Tumor Hospital of Harbin Medical University, the First Affiliated Hospital of Harbin Medical University and the Second Affiliated Hospital of Harbin Medical University, from January 2000 to December 2011. All patients received informed consent before the collection of specimens. None of the patients received adjuvant chemotherapy, radiotherapy or immunotherapy before surgery. The pathological diagnosis was confirmed by two senior pathologists (Jinshu Geng, Jiping Qi). Clinical data of the patients was collected from the departments to provide follow-up care of the Tumor Hospital of Harbin Medical University. Table 1 summarizes the characteristics of the 222 resected SCLCs. The patients consisted of 140 males and 82 females ranging from 26 to 75 years (median, 54 years) of age. Clinicopathological factors, including age, gender, smoking history, ECOG scores, lymph node involvement, tumor status, and p-TNM stage were evaluated. The p-TNM staging system of the International Union Against Cancer (7th Edition) was used to classify specimens as stages I (n = 102), II (n = 71), III (n = 35), IV (n = 14). All patients with SCLC were followed-up from 1.0 to 116.0 months (median, 17.8 months) after diagnosis. The study was approved by Ethical Review Committee of Harbin Medical University, Harbin, China.
Table 1.
Clinicopathological variables and p300, CBP and MLL expression
| Variables | No. | p300 expression | CBP expression | MLL expression | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| negative (-) | positive (+) | p value | negative (-) | positive (+) | p value | negative (-) | positive (+) | p value | ||
| Age (years) | ||||||||||
| < 60 | 162 | 148 | 14 | 0.174 | 74 | 88 | 0.449 | 128 | 34 | 0.522 |
| ≥ 60 | 60 | 58 | 2 | 24 | 36 | 45 | 15 | |||
| Gender | ||||||||||
| Male | 140 | 128 | 12 | 0.304 | 65 | 75 | 0.37 | 104 | 36 | 0.087 |
| Female | 82 | 78 | 4 | 33 | 49 | 69 | 13 | |||
| Smoking history | ||||||||||
| No | 103 | 97 | 6 | 0.459 | 44 | 59 | 0.691 | 86 | 17 | 0.063 |
| Yes | 119 | 109 | 10 | 54 | 65 | 87 | 32 | |||
| ECOG scores | ||||||||||
| < 2 | 194 | 180 | 14 | 0.989 | 89 | 105 | 0.171 | 150 | 44 | 0.565 |
| ≥ 2 | 28 | 26 | 2 | 9 | 19 | 23 | 5 | |||
| Lymph node involvement | ||||||||||
| No | 111 | 107 | 4 | 0.038 | 49 | 62 | 1 | 90 | 21 | 0.257 |
| Yes | 111 | 99 | 12 | 49 | 62 | 83 | 28 | |||
| Tumor status | ||||||||||
| ≥ 3 cm | 139 | 126 | 13 | 0.11 | 61 | 78 | 0.92 | 105 | 34 | 0.267 |
| < 3 cm | 83 | 80 | 3 | 37 | 46 | 68 | 15 | |||
| p TNM stage | ||||||||||
| I + II | 173 | 161 | 12 | 0.769 | 81 | 92 | 0.131 | 133 | 40 | 0.479 |
| III + IV | 49 | 45 | 4 | 17 | 32 | 40 | 9 | |||
Primary antibodies
The mouse monoclonal anti-p300 antibody (Abcam, ab3164) was used at a 1:50 dilution; rabbit polyclonal anti-CBP antibody (Abcam, ab2832) at a 1:200 dilution; Mouse monoclonal anti-MLL (Abcam, ab32400) at a 1:200 dilution.
Immunohistochemistry
Paraffin sections of surgically resected specimens were routinely deparaffinized through a series of xylene baths and were rehydrated through graded alcohols. Sections were pre-treated by high-pressure mediated antigen retrieval with 0.01 M sodium citrate buffer (pH6) at 100 Kpa for 5 mins. Slides were peroxidase blocked in 3% H2O2 for 20 min. The sections were incubated with a primary antibody overnight at 4°C, and then visualized with HistostainTM-SP (streptavidin/peroxidase) kit (LAB-SA detection method, Beijing Zhongshan Biotechnology Co., Ltd) according to the instruction manual. Normal tonsil tissue sections were used as positive controls for EP300, human pancreas tissue sections for CBP, and human lung adenocarcinoma tissue sections for MLL. Parallel negative controls omitting primary antibody were also performed, and they did not show appreciable background staining. All the immunoreactions were separately evaluated by two investigators (Jinshu Geng, Jiping Qi) without knowledge of patients’ clinical records. Five views were examined per slide, and 100 cells were observed per view at 400 × magnification. Immunostaining of p300, CBP and MLL was scored following a semi-quantitative scale by evaluating representative tumor areas, intensity and percentage of cells. We classified staining as positive or negative, which were scored as follows: intensity (0 = negative, 1 = weak, 2 = intense), and percentage of positive tumor cells (1 = 1-25%, 2 = 26-50%, 3 = 51-75%, 4 = 76-100%). The scores of each sample were multiplied to give a final score of 0-8 and the total expression of p300, CBP and MLL was determined as either negative or low expression (-): score ≤ 2; positive expression or high expression (+): score ≥ 3.
Statistical analysis
Chi-square test or two-tailed Fisher’s exact test was used to analyze possible associations between qualitative clinicopathological variables and p300, CBP or MLL immunoreactivity. Spearman’s rank correlation coefficient was used to analyze the correlation between the expression of any two of p300, CBP and MLL. Disease-free survival times were measured from the date of surgery to the appearance of local or distant tumor progression. Overall survival times were measured from the date of surgery to death or last follow-up. Disease-free survival and overall survival were evaluated using the Kaplan-Meier method with the log-rank test. The Cox proportional hazard model was used to analyze univariate and multivariate hazards ratios for the study parameters. Variables with significant P values in univariate analysis were used for multivariates analysis. P values less than 0.05 were considered to be statistically significant. Statistical analysis was carried out using SPSS software version 9.0.
Results
p300, CBP and MLL expression in SCLC tissue and the correlations with clinicopathological factors
p300, CBP and MLL were primarily nuclear and, less frequently, cytoplasmic. Representative images of positive and negative expression of p300, CBP and MLL were shown in Figure 1. Positive rate of p300, CBP and MLL expression was 7.2% (16/222), 58.6% (124/222), and 22.1% (49/222) in SCLC samples, respectively. However, we noted that no significant correlation coefficient between the expression of any two of p300, CBP and MLL in lung cancer used Spearman’s rank correlation coefficient. As shown in Table 1, high expression of p300 was correlated with lymph node involvement (p = 0.038), while high expression of CBP and MLL was not (p = 1.000, p = 0.257, respectively). No statistical differences were found between clinicopathological factors (age, gender, smoking history, ECOG score, tumor status, and p-TNM stage) and p300, CBP or MLL expression.
Figure 1.
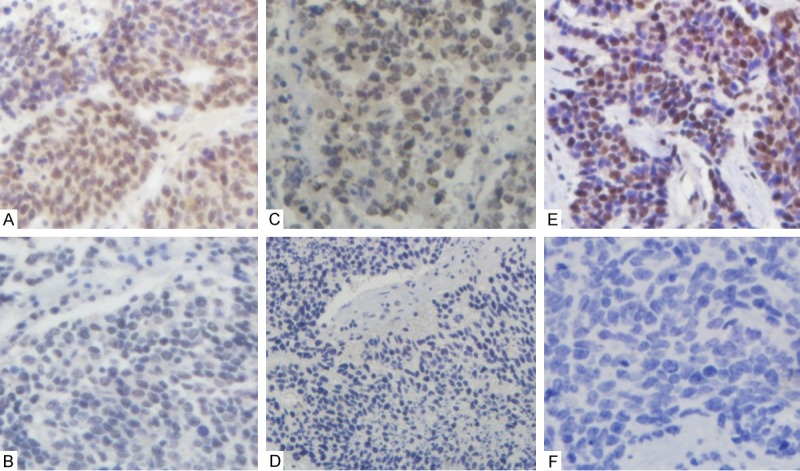
p300, CBP and MLL expression in SCLC specimens. A: p300 positive expression; B: p300 negative expression. C: CBP positive expression; D: CBP negative expression. E: MLL positive expression; F: MLL negative expression, p300 and CBP expression are primarily cytoplasmic and nuclear, however, MLL was primarily nuclear and less frequently, cytoplasmic. Photographs from A to F were taken at × 40 magnification.
Association among expression of p300, CBP, MLL and overall survival and disease-free survival in SCLC
Patients were divided into p300-positive or -negative groups, CBP-positive or -negative groups, and also into MLL-positive or -negative groups. Kaplan-Meier curves for disease-free survival and overall survival are shown in Figure 2. Log-rank tests were used for statistical analysis. Patients with high p300 expression had shorter OS and DFS (mean ± SD, 13.54 ± 2.01 months and 8.23 ± 1.55 months, respectively) than those with low p300 expression (mean ± SD, 21.60 ± 1.28 months and 14.52 ± 1.02 months, respectively) (Figure 2A, p = 0.01 and p = 0.009, respectively). The overall survival rate of the patients with CBP-positive tumors (mean ± SD, 17.71 ± 0.90 months) was significantly lower than that of the patients with CBP-negative tumors (mean ± SD, 24.82 ± 2.28 months) (Figure 2B, p = 0.005). The disease-free survival rate of the patients with CBP-positive tumors (mean ± SD, 11.40 ± 0.72 months) was also significantly lower than that of the patients with CBP-negative tumors (mean ± SD, 17.11 ± 1.82 months, p = 0.007). There were no significant correlations between expression and disease-free survival and overall survival. Moreover, we tried to identify subgroups with better or poor prognosis by double stratification for p300 expression and CBP expression. We divided 222 patients into three groups, double-negative (-), p300- or CBP-positive (+), and double-positive (++). The double-positive group had a significantly poor overall survival and disease-free survival (mean ± SD, 10.39 ± 2.06 months and 5.73 ± 1.41 months, respectively) and the double-negative group had the best overall survival and disease-free survival (mean ± SD, 25.10 ± 2.41 months and 17.31 ± 1.92 months, respectively) (Figure 2C, p <; 0.001 and p < 0.001, respectively). The p300- or CBP-positive groups showed medium over-all survival among the three groups.
Figure 2.
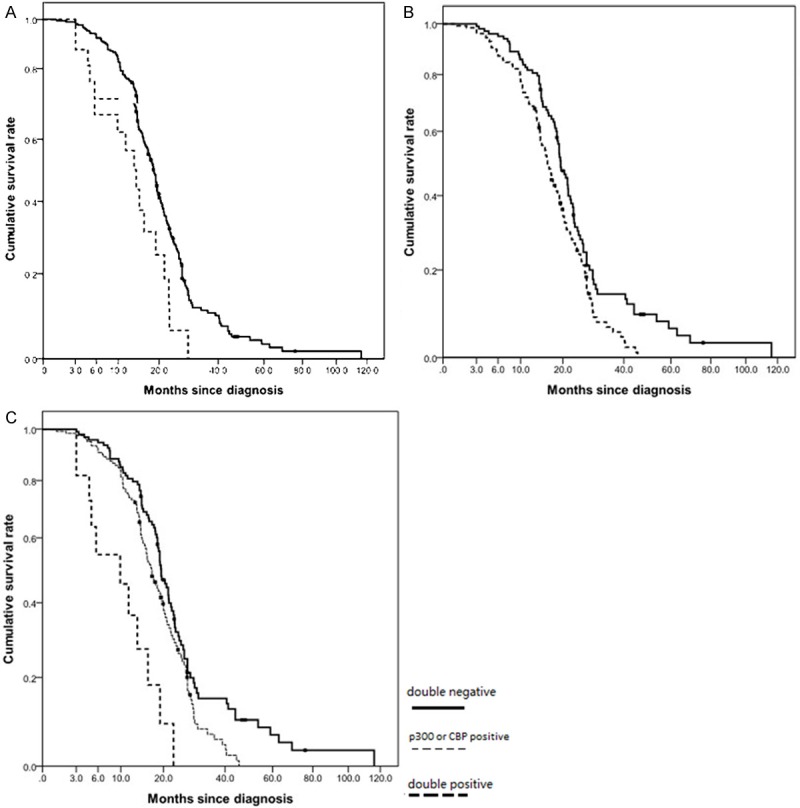
Kaplan-Meier cumulative survival analyses for 220 SCLC patients with different p300, CBP and MLL status. A: Difference in overall survival for negative p300 expression and positive p300 expression was statistically by Log-rank (P = 0.01). Solid black line, patients in negative p300 expression; dashed black line, patients in positive p300 expression. B: Difference in overall survival for negative CBP expression and positive CBP expression was statistically by Log-rank (P = 0.005). Solid black line, patients in negative CBP expression; dashed black line, patients in positive CBP expression. C: Patients were divided into three groups according to p300 expression (positive or negative) and CBP expression (positive or negative); (-): double negative, (+): p300 or CBP positive, (++): double positive. The double-positive group had a significantly poor overall survival and the double-negative group had the best overall survival (p < 0.001).
Univariate and multivariate analysis of prognostic factors in SCLC patients
Through Cox proportional hazard model, univariate analysis revealed that positive p300, positive CBP, lymph node involvement, and p-TNM stage were significantly correlated with overall survival (Table 2). Multivariate analysis with factors proven to be significant in the univariate analysis revealed that p300 and CBP expression were independent prognostic factors for overall survival (HR 1.488, 95% C.I. 1.118-1.980, p = 0.006; HR 1.879, 95% C.I. 1.119-3.156, p = 0.017, respectively) by the Cox hazard model.
Table 2.
Univariate and multivariate analysis of clinicopathological factors for the overall survival of 222 patients with SCLC
| Factors | B | S.E. | Wald | p-value | Exp (B) | 95% CI for Exp (B) | |
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Lower | Upper | ||||||
| Univariate analysis | |||||||
| Age | 0.032 | 0.158 | 0.041 | 0.840 | 1.032 | 0.757 | 1.408 |
| Gender | 0.122 | 0.145 | 0.707 | 0.401 | 1.130 | 0.850 | 1.502 |
| Smoking history | 0.282 | 0.144 | 3.850 | 0.050 | 1.326 | 1.000 | 1.757 |
| ECOG scores | -0.091 | 0.219 | 0.170 | 0.680 | 0.913 | 0.594 | 1.404 |
| Lymph node involvement | 0.402 | 0.143 | 7.877 | 0.005* | 1.495 | 1.129 | 1.980 |
| Tumor status | 0.186 | 0.146 | 1.632 | 0.201 | 1.205 | 0.905 | 1.604 |
| p-TNM stage | -0.584 | 0.166 | 12.298 | 0.000* | 0.558 | 0.403 | 0.773 |
| p300 expression | 0.658 | 0.263 | 6.281 | 0.012* | 1.931 | 1.154 | 3.231 |
| CBP expression | 0.402 | 0.145 | 7.647 | 0.006* | 1.495 | 1.124 | 1.988 |
| MLL expression | 0.263 | 0.172 | 2.346 | 0.126 | 1.301 | 0.929 | 1.821 |
| Multivariate analysis | |||||||
| Lymph node involvement | 0.206 | 0.172 | 1.435 | 0.231 | 1.229 | 0.817 | 1.721 |
| p-TNM stage | 0.392 | 0.200 | 3.835 | 0.050 | 1.479 | 1.000 | 2.189 |
| p300 expression | 0.379 | 0.146 | 7.422 | 0.006* | 1.488 | 1.118 | 1.980 |
| CBP expression | 0.631 | 0.265 | 5.685 | 0.017* | 1.879 | 1.119 | 3.156 |
B, partial regression coefficient; S.E., standard error of partial regression coefficient; Wald, X2 value which was used to compare if there was difference between total partial regression coefficient and 0; CI, confidence interval; Exp (B), relative hazard ratio.
p < 0.05.
Discussion
p300 and CBP are involved in a number of cellular processes including proliferation, cell cycle regulation, apoptosis, differentiation and DNA damage response [10,13], and function as transcriptional co-factors and histone acetyltransferases. Studies showed that p300 and CBP play an important role in the tumorigenesis of a variety of malignancies including colorectal, breast, hepatocellular and non-small cell lung carcinomas [21-24]. Mixed lineage leukemia (MLL) is involved in chromosome translocations resulting in acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), or leukemia of mixed immunophenotype. However, the significance of p300, CBP and MLL expression in a clinical settings and its prognostic value in SCLC remain to be elucidated.
To the best of our knowledge, this is the first report demonstrating the expression level of MLL in SCLC. p300 expression was correlated with lymph node metastasis. Similar results were found in colorectal cancer and hepatocellular carcinoma [25]. Furthermore, overall survival and disease-free survival were poor for patients with p300 and CBP overexpression. Through double stratification for p300 expression and CBP expression, we identified that double-positive group had a significantly poor prognosis and the double-negative group had the best prognosis. A Cox multivariate regression analysis also showed the significance of the overexpression p300 and CBP, such as smoking status, lymph node involvement and p-TNM stage. Therefore, p300 and CBP expression might be a reliable new prognostic biomarker for tumour recurrence or metastasis in SCLC patients.
It is now known that the expression of p300 and CBP may be associated with the progression of SCLC. There are several possible mechanisms. One potential reason is that the overexpression of p300 and CBP, as transcriptional coactivators for a number of nuclear proteins, might stimulate the expression of nuclear oncoproteins, such as myb, jun and fos, and transforming viral proteins (such as E1A) [6,26-28]. The involvement of these proteins in vital tumorigenic pathways provides a mechanistic route as to how their activation could result in cancer. p300 and CBP are involved in chromosomal translocation, which is associated with hematologic malignancies [29]. Thus, we assume that the poor survival of SCLC patients with increased expression of p300 and CBP is correlated with the inhibition of apoptosis and the enhanced propensity for lymph vessel and/or hematogenous formation. Further investigation, however, is needed to clarify the potential function of p300 and CBP in SCLC carcinogenesis.
Limitations of this study
There are several limitations in our study. At first, due to its retrospective nature, selection bias may exist in the study. Furthermore, other significant molecular markers in SCLC, such as FHIT, MAD1L1, TP53 and RB1 expression have not been investigated in current research. We believe that the correlations between these markers and p300, CBP would be interesting to pursue if further studies are conducted.
In conclusion, the present study indicates that expression levels of p300, CBP and MLL are up-regulated in human small cell lung cancers. p300 and CBP overexpression are indicators of poor prognosis for these patients. Further studies will be needed to clarify the downstream mechanisms involved in controlling the biological behavior of SCLC via histone-modifying molecules.
Disclosure of conflict of interest
None.
References
- 1.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378:1741–1755. doi: 10.1016/S0140-6736(11)60165-7. [DOI] [PubMed] [Google Scholar]
- 2.Oze I, Hotta K, Kiura K, Ochi N, Takigawa N, Fujiwara Y, Tabata M, Tanimoto M. Twenty-seven years of phase III trials for patients with extensive disease small-cell lung cancer: disappointing results. PLoS One. 2009;4:e7835. doi: 10.1371/journal.pone.0007835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lally BE, Urbanic JJ, Blackstock AW, Miller AA, Perry MC. Small cell lung cancer: have we made any progress over the last 25 years? Oncologist. 2007;12:1096–104. doi: 10.1634/theoncologist.12-9-1096. [DOI] [PubMed] [Google Scholar]
- 4.van Holde KE. In: Chromatin. Rich A, editor. New York: Springer; 1988. pp. 111–148. [Google Scholar]
- 5.Popovic R, Licht JD. Emerging Epigenetic Targets and Therapies in Cancer Medicine. Cancer Discov. 2012;2:405–13. doi: 10.1158/2159-8290.CD-12-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckner R, Ewen ME, Newsome D, Gerdes M, DeCaprio JA, Lawrence JB, Livingston DM. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 7.Arany Z, Sellers WR, Livingston DM, Eckner R. E1A-associated p300 and CREB associated CBP belong to a conserved family of coactivators. Cell. 1994;77:799–800. doi: 10.1016/0092-8674(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 8.Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 9.Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 10.Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- 11.Muraoka M, Konishi M, Kikuchi-Yanoshita R, Tanaka K, Shitara N, Chong JM, Iwama T, Miyaki M. p300 gene alterations in colorectal and gastric carcinomas. Oncogene. 1996;12:1565–1569. [PubMed] [Google Scholar]
- 12.Borrow J, Stanton VP Jr, Andresen JM, Becher R, Behm FG, Chaganti RS, Civin CI, Disteche C, Dubé I, Frischauf AM, Horsman D, Mitelman F, Volinia S, Watmore AE, Housman DE. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14:33–41. doi: 10.1038/ng0996-33. [DOI] [PubMed] [Google Scholar]
- 13.Giles RH, Peters DJ, Breuning MH. Conjunction dysfunction: CBP/p300 in human disease. Trends Genet. 1998;14:178–183. doi: 10.1016/s0168-9525(98)01438-3. [DOI] [PubMed] [Google Scholar]
- 14.Peifer M, Fernández-Cuesta L, Sos ML, George J, Seidel D, Kasper LH, Plenker D, Leenders F, Sun R, Zander T, Menon R, Koker M, Dahmen I, Müller C, Di Cerbo V, Schildhaus HU, Altmüller J, Baessmann I, Becker C, de Wilde B, Vandesompele J, Böhm D, Ansén S, Gabler F, Wilkening I, Heynck S, Heuckmann JM, Lu X, Carter SL, Cibulskis K, Banerji S, Getz G, Park KS, Rauh D, Grütter C, Fischer M, Pasqualucci L, Wright G, Wainer Z, Russell P, Petersen I, Chen Y, Stoelben E, Ludwig C, Schnabel P, Hoffmann H, Muley T, Brockmann M, Engel-Riedel W, Muscarella LA, Fazio VM, Groen H, Timens W, Sietsma H, Thunnissen E, Smit E, Heideman DA, Snijders PJ, Cappuzzo F, Ligorio C, Damiani S, Field J, Solberg S, Brustugun OT, Lund-Iversen M, Sänger J, Clement JH, Soltermann A, Moch H, Weder W, Solomon B, Soria JC, Validire P, Besse B, Brambilla E, Brambilla C, Lantuejoul S, Lorimier P, Schneider PM, Hallek M, Pao W, Meyerson M, Sage J, Shendure J, Schneider R, Büttner R, Wolf J, Nürnberg P, Perner S, Heukamp LC, Brindle PK, Haas S, Thomas RK. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet. 2012;44:1104–1110. doi: 10.1038/ng.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishihama K, Yamakawa M, Semba S, Takeda H, Kawata S, Kimura S, Kimura W. Expression of HDAC1 and CBP/p300 in human colorectal carcinomas. J Clin Pathol. 2007;60:1205–1210. doi: 10.1136/jcp.2005.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green AR, Burney C, Granger CJ, Paish EC, El-Sheikh S, Rakha EA, Powe DG, Macmillan RD, Ellis IO, Stylianou E. The prognostic significance of steroid receptor co-regulators in breast cancer: co-repressor NCOR2/SMRT is an independent indicator of poor outcome. Breast Cancer Res Treat. 2008;110:427–437. doi: 10.1007/s10549-007-9737-y. [DOI] [PubMed] [Google Scholar]
- 17.Isharwal S, Miller MC, Marlow C, Makarov DV, Partin AW, Veltri RW. p300 (histone acetyltransferase) biomarker predicts prostate cancer biochemical recurrence and correlates with changes in epithelia nuclear size and shape. Prostate. 2008;68:1097–1104. doi: 10.1002/pros.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrij F, Giles RH, Dauwerse HG, Saris JJ, Hennekam RC, Masuno M, Tommerup N, van Ommen GJ, Goodman RH, Peters DJ. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature. 1995;376:348–351. doi: 10.1038/376348a0. [DOI] [PubMed] [Google Scholar]
- 19.Sakai K, Nagahara H, Abe K, Obata H. Loss of heterozygosity on chromosome 16 in hepatocellular carcinoma. J Gastroenterol Hepatol. 1992;7:288–292. doi: 10.1111/j.1440-1746.1992.tb00982.x. [DOI] [PubMed] [Google Scholar]
- 20.Rowley JD. The critical role of chromosome translocations in human leukemias. Annu Rev Genet. 1998;32:495–519. doi: 10.1146/annurev.genet.32.1.495. [DOI] [PubMed] [Google Scholar]
- 21.Hou X, Li Y, Luo RZ, Fu JH, He JH, Zhang LJ, Yang HX. High expression of the transcriptional co-activator p300 predicts poor survival in resectable non-small cell lung cancers. Eur J Surg Oncol. 2012;38:523–530. doi: 10.1016/j.ejso.2012.02.180. [DOI] [PubMed] [Google Scholar]
- 22.Chen LC, Kurisu W, Ljung BM, Goldman ES, Moore D 2nd, Smith HS. Heterogeneity for allelic loss in human breast cancer. J Natl Cancer Inst. 1992;84:506–510. doi: 10.1093/jnci/84.7.506. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Kudo J, Ishibashi H, Hirata Y, Niho Y. Frequent loss of heterozygosity on chromosome 22 in hepatocellular carcinoma. Hepatology. 1993;17:794–799. [PubMed] [Google Scholar]
- 24.Duriez C, Schmitz A, Fouchet P, Buecher B, Thuille B, Lerebours F, Léger R, Boman F, Fléjou JF, Monges G, Paraf F, Bedossa P, Sabourin JC, Salmon RJ, Laurent-Puig P, Thomas G, Olschwang S. Localization of a tumor suppressor gene distal to D22S270 in colorectal cancers. Gastroenterol Clin Biol. 1997;21:358–364. [PubMed] [Google Scholar]
- 25.Li M, Luo RZ, Chen JW, Cao Y, Lu JB, He JH, Wu QL, Cai MY. High expression of transcriptional coactivator p300 correlates with aggressive features and poor prognosis of hepatocellular carcinoma. J Transl Med. 2011;9:5. doi: 10.1186/1479-5876-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bannister AJ, Kouzarides T. CBP-induced stimulation of c-Fos activity is abrogated by E1A. EMBO J. 1995;14:4758–762. doi: 10.1002/j.1460-2075.1995.tb00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bannister AJ, Oehler T, Wilhelm D, Angel P, Kouzarides T. Stimulation of c-Jun activity by CBP: c-Jun residues Ser63/73 are required for CBP induced stimulation in vivo and CBP binding in vitro. Oncogene. 1995;11:2509–2514. [PubMed] [Google Scholar]
- 28.Tomita A, Towatari M, Tsuzuki S, Hayakawa F, Kosugi H, Tamai K, Miyazaki T, Kinoshita T, Saito H. c-Myb acetylation at the carboxyl-terminal conserved domain by transcriptional co-activator p300. Oncogene. 2000;19:444–451. doi: 10.1038/sj.onc.1203329. [DOI] [PubMed] [Google Scholar]
- 29.Shigeno K, Yoshida H, Pan L, Luo JM, Fujisawa S, Naito K, Nakamura S, Shinjo K, Takeshita A, Ohno R, Ohnishi K. Disease-related potential of mutations in transcriptional cofactors CREB-binding protein and p300 in leukemias. Cancer Lett. 2004;213:11–20. doi: 10.1016/S0304-3835(03)00442-7. [DOI] [PubMed] [Google Scholar]


