Abstract
Systemic mastocytosis is a neoplastic proliferation of mast cells that frequently presents with associated clonal hematological non-mast cell lineage disease. Myeloid and lymphoid neoplasms with abnormalities of the FGFR1 gene are a heterogenous group of rare and aggressive hematopoietic stem cell disorders. About a dozen of chromosome changes involving the FGFR1 gene, presenting as myeloid or lymphoid neoplasms, have been described in the literature. To date, only 2 cases of myeloid and lymphoid neoplasms with abnormalities of the FGFR1 gene have been reported in association with systemic mastocytosis, one with t(8;13) and one with t(8;17) involving the FGFR1 gene. Here we describe another case of myeloproliferative neoplasm with chromosome translocation t(8;19) involving FGFR1 gene associated with systemic mastocytosis.
Keywords: Myeloproliferative neoplasm, systemic mastocytosis associated with clonal hematological non-mast cell lineage disease, FGFR1, eosinophilia
Introduction
Systemic mastocytosis, defined as a clonal proliferation of mast cells characterized by the accumulation of multifocal clusters of abnormal mast cells within multiple organ systems, is a distinct entity of myeloproliferative neoplasm in the 2008 World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues [1]. As many as 40% cases of systemic mastocytosis are associated with clonal hematological, non-mast cell lineage disease (SM-AHNMD) [2]. Genetically, a somatic activating point mutation of the KIT proto-oncogene is seen in the majority of cases of systemic mastocytosis. The prognosis of SM-AHNMD is dependent on the associated hematological neoplasm, therefore, recognizing the AHNMD is critical when diagnosing systemic mastocytosis [2,3].
An uncommon group of myeloid and lymphoid neoplasms have been associated with eosinophilia and chromosome rearrangements involving the platelet derived growth factor receptor alpha (PDGFRA), platelet derived growth factor receptor beta (PDGFRB), or fibroblast derived growth factor receptor 1 (FGFR1) gene, each encoding a tyrosine kinase. The tyrosine kinase becomes constitutively activated when a fusion gene is formed due to a chromosome translocation or insertional mutation. To help recognize this unique group of neoplasm for better clinical management, they are separately classified in the 2008 WHO as “myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB and FGFR1”, although the peripheral blood and bone marrow eosinophilia is not invariably present [4].
Abnormalities of FGFR1 on chromosome 8p11-12 result in rare and aggressive disorders presenting as B or T lymphoblastic leukemia/lymphoma, acute myeloid leukemia, mix phenotype acute leukemia or myeloproliferative neoplasms. The majority of these patients also have blood or bone marrow eosinophlia. These disorders are sometimes referred to as the 8p11-12 myeloproliferative syndrome (EMS) [5]. Patients affected are typically young adults, with a slight male predominance. The peripheral blood usually shows leukocytosis, and blood or bone marrow eosinophilia is seen in 80-90% of cases. The bone marrow generally shows increased cellularity; the liver, spleen, and lymph nodes often contain infiltration by neoplastic cells.
While neoplasms associated with abnormalities PDGFRA and PDGFRB have shown a good response to tyrosine kinase inhibitors, the neoplasms associated with FGFR1 abnormalities appear to be refractory to this mode of treatment. Although rare reports have shown that interferon [6] and tyrosine kinase inhibitors such as PKC142 [7] may potentially be effective in some patients, the prognosis is poor with traditional chemotherapy. Therefore, hematopoietic stem cell transplantation is an earlier consideration for these patients, even for those in the chronic phase of the disease. Here we report the unique case of myeloproliferative neoplasm with chromosome translocation t(8;19)(p12;q13.1) involving the FGFR1 presenting as AHNMD of SM.
Case report
A 68-year-old male presented to his primary care physician with a two year history of fatigue, night sweats, early satiety, and a 45 pound weight loss. He denied any history of infection, allergies, autoimmune diseases, or medication use. Blood counts revealed leukocytosis [white blood cell count (WBC) 41,100/μL], erythrocytosis and thrombocytopenia; and an ultrasound showed splenomegaly (16.7 cm). The patient was referred to Emory University Hospital for further workup and management. A repeat complete blood count showed WBC 41,300 cells/μL, with 26% myelocytes, 2% metamyelocytes, 16% band form neutrophils, 49% segmented neutrophils, 4% lymphocytes, 3% monocytes; hemoglobin 16.6 g/dL, hematocrit 49.6%; and platelet count 106,000/μL. The peripheral blood showed mild red blood cell anisopoikilocytosis, and no significant dysgranulopoiesis. The myeloid to erythroid ratio on bone marrow aspirate smear was 16:1; eosinophils, including eosinophilic myelocytes, as well as mast cells were slightly increased but blasts were not. Flow cytometric immunophenotyping failed to reveal any abnormal cell populations. The bone marrow biopsy showed a cellularity of more than 95%; megakaryocytes were mildly increased with slight nuclear atypia (Figure 1A and 1B). There was no significant fibrosis. Eosinophils were mildly increased. Multiple foci of spindle-shaped cells with moderate amount of eosinophilic cytoplasm were identified (Figure 2A). Immunohistochemistry revealed clusters of spindle-shaped cells to be CD117 (DAKO, Carpinteria, CA) positive mast cells that co-express CD25 (Leica Microsystems, Buffalo Grove, IL), consistent with systemic mastocytosis (Figure 2B, 2C). A KIT D816V activating point mutation was detected by allele-specific polymerase chain reaction (PCR) analysis (Roche Molecular Systems, Inc.). Real time quantitative allele-specific PCR analysis for JAK2 V617F mutation was negative. PCR analysis of BCR/ABL1 (reverse transcription real-time quantitative PCR) and BCL2/IGH translocation (real-time PCR with capillary electrophoresis) were both negative.
Figure 1.
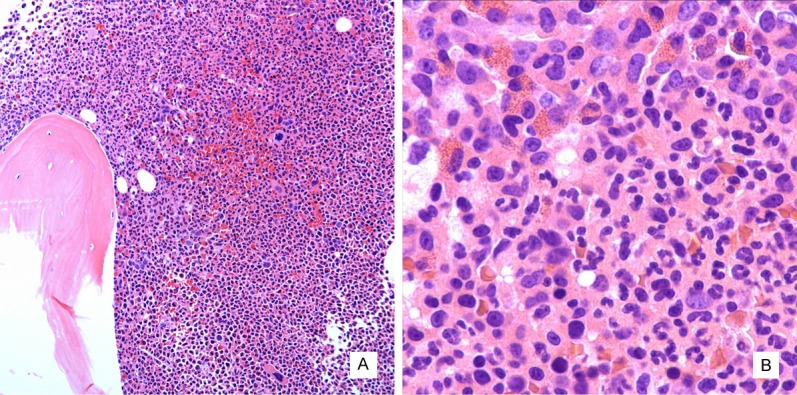
Histomorphology of bone marrow biopsy. The marrow is hypercellular with increased myeloid to erythroid ratio and atypical megakaryocytes (A, hematoxylin and eosin stain, 100x). Eosinophils including immature eosinophilic myelocytes are focally increased (B, hematoxylin and eosin stain, 400x).
Figure 2.
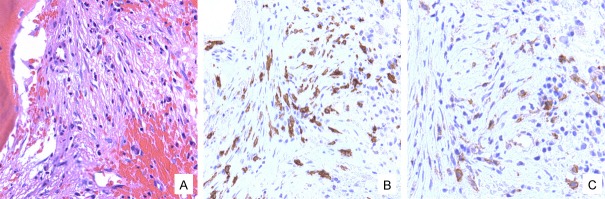
A representative aggregate of atypical spindle-shaped mast cells in the bone marrow (A, hematoxylin and eosin stain, 200x) that are positive for CD117 (B, 400x) and CD25 (C, 400x).
Chromosome analysis demonstrated the presence of abnormal metaphases with a reciprocal translocation between the short arm of chromosome 8 and the long arm of chromosome 19, consistent with t(8;19)(p12;q13.1) (Figure 3A). Fluorescence in-situ hybridization (FISH) testing on directly-prepared bone marrow aspirate smear with FGFR1 dual color breakapart probe (Kreatech Diagnostics North America, Durham, NC) confirmed the presence of FRGR1 gene rearrangement secondary to chromosome translocation t(8;19). FISH analysis using a panel of 6 DNA probes [D5S630 and EGR1 for deletion 5q or monosomy 5; D7Z1 and D7S486 for deletion 7q or monosomy 7; D8Z2 for trisomy 8; and MLL for translocation involving 11q23 (Abbott Molecular, Inc., Cytocell, Ltd.)] failed to detect these common abnormalities associated with myeloid neoplasms.
Figure 3.
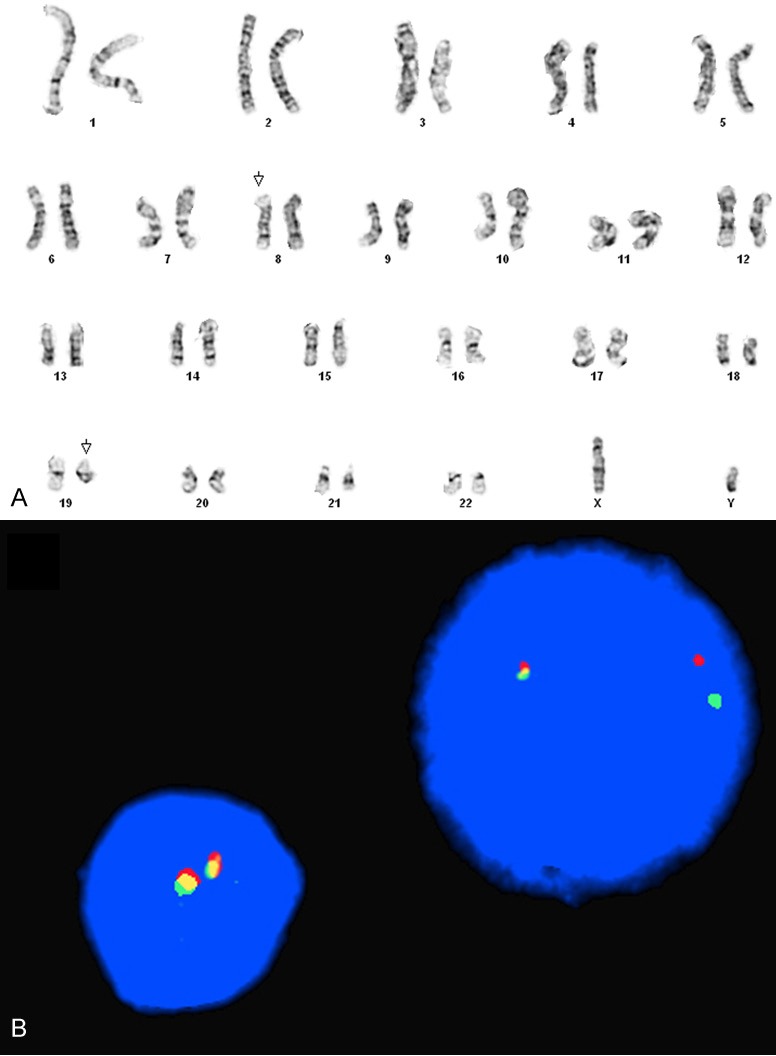
Chromosome analysis of the bone marrow aspirate demonstrated an abnormal karyotype with chromosome translocation t(8;19)(p12;q13.1) (A). FISH analysis confirmed the presence of FGFR1 gene rearrangement as shown by an interphase nucleus with one red, one green and one fusion signal pattern (B).
Taken together, in the background of a myeloproliferative neoplasm associated with t(8;19), this patient concurrently had systemic mastocytosis best diagnosed SM-AHNMD. The patient has since undergone HLA-matched, allogeneic stem cell transplantation following conditioning with fludarabine/melphalan. He is currently in clinical and cytogenetic remission, eighteen months after transplantation and two and half years after initial diagnosis.
Discussion
Systemic mastocytosis is a clonal neoplastic proliferation of mast cells involving multiple organs. According to the 2008 WHO, its diagnosis requires one major criterion and one minor criterion or at least three minor criteria [1]. The major criterion is defined as the presence of multifocal dense mast cell infiltrates (15 or more mast cells per aggregate) detected histologically in the bone marrow or another extracutaneous organ(s). The three minor criteria include: (1) 25% or more of the mast cells in the infiltrate are spindle-shaped or atypical or 25% or more of the mast cells are immature or atypical on bone marrow aspirate smear; (2) persistent elevation of serum tryptase level greater than 20 ng/mL; (3) demonstration of the characteristic KIT D816V mutation; and (4) expression of CD2, CD25 or both in the neoplastic mast cells. Systemic mastocytosis associated with clonal hematological, non-mast cell lineage disease (SM-AHNMD) is the most common subtype [1-3,8], comprising up to 40% of systemic mastocytosis.
Myeloid and lymphoid neoplasms with FGFR1 abnormalities are rare, with approximately 80 cases reported in the literature. The translocation partners of FGFR1 are quite variable. About a dozen of fusion partners have been identified deriving from 8p11-12 translocations. These include TPR at 1q25, RANBP2 (NUP358) at 2q12, LRRFIP1 at 2q37, FGFR1OP1 (POP) at 6q27, CUX1 at 7q22, TRIM24 (TIF) at 7q34, CEP110 at 9q33, NUP98 at 11p15, FGFR1OP2 at 12p11, CPSF6 at 12q15, ZMYM2 (ZNF198) at 13q12, MYO18A at 17q23, HERVK at 19q13 and BCR at 22q11 [5,8-19]. The transforming potential of the abnormal FGFR1 fusion proteins has been confirmed by experimental studies in several fusion proteins derived from the commonly associated rearrangements: ZMYM2(ZNF198)-FGFR1 from t(8;13)(p11;q12), CEP110-FGFR1 from t(8;9)(p11;q33), FGFR1OP1-FGFR1 from t(6;8)(q27;p11-12), and BCR-FGFR1 from t(8;22)(p11;q11) [20-25]. The other fusion partners are rarer, with only one or two reported cases associated with each of them. Most cases of myeloid and lymphoid neoplasms associated with FGFR1 rearrangement were diagnosed by cytogenetic analysis; some were confirmed by molecular studies. Only one case of a myeloid neoplasm with t(8;19)(p12;q13.3) has been reported thus far. The patient presented with a paraneoplastic syndrome and was diagnosed with acute myeloid leukemia with minimal differentiation (AML-M0), probably secondary to a myeloproliferative disorder, with a high hemoglobin level (17 g/dL), but without leukocytosis or eosinophilia. The chromosome location was identified as 8p12, nonetheless, the involvement of FGFR1 in the reciprocal translocation was confirmed by dual-color FISH with the FGFR1-specific probes [15]. The partner gene located at 19q13.3 was later recognized as human endogen retrovirus gene (HERVK), although the transforming activity has not yet been confirmed in any study [16].
Myeloid and lymphoid neoplasms with FGFR1 abnormalities presenting as clonal hematological, non-mast cell lineage disease of systemic mastocytosis are extremely rare. Lewis et al reported the first association in a 29-year-old woman who initially presented with urticaria pigmentosa [26]. She then progressed to systemic mastocytosis about 3-4 years later, and shortly after that she was diagnosed with atypical chronic myeloid leukemia, BCR-ABL1-negative. Cytogenetic studies demonstrated the presence of chromosome translocation t(8;17) in all 20 bone marrow metaphases analyzed, presumably involving FGFR1 gene at 8p11-12. More recently, Mayeur-Rousse et al reported another case of systemic mastocytosis with associated myeloproliferative neoplasm in blast crisis and chromosomal translocation t(8;13)(p11;q12) [ZMYM2(ZNF198)-FGFR1] [27]. The patient was a 31-years-old man who presented with leukocytosis and absolute eosinophilia. A bone marrow biopsy demonstrated myeloproliferative neoplasm in myeloblast crisis. Cytogenetic analysis demonstrated chromosome translocation t(8;13)(p11;q12). Molecular studies confirmed the presence of ZMYM2(ZNF198)-FGFR1 fusion gene. In addition, a spindled mast cell population was identified in the bone marrow as well as in a cervical lymph node. These atypical mast cells were positive for CD25. Though KIT D816V mutation was not detected, the morphologic and immunohistochemical findings were diagnostic of systemic mastocytosis. The patient expired shortly after initial diagnosis.
Our case was diagnosed as a myeloproliferative neoplasm based on the clinical presentation of splenomegaly, and laboratory findings of leukocytosis with myeloid precursors in the peripheral blood, hypercellular bone marrow with myeloid hyperplasia, and clonal evidence of reciprocal chromosome translocation t(8;19)(p12;q13.1). Blasts and eosinophils were not significantly increased in either blood or bone marrow though focal increase in eosinophils was noted on the bone marrow core biopsy. Systemic mastocytosis was diagnosed according to published WHO criteria; one major criterion and two minor criteria (abnormal phenotype of mast cells, and KIT D816V mutation) were present at diagnosis.
Our case reported here has a reciprocal translocation t(8;19)(p12;q13.1) with involvement of FGFR1 gene confirmed by FISH analysis. The breakpoint on chromosome 19 is at q13.1, suggesting that the same fusion gene FGFR1-HERVK would most likely be derived from the reciprocal translocation. Whether the concurrent systemic mastocytosis is biologically associated with t(8;19)(p12;q13.1) or is simply coincidental has yet to be determined. However, the question cannot be answered until there are more clinical cases diagnosed with this specific translocation.
Myeloid and lymphoid neoplasms with FGFR1 abnormalities usually have poor prognoses. It is predicted that most patients with the myeloproliferative neoplasm will eventually transform to acute leukemia. Hematopoietic stem cell transplantation is currently the only chance to cure the neoplasm. The patient described in this case report received HLA-matched related donor allogeneic stem cell transplantation and is currently in clinical and cytogenetic remission. Long term followup will be required to monitor whether the malignant clone is actually eradicated by transplantation.
Disclosure of conflict of interest
None.
References
- 1.Horny HP, Bennett JM, Bain BJ. Mastocytosis. In: Swerdlow SH, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th edition. Lyon, France: IARC press; 2008. pp. 54–63. [Google Scholar]
- 2.Pardanani A, Lim KH, Lasho TL, Finke C, McClure RF, Li CY, Tefferi T. Prognostically relevant breakdown of 123 patients with systemic mastocytosis associated with other myeloid malignancies. Blood. 2009;114:3769–72. doi: 10.1182/blood-2009-05-220145. [DOI] [PubMed] [Google Scholar]
- 3.Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, McClure RF, Li CY, Pardanani A. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113:5727–36. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 4.Bain BJ, Horny HP, Vardiman JW. Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB or FGFR1. In: Swerdlow SH, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th edition. Lyon, France: IARC Press; 2008. pp. 68–78. [Google Scholar]
- 5.Jackson CC, Medeiros LJ, Miranda RN. 8p11 myeloproliferative syndrome: a review. Hum Pathol. 2010;41:461–76. doi: 10.1016/j.humpath.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Climent JA, Vizcarra E, Benet I, Marugan I, Terol MJ, Solano C, Arbona C, Tormo M, Comes AM, Garcia-Conde J. Cytogenetic response induced by interferon alpha in the myeloproliferative disorder with eosinophilia, T cell lymphoma and the chromosomal translocation t(8;13)(p11;q12) Leukemia. 1998;12:999–1000. doi: 10.1038/sj.leu.2401029. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Deangelo DJ, Kutok JL, Williams IR, Lee BH, Wadleigh M, Duclos N, Cohen S, Adelsperger J, Okabe R, Coburn A, Galinsky I, Huntly B, Cohen PS, Meyer T, Fabbro D, Roesel J, Banerji L, Griffin JD, Xiao S, Fletcher JA, Stone RM, Gilliland DG. PKC412 inhibits the zinc finger 198-fibroblast growth factor receptor 1 fusion tyrosine kinase and is active in treatment of stem cell myeloproliferative disorder. Proc Natl Acad Sci U S A. 2004;101:14479–84. doi: 10.1073/pnas.0404438101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SY, Oh B, She CJ, Kim HK, Jeon YK, Shin MG, Yoon SS, Lee DS. 8p11 Myeloproliferative syndrome with BCR-FGFR1 rearrangement presenting with T-lymphoblastic lymphoma and bone marrow stromal cell proliferation: a case report and review of the literature. Leuk Res. 2011;35:e30–4. doi: 10.1016/j.leukres.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Wakim JJ, Tirado CA, Chen W, Collins R. t(8;22)/BCR-FGFR1 myeloproliferative disorder presenting as B-acute lymphoblastic leukemia: report of a case treated with sorafenib and review of the literature. Leuk Res. 2011;35:e151–3. doi: 10.1016/j.leukres.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Onozawa M, Ohmura K, Ibata M, Iwasaki J, Okada K, Kasahara I, Yamaguchi K, Kubota K, Fujisawa S, Shigematsu A, Endo T, Kondo T, Hashino S, Tanaka J, Matsuno Y, Asaka M, Imamura M. The 8p11 myeloproliferative syndrome owing to rare FGFR1OP2-FGFR1 fusion. Eur J Haematol. 2011;86:347–9. doi: 10.1111/j.1600-0609.2010.01568.x. [DOI] [PubMed] [Google Scholar]
- 11.Hu S, He Y, Zhu X, Li J, He H. Myeloproliferative disorders with t(8;9)(p12;q33): a case report and review of the literature. Pediatr Hematol Oncol. 2011;28:140–6. doi: 10.3109/08880018.2010.528170. [DOI] [PubMed] [Google Scholar]
- 12.Patnaik MM, Gangat N, Knudson RA, Keefe JG, Hanson CA, Pardanani A, Ketterling RP, Tefferi A. Chromosome 8p11.2 translocations: prevalence, FISH analysis for FGFR1 and MYST3, and clinicopathologic correlates in a consecutive cohort of 13 cases from a single institution. Am J Hematol. 2010;85:238–42. doi: 10.1002/ajh.21631. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Zhang Y, Li Y, Lei P, Zhai Y, Liu L. Biphenotypic hematologic malignancy: a case report of the 8p11 myeloproliferative syndrome in a child. J Pediatr Hematol Oncol. 2010;32:501–3. doi: 10.1097/MPH.0b013e3181e413fa. [DOI] [PubMed] [Google Scholar]
- 14.Baldazzi C, Iacobucci I, Luatti S, Ottaviani E, Marzocchi G, Paolini S, Stacchini M, Papayannidis C, Gamberini C, Martinelli G, Baccarani M, Testoni N. B-cell acute lymphoblastic leukemia as evolution of a 8p11 myeloproliferative syndrome with t(8;22)(p11;q11) and BCR-FGFR1 fusion gene. Leuk Res. 2010;34:e282–5. doi: 10.1016/j.leukres.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Mugneret F, Chaffanet M, Maynadie M, Guasch G, Favre B, Casasnovas O, Birnbaum D, Pebusque MJ. The 8p12 myeloproliferative disorder. t(8;19)(p12;q13.3): a novel translocation involving the FGFR1 gene. Br J Haematol. 2000;111:647–9. doi: 10.1046/j.1365-2141.2000.02355.x. [DOI] [PubMed] [Google Scholar]
- 16.Guasch G, Popovici C, Mugneret F, Chaffanet M, Pontarotti P, Birnbaum D, Pebusque MJ. Endogenous retroviral sequence is fused to FGFR1 kinase in the 8p12 stem-cell myeloproliferative disorder with t(8;19)(p12;q13.3) Blood. 2003;101:286–8. doi: 10.1182/blood-2002-02-0577. [DOI] [PubMed] [Google Scholar]
- 17.Gervais C, Dano L, Perrusson N, Helias C, Jeandidier E, Galoisy AC, Ittel A, Herbrecht R, Bilger K, Mauvieux L. A translocation t(2;8)(q12;p11) fuses FGFR1 to a novel partner gene, RANBP2/NUP358, in a myeloproliferative/myelodysplastic neoplasm. Leukemia. 2013;27:1186–8. doi: 10.1038/leu.2012.286. [DOI] [PubMed] [Google Scholar]
- 18.Li F, Zhai YP, Tang YM, Wang LP, Wan PJ. Identification of a novel partner gene, TPR, fused to FGFR1 in 8p11 myeloproliferative syndrome. Genes Chromosomes Cancer. 2012;51:890–7. doi: 10.1002/gcc.21973. [DOI] [PubMed] [Google Scholar]
- 19.Lee H, Kim M, Lim J, Kim Y, Han K, Cho BS, Kim HJ. Acute myeloid leukemia associated with FGFR1 abnormalities. Int J Hematol. 2013;97:808–12. doi: 10.1007/s12185-013-1337-5. [DOI] [PubMed] [Google Scholar]
- 20.Ollendorff V, Guasch G, Isnardon D, Galindo R, Birnbaum D, Pebusque MJ. Characterization of FIM-FGFR1, the fusion product of the myeloproliferative disorder-associated t(8;13) translocation. J Biol Chem. 1999;274:26922–30. doi: 10.1074/jbc.274.38.26922. [DOI] [PubMed] [Google Scholar]
- 21.Guasch G, Mack GJ, Popovici C, Dastugue N, Birnbaum D, Rattner JB, Pebusque MJ. FGFR1 is fused to the centrosome-associated protein CEP110 in the 8p12 stem cell myeloproliferative disorder with t(8;9)(p12;q33) Blood. 2000;95:1788–96. [PubMed] [Google Scholar]
- 22.Guasch G, Ollendorff V, Borg JP, Birnbaum D, Pebusque MJ. 8p12 stem cell myeloproliferative disorder: the FOP-fibroblast growth factor receptor 1 fusion protein of the t(6;8) translocation induces cell survival mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt/mTOR pathways. Mol Cell Biol. 2001;21:8129–42. doi: 10.1128/MCB.21.23.8129-8142.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demiroglu A, Steer EJ, Heath C, Taylor K, Bentley M, Allen SL, Koduru P, Brody JP, Hawson G, Rodwell R, Doody ML, Carnicero F, Reiter A, Goldman JM, Melo JV, Cross NC. The t(8;22) in chronic myeloid leukemia fuses BCR to FGFR1: transforming activity and specific inhibition of FGFR1 fusion proteins. Blood. 2001;98:3778–83. doi: 10.1182/blood.v98.13.3778. [DOI] [PubMed] [Google Scholar]
- 24.Agerstam H, Jaras M, Andersson A, Johnels P, Hansen N, Lassen C, Rissler M, Gisselsson D, Olofsson T, Richter J, Fan X, Ehinger M, Fioretos T. Modeling the human 8p11-myeloproliferative syndrome in immunodeficient mice. Blood. 2010;116:2103–11. doi: 10.1182/blood-2009-05-217182. [DOI] [PubMed] [Google Scholar]
- 25.Ren M, Cowell JK. Constitutive Notch pathway activation in murine ZMYM2-FGFR1-induced T-cell lymphomas associated with atypical myeloproliferative disease. Blood. 2011;117:6837–47. doi: 10.1182/blood-2010-07-295725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis JP, Welborn JL, Meyers FJ, Levy NB, Roschak T. Mast cell disease followed by leukemia with clonal evolution. Leuk Res. 1987;11:769–73. doi: 10.1016/0145-2126(87)90060-9. [DOI] [PubMed] [Google Scholar]
- 27.Mayeur-Rousse C, Sorel N, Voldoire M, Canioni D, Brizard F, Randriamalala E, Turhan AG, Chomel JC. Unique association of systemic mastocytosis and myeloid/lymphoid neoplasm in blast crisis with abnormality of FGFR1 gene. Leuk Res. 2012;36:377–81. doi: 10.1016/j.leukres.2011.10.009. [DOI] [PubMed] [Google Scholar]


