Abstract
IgG4-related sclerosing disease is an established disease entity with characteristic clinicopathological features. Some recent reports have demonstrated that this disease can occur in the respiratory system including the pleura. Herein, we describe the first documented case of concomitant occurrence of IgG4-related pleuritis and periaortitis. A 71-year-old Japanese female with a history of essential thrombocythemia presented with persistent cough and difficulty in breathing. Computed tomography demonstrated thickening of the right parietal pleura, pericardium, and periaortic tissue and pleural and cardiac effusions. Histopathological study of the surgical biopsy specimen of the parietal pleura revealed marked fibrous thickening with lymphoplasmacytic infiltration. Phlebitis was noted, however, only a few eosinophils had infiltrated. Immunohistochemical study revealed abundant IgG4-positive plasma cell infiltration and high ratio of IgG4-/IgG-positive plasma cells (84%). Therefore, a diagnosis of IgG4-related pleuritis was made with consideration of the elevated serum IgG4 level (684 mg/dL). Recently, the spectrum of IgG4-related sclerosing disease has expanded, and this disease can occur in the pleura, pericardium, and periaortic tissue. Although histopathological analysis of the pericardium and periaortic tissue was not performed in the present case, it was suspected that thickening of the pericardium and periaortic tissue was clinically due to IgG4-related sclerosing disease. Our clinicopathological analyses of IgG4-related pleuritis and pericarditis reveal that this disease can present as dyspnea and pleural and pericardial effusion as seen in the present case, therefore, it is important to recognize that IgG4-related sclerosing disease can occur in these organs for accurate diagnosis and treatment.
Keywords: IgG4-related sclerosing disease, pleuritis, periaortitis
Introduction
IgG4-related sclerosing disease is an established systemic fibroinflammatory disease characterized clinically by the formation of tumor-like lesions and elevated serum IgG4 concentration [1-3], and histopathologically by the presence of fibrosclerosis and dense lymphoplasmacytic infiltration with abundant IgG4-positive plasma cells and high IgG4/IgG-positive plasma cell ratio accompanied by eosinophilic infiltration and obliterative phlebitis [2-5]. This disease entity was first established in sclerosing pancreatitis (autoimmune pancreatitis) [1]. Since then, it has been recognized that this disorder can occur in various organs including the liver, bile duct, gallbladder, nasal cavity, salivary gland, lacrimal gland, aorta, kidney, pituitary gland, and retroperitoneum [4-20]. Recently, this disease was also demonstrated in the respiratory system [21-26]. Taniguchi et al. reported the first documented case of interstitial pneumonia associated with IgG4-related autoimmune pancreatitis, and they demonstrated an infiltration of IgG4-positive plasma cells in the alveolar septum [21]. Zen et al. reported that inflammatory pseudotumor (plasma cell granuloma) of the lung shows the same histopathological and immunohistochemical features of IgG4-related sclerosing pancreatitis, and this condition is also included in the spectrum of IgG4-related sclerosing disease [22]. Subsequently, Shrestha et al. analyzed the histopathological features of the pulmonary lesions of 6 cases with autoimmune pancreatitis [23]. They demonstrated that these lesions were characterized by the presence of lymphangitic distribution of plasma cell-rich inflammatory infiltration, active fibrosis, remarkable intimal inflammation involving both pulmonary veins and arteries, fibrinous pleuritis, and dense peribronchial inflammation [23]. Zen et al. also analyzed the clinicopathological features of 16 cases of IgG4-related pulmonary disease and 5 cases of pleural lesions, and showed that three of 5 cases of pleural IgG4-related disease had extrapulmonary IgG4-related lesions [24], however, concomitant occurrence of periaortitis has not been reported yet. In this report, we document the first case of concomitant IgG4-related pleuritis and periaortitis, and review the literature.
Case report
A 71-year-old Japanese female, who had never smoked, presented with persistent cough and difficulty in breathing on exertion. She had been suffering from thrombocytosis for approximately 20 years, which was diagnosed as essential thrombocythemia 5 years earlier. She had been administered aspirin and hydroxycarbamide for essential thrombocythemia. A contrast-enhanced computed tomography demonstrated thickening of the right parietal pleura and pericardium, pleural effusion in the right thoracic cavity, and cardiac effusion (Figure 1). Thickening of the periaortic tissue from the aortic arch to the thoracic aorta was also detected, which was suggestive of periaortitis (Figure 1). Mild mediastinal lymph node swelling was also observed. Neither tumorous lesions nor features suggestive of interstitial pneumonia were present in the lung. She had no clinical history of pancreatitis, cholangitis, allergic diseases, and asthma.
Figure 1.
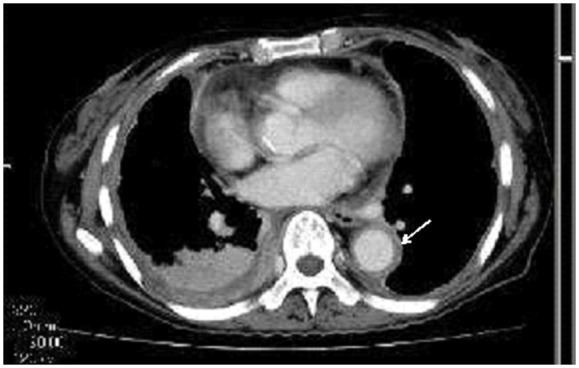
Contrast-enhanced computed tomography showing thickening of the right pleura, pericardium, and periaortic tissue (arrow), as well as pleural and cardiac effusions.
Laboratory tests revealed anemia, thrombocytosis, and elevated soluble interleukin-2 receptor (red blood cells 2.89 × 1012/L (range 3.8-4.8), hemoglobin 8.1 g/L (11.3-15.0), white blood cells 14.9 × 109/L (3.0-8.0), eosinophils 2.6% (<7%), platelets 1047 × 109/L (150-400), soluble interleukin-2 receptor 3,170 U/mL (135-483). Serum IgG was slightly elevated (1,756 mg/dL (range 870-1,700)), and IgG4 level was elevated (684 mg/dL (4.8-105)).
She underwent surgical biopsy of the right parietal pleura to confirm the diagnosis.
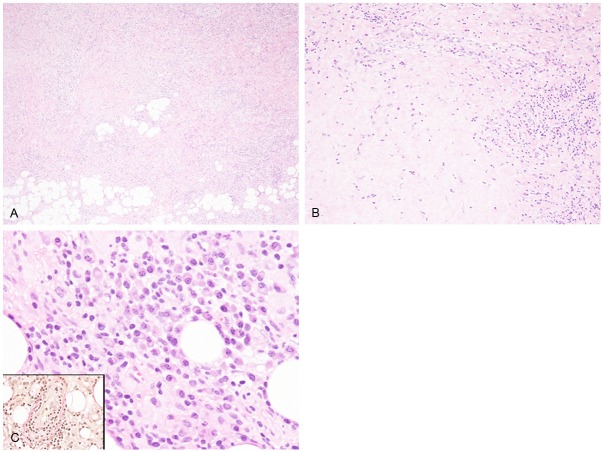
Histopathological study demonstrated marked fibrous thickening of the parietal pleura (Figure 2A, 2B). Dense fibrosis was observed, which extended into the fatty tissue (Figure 2A). Lymphoplasmacytic infiltration was present within the fibrous lesion (Figure 2A, 2B), and small lymphoid follicles were also observed. Lymphocytes were small in size and plasma cells also appeared mature (Figure 2C). Although typical obliterative phlebitis was not noted, phlebitis with subendothelial lymphoplasmacytic infiltration was observed (Figure 2C, inset). Only a few eosinophils had infiltrated into the lesion (1-2 eosinophils/10 high power fields).
Figure 2.
Histopathological features of the right parietal pleura. (A, B) Dense fibrosis of the pleura extending into the fatty tissue. Lymphoplasmacytic infiltration is also observed, HE, × 40 (A), × 100 (B). (C) Lymphocytes and plasma cells are without atypia. HE, × 200. Phlebitis with subendothelial lymphoplasmacytic infiltration is also noted, elastic van Gieson stain, × 200 (inset).
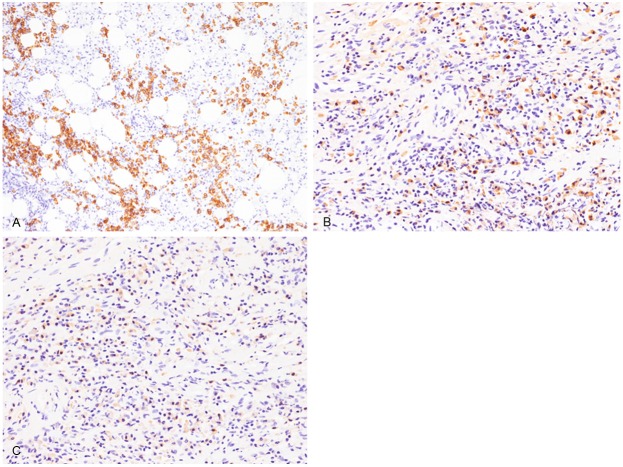
Immunohistochemical and in situ hybridization studies were performed using an autostainer (Ventana) by the same method as previously reported [27-32]. Many CD138-positive plasma cells had infiltrated into the lesion (Figure 3A), as well as relatively abundant CD3-positive T-lymphocytes, and a few CD20-positive B-cells. There was also abundant IgG4- and IgG-positive plasma cells infiltration (Figure 3B, 3C). The IgG4-positive plasma cell count was 273/10 high-power fields and the ratio of IgG4-/IgG-positive plasma cells was 84%. Kappa- and lambda chain-positive plasma cells were evenly distributed by in situ hybridization analyses. Moreover, no EBER-positive cells were detected by in situ hybridization.
Figure 3.
Immunohistochemical features of the right parietal pleura. (A) Abundant CD138-positive cell infiltration, × 100. (B, C) Profuse infiltration of IgG4- (B) and IgG (C)-positive plasma cells, × 200 (A-C).
Accordingly, an ultimate diagnosis of IgG4-related pleuritis was made. Although no biopsy or surgical resection of the pericardium and aorta was performed, the thickening of the pericardium and periaortic tissue was suspected to be clinically due to IgG4-related sclerosing disease.
Thus, steroid therapy (40 mg/day) was administered, which immediately improved the cough and difficulty in breathing. Follow-up computed tomography revealed a mild decrease in the pleural thickening, and the pleural and cardiac effusions were no longer observed.
Discussion
IgG4-related sclerosing disease has been recognized as a distinct clinicopathological disease entity, which has been demonstrated to occur in various organs [4,6]. Recently, this disease has been shown to also involve the respiratory system [21-26], and that IgG4-related lung lesions can occur in the form of tumorous lesions or interstitial pneumonia [21-25]. Moreover, some recent reports revealed that IgG4-related sclerosing disease can occur in the pleura. Table 1 summarizes the clinicopathological features of the previously reported cases of IgG4-related pleuritis as well as the present one. Zen et al. reported a series of 5 cases of IgG4-related pleuritis [24], and the remaining cases were single case reports [25,26,33-35]. IgG4-related sclerosing disease frequently affected middle-aged males (average 62.7 years, range from 29 to 78 years; male: female 8: 3), however, this disease can also occur in young female (29-years-old) [35]. The most common complaint was dyspnea, and pleural thickening and effusion were the most frequent signs of this disease. Mediastinal lymph node swelling and lung nodules were observed in 3 cases. Pericardial thickening and effusion, and pericarditis were observed only in the present case, although 5 cases had non-pleural and non-pulmonary IgG4-related sclerosing diseases. Steroid therapy was effective in all cases, in whom information regarding therapeutic effects was available. The characteristic histopathological features of IgG4-related pleuritis are as follows: i) the pleura is severely thickened by diffuse sclerosing inflammation, and extends into the subpleural fibrous and fatty tissue, ii) inflammation consists of lymphocytes and plasma cells, iii) eosinophilic infiltration is frequently seen, but neutrophilic infiltration is rare, iv) obliterative phlebitis is commonly found, and v) abundant IgG4-positive plasma cell infiltration is observed, and the ratio of IgG4-/IgG-positive plasma cells is high (>40%) [24]. Although only a small number of eosinophils had infiltrated and typical obliterative phlebitis was not present (but phlebitis with subendothelial lymphoplasmacytic infiltration was noted) in the present case, the remaining typical features were observed. Therefore, an ultimate diagnosis of IgG4-related pleuritis was made with consideration of the elevated serum IgG4 (684 mg/dL).
Table 1.
Clinicopathological features of IgG4-related pleuritis
| Case No. | Age | Gender | Initial presentation | Location | Associated diseases | Serum IgG-4 (mg/dL) | Steroid treatment | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 65 | Male | Pleural effusion and swelling of the mediastinal lymph nodes | Left pleural effusion, lung nodules, and mediastinal lymph node swelling. | Mikulicz’s disease | 1,194 | Effective | [33] |
| 2 | 63 | Female | Dyspnea | Bilateral pleural and pericardium effusions with pericardial thickening. Mediastinal lymph node swelling. | Autoimmune pancreatitis, Hashimoto thyroiditis | 420 | Effective | [34] |
| 3 | 74 | Male | Dyspnea | Pleural effusion, nodular lesion in the lung, as well as extensive pleural thickening and adhesion. | None | Not available | Not available | [25] |
| 4-8 | 49-76 | Males | Not available | Pleura in 4 cases, lung and pleura in 1 case. | 3/5 cases (+). | Not available | Not available | [24] |
| 9 | 78 | Male | General fatigue and fever | Bilateral pleural effusion and thickening. | None | 483 | Not performed | [26] |
| 10 | 29 | Female | Chest pain and dyspnea | Bilateral pleural thickening and effusion | None | 136 | Effective | [35] |
| Present case | 71 | Female | Cough and difficulty in breathing | Pleural thickening and effusion, pericardial thickening and effusion, and mediastinal lymph node swelling. | None | 684 | Effective |
In 2008, Kasashima et al. reported a close relationship between IgG4-related sclerosing disease and inflammatory abdominal aortic aneurysm, and they concluded that inflammatory abdominal aortic aneurysm can be classified as IgG4-related and non-IgG4-related [11]. Subsequently, it has been demonstrated that IgG4-related inflammatory aortic aneurysm can occur in the aortic arch [13]. The characteristic histopathological features of IgG4-related aortic aneurysm are as follows: i) diffuse fibrous thickening of the adventitia of the aorta (>4 mm), ii) abundant lymphoplasmacytic infiltration with frequent eosinophilic infiltration, iii) abundant IgG4-positive plasma cell infiltration, and iv) the ratio of IgG4-/IgG-positive plasma cells is usually more than 60% [11,13,36]. This disease accounts for 5% of all surgically resected abdominal aortic aneurysm, and 57% of inflammatory abdominal aortic aneurysm [11], as well as 7% of thoracic aortic aneurysms [37]. Moreover, IgG4-related aortic aneurysm has been recognized as a manifestation of IgG4-related chronic periaortitis, which encompasses idiopathic retroperitoneal fibrosis, inflammatory aortic aneurysm, and perianeurysmal retroperitoneal fibrosis [38]. Both retroperitoneal fibrosis and IgG4-related inflammatory abdominal aortic aneurysm are recognized as IgG4-related chronic periaortitis, together with mediastinal fibrosis and IgG4-related inflammatory aortic aneurysm of the thoracic aorta [11,13,36]. In the present case, although no histopathological analysis of the aorta was performed, the periaortic lesion was suspected clinically to be IgG4-related chronic periaortitis.
In the present case, pericarditis was also observed in the clinical imaging analysis. IgG4-related pericarditis has also been reported as a spectrum of IgG4-related sclerosing disease [39-41], and the pericardium showed the same histopathological and immunohistochemical features as other IgG4-related sclerosing diseases [39,41]. Moreover, a case with massive pleural and pericardial effusions and thickening of the pericardium has been reported [41]. Therefore, thickening of the pericardium of the present case is suspected to be due to IgG4-related sclerosing disease.
In conclusion, we describe the first documented case of IgG4-related pleuritis and periaortitis. It is important to recognize that IgG4-related sclerosing disease can occur in the pleura, periaortic tissue, and pericardium, and can clinically present as dyspnea and pleural and pericardial effusions. Therefore, detailed histopathological and immunohistochemical analyses and measurement of serum IgG4 are required for correct diagnosis and treatment.
References
- 1.Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 2.Sato Y, Notohara K, Kojima M, Takata K, Masaki Y, Yoshino T. IgG4-related disease: historical overview and pathology of hematological disorders. Pathol Int. 2010;60:247–258. doi: 10.1111/j.1440-1827.2010.02524.x. [DOI] [PubMed] [Google Scholar]
- 3.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–551. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 4.Zen Y, Nakanuma Y. IgG4-related disease. A cross-sectional study of 114 cases. Am J Surg Pathol. 2010;34:1812–1819. doi: 10.1097/PAS.0b013e3181f7266b. [DOI] [PubMed] [Google Scholar]
- 5.Zen Y, Nakanuma Y. Pathogenesis of IgG4-related disease. Curr Opin Rheumatol. 2011;23:114–118. doi: 10.1097/BOR.0b013e3283412f4a. [DOI] [PubMed] [Google Scholar]
- 6.Kamisawa T, Okamoto A. IgG4-related sclerosing disease. World J Gastroenterol. 2008;14:3948–3955. doi: 10.3748/wjg.14.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Morimoto H, Miwa A, Uchiyama A, Portmann BC, Nakanuma Y. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004;28:1193–1203. doi: 10.1097/01.pas.0000136449.37936.6c. [DOI] [PubMed] [Google Scholar]
- 8.Hamano H, Kawa S, Ochi Y, Unno H, Shiba N, Wajiki M, Nakazawa K, Shimojo H, Kiyosawa K. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. Lancet. 2002;359:1403–1404. doi: 10.1016/s0140-6736(02)08359-9. [DOI] [PubMed] [Google Scholar]
- 9.Kitagawa S, Zen Y, Harada K, Sasaki M, Sato Y, Minato H, Watanabe K, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Tsuneyama K, Saito K, Haratake J, Takagawa K, Nakanuma Y. Abundant IgG4-positive plasma cell infiltration characterizes chronic sclerosing sialadenitis (Küttner’s tumor) Am J Surg Pathol. 2005;29:783–791. doi: 10.1097/01.pas.0000164031.59940.fc. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M, Takahashi H, Ohara M, Suzuki C, Naishiro Y, Yamamoto H, Shinomura Y, Imai K. A new conceptualization for Mikulicz’s disease as an IgG4-related plasmacytic disease. Mod Rheumatol. 2006;16:335–340. doi: 10.1007/s10165-006-0518-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasashima S, Zen Y, Kawashima A, Konishi K, Sasaki H, Endo M, Matsumoto Y, Kawakami K, Kasashima F, Moriya M, Kimura K, Ohtake H, Nakanuma Y. Inflammatory abdominal aortic aneurysm: close relationship to IgG4-related periaortitis. Am J Surg Pathol. 2008;32:197–204. doi: 10.1097/PAS.0b013e3181342f0d. [DOI] [PubMed] [Google Scholar]
- 12.Kasashima S, Zen Y, Kawashima A, Endo M, Matsumoto Y, Kasashima F. A new clinicopathological entity of IgG4-related inflammatory abdominal aortic aneurysm. J Vasc Surg. 2009;49:1264–1271. doi: 10.1016/j.jvs.2008.11.072. [DOI] [PubMed] [Google Scholar]
- 13.Ishida M, Hotta M, Kushima R, Asai T, Okabe H. IgG4-related inflammatory aneurysm of the aortic arch. Pathol Int. 2009;59:269–273. doi: 10.1111/j.1440-1827.2009.02363.x. [DOI] [PubMed] [Google Scholar]
- 14.Kasashima S, Zen Y, Kawashima A, Endo M, Matsumoto Y, Kasashima F, Ohtake H, Nakanuma Y. A clinicopathologic study of immunoglobulin G4-related sclerosing disease of the thoracic aorta. J Vasc Surg. 2010;52:1587–1595. doi: 10.1016/j.jvs.2010.06.072. [DOI] [PubMed] [Google Scholar]
- 15.Kasashima S, Zen Y. IgG4-related inflammatory abdominal aortic aneurysm. Curr Opin Rheumatol. 2011;23:18–23. doi: 10.1097/BOR.0b013e32833ee95f. [DOI] [PubMed] [Google Scholar]
- 16.Zen Y, Kasashima S, Inoue D. Retroperitoneal and aortic manifestations of immunoglobulin G4-related disease. Semin Diagn Pathol. 2012;29:212–218. doi: 10.1053/j.semdp.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Kawano M, Mizushima I, Yamaguchi Y, Imai N, Nakashima H, Nishi S, Hisano S, Yamanaka N, Yamamoto M, Takahashi H, Umehara H, Saito T, Saeki T. Immunohistochemical characteristics of IgG4-related tubulointerstitial nephritis: Detailed analysis of 20 Japanese cases. Int J Rheumatol. 2012;2012:609795. doi: 10.1155/2012/609795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong S, Lam WY, Wong WK, Lee KC. Hypophysitis presented as inflammatory pseudotumor in immunoglobulin G4-related systemic disease. Hum Pathol. 2007;38:1720–1723. doi: 10.1016/j.humpath.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Cheuk W, Yuen HK, Chan JK. Chronic sclerosing dacryoadenitis: Part of the spectrum of IgG4-related sclerosing disease? Am J Surg Pathol. 2007;31:643–645. doi: 10.1097/01.pas.0000213445.08902.11. [DOI] [PubMed] [Google Scholar]
- 20.Ishida M, Hotta M, Kushima R, Shibayama M, Shimizu T, Okabe H. Multiple IgG4-related sclerosing lesions in the maxillary sinus, parotid gland and nasal septum. Pathol Int. 2009;59:670–675. doi: 10.1111/j.1440-1827.2009.02425.x. [DOI] [PubMed] [Google Scholar]
- 21.Taniguchi T, Ko M, Seko S, Nishida O, Inoue F, Kobayashi H, Saiga T, Okamoto M, Fukuse T. Interstitial pneumonia associated with autoimmune pancreatitis. Gut. 2004;53:770. [PMC free article] [PubMed] [Google Scholar]
- 22.Zen Y, Kitagawa S, Minato H, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Fujimura M, Nakanuma Y. IgG4-positive plasma cells in inflammatory pseudotumor (plasma cell granuloma) of the lung. Hum Pathol. 2005;36:710–717. doi: 10.1016/j.humpath.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Shrestha B, Sekiguchi H, Colby TV, Graziano P, Aubry MC, Smyrk TC, Feldman AL, Cornell LD, Ryu JH, Chari ST, Dueck AC, Yi ES. Distinctive pulmonary histopathology with increased IgG4-positive plasma cells in patients with autoimmune pancreatitis: Report of 6 and 12 cases with similar histopathology. Am J Surg Pathol. 2009;33:1450–1462. doi: 10.1097/PAS.0b013e3181ac43b6. [DOI] [PubMed] [Google Scholar]
- 24.Zen Y, Inoue D, Kitao A, Onodera M, Abo H, Miyayama S, Gabata T, Matsui O, Nakanuma Y. IgG4-related lung and pleural disease: A clinicopathologic study of 21 cases. Am J Surg Pathol. 2009;33:1886–1893. doi: 10.1097/PAS.0b013e3181bd535b. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita K, Haga H, Kobashi Y, Miyagawa-Hayashi A, Yoshizawa A, Manabe T. Lung involvement in IgG4-related lymphoplasmacytic vasculitis and interstitial fibrosis: Report of 3 cases and review of the literature. Am J Surg Pathol. 2008;32:1620–1626. doi: 10.1097/PAS.0b013e318172622f. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto H, Suzuki T, Yasuo M, Kobayashi O, Tsushima K, Ito M, Urushihata K, Yamazaki Y, Hanaoka M, Koizumi T, Uehara T, Kawaskami S, Hamano H, Kawa S, Kubo K. IgG4-related pleural disease diagnosed by a re-evaluation of chronic bilateral pleuritis in a patient who experienced occasional acute left bacterial pleuritis. Intern Med. 2011;50:893–897. doi: 10.2169/internalmedicine.50.4726. [DOI] [PubMed] [Google Scholar]
- 27.Ishida M, Fukami T, Nitta N, Iwai M, Yoshida K, Kagotani A, Nozaki K, Okabe H. Xanthomatous meningioma: a case report with review of the literature. Int J Clin Exp Pathol. 2013;6:2242–2246. [PMC free article] [PubMed] [Google Scholar]
- 28.Ishida M, Hodohara K, Yoshii M, Okuno H, Nakanishi R, Horinouchi A, Nakanishi R, Harada A, Iwai M, Yoshida K, Kagotani A, Yoshida T, Okabe H. Methotrexate-related Epstein-Barr virus-associated lymphoproliferative disorder occurring in the gingiva of a patient with rheumatoid arthritis. Int J Clin Exp Pathol. 2013;6:2237–2241. [PMC free article] [PubMed] [Google Scholar]
- 29.Ishida M, Kodama N, Takemura Y, Iwai M, Yoshida K, Kagotani A, Matsusue Y, Okabe H. Primary bone carcinosarcoma of the fibula with chondrosarcoma and squamous cell carcinoma components. Int J Clin Exp Pathol. 2013;6:2216–2223. [PMC free article] [PubMed] [Google Scholar]
- 30.Ishida M, Igarashi T, Teramoto K, Hanaoka J, Iwai M, Yoshida K, Kagotani A, Tezuka N, Okabe H. Mucinous brobchioloalveolar carcinoma with K-ras mutation arising in type 1 congenital cystic adenomatous malformation: a case report with review of the literature. Int J Clin Exp Pathol. 2013;6:2597–2602. [PMC free article] [PubMed] [Google Scholar]
- 31.Ishida M, Yoshida K, Kagotani A, Iwai M, Yoshii M, Okuno K, Horinouchi A, Nakanishi R, Harada A, Yoshida T, Okuno T, Hodohara K, Okabe H. Anaplastic lymphoma kinase-positive large B-cell lymphoma: A case report with emphasis on the cytological features of the pleural effusion. Int J Clin Exp Pathol. 2013;6:2631–2635. [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshii M, Ishida M, Hodohara K, Okuno Y, Nakanishi R, Yoshida T, Okabe H. Systemic Epstein-Barr virus-positive T-cell lymphoproliferative disease of childhood: Report of a case with review of the literature. Oncol Lett. 2012;4:381–384. doi: 10.3892/ol.2012.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyake K, Moriyama M, Aizawa K, Nagano S, Inoue Y, Sadanaga A, Nakashima H, Nakamura S. Peripheral CD4+ T cells showing a Th2 phenotype in a patient with Mikulicz’s disease associated with lymphadenopathy and pleural effusion. Mod Rheumatol. 2008;18:86–90. doi: 10.1007/s10165-007-0010-3. [DOI] [PubMed] [Google Scholar]
- 34.Rossi G, Marchioni A, Guicciardi N, Cadioli A, Cavazza A. Recurrent pleural and pericardium effusions in a white woman with IgG4-related syndrome. Am J Surg Pathol. 2009;33:802–803. doi: 10.1097/PAS.0b013e31819841df. [DOI] [PubMed] [Google Scholar]
- 35.Sekiguchi H, Horie R, Utz JP, Ryu JH. IgG4-related systemic disease presenting with lung entrapment and constrictive pericarditis. Chest. 2012;142:781–783. doi: 10.1378/chest.11-2608. [DOI] [PubMed] [Google Scholar]
- 36.Ishida M, Okabe H. Immunoglobulin G4-related inflammatory aortic aneurysm. In: Grundmann RT, editor. Etiology, pathogenesis and pathophysiology of aortic aneurysm and aneurysmal rupture. Croatia: InTech; 2011. pp. 91–104. [Google Scholar]
- 37.Kasashima S, Zen Y, Kawashima A, Endo M, Matsumoto Y, Kasashima F, Ohtake H, Nakanuma Y. A clinicopathologic study of immunoglobulin G4-related sclerosing disease of the thoracic aorta. J Vasc Surg. 2010;52:1587–1595. doi: 10.1016/j.jvs.2010.06.072. [DOI] [PubMed] [Google Scholar]
- 38.Jois RN, Gaffney K, Marshall T, Scott DG. Chronic periaortitis. Rheumatology. 2004;43:1141–1146. doi: 10.1093/rheumatology/keh326. [DOI] [PubMed] [Google Scholar]
- 39.Sekiguchi H, Horie R, Suri RM, Yi ES, Ryu JH. Constrictive pericarditis caused by immunoglobulin G4-related disease. Circ Heart Fail. 2012;5:e30–31. doi: 10.1161/CIRCHEARTFAILURE.111.966408. [DOI] [PubMed] [Google Scholar]
- 40.Ishizaka N, Sakamoto A, Imai Y, Terasaki F, Nagai R. Multifocal fibrosclerosis and IgG4-related disease involving the cardiovascular system. J Cardiol. 2012;59:132–138. doi: 10.1016/j.jjcc.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Sakamoto A, Nagai R, Saito K, Imai Y, Takahashi M, Hosoya Y, Takeda N, Hirano K, Koike K, Enomoto Y, Kume H, Homma Y, Maeda D, Yamada H, Fukayama M, Hirata Y, Ishizaka N. Idiopathic retroperitoneal fibrosis, inflammatory aortic aneurysm, and inflammatory pericarditis-Retrospective analysis of 11 case histories. J Cardiol. 2012;59:139–146. doi: 10.1016/j.jjcc.2011.07.014. [DOI] [PubMed] [Google Scholar]