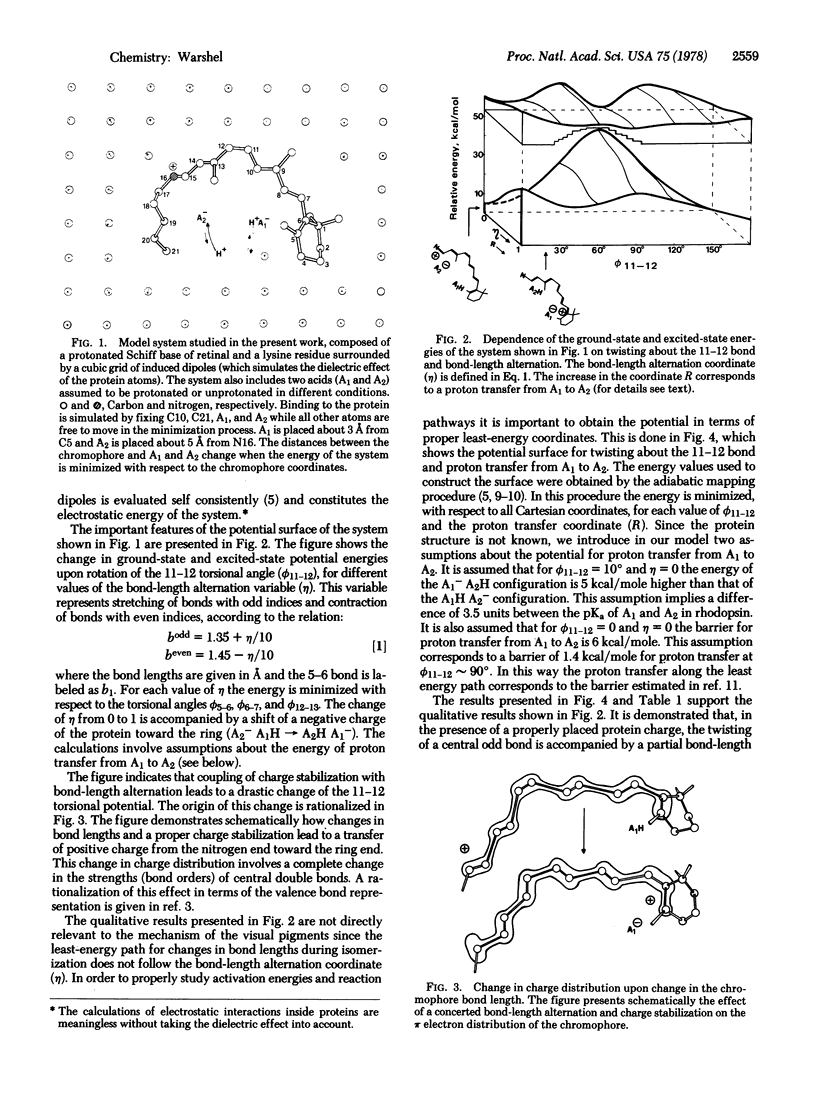
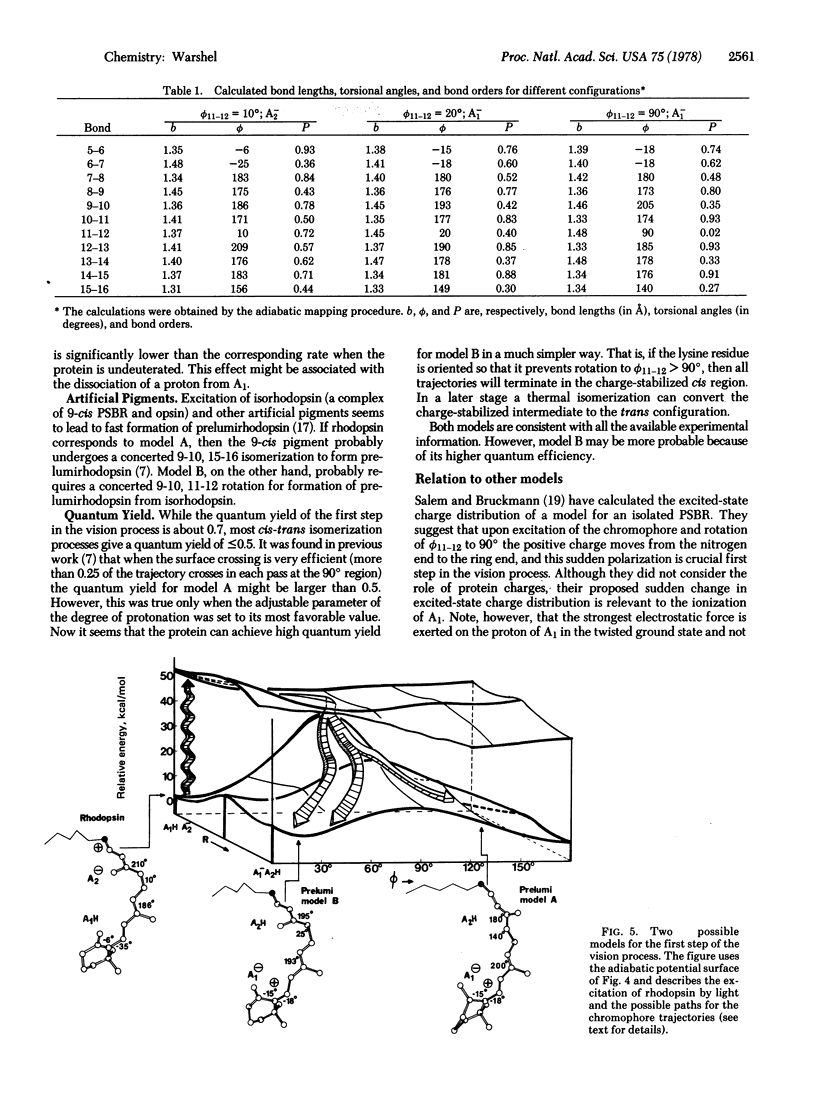
Abstract
The effects of charged groups of rhodopsin and bacteriorhodopsin on the potential energy surface of their chromophore are examined, taking into account the protein dielectric effect. It is found that the barriers for twisting double bonds of an isolated chromophore can be drastically reduced when the chromophore interacts with the protein charges. New types of local minima are found in the ground-state potential surface of the protein-chromophore complex. These minima correspond to "charge-stabilized intermediates" which are formed when a shift of the chromophore positive charge to the ring is stabilized by the ionization of a properly placed acidic group of the protein and by partial alternation of the bond lengths of the chromophore. It is suggested that the absorption of light by rhodopsin and bacteriorhodopsin may be used not only for isomerization about double bonds, but also for trapping such charge-stabilized intermediates. Thus, for example, it is concluded that prelumirhodopsin might be still in the cis configuration. Both the mechanism of the proton pump system of the purple membrane and the dark reaction of the visual and purple membrane pigments are considered. The connection between the finding of the present work and the mechanism of storage of light energy in photobiology is indicated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busch G. E., Applebury M. L., Lamola A. A., Rentzepis P. M. Formation and decay of prelumirhodopsin at room temperatures. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2802–2806. doi: 10.1073/pnas.69.10.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrey T. G., Honig B. Molecular aspects of photoreceptor function. Q Rev Biophys. 1975 May;8(2):129–184. doi: 10.1017/s0033583500001785. [DOI] [PubMed] [Google Scholar]
- Gelin B. R., Karplus M. Mechanism of tertiary structural change in hemoglobin. Proc Natl Acad Sci U S A. 1977 Mar;74(3):801–805. doi: 10.1073/pnas.74.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. H., Monger T. G., Alfano R. R., Aton B., Callender R. H. Cis-trans isomerisation in rhodopsin occurs in picoseconds. Nature. 1977 Sep 8;269(5624):179–180. doi: 10.1038/269179a0. [DOI] [PubMed] [Google Scholar]
- Henderson R. The purple membrane from Halobacterium halobium. Annu Rev Biophys Bioeng. 1977;6:87–109. doi: 10.1146/annurev.bb.06.060177.000511. [DOI] [PubMed] [Google Scholar]
- Huppert D., Rentzepis P. M., Kliger D. S. Picosecond and nanosecond isomerization kinetics of protonated 11-cis retinylidene Schiff bases. Photochem Photobiol. 1977 Feb;25(2):193–197. doi: 10.1111/j.1751-1097.1977.tb06897.x. [DOI] [PubMed] [Google Scholar]
- Lewis A. The molecular mechanism of excitation in visual transduction and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1978 Feb;75(2):549–553. doi: 10.1073/pnas.75.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathies R., Stryer L. Retinal has a highly dipolar vertically excited singlet state: implications for vision. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2169–2173. doi: 10.1073/pnas.73.7.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseroff A. R., Callender R. H. Resonance Raman spectroscopy of rhodopsin in retinal disk membranes. Biochemistry. 1974 Sep 24;13(20):4243–4248. doi: 10.1021/bi00717a027. [DOI] [PubMed] [Google Scholar]
- Peters K., Applebury M. L., Rentzepis P. M. Primary photochemical event in vision: proton translocation. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3119–3123. doi: 10.1073/pnas.74.8.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem L., Bruckmann P. Conversion of a photon to an electrical signal by sudden polarisation in the N-retinylidene visual chromophore. Nature. 1975 Dec 11;258(5535):526–528. doi: 10.1038/258526a0. [DOI] [PubMed] [Google Scholar]
- Warshel A. Bicycle-pedal model for the first step in the vision process. Nature. 1976 Apr 22;260(5553):679–683. doi: 10.1038/260679a0. [DOI] [PubMed] [Google Scholar]
- Warshel A. Energy-structure correlation in metalloporphyrins and the control of oxygen binding by hemoglobin. Proc Natl Acad Sci U S A. 1977 May;74(5):1789–1793. doi: 10.1073/pnas.74.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A. Interpretation of resonance Raman spectra of biological molecules. Annu Rev Biophys Bioeng. 1977;6:273–300. doi: 10.1146/annurev.bb.06.060177.001421. [DOI] [PubMed] [Google Scholar]
- Warshel A., Levitt M. Theoretical studies of enzymic reactions: dielectric, electrostatic and steric stabilization of the carbonium ion in the reaction of lysozyme. J Mol Biol. 1976 May 15;103(2):227–249. doi: 10.1016/0022-2836(76)90311-9. [DOI] [PubMed] [Google Scholar]
- YOSHIZAWA T., WALD G. Pre-lumirhodopsin and the bleaching of visual pigments. Nature. 1963 Mar 30;197:1279–1286. doi: 10.1038/1971279a0. [DOI] [PubMed] [Google Scholar]



