Abstract
BackgroundEvidence suggests that the gut microbiota play an important role in gastrointestinal problems.
AimTo give clinicians a practical reference guide on the role of specified probiotics in managing particular lower gastrointestinal symptoms/problems by means of a systematic review-based consensus.
MethodsSystematic literature searching identified randomised, placebo-controlled trials in adults; evidence for each symptom/problem was graded and statements developed (consensus process; 10-member panel). As results cannot be generalised between different probiotics, individual probiotics were identified for each statement.
ResultsThirty seven studies were included; mostly on irritable bowel syndrome [IBS; 19 studies; treatment responder rates: 18–80% (specific probiotics), 5–50% (placebo)] or antibiotic-associated diarrhoea (AAD; 10 studies). Statements with 100% agreement and ‘high’ evidence levels indicated that: (i) specific probiotics help reduce overall symptom burden and abdominal pain in some IBS patients; (ii) in patients receiving antibiotics/Helicobacter pylori eradication therapy, specified probiotics are helpful as adjuvants to prevent/reduce the duration/intensity of AAD; (iii) probiotics have favourable safety in patients in primary care. Items with 70–100% agreement and ‘moderate’ evidence were: (i) specific probiotics help relieve overall symptom burden in some patients with diarrhoea-predominant IBS, and reduce bloating/distension and improve bowel movement frequency/consistency in some IBS patients and (ii) with some probiotics, improved symptoms have led to improvement in quality of life.
ConclusionsSpecified probiotics can provide benefit in IBS and antibiotic-associated diarrhoea; relatively few studies in other indications suggested benefits warranting further research. This study provides practical guidance on which probiotic to select for a specific problem.
Introduction
Gastrointestinal (GI) problems are a major reason for consultation.1 Symptom management of GI problems often begins in primary care with adjustment of lifestyle factors that may cause or worsen symptoms, such as diet.2 Pharmacological treatments for patients with functional GI disorders (FGID) have limited efficacy and may cause side effects.3–4 Given that changes in the gut microbiota have been implicated in the pathogenesis of GI disorders [such as irritable bowel syndrome (IBS)],5–8 there is growing interest in therapies that might influence these changes, such as probiotics.
Probiotics are defined as ‘live microorganisms which when administered in adequate amounts confer a health benefit on the host'.9 These are distinct from prebiotics (dietary substances such as indigestible oligosaccharides that provide a health benefit by selectively promoting the growth of beneficial bacteria in the gut) and synbiotics (products containing a synergistic combination of prebiotics and probiotics). The remainder of this article will focus on probiotics. Despite their long history, wide availability and substantial publication record, the clinical role of probiotics has, in general, been inadequately characterised and remains ill-defined. Attempts to summarise probiotic research are complicated by the wide variety of probiotic strains that are available, as results obtained with one strain are not generalisable to others.10 The range of different formulations (capsules, sachets, yoghurts and fermented milks or fruit drinks), the dose and the presence of supporting substrates add further sources of variation.11,12 Effects, moreover, may be different according to age and health status of the target group.14–15
Many gastroenterologists recommend probiotics,16–17 and primary care physicians are increasingly confronted with questions about the suitability (or otherwise) of probiotics, but their familiarity with probiotics is limited.18–19 All clinicians are faced with an increasingly broad range of products, and deciding whether or not to recommend one of these to a particular patient is a major challenge. At the same time, the public is exposed to widespread claims for probiotics with a variety of products in shops, without clear guidance as to which might be useful. Clear, evidence-based guidance is therefore needed regarding the effectiveness of different probiotics and their clinical use.
Clinical guidelines usually focus on specific disease entities, but primary care physicians and gastroenterologists working in the field of FGID generally have to deal with overlapping symptom complexes.20 Consequently, the aim of this study was to provide practical advice to clinicians regarding the use of probiotics in the treatment of lower GI symptoms in adults in clinical practice. This advice was based on an extensive review of the literature followed by a validated approach to developing consensus that crosses international boundaries. The findings were translated into a reference tool identifying available probiotics with evidence for/against a beneficial effect for different GI symptoms/problems, to help clinicians make appropriate, evidence-based treatment decisions.
Methods
Systematic literature searches
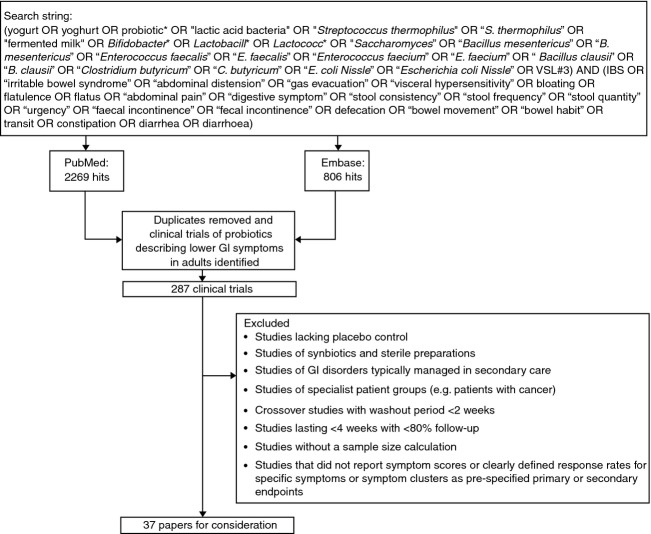
Systematic literature searches were performed (based on AGREE II criteria21) to answer the following question: in high-quality clinical studies performed in adults, what effects do probiotics have on lower GI symptoms/problems that are typically managed in primary care? PubMed and Embase (which together provide extensive coverage of the biomedical literature) were searched to identify all studies that assessed the effect of probiotics on lower GI symptoms, using the search string shown in Figure 1. The search results were combined and duplicates were removed. An initial screen of article titles and abstracts was then performed to identify clinical trials of probiotics that studied lower GI symptoms in adults (≥18 years old). Only studies of adults were included because the intestinal microbiota differ between children and adults.14 In addition, trials that evaluated only synbiotics were excluded. Studies of patients with IBS or other FGID; diarrhoea as a side effect of antibiotic treatment; lactose intolerance; or no specific GI diagnosis were included. Studies of well-defined disorders such as inflammatory bowel disease and diverticular disease were excluded. Studies of specialist populations (e.g. patients with any type of cancer) were also excluded.
Figure 1.
Flow diagram of literature searches. The initial PubMed and Embase searches were performed on 31 January 2012, and were limited to English language publications. GI, gastrointestinal.
The output of the systematic literature searches was discussed by the Consensus Group in a face-to-face workshop. To ensure a high-quality evidence base, they agreed to exclude the following: (i) studies without a placebo control group; (ii) crossover studies with a washout period of less than 2 weeks; (iii) studies in which fewer than 80% of participants were followed up unless the study duration exceeded 4 weeks; (iv) studies that did not perform a sample size calculation; (v) studies that did not report symptom scores or clearly defined response rates for specific symptoms or symptom clusters as prespecified primary or secondary end points. Prespecified primary/secondary end points were to be listed as such in the section or in the study objectives at the end of the section of the article under consideration.
Data for the following lower GI symptoms/problems were extracted from the included articles: IBS; abdominal pain; bloating/distension; flatus; constipation; bowel habit (e.g. frequency and/or consistency of bowel movements); diarrhoea (as part of IBS or associated with use of antibiotics including Helicobacter pylori eradication therapy). Health-related quality of life data were also extracted. As it was evident from previous publications that different probiotic strains will have different effects,10 the identities of the probiotic strains used in each study were recorded. Results of adverse event monitoring were also recorded if available.
Consensus development
A modified Delphi process was used to develop consensus statements. The Delphi process is an increasingly widely used technique for reaching expert consensus.22,23 It uses a process of anonymous and iterative feedback and voting to achieve consensus among a panel of independent experts by means of stepwise refinement of responses.
The Consensus Group consisted of primary care physicians with an interest in gastroenterology drawn from the European Society for Primary Care Gastroenterology (ESPCG), with the addition of one primary care physician from Belgium, two members from secondary care and a microbiologist. The Group was led by a nonvoting Chair (APSH, ESPCG Research Officer) who, in common with other members of the Consensus Group, has experience of systematic reviews and guideline development. Statements were developed (based on evidence and clinical experience) by the Chair in collaboration with a Steering Committee (BP, NdW and PW).
Development and grading of statements
Statements were prepared for each of the categories outlined above. The level of supporting evidence and strength of each statement were rated by the Chair and Steering Committee using the Grades of Recommendation Assessment, Development and Evaluation (GRADE) system25 as follows: high – further research is unlikely to change our confidence in the estimate of effect; moderate – further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low – further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; and very low – any estimate of effect is very uncertain.
Refinement of statements
The statements were worded to reflect the grade of available evidence. For example, the phrase ‘probiotics may help to…’ was used to distinguish statements with a low grade of evidence from those with a moderate or high grade of evidence (‘probiotics help to…’). The proportion of patients with IBS who responded to treatment did not exceed 80% in the included studies (despite ‘responders’ being defined very broadly as patients showing any improvement from baseline in some studies). Therefore, statements relating to potential benefits in IBS used the phrase ‘in some patients with IBS’ rather than ‘in patients with IBS’. Two rounds of anonymous voting on the statements were performed. Votes were cast using an online platform (INSINC Consulting, Guelph, ON, Canada and ECD Solutions, Atlanta, GA, USA), with each round being analysed by the nonvoting Chair (APSH). For each statement, voters indicated their level of agreement on a scale from 1 to 6 (1: strongly disagree; 2: disagree with major reservation; 3: disagree with minor reservation; 4: agree with major reservation; 5: agree with minor reservation; and 6: strongly agree). Consensus was defined a priori as agreement by at least 67% of respondents.
Results
In total, 37 publications were identified and used to develop statements. Table 1 provides a summary of the symptoms and indications examined in the 37 studies, all of which had a placebo control group or placebo-controlled period. Collectively, the 37 studies investigated a total of 32 different probiotics at doses of 1 × 106–4.5 × 1011 colony forming units (CFU) administered once, twice or three times daily. They predominantly contained bacteria (mostly lactobacilli and/or bifidobacteria); a few contained Saccharomyces. Of note, the term ‘probiotics’ has been used throughout this section to refer to products that contain probiotics, regardless of whether the product contains a single strain or multiple strains.
Table 1.
Indications and symptoms examined in included studies
| Number of studies | Indication | ||||||
|---|---|---|---|---|---|---|---|
| Symptom | IBS | Functional GI disorders | Antibiotic treatment | Helicobacter pylori eradication | Lactose intolerance | Healthy/minor GI symptoms | Total |
| IBS (global symptoms) | 16 | 0 | 0 | 0 | 0 | 0 | 16 |
| Abdominal pain | 18 | 2 | 0 | 0 | 1 | 2 | 23 |
| Bloating/distension | 15 | 1 | 0 | 0 | 1 | 2 | 19 |
| Flatus | 10 | 2 | 0 | 0 | 1 | 2 | 15 |
| Diarrhoea (treatment) | 3 | 2 | 0 | 0 | 1 | 1 | 7 |
| Diarrhoea (prevention) | 0 | 0 | 6 | 4 | 0 | 0 | 10 |
| Constipation | 2 | 2 | 0 | 0 | 0 | 0 | 4 |
| Bowel habit | 17 | 1 | 0 | 0 | 0 | 3 | 21 |
| Health-related quality of life | 12 | 1 | 0 | 0 | 0 | 2 | 15 |
| Total | 19 | 2 | 6 | 4 | 1 | 5 | 37 |
GI, gastrointestinal; IBS, irritable bowel syndrome.
Treatment adherence was addressed in 29 of the included studies. In 27 of the included studies, adherence to treatment was assessed by counting empty containers/unused test substance returned at the end of the study and/or by participant self-reporting (in treatment diaries or during investigator visits). Faecal recovery of probiotic strains was used as a measure of adherence in three of the studies. Where adherence data were reported (21 studies), the level of adherence was generally high. In the active treatment groups, the proportion of participants who were adherent to treatment was >75–100%. In the faecal recovery analyses, 79–92% of participants in the active treatment groups tested positive for the specific probiotic strain(s).
The majority of the studies focused on IBS (based on Rome I, II or III criteria or physician diagnosis; 19 studies) or antibiotic-associated diarrhoea (AAD; 10 studies, of which four examined H. pylori eradication therapy). The studies in IBS tended to include all IBS subtypes, with only two studies focusing on constipation-predominant IBS (IBS-C), and three studies focusing on diarrhoea-predominant IBS (IBS-D). The IBS studies employed different definitions of treatment response and reported a correspondingly wide range of ‘responder’ rates (18–80% and 5–50% in groups receiving specific probiotics and placebo respectively). Table 2 provides an overview of the 37 studies, including the indication studied, the probiotic treatment regimens used, study design and the number of patients analysed.
Table 2.
Overview of probiotic treatment regimens and results in included studies
| Diagnosis | Patients analysed (n) | Study design | Probiotic strain(s)* (brand name)Formulation and regimen | Primary end point | Results for primary end point | P value | Reference |
|---|---|---|---|---|---|---|---|
| Marketed products | |||||||
| IBS (Rome II) | 100 | DBRCT | Bifidobacterium longum subsp. longum LA 101, Lactobacillus acidophilus LA 102, L. delbrueckii subsp. lactis LA 103, Streptococcus salivarius subsp. thermophilus LA 104 (Lactibiane)Sachets, 1 × 1010 CFU o.i.d. for 4 weeks | Satisfactory relief of overall IBS symptoms, and abdominal pain/discomfort score | Proportion with satisfactory relief: specific probiotic, 42.6%; placebo, 42.3%.Reduction in abdominal pain score from first to fourth week of treatment: specific probiotic, −41.9%; placebo, −24.2% | > 0.050.048 | Drouault-Holowacz et al.26 |
| IBS, including abdominal pain (diagnosed by a primary care physician) | 298 | DBRCT | Escherichia coli DSM17252 (Symbioflor-2)Oral liquid, 1.5–4.5 × 107 CFU/mL for 8 weeks (0.75 mL t.i.d. for week 1; 1.5 mL t.i.d. for weeks 2–8) | Abdominal pain and overall IBS symptom scores (treatment response: absence of symptoms at ≥1 visit during treatment) | Abdominal pain response rate: specific probiotic, 18.9%; placebo, 6.7%Overall GI symptom response rate: specific probiotic, 18.2%; placebo, 4.7% | 0.00160.0004 | Enck et al.27 |
| IBS (Rome II) | 86 | DBRCT | L. rhamnosus GG, L. rhamnosus Lc705, Propionibacterium freudenreichii subsp. shermanii JS, B. animalis subsp. lactis Bb12 (Gefilus MAX)Milk-based drink, 1.2 dL (1 × 107 CFU/mL of each strain) o.i.d. for 5 months | Change in composite IBS symptom score (abdominal pain, distension, flatulence and rumbling) | Decrease from baseline: specific probiotic, 37%; placebo, 9% | 0.0083 | Kajander et al.28 |
| IBS (Rome II) with abdominal bloating | 48 | DBRCT | B. longum subsp. longum, B. infantis subsp. infantis,B. breve,L. acidophilus,L. paracasei subsp. paracasei,L. delbrueckii subsp. bulgaricus,L. plantarum, Streptococcus salivarius subsp. thermophilus (VSL#3)One sachet (450 billion lyophilised bacteria) b.i.d. for 4–8 weeks | Bloating severity score | Posttreatment score: specific probiotic, 31.3; placebo, 38.5 | 0.11 | Kim et al.29 |
| IBS (Rome criteria) | 12 | DBRCT, crossover | L. plantarum 299v (ProViva)Fermented oatmeal gruel, 6.25 × 109 CFU o.i.d. (125 mL) for 4 weeks | Gas production | Gas production in 24 h: specific probiotic, 249 mL; placebo, 245 mL | >0.05 | Sen et al.30 |
| IBS (Rome II) | 74 | DBRCT | L. paracasei subsp. paracasei F19, L. acidophilus La5, B. animalis subsp. lactis Bb12 (Cultura)Fermented milk, 5 × 107 CFU/mL, 200 mL b.i.d. for 8 weeks | Proportion of patients reporting adequate relief of their IBS symptoms at least 50% of the weeks during the treatment period (‘responders’) | Proportion of responders: specific probiotic, 38%; placebo, 27% | 0.3 | Simrén et al.31 |
| IBS (Rome II) | 52 | DBRCT | L. paracasei subsp. paracasei F19, L. acidophilus La5, B. animalis subsp. lactis Bb 12 (Cultura)Fermented milk, 250 mL b.i.d. (7.5 × 1010 CFU/day) for 8 weeks | Proportion reporting adequate symptom relief, and total IBS-SSI score | No significant differences between specific probiotic and placebo | >0.05 | Sondergaard et al.32 |
| IBS (Rome II; females) | 362 | DBRCT | B. longum subsp. infantis 35624 (Bifantis/Align)Capsules, three dose groups: 1 × 106, 1 × 108 or 1 × 1010 CFU o.i.d. for 4 weeks | Abdominal pain/discomfort score | Change from baseline: −0.89 in the group receiving specific probiotic 1 × 108 CFU o.i.d. compared to −0.58 in the placebo group | 0.023 | Whorwell et al.33 |
| IBS (Rome II) | 52 | DBRCT | L. acidophilus (CUL60 and CUL21), B. animalis subsp. lactis CUL34, B. bifidum CUL20 (LAB4)Capsules, 2.5 × 1010 CFU o.i.d. for 8 weeks | IBS-SSI score | Difference (specific probiotic vs. placebo): 6 weeks: –47.82 8 weeks: –52.7310 weeks: no significant difference | 0.03470.0217>0.05 | Williams et al.34 |
| IBS-C (Rome III) | 34 | DBRCT | B. animalis subsp. lactis DN-173 010 (Activia)Fermented milk, 125 g (1.25 × 1010 CFU) b.i.d. for 4 weeks | Abdominal distension (measured by abdominal inductance plethysmography) | Median percentage change in maximal distension (specific probiotic vs. placebo): −39%Mean abdominal distension (specific probiotic vs. placebo): −1.52 cm | 0.020.096 | Agrawal et al.35 |
| IBS-C (Rome II) | 267 | DBRCT | B. animalis subsp. lactis DN-173 010 (Activia)Fermented milk, 125 g (1.25 × 1010 CFU) b.i.d. for 6 weeks | ‘Discomfort’ dimension of the validated FDDQOL questionnaire (response: improvement of ≥ 10% vs. baseline) | Responder rate (week 3): specific probiotic, 65.2%; placebo, 47.7%Responder rate (week 6): specific probiotic, 63.0%; placebo, 56.8%)Change in score from baseline: no significant difference between groups | 0.003>0.05>0.05 | Guyonnet et al.36 |
| IBS-D (Rome III) | 50 | DBRCT | L. acidophilus LH5, L. plantarum LP1, L. rhamnosus LR3, B. breve BR2, B. animalis subsp. lactis BL2, B. longum subsp. longum BG3, Streptococcus salivarius subsp. thermophilus ST3 (bacterial component of Duolac7)One capsule b.i.d. (1 × 1010 cells/day) for 8 weeks | Adequate relief of IBS symptoms for ≥50% of weeks during treatment and 2-week follow-up | Proportion with adequate symptom relief: specific probiotic, 48%; placebo, 12% | 0.01 | Ki Cha et al.37 |
| IBS-D (Rome II) | 25 | DBRCT | B. longum,B. longum subsp. infantis,B. breve,L. acidophilus,L. paracasei subsp. paracasei,L. delbrueckii subsp. bulgaricus,L. plantarum,Streptococcus salivarius subsp. thermophilus (VSL#3)One sachet (225 billion lyophilised bacteria) b.i.d. for 8 weeks (total daily dose 450 billion bacteria) | Transit time and global satisfaction (treatment response: satisfactory relief of overall IBS symptoms on ≥4 of 8 weekly assessments) | GI transit: no difference between the two treatment groups.Proportion of responders: specific probiotic, 33%; placebo, 38% | 0.41–0.991.00 | Kim et al.38 |
| IBS-D (Rome II) | 29 | SBRCT | Streptococcus salivarius subsp. thermophilus (1 × 108 CFU/mL), L. delbrueckii subsp. bulgaricus (1 × 107 CFU/mL), L. acidophilus (1 × 107 CFU/mL), B. longum subsp. longum (1 × 107 CFU/mL) (AB100 Jianneng)Fermented milk, 200 g b.i.d. for 4 weeks (each mL contained at least 1.3 × 108 CFU total) | Improvement in proportion with abnormal intestinal permeability | N/A – primary end point data were not GI symptoms/HRQoL measures (IBS symptoms were assessed as secondary end points) | – | Zeng et al.39 |
| AAD | 89 | DBRCT | L. acidophilus CL1285, L. paracasei subsp. paracasei LBC80R (Bio-K+ CL1285)Fermented milk, half container (49 g) o.i.d. for 2 days, then full container (98 g; 50 × 109 CFU) o.i.d., starting within 48 h of initiating antibiotic treatment and continuing for duration of antibiotic treatment | AAD (≥3 liquid stools in a 24-h period) | Incidence of AAD: specific probiotic, 15.9%; placebo, 35.6%; OR, 0.343 | 0.05 | Beausoleil et al.40 |
| AAD | 255 | DBRCT | L. acidophilus CLl285, L. paracasei subsp. paracasei LBC80R (Bio-K+ CL1285)Capsules, two dose groups: One or two probiotic capsules (50 or 100 billion CFU)/day, initiated within 36 h of starting antibiotic treatment and continued for 5 days after completing antibiotic treatment (duration of antibiotic treatment was 3–14 days) | AAD (≥3 liquid stools in a 24-h period) | Incidence of AAD: specific probiotic (two capsules), 15.5%; specific probiotic (one capsule); 28.2%; placebo, 44.1%Duration of AAD: specific probiotic (two capsules), 2.8 days; specific probiotic (one capsule); 4.1 days; placebo, 6.4 days | ≤0.02<0.001 | Gao et al.41 |
| AAD | 437 | DBRCT | L. acidophilus CL1285, L. paracasei subsp. paracasei LBC80R (Bio-K+ CL1285)Fermented milk, half container (49 g) o.i.d. for 2 days, then full container (98 g; 50 × 109 CFU) o.i.d. for 29–40 days (started within 24 h after the first dose of antibiotic, and continued until 5 days after the last dose of antibiotic) | AAD (≥1 episodes of unformed or liquid stool in a 24-h period) severity and incidence | Mean number of days with AAD: specific probiotic, 0.67 days; placebo, 1.19 daysProportion of patients with AAD: specific probiotic, 21.8%; placebo, 29.4% (note study was underpowered).OR of AAD (multivariate logistic regression, specific probiotic vs. placebo): 0.627 | 0.0400.0670.037 | Sampalis et al.42 |
| AAD | 113 | DBRCT | L. paracasei subsp. paracasei DN-114 001 (L. paracasei subsp. paracasei immunitass) (Actimel)Yoghurt drink, 100 g (97 mL; 1 × 108 CFU/mL) b.i.d. started within 48 h of starting antibiotic treatment and continued for 1 week after stopping antibiotic treatment (publication does not state duration of antibiotics). Follow-up was 4 weeks later | AAD (>2 liquid stools a day for ≥3 days in quantities in excess of normal for the patient) | Incidence of AAD: specific probiotic, 12%; placebo, 34%OR of diarrhoea (adjusted logistic regression, specific probiotic vs. placebo): 0.25 | 0.007 | Hickson et al.43 |
| AAD, Clostridium difficile-associated diarrhoea | 138 | DBRCT | L. acidophilus (CUL60 and CUL21), B. animalis subsp. lactis CUL34, B. bifidum CUL20 (LAB4; strains not given in publication; information obtained from company website)Capsules, 2 × 1010 CFU o.i.d. started within 36 h of antibiotic prescription (duration 20 days) | C. difficile-associated diarrhoea | N/A – C. difficile-associated diarrhoea was not covered in this consensus (AAD was assessed as a secondary end point) | – | Plummer et al.44 |
| AAD | 214 | DBRCT | L. rhamnosus R0011, L. acidophilus R0052 (Lacidofil cap)One capsule (2 × 109 CFU) b.i.d. starting within 48 h of initiating antibiotic treatment (duration 2 weeks) | AAD (loose or watery stools >3× per day for ≥2 days within 14 days of enrolment) | Incidence of AAD: specific probiotic, 3.9%; placebo, 7.2% (note study was underpowered) | 0.44 | Song et al.45 |
| H. pylori therapy-associated side effects | 124 | DBRCT | Saccharomyces boulardii (Reflor)500 mg (two sachets) b.i.d. for 2 weeks during 2-week H. pylori eradication therapy. Patients were followed up for a further 2 weeks | H. pylori eradication therapy-associated side effects | Diarrhoea occurred in 14.5% of the specific probiotic group and 30.6% of the placebo group | <0.05 | Cindoruk et al.46 |
| H. pylori therapy-associated side effects | 64 | TBRCT | (1) L. rhamnosus GG (Giflorex)Saccharomyces boulardii† (Codex)(2) One sachet b.i.d. for 2 weeks (during and for 1 week after 1-week H. pylori eradication therapy). Each sachet contained 6 × 109 CFU (1) or 5 × 109 CFU (2) | H. pylori eradication therapy-associated side effects | Diarrhoea occurred in 5% of each specific probiotic group, compared to 30% of the placebo group | 0.018 | Cremonini et al.47 |
| H. pylori therapy-associated side effects | 88 | DBRCT | L. acidophilus LA-5, B. animalis subsp. lactis Bb12, Streptococcus salivarius subsp. thermophilus (ABT-21 culture)Fermented milk, 125 g b.i.d. (≥1 × 106 CFU/g of each strain) for 5 weeks (eradication triple therapy during fifth week of study intervention) | H. pylori eradication therapy-associated diarrhoea episodes (≥3 watery stools per day, with ≥1 day in the eradication week) | Number of diarrhoea episodes: active specific probiotic, 4; pasteurised specific probiotic, 2; acidified milk, 3Number of days with watery stools: active specific probiotic, 4; pasteurised specific probiotic, 10; acidified milk, 10Mean duration of diarrhoea episodes: active specific probiotic, 1.0 day; pasteurised specific probiotic, 5.0 days; acidified milk, 4.7 days | >0.05<0.05<0.05 | de Vrese et al.48 |
| H. pylori therapy-associated side effects | 106 | DBRCT | Bacillus clausii strains O/C, N/R, T and SIN (Enterogermina)One vial (each vial contains 2 × 109 spores of Bacillus clausii) t.i.d., taken during 1 week of H. pylori eradication therapy and continued for 1 further week | H. pylori eradication therapy-associated side effects | Incidence of diarrhoea after 1 week: specific probiotic, 9.3%; placebo, 30.8%; RR: 0.30. Mean intensity and frequency of diarrhoea episodes were also reduced | <0.01 | Nista et al.49 |
| Functional GI symptoms | 87 | TBRCT | B. animalis subsp. lactis HN019 (HOWARU Bifido/DR10)Capsules, two dose groups: 1.8 billion or 17.2 billion CFU/day for 2 weeks | Whole-gut transit time | Change from baseline: specific probiotic groups, −25% and −33%; placebo group, +17% | <0.001 | Waller et al.50 |
| Women with mild digestive symptoms | 197 | DBRCT | B. animalis subsp. lactis DN-173 010 (Activia)Fermented milk, 125 g (1.25 × 1010 CFU) b.i.d. for 4 weeks | GI well-being | Proportion reporting improved GI well-being at weeks 1–4: specific probiotic, 37–41%; placebo, 22–34%; OR, 1.7 | 0.006 | Guyonnet et al.51 |
| Healthy, postprandial intestinal gas-related symptoms | 61 | DBRCT | Bacillus coagulans GBI-30, 6086 (Digestive Advantage Gas Defense Formula)One capsule (2 × 109 CFU) o.i.d. for 4 weeks | GSRS abdominal pain, distension and flatus subscores, and SODA bloating and gas subscores | Difference between specific probiotic and placebo groups at 4 weeks:Abdominal pain (GSRS), −0.627Abdominal distension (GSRS), −0.572Flatus (GSRS), −0.511Bloating (SODA): −0.229Gas (SODA): − 0.348 | 0.0460.0610.1540.2940.118 | Kalman et al.52 |
| Lactose-intolerant individuals | 60 | RCT | L. reuteri DSM17938 (Reuterin)Two pills b.i.d. (4 × 108 CFU/day) for 10 days preceding lactose breath test | Lactose breath test normalisation rate | N/A – primary end point data were not GI symptoms/HRQoL measures (bloating, abdominal pain, flatus and diarrhoea were assessed as secondary end points) | – | Ojetti et al.53 |
| Elderly nursing home residents | 179 | DBRCT | (1) B. longum subsp. longum 46 and 2C, (2) B. animalis subsp. lactis Bb12 (Yosa)Fermented oat drinks, 1 × 109 CFU/day (200 mL) for 7 months | Proportion of participants with bowel functioning on >30% of days | Bowel functioning on ≥30% of days: placebo, 49%specific probiotic 1, 70%specific probiotic 2, 59%Normal bowel functioning (solid or normal consistency of stools) on ≥30 days: placebo, 14%specific probiotic 1, 37%specific probiotic 2, 30% | 0.0440.2530.0200.036 | Pitkala et al.54 |
| Healthy individuals (competitive cyclists) | 88 | DBRCT | L. fermentum VRI-003 (PCC) (ProBioPCC)One capsule (≥1 × 109 CFU) o.i.d. for 11 weeks (mean) | Self-reported upper respiratory tract and GI symptoms | GI symptoms Ratio (99% CI) of number of episodes (specific probiotic/placebo): men, 2.06 (0.51–11); women, 3.02 (0.76–17)Ratio (99% CI) of duration of episodes (specific probiotic/placebo): men, 2.57 (0.53–17); women, 1.85 (0.35–27)Difference (99% CI) in severity (specific probiotic − placebo): men, −0.47 (−1.21 to 0.28); women, −0.31 (−1.39 to 0.79)Upper respiratory tract symptoms: no clear difference between groups | – | West et al.55 |
| Investigative strains | |||||||
| IBS (mild-to-moderate; Rome III) | 122 | DBRCT | B. bifidum MIMBb75One capsule (1 × 109 CFU) o.i.d. for 4 weeks | Global IBS symptoms (7-point Likert scale) | Improvement from baseline: specific probiotic, −0.88; placebo, −0.16 | <0.0001 | Guglielmetti et al.56 |
| IBS (Rome III) | 70 | DBRCT | B. bifidum BGN4, B. animalis subsp. lactis AD011, L. acidophilus AD031, L. paracasei subsp. paracasei IBS041One sachet b.i.d. (10 billion bacteria of each strain/day) for 8 weeks | Abdominal pain, flatus, defecation discomfort individual and sum scores | Abdominal pain (vs. baseline): Week 4: specific probiotic, −23.9; placebo, −10.9Week 8: specific probiotic, −31.9; placebo, −17.7Flatus (vs. baseline): Week 4: specific probiotic, −18.5; placebo, −18.4Week 8: specific probiotic, −27.0; placebo, −21.3 Defecation discomfort (vs. baseline): Week 4: specific probiotic, −29.2; placebo, −13.5Week 8: specific probiotic, −30.5; placebo, −18.4Sum score (vs. baseline): Week 4: specific probiotic, −71.7; placebo, −42.8Week 8: specific probiotic, −89.5; placebo, −57.5 | 0.0610.0450.9820.4370.0430.1220.1150.064 | Hong et al.57 |
| IBS (Rome I) | 81 | DBRCT | L. rhamnosus GG, L. rhamnosus Lc705, Propionibacterium freudenreichii subsp. shermanii JS, B. breve Bb99One capsule (8–9 × 109 CFU total; equal amount of each strain) o.i.d. for 6 months | Change in weekly sum score of each of four symptoms (abdominal pain, distension, flatus and borborygmi), total symptom score and bowel habit | Baseline-adjusted symptom score, difference between specific probiotic and placebo: Abdominal pain: −1.5Distension: −1.6 Flatus: −1.2 Borborygmi: −2.2Total symptom score: −6.5Weekly defecation frequency: 1.3 | 0.1100.0830.2320.0080.0370.102 | Kajander et al.58 |
| IBS (Rome II) | 16 | DBRCT, crossover | L. plantarum MF1298One capsule (1 × 1010 CFU) o.i.d. for 3 weeks | Treatment preference | 13 participants (81%) preferred placebo to the specific probiotic | 0.012 | Ligaarden et al.59 |
| IBS (Rome III) | 40 | SBRCT | L. acidophilus-SDC 2012, L. acidophilus-SDC 2013 2 × 109 CFU/mL in one capsule taken b.i.d. for 4 weeks | Abdominal pain (responder rate) | Proportion with improvement in abdominal pain/discomfort: specific probiotic, 80%; placebo, 35% | 0.011 | Sinn et al.60 |
| Functional GI disorders | 72 | DBRCT | (1) L. acidophilus,B. bifidum,Bacillus subtilis,L. delbrueckii subsp. bulgaricus,L. delbrueckii subsp. lactis and Bacillus lichenformis, or (2) L. acidophilus,B. bifidum,L. delbrueckii subsp. bulgaricus,L. delbrueckii subsp. lactis,L. brevis,L. caucasicus (nomina rejicienda; now L. delbrueckii subsp. delbrueckii), L. fermentum,L. leichmanii,L. paracasei subsp. paracasei,L. plantarum,L. helveticus and Saccharomyces boulardiiCaplets, each containing 5 × 107 bacteria, taken for12 weeks (Week 1: one caplet od; Week 2: one caplet t.i.d.; Week 3: two caplets t.i.d.; Week 4: three caplets t.i.d.; Weeks 5–12: four caplets t.i.d.) | HRQoL (GIQLI) | GIQLI total score and well-being subscales (physical, social and mental) showed no significant change from baseline at 4, 8 and 12 weeks | >0.05 | Kim et al.61 |
| Healthy young adults | 71 | DBRCT | B. animalis subsp. lactis Bb12, L. paracasei subsp. paracasei CRL-431Capsules, four dose groups: 1 × 108, 109, 1010 or 1011 CFU o.i.d. for 3 weeks | Granulocyte activity | N/A – primary end point data were not GI symptoms/HRQoL measures (bowel habit was assessed as a secondary end point) | – | Larsen et al.62 |
AAD, antibiotic-associated diarrhoea; b.i.d., twice daily; CFU, colony forming units; CI, confidence interval; DBRCT double-blind randomised controlled trial; FDDQOL, Functional Digestive Disorders Quality of Life; GI, gastrointestinal; GIQLI, Gastrointestinal Quality of Life Index; GSRS, Gastrointestinal Symptom Rating Scale; HRQoL, health-related quality of life; IBS, irritable bowel syndrome; IBS-C, constipation-predominant IBS; IBS-D, diarrhoea-predominant IBS; o.i.d., once daily; OR, odds ratio; N/A, not applicable; RCT, randomised controlled trial; RR, relative risk; SBRCT, single-blind randomised controlled trial; SODA, Severity of Dyspepsia Assessment; SSI, Symptom Severity Index; TBRCT, triple-blind randomised controlled trial; t.i.d., three times daily.
In some cases, the specific strain was not identified in the publication and could not be found elsewhere (e.g. it may be proprietary information).
This study tested a third product (Ferzym) that was excluded from the current analysis because online information indicated that it was a synbiotic.
Sixteen statements were developed, covering nine symptoms or problems plus general considerations relating to probiotic use. Of the 16 statements, 11 achieved consensus in the first round of voting and 15 achieved consensus in the second round (see Figure 2). Table 3 summarises the studies and specific probiotics with supportive or nonsupportive evidence for each consensus statement, together with an indication of whether the result was a primary or secondary end point, or part of a subanalysis. Table S1 shows probiotic availability in Europe, the USA and China.
Figure 2.
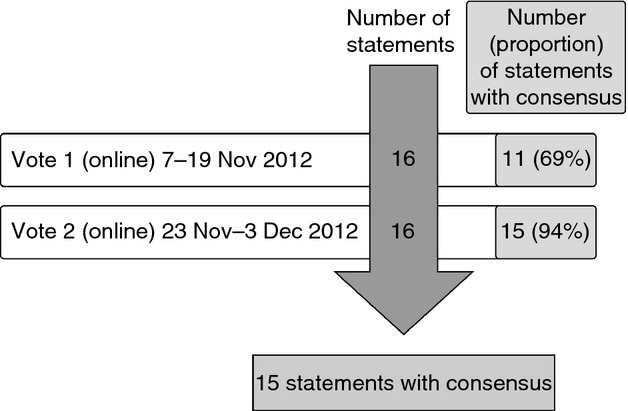
Overview of Delphi consensus development process and voting results.
Table 3.
Overview of statements, grading and probiotics (marketed products and investigative strains) with supportive evidence. For many statements, the majority of the evidence came from populations with IBS, and the statements were therefore focused on IBS. Probiotics studied in indications other than IBS are still included below, but are placed in square brackets
| Statement | Grade of evidence for effect | Level of agreement (%) | Probiotics for which studies show supportive evidence of benefit* – (bold font indicates primary end point data) | Probiotics for which studies suggest a lack of significant benefit* –(bold font indicates primary end point data) |
|---|---|---|---|---|
| 1: Specific probiotics help relieve overall symptom burden in some patients with IBS | High | 100 | Bifidobacterium bifidum MIMBb75,56 B. longum subsp. infantis 35624 (Bifantis/Align),33 Escherichia coli DSM17252 (Symbioflor-2),27 investigative combinations (BIFIDO,57 Valio Bb9958), marketed combinations (Gefilus MAX,28 LAB434) | Lactobacillus plantarum MF1298,59 marketed combinations (Cultura,31–32 Lactibiane26) |
| 2: Specific probiotics may help relieve overall symptom burden in some patients with IBS-C | Low | 80 | B. animalis subsp. lactis DN-173 010 (Activia)35–36 | B. longum subsp. infantis 35624 (Bifantis/Align)33 |
| 3: Specific probiotics help relieve overall symptom burden in some patients with IBS-D | Moderate | 100 | B. longum subsp. infantis 35624 (Bifantis/Align),33 marketed combinations (AB100 Jianneng,39 Duolac737) | Marketed combination (VSL#3)38 |
| 4: Specific probiotics help reduce abdominal pain in some patients with IBS | High | 100 | [Bacillus coagulans GBI-30, 6086 (Digestive Advantage Gas Defense Formula)52], B. animalis subsp. lactis DN-173 010 (Activia),35 [B. animalis subsp. lactis HN019 (HOWARU Bifido/DR10)50], B. bifidum MIMBb75,56 B. longum subsp. infantis 35624 (Bifantis/Align),33 Escherichia coli DSM17252 (Symbioflor-2),27 investigative combinations (BIFIDO,57 SDC,60 Valio Bb9958), [L. reuteri DSM17938 (Reuterin)53], marketed combinations (AB100 Jianneng,39 Lactibiane26) | B. animalis subsp. lactis DN-173 010 (Activia),36 [B. animalis subsp. lactis DN-173 010 (Activia)51], investigative combinations ([GoL661], [GoL1261]), L. plantarum MF1298,59 marketed combinations (Cultura,31–32 Duolac7,37 Gefilus MAX,28 LAB4,34 VSL#329–38) |
| 5: Specific probiotics help reduce bloating/distension in some patients with IBS | Moderate | 70 | B. animalis subsp. lactis DN-173 010 (Activia),35 B. animalis subsp. lactis DN-173 010 (Activia),36 B. bifidum MIMBb75,56 B. longum subsp. infantis 35624 (Bifantis/Align),33 Escherichia coli DSM17252 (Symbioflor-2),27 [L. reuteri DSM17938 (Reuterin)53], marketed combinations (Gefilus MAX,28 LAB434) | [Bacillus coagulans GBI-30, 6086 (Digestive Advantage Gas Defense Formula)52], [B. animalis subsp. lactis DN-173 010 (Activia)51], investigative combinations ([GoL661], [GoL1261], Valio Bb9958), L. plantarum MF1298,59 marketed combinations (AB100 Jianneng,39 Cultura,31–32 Duolac7,37 VSL#3,29 VSL#338) |
| 6: Probiotics tested to date do not reduce flatus in patients with IBS | Low | 90 | [B. animalis subsp. lactis DN-173 010 (Activia)51], [B. animalis subsp. lactis HN019 (HOWARU Bifido/DR10)50], B. longum subsp. infantis 35624 (Bifantis/Align),33 [L. reuteri DSM17938 (Reuterin)53], marketed combinations (AB100 Jianneng,39 VSL#329) | [Bacillus coagulans GBI-30, 6086 (Digestive Advantage Gas Defense Formula)52], B. animalis subsp. lactis DN-173 010 (Activia),35 investigative combinations (BIFIDO,57 [GoL661], [GoL1261], Valio Bb9958), L. plantarum 299v (ProViva),30 marketed combinations (Duolac7,37 Gefilus MAX,28 VSL#338) |
| 7: Specific probiotics may help reduce constipation in some patients with IBS | Low | 60 (no consensus) | B. animalis subsp. lactis DN-173 010 (Activia),35 [B. animalis subsp. lactis HN019 (HOWARU Bifido/DR10)50] | B. bifidum MIMBb75,56 investigative combinations ([GoL661], [GoL1261]) |
| 8: Specific probiotics help improve frequency and/or consistency of bowel movements in some patients with IBS | Moderate | 70 | [B. animalis subsp. lactis Bb12 (Yosa)54], B. animalis subsp. lactis DN-173 010 (Activia),35–36 [B. animalis subsp. lactis DN-173 010 (Activia)51], [B. animalis subsp. lactis HN019 (HOWARU Bifido/DR10)50], B. bifidum MIMBb75,56 B. longum subsp. infantis 35624 (Bifantis/Align),33 Escherichia coli DSM17252 (Symbioflor-2),27 investigative combinations ([Bioferme54], [CH62], SDC,60 Valio Bb9958), marketed combinations (Duolac7,37 LAB4,34 Lactibiane26) | Investigative combination (BIFIDO57), L. plantarum MF129859, marketed combinations (Cultura,31–32 Gefilus MAX,28 VSL#3,38 VSL#329) |
| 9: Probiotics tested to date do not reduce diarrhoea in patients with IBS | Very low | 80 | [L. reuteri DSM17938 (Reuterin)53] | [B. animalis subsp. lactis Bb12 (Yosa)54], [B. animalis subsp. lactis HN019 (HOWARU Bifido/DR10)50], investigative combinations ([Bioferme54], [GoL661], [GoL1261]), L. plantarum MF1298,59 marketed combinations (Duolac7,37 Gefilus MAX28) |
| 10: In patients receiving antibiotic therapy, specific probiotics are helpful as adjuvant therapy to prevent, or reduce the duration of, associated diarrhoea | High | 100 | L. paracasei subsp. paracasei DN-114 001 (Actimel),43 marketed combination (Bio-K+ CL1285)40,41 | Marketed combinations (LAB4,44 Lacidofil cap – underpowered45) |
| 11: In patients receiving Helicobacter pylori eradication therapy, specific probiotics are helpful as adjuvant therapy to prevent or reduce the duration/intensity of associated diarrhoea | High | 100 | L. rhamnosus GG (Giflorex),47 marketed combinations (ABT-21 culture,48 Enterogermina49), Saccharomyces boulardii (Codex,47 Reflor46) | |
| 12: With specific probiotics, improvement of symptoms has been shown to lead to improvement in some aspects of health-related quality of life | Moderate | 80 | B. animalis subsp. lactis DN-173 010 (Activia),51 B. animalis subsp. lactis DN-173 010 (Activia),36 B. bifidum MIMBb75,56 Escherichia coli DSM17252 (Symbioflor-2),27 investigative combination (GoL12),61 marketed combinations (Duolac7,37 LAB4,34 Lactibiane26) | Bacillus coagulans GBI-30, 6086 (Digestive Advantage Gas Defense Formula),52 B. longum subsp. infantis 35624 (Bifantis/Align),33 investigative combinations (BIFIDO,57 GoL6,61 Valio Bb9958), marketed combinations (Cultura,31–32 Gefilus MAX28) |
| 13: Probiotics have a favourable safety profile in patients with a range of lower GI symptoms typically managed in primary care or general practice | High | 100 | B. animalis subsp. lactis DN-173 010 (Activia),36 B. animalis subsp. lactis HN019 (HOWARU Bifido/DR10),50 B. bifidum MIMBb75,56 B. longum subsp. infantis 35624 (Bifantis/Align),33 investigative combinations (BIFIDO,57 CH,62 GoL6,61 GoL12,61 SDC,60 Valio Bb9958), L. paracasei subsp. paracasei DN-114 001 (Actimel),43 L. rhamnosus GG (Giflorex),47 marketed combinations (AB100 Jianneng,39 ABT-21 culture,48 Bio-K+ CL1285,40,41 Cultura, Duolac7,37 Gefilus MAX,28 LAB4,34 Lacidofil cap,45 VSL#329–38), Saccharomyces boulardii (Codex,47 Reflor46) | Escherichia coli DSM17252 (Symbioflor-2),27 L. fermentum VRI-003 PCC,55 L. plantarum MF129859 |
| 14: Specific probiotics have a role in the management of some IBS symptoms and can also be used as an adjunct to conventional treatment | NA | 90 | – | – |
| 15: Probiotic strains should be selected based on the patient's symptoms, the clinical indication and the available evidence; no probiotic alleviates the full range of symptoms in IBS | NA | 80 | – | – |
| 16: When trying a probiotic therapy for a chronic GI problem, the product should be taken for 1 month; dose selection should be based on available evidence and manufacturers' recommendations | NA | 80 | – | – |
IBS, irritable bowel syndrome; IBS-C, constipation-predominant IBS; IBS-D, diarrhoea-predominant IBS.
For simplicity, single-strain probiotics are identified by the name of the strain (and the brand name where available), and multi-strain products are identified as ‘combination (X)’ and listed in full below.
Investigative combinations: Valio Bb99: Lactobacillus rhamnosus GG, L. rhamnosus Lc705, Propionibacterium freudenreichii subsp. shermanii JS and Bifidobacterium breve Bb99 (Valio Ltd, Helsinki, Finland).
BIFIDO: Bifidobacterium bifidum BGN4, B. animalis subsp. lactis AD011, Lactobacillus acidophilus AD031 and L. paracasei subsp. paracasei IBS041 (BIFIDO Co. Ltd, Hongchun, Korea).
Bioferme: Bifidobacterium longum subsp. longum 46 and B. longum subsp. longum 2C (Bioferme Ltd, Kaarina, Finland).
CH: Bifidobacterium animalis subsp. lactis Bb12 and Lactobacillus paracasei subsp. paracasei CRL-431 (Chr. Hansen A/S, Hoersholm, Denmark).
GoL6 contains nonspecified strains from the species: Lactobacillus acidophilus,Bifidobacterium bifidum,Bacillus subtilis,L. delbrueckii subsp. bulgaricus,L. delbrueckii subsp. lactis and Bacillus lichenformis (Garden of Life, West Palm, FL, USA).
GoL12 contains nonspecified strains from the species: Lactobacillus acidophilus, Bifidobacterium bifidum, L. delbrueckii subsp. bulgaricus,L. delbrueckii subsp. lactis,L. brevis,L. caucasicus (nomina rejicienda; now L. delbrueckii subsp. delbrueckii), L. fermentum, L. leichmanii, L. paracasei subsp. paracasei, L. plantarum, L. helveticus and Saccharomyces boulardii (Garden of Life, West Palm, FL, USA).
SDC: Lactobacillus acidophilus-SDC 2012 and L. acidophilus-SDC 2013 (Seoul Dairy Cooperative, Seoul, Korea).
Marketed combinations: AB100 Jianneng: Streptococcus salivarius subsp. thermophilus,Lactobacillus delbrueckii subsp. bulgaricus,L. acidophilus and Bifidobacterium longum subsp. longum (Bright Dairy, Shanghai, China).
ABT-21 culture: Lactobacillus acidophilus LA-5, Bifidobacterium animalis subsp. lactis Bb12 and Streptococcus salivarius subsp. thermophilus (Christian Hansen, Nienburg, Germany).
Bio-K+ CL1285: Lactobacillus acidophilus CL1285 and L. paracasei subsp. paracasei LBC80R (Bio-K+ International Inc., Quebec, QC, Canada).
Cultura: Lactobacillus paracasei subsp. paracasei F19, L. acidophilus La5 and Bifidobacterium animalis subsp. lactis Bb12 (Arla Foods Innovation, Stockholm, Sweden).
Duolac7: Lactobacillus acidophilus LH5, L. plantarum LP1, L. rhamnosus LR3, Bifidobacterium breve BR2, B. animalis subsp. lactis BL2, B. longum subsp. longum BG3 and Streptococcus salivarius subsp. thermophilus ST3 (Cell Biotech, Co. Ltd, Seoul, Korea).
Enterogermina: Bacillus clausii strains O/C, N/R, T and SIN (Sanofi Synthelabo OTC, Milan, Italy).
Gefilus MAX: Lactobacillus rhamnosus GG, L. rhamnosus Lc705, Propionibacterium freudenreichii subsp. shermanii JS and Bifidobacterium animalis subsp. lactis Bb12 (Valio Ltd, Helsinki, Finland).
LAB4: Lactobacillus acidophilus (CUL60 and CUL21), Bifidobacterium animalis subsp. lactis CUL34 and B. bifidum CUL20 (Cultech, Port Talbot, UK).
Lacidofil cap: Lactobacillus rhamnosus R0011 and L. acidophilus R0052 (Lallemand Inc., Montreal, QC, Canada).
Lactibiane: Bifidobacterium longum subsp. longum LA 101, Lactobacillus acidophilus LA 102, L. delbrueckii subsp. lactis LA 103 and Streptococcus salivarius subsp. thermophilus LA 104 (PiLeJe, Paris, France).
VSL#3: Bifidobacterium longum subsp. longum, B. infantis subsp. infantis,B. breve,Lactobacillus acidophilus,L. paracasei subsp. paracasei,L. delbrueckii subsp. bulgaricus,L. plantarum and Streptococcus salivarius subsp. thermophilus (VSL Pharmaceuticals Inc., Gaithersburg, MD, USA).
For each consensus statement, the result of the second (final) vote and the grade of supporting evidence are given, followed by a discussion of the evidence. In some cases, the consensus statement is indication-specific; however, studies in other indications that provide relevant data are also described for completeness. In the following discussion, ‘significant’ refers to a statistically significant result (P < 0.05). Sometimes, a particular probiotic yielded conflicting results for a symptom/problem when it was investigated in different studies (see Table 3).
Irritable bowel syndrome (global symptom assessment)
Statement 1: specific probiotics help relieve overall symptom burden in some patients with IBS
Agreement: 100% (6, 40%; 5, 50%; 4, 10%; grade of evidence for effect: high).
Supportive evidence: Eleven studies of 10 different probiotics evaluated overall symptoms in 1313 patients with IBS. Of these studies, nine evaluated overall IBS symptoms as a primary end point, with five reporting a significant beneficial effect of five different probiotic treatments compared with placebo27–58 and three reporting no significant differences between two specific probiotic treatments and placebo.26,31 One of the nine studies reported a significant improvement vs. placebo in a subanalysis of patients with a Bristol stool scale score of 3 or more at baseline, but no significant effect was seen in the overall study population.57 Two studies of two different probiotics evaluated overall IBS symptoms as a secondary end point only, with one reporting a negative effect of the specific probiotic treatment compared with placebo,59 and one dose-ranging study33 reporting a beneficial effect of the specific probiotic treatment at the 1 × 108 CFU dose, but not at the lower and higher doses tested (1 × 106 and 1 × 1010 CFU).
Statement 2: specific probiotics may help relieve overall symptom burden in some patients with IBS-C
Agreement: 80% (6, 10%; 5, 30%; 4, 40%; 3, 10%; 2, 10%; grade of evidence for effect: low).
Supportive evidence: Three studies of two different probiotics evaluated overall IBS symptoms as a secondary end point in 376 patients with IBS-C. One study reported a beneficial effect of the specific probiotic treatment vs. placebo,35 and another study of the same probiotic reported a significant improvement from baseline in the probiotic group, but not in the placebo group, in a subanalysis of patients with fewer than three bowel movements/week.36 One study of a different probiotic reported no significant improvement in symptoms vs. placebo.33
Statement 3: specific probiotics help relieve overall symptom burden in some patients with IBS-D
Agreement: 100% (6, 10%; 5, 70%; 4, 20%; grade of evidence for effect: moderate).
Supportive evidence: Four studies of four different probiotics evaluated overall IBS symptoms in 305 patients with IBS-D. Two studies evaluated overall IBS symptoms as a primary end point, with one reporting a significant beneficial effect of the specific probiotic treatment compared with placebo,37 and one reporting no significant difference.38 Two studies evaluated overall IBS symptoms as a secondary end point only, and both reported a significant beneficial effect of the specific probiotic treatments33–39; one of these was a post hoc analysis of the most effective dose in a subset of patients with IBS-D.33
Abdominal pain
Statement 4: specific probiotics help reduce abdominal pain in some patients with IBS
Agreement: 100% (6, 30%; 5, 50%; 4, 20%, grade of evidence for effect: high).
Supportive evidence: Eighteen studies of 15 different probiotics evaluated abdominal pain in 1806 patients with IBS. Of these studies, six (each examining a different probiotic) evaluated abdominal pain as a primary end point, with four showing a significant beneficial effect of specific probiotic treatments compared with placebo,27–60 one58 showing a trend towards a beneficial effect in the weekly symptom score for abdominal pain (in a secondary analysis, abdominal pain was reduced in a significantly greater proportion of the probiotic group than the placebo group) and one26 showing no significant increase in the proportion of patients reporting symptom relief, but a significantly greater decrease in the abdominal pain score in the probiotic group than the placebo group. Twelve studies evaluated abdominal pain as a secondary end point only. Results from these studies were mixed: one reported a negative effect of the specific probiotic treatment,59 eight (examining six different probiotics) reported no significant effect28–38 and three reported a significant beneficial effect of three different probiotics35,39 (one of which35 also showed no significant effect in another study36).
Abdominal pain was examined in indications other than IBS in five studies of six different probiotics. One study examined abdominal pain as a primary end point in individuals with symptoms related to postprandial intestinal gas, and found a significant improvement in the probiotic group compared with the placebo group.52 Four studies examined abdominal pain as a secondary end point only, with two reporting no significant difference between three different probiotic treatments and placebo,51–61 and two (examining one probiotic in lactose-intolerant individuals undergoing a hydrogen breath test53 and a different probiotic in patients with functional GI symptoms50) reporting significantly improved abdominal pain vs. baseline in the probiotic group, but not in the placebo group.
Bloating/distension
Statement 5: specific probiotics help reduce bloating/distension in some patients with IBS
Agreement: 70% (6, 10%; 5, 30%; 4, 30%; 3, 20%; 2, 10%; grade of evidence for effect: moderate).
Supportive evidence: Fifteen studies of 12 different probiotics evaluated treatment of bloating/distension in 1596 patients with IBS. Of these studies, three (examining three different probiotics) evaluated bloating/distension as a primary end point, with one reporting a significant, beneficial effect of the specific probiotic treatment vs. placebo,35 and two reporting no significant differences.29–58 Twelve studies evaluated bloating/distension as a secondary end point only, with six reporting a significant, beneficial effect of six different probiotic treatments27–56 (one of which36 also showed a beneficial effect as a primary end point in another study35). In one study,36 the significant effect was seen at week 3, but not at week 6, and in another,33 the significant effect was seen at one specific dose only. The remaining six studies reported no significant difference between five different probiotic treatments and placebo31–59 (one of these probiotics38 also showed no significant effect as a primary end point29).
Four studies investigated the effect of five different probiotics on distension/bloating in indications other than IBS. One study evaluated symptoms related to postprandial intestinal gas as a primary end point in healthy individuals and reported no significant differences between the probiotic and placebo groups.52 The remaining three studies (of four different probiotics) evaluated distension/bloating as a secondary end point, with single studies reporting no significant differences between the probiotic and control groups in women with mild digestive symptoms51 and patients with FGID.61 The third study, in individuals with lactose intolerance undergoing a hydrogen breath test, reported significantly reduced bloating in the group receiving the specific probiotic treatment, but no significant improvement in the placebo group.53
Flatus
Statement 6: probiotics tested to date do not help reduce flatus in patients with IBS
Agreement: 90% (6, 20%; 5, 30%; 4, 40%; 2, 10%; grade of evidence for effect: low).
Supportive evidence: Overall, 10 studies, using nine different probiotics, evaluated flatus in 797 patients with IBS. The statement on flatus had to be formulated in the negative as there was a low level of agreement when it was formed in the positive. This was because the evidence in IBS studies was weak: all three studies that examined flatus as a primary end point,30,57 and four of seven studies in which flatus was a secondary end point only,28–38 showed no significant difference between seven specific probiotic treatments and control. The remaining three studies that evaluated flatus as a secondary end point reported a significant beneficial effect of three different probiotic treatments29,33 (one of which29 also showed no significant effect in another study38). In one of these studies,33 the significant effect was seen at one specific dose only.
Five studies examined the effect of six different probiotics on flatus in indications other than IBS. In two studies, no significant effects on flatus (primary end point for one probiotic52; secondary end point for two other probiotics61) were reported. In three studies, a significant benefit of three different probiotic treatments on flatus (secondary end point) was noted; these studies were in women with mild digestive symptoms,51 patients with functional GI symptoms50 and individuals with lactose intolerance undergoing a lactose breath test.53
Constipation
Statement 7: specific probiotics may help reduce constipation in some patients with IBS
Agreement: 60% (5, 20%; 4, 40%; 3, 30%; 1, 10%; grade of evidence for effect: low).
Supportive evidence: Two studies of two different probiotics examined treatment of constipation as a secondary end point in 156 patients with IBS. One study (specifically in patients with IBS-C) reported significant improvements with the specific probiotic treatment vs. control for some of the end points (orocaecal transit time, colonic transit time and urgency), but not others (stool frequency and consistency, straining during evacuation and feelings of incomplete evacuation).35 The second study did not detect any effects of the specific probiotic treatment on the frequency of bowel movements and feelings of incomplete evacuation.56
Two studies of three different probiotics examined constipation in patients with broader FGID. Of these studies, one61 reported no significant effect of two different probiotic treatments, and the other study50 did not report a between-group statistical analysis; however, the decrease in constipation frequency score was approximately twofold greater in the probiotic groups than in the placebo groups.
Bowel habit
Statement 8: specific probiotics help improve frequency and/or consistency of bowel movements in some patients with IBS
Agreement: 70% (6, 10%; 5, 40%; 4, 20%; 3, 20%; 2, 10%; grade of evidence for effect: moderate).
Supportive evidence: Seventeen studies of 14 different probiotics evaluated bowel habit in 1777 patients with IBS. Of these, two studies of two different probiotics evaluated bowel habit as a primary end point, with one study reporting no difference in GI transit measurements between the probiotic and placebo groups,38 and one reporting no significant difference in weekly defecation frequency between the probiotic and placebo groups, but a significant positive effect of the specific probiotic treatment vs. placebo on the secondary end points of urgency and feelings of incomplete evacuation.58
Fifteen of the 17 studies in patients with IBS evaluated bowel habit as a secondary end point only. The main end points assessed were stool frequency, stool consistency and satisfaction with bowel habits. One or more of these end points were evaluated in 14 studies, with seven reporting significant beneficial effects of seven different probiotics,26–56 six reporting no significant effects of five different probiotics28–60 (one of which29 showed no significant benefit as a primary end point in another study38) and one reporting a significant negative effect of the specific probiotic treatment.59 In addition, one study reported significant improvements in the secondary end points of transit time (see section) and urgency in patients with IBS-C, but no significant effects on straining and feelings of incomplete evacuation.35
Four studies of five different probiotic treatments assessed bowel habit in indications other than IBS, with all five probiotics showing significant effects on measures of bowel habit (see Table 3).50–62
Diarrhoea
Statement 9: probiotics tested to date do not reduce diarrhoea in patients with IBS
Agreement: 80% (6, 30%; 5, 30%; 4, 20%; 2, 20%; grade of evidence for effect: very low).
Supportive evidence: Three studies of three different probiotics evaluated, as a secondary end point, the treatment of diarrhoea in 152 patients with IBS. Two studies reported no difference between specific probiotic treatments and placebo,28–37 and one study reported a significant worsening of diarrhoea with the specific probiotic treatment compared with placebo.59
Four studies of six different probiotics evaluated diarrhoea as a secondary end point in indications other than IBS. Specific probiotic treatment had no significant effect on diarrhoea in elderly nursing home residents,54 individuals with a functional bowel disorder61 and individuals with functional GI symptoms.50 The only identified study to show a beneficial effect was a study of one specific probiotic in patients with lactose intolerance53; in this study, diarrhoea improved significantly in the probiotic group, but not in the placebo group.
Statement 10: in patients receiving antibiotic therapy, specific probiotics are helpful as adjuvant therapy to prevent or reduce the duration of associated diarrhoea
Agreement: 100% (6, 60%; 5, 40%; grade of evidence for effect: high).
Supportive evidence: Six studies of four different probiotics examined prevention of AAD and/or reduction in AAD in 1246 patients who received antibiotics. Although initiated in a hospital setting, these studies were included because of the relevance of AAD to primary care. Five studies examined AAD as a primary end point, with four studies of two different probiotics showing a significant reduction in AAD,40–43 and one underpowered study of another probiotic showing a nonsignificant reduction.45 One study assessed AAD as a secondary end point only and found no difference between the probiotic and placebo groups.44
Statement 11: in patients receiving H. pylori eradication therapy, specific probiotics are helpful as adjuvant therapy to prevent or reduce the duration/intensity of associated diarrhoea
Agreement: 100% (6, 60%; 5, 40%; grade of evidence for effect: high).
Supportive evidence: Four studies, which evaluated five different probiotics, had a primary objective to investigate the occurrence of diarrhoea as a side effect of H. pylori eradication triple therapy in 382 patients. All four studies reported a significant benefit of specific probiotic treatments compared with placebo.46–49 However, the results for two of the probiotic treatments were mixed, with a significant benefit of the specific probiotic treatment seen after 1 week, but not 2 weeks, in one study,49 and significantly fewer days with diarrhoea and shorter mean duration of diarrhoea episodes, but no significant difference in frequency of diarrhoea episodes, in the probiotic group compared with the placebo group in another study.48
Health-related quality of life
Statement 12: with specific probiotics, improvement of symptoms has been shown to lead to improvement in some aspects of health-related quality of life
Agreement: 80% (6, 10%; 5, 30%; 4, 40%; 3, 20%; grade of evidence for effect: moderate).
Supportive evidence: Health-related quality of life was assessed as a primary end point in three studies of three different probiotics. One study in patients with IBS-C36 reported no significant difference between the probiotic and placebo groups for the change from baseline in the discomfort dimension score of the Functional Digestive Disorders Quality of Life (FDDQL) questionnaire (primary end point); however, the probiotic group had a significantly greater proportion of responders for the discomfort dimension score than the placebo group at week 3. Another study of the same probiotic was performed in women with minor GI symptoms, and reported a significantly greater improvement in ‘GI well-being’ (primary end point) in the probiotic group than in the placebo group.51 The remaining study assessed two different probiotics in patients with FGID61 and reported no significant differences between the probiotic and control groups for the Gastrointestinal Quality of Life Index (GIQLI) total score and well-being subscales (physical, social and mental; primary end point); however, the 36-item Short-Form Health Survey (SF-36; secondary end point) showed significant changes in the probiotic groups for physical functioning and/or ‘role–physical’ domains, but no significant changes in the control groups.
Twelve studies assessed aspects of health-related quality of life as secondary end points only. Of these, seven (evaluating six different probiotics) found no difference between treatment groups in measures of health-related quality of life.28–58 The remaining five studies (all in patients with IBS) reported significant benefits of five different probiotic treatments for some aspects of health-related quality of life.26–56
Adverse events
Statement 13: probiotics have a favourable safety profile in patients with a range of lower GI symptoms typically managed in primary care or general practice
Agreement: 100% (6, 80%; 5, 20%; grade of evidence for effect: high).
Supportive evidence: Safety data were reported in 28 studies, none of which revealed significant treatment-emergent adverse events that were attributed to probiotic use. Of the 28 studies, 25 reported no relevant differences in safety between 23 specific probiotic treatments and placebo.28–62 The remaining three studies (each examining a different probiotic) are summarised below.
In a study of patients with IBS, two patients in the probiotic group discontinued from the study because of adverse events (moderate nausea and severe exanthema). However, the most frequent adverse events (fatigue, pruritus and diarrhoea) occurred equally in the probiotic and placebo groups.27 In a study of patients with IBS, one participant had a short stay in hospital for cervicobrachialgia 2 weeks after the end of the specific probiotic treatment; however, there was no organic explanation and the patient continued in the trial.59 In a study of healthy athletes, there was a twofold increase in the number and duration of mild GI symptoms in the probiotic group compared with the placebo group, although severity tended to be lower.55
General considerations
Statement 14: specific probiotics have a role in the management of some IBS symptoms and can also be used as an adjunct to conventional treatment
Agreement: 90% (6, 60%; 5, 20%; 4, 10%; 2, 10%; grade of evidence for effect: NA).
Statement 14 was derived from the evidence collated during this international consensus and from the clinical experience of the Consensus Group. It was presented for voting with the following explanations: for patients with IBS who are responding positively to conventional therapy, probiotics should be considered as an adjunct rather than a replacement for conventional treatment; for patients with IBS who are not responding to conventional therapy, replacement of the ineffective conventional treatment with a probiotic may be considered.
Statement 15: probiotic strains should be selected based on the patient's symptoms, the clinical indication and the available evidence; no probiotic alleviates the full range of symptoms in IBS
Agreement: 80% (6, 20%; 5, 50%, 4, 10%; 3, 10%; 2, 10%; grade of evidence for effect: NA).
Statement 15 was based on the observation that some studies in patients with IBS showed a beneficial effect of a given probiotic on some symptoms, but not on others. For example, one study reported that Bifidobacterium bifidum MIMBb75 was beneficial for improving global IBS symptoms, bloating and aspects of health-related quality of life (physical and mental health), but not for frequency of bowel movement and feeling of incomplete bowel evacuation.56 In another study, Bifidobacterium longum subsp. infantis 35624 (1 × 108 CFU once daily) significantly improved all IBS symptoms assessed, except urgency.33 Studies of multi-strain probiotics provide further examples.28,57
Statement 16: when trying a probiotic therapy for a chronic GI problem, the product should be taken for 1 month; dose selection should be based on available evidence and manufacturers' recommendations
Agreement: 80% (6, 30%; 5, 40%; 4, 10%; 2, 20%; grade of evidence for effect: NA).
Statement 16 was based on the observation that the treatment duration was at least 4 weeks in most studies (21/24) that examined probiotics for the treatment of chronic GI problems.
Discussion
This is the first practical consensus on the role of probiotics in the management of the full range of lower GI symptoms in adults consulting clinicians in a pragmatic setting (particularly in primary care). The outcome of this consensus (summarised in Tables 3 and 4) is relevant to both primary care physicians and gastroenterologists, and is important because patients as well as the general public are becoming increasingly aware of probiotics as a result of considerable media interest and intensive advertising campaigns. Consequently, there is a need for physicians to be in a position to provide advice on whether probiotics might be helpful for patients with specific lower GI symptoms/problems, and, if so, which ones might be appropriate to recommend. What is evident is that there is no clear, simple guidance possible and that research linking specific probiotics with particular symptoms or problems is complex to interpret, partly because of the widespread types of studies and end points. However, our research confirms that there is positive evidence for the role of probiotics in lower GI problems.
Table 4.
Practical implications of consensus statements for physicians
| Grade of evidence for effect | Symptoms/indications | Meaning for physicians |
|---|---|---|
| High | Overall symptoms and abdominal pain in IBSPrevention or reduction of diarrhoea in patients receiving antibiotics, including Helicobacter pylori eradication therapy | Probiotics with supportive evidence for benefit should be tried |
| Moderate | Overall symptoms in IBS-DBowel movements and bloating/distension in IBS | Probiotics with supportive evidence for benefit could be tried |
| Low | Overall symptoms in IBS-C | Probiotics with supportive evidence for benefit could be considered |
| Very low | Flatus in IBS*Diarrhoea in IBS | Currently no evidence to support use of probiotics |
Constipation in IBS is not addressed in this table because consensus was not achieved for this statement.
IBS, irritable bowel syndrome; IBS-C, constipation-predominant IBS; IBS-D, diarrhoea-predominant IBS.
The grade of evidence was initially deemed to be low (rather than very low) for flatus in IBS, but the statement was revised to be negative in response to voter feedback during the Delphi process.
Consensus findings in comparison with other research
A strong consensus was reached on the positive role of probiotics in the prevention of AAD or diarrhoea associated with H. pylori eradication therapy. Although the strain-/formulation-specific properties of different probiotics mean that meta-analyses of probiotics should be interpreted with caution, our findings are consistent with several previous meta-analyses,63–67 and the potential of probiotics to reduce side effects is also noted in the Maastricht IV/Florence consensus report on the management of H. pylori infection.68 Furthermore, our finding is consistent with the proposed role of probiotics in maintaining the gut microbiota,69 which are typically disturbed during oral antibiotic treatment. However, the current consensus does not support a role for probiotics in the treatment of diarrhoea in adults with IBS. The role of probiotics in the prevention of traveller's diarrhoea is also a topic of interest,70–71 but was not addressed in any of the studies eligible for the current analysis.
The Consensus Group concluded, with a high level of evidence, that specific probiotics help reduce overall symptom burden and abdominal pain in IBS (Statements 1 and 4: 100% agreement among voters), consistent with previous meta-analyses and reviews.2,8 There was a moderate level of evidence, with 70% agreement, for a role of specific probiotics in reducing bloating and improving the frequency and/or consistency of bowel movements in IBS (Statements 5 and 8). The level of evidence was also moderate for a role of probiotics in improving some aspects of health-related quality of life (Statement 12: 80% agreement); there is a need for more research on the effects of probiotics on health-related quality of life, because this has clear implications for the day-to-day functioning of the patient.
There was a low level of evidence for a role of specific probiotics in reducing constipation [Statement 7: 60% agreement (no consensus)] and for the statement that probiotics tested to date do not reduce flatus in patients with IBS (Statement 6: 90% agreement). An overview of meta-analyses in IBS showed a similar trend, with more supportive evidence available for overall symptom burden and abdominal pain than for flatus.8 Nevertheless, a meta-analysis of studies in adults with IBS did report a significant reduction in flatus with probiotic treatment.72
The lack of consensus on the role of probiotics in the management of constipation is consistent with the World Gastroenterology Organisation guideline on prebiotics and probiotics, which recommends certain prebiotics, but not probiotics, for the treatment of constipation.10 The grade of evidence and level of agreement were higher for bowel habit than for constipation; a possible explanation is that improvement in individual measures of function might be easier to achieve (or measure) than improvement in multiple facets of a GI problem. However, other confounding factors may play a role: besides a potential gender effect, bifidobacteria, attaining their highest counts in the colon, are more likely to have an effect on the colonic transit time than lactobacilli, which occur mainly in the small bowel.
Oral probiotics had a favourable safety profile in the included studies overall; the majority of the studies found no differences in safety between probiotic and placebo, and none of the studies identified significant adverse events attributed to probiotic use. The statement on the safety of probiotics achieved the highest degree of consensus. However, safety should not be generalised to untested situations, including other probiotics and different modes of administration, such as delivery by enteral tube.73 In addition, their use in certain patient groups, such as those who are immuno-compromised, needs to be considered on a case-by-case basis – at present, there are few data on the safety of probiotics in such patients. Immune compromise (including a debilitated state or malignancy) has been identified as a risk factor for rare cases of bacteraemia or fungaemia in patients taking certain probiotics (most commonly Saccharomyces boulardii).74,75
Strengths and limitations
Suboptimal trial design has been highlighted as an important issue in studies of probiotics.77 To address this, we applied strict quality criteria in the selection of papers: only randomised, placebo-controlled clinical trials of probiotics that had suitable follow-up were included in the analysis. However, a limitation of the current consensus, similar to any systematic review, is the potential for publication bias – inconclusive or negative results are less likely to be published than positive results. Furthermore, studies designed to assess one end point can show positive effects on other end points by chance. We have therefore only included studies with a sample size calculation and we have distinguished between results that were assessed as primary and secondary end points to limit the influence of chance findings in secondary end points. Challenges identified by the Consensus Group during the voting process included the small number of high-quality studies, small study populations and diverse results, with some members noting that the evidence was insufficient to fully support Statements 5 and 8, and that there is currently insufficient evidence to personalise choices in probiotic treatment (in response to Statement 15). The Consensus Group included representatives from many European countries, but the relevance of the statements to all European primary care/gastroenterology settings cannot be determined.
Factors determining the response to treatment include probiotic strain(s), dose and mode of administration, health status of the patient, diet and concomitant medications (e.g. antibiotics and antacids). The variable results noted across some of the studies included in our analysis could have been affected by any of these factors, as well as the different patient groups enrolled in the studies and different levels of placebo response. Furthermore, this consensus focused on adults, and the statements cannot be extended to children. Additional points to consider are that probiotic research is evolving rapidly, and that the current statements reflect physicians', rather than patients', perspectives. Many patients have an interest in probiotics and their potential to reduce their symptoms,78–79 and may take probiotics (or products incorrectly identified as probiotics) before consulting their physician. Therefore, educational materials for the general public are also needed to improve understanding and to ensure appropriate use of probiotics (see the consumer guidelines of the International Scientific Association for Probiotics and Prebiotics for an example15–80). To address these points, the ESPCG intends to update this consensus publication with new research and input from patient groups in 3 years.
Most of the studies eligible for inclusion in this consensus focused on IBS or AAD; only a small subset of the studies examined probiotics in healthy individuals or patients with lactose malabsorption, other functional GI problems or mild GI symptoms, and therefore specific statements were not prepared for these groups. Nevertheless, this small subset of studies was still included alongside the statements in Table 3 for completeness. The prophylactic application of probiotics is a potentially interesting area for future research, although it will be challenging in terms of study design.
Conclusions and clinical implications
The practical clinical implications of the consensus statements are summarised for each grade of evidence in Table 4. It should be noted that effects are strain-/formulation-specific and cannot be extrapolated from one probiotic to another. Furthermore, specific probiotics will have different effects in different patients; a probiotic that does not work in one indication may have evidence supporting a beneficial effect in a different indication or for a different symptom. When trying a probiotic therapy for a chronic GI problem, it is critically important that the product is taken in adequate doses on a regular basis (e.g. just before a meal) for a reasonable period of time, which should be at least a month, unless it cannot be tolerated for any reason. Regular consumption is important because probiotic strains are transient and will generally be washed out within days, although strain-specific differences occur, for example, linked to the production of pili81–82 or mucus-binding proteins83 by the probiotic bacteria.
The need for objective, evidence-based guidance on the role of probiotics is becoming increasingly important as public awareness of probiotics grows. This consensus is intended as a practical reference to help physicians make appropriate, evidence-based recommendations to patients who might benefit from probiotic treatment. Overall, the randomised, placebo-controlled trials included in our analysis support, with a high evidence level, a role for specific probiotics in the management of overall symptoms and abdominal pain in patients with IBS, and for preventing or reducing diarrhoea in patients receiving antibiotics or H. pylori eradication triple therapy. The trials support, with a moderate evidence level, a role for specific probiotics in managing overall symptoms in patients with IBS-D; improving bowel movements and bloating/distension in patients with IBS; and improving some aspects of health-related quality of life.
Authorship
Guarantor of the article: APSH initiated the project.
Author contributions: APSH, BP, CM, PW, CW, NdW, Lamya Moulay and Jane Mason developed the initial literature search strategy. APSH, BP, CM, PW, LA, PF, CL, JM, J-MPdF, GR, CW, NdW, Lamya Moulay, Jane Mason and Bohumil Seifert took part in the screening and analysis of the retrieved references. APSH, BP, CM, PW, LA, PF, CL, JM, J-MPdF, GR, CW, NdW and Bohumil Seifert attended a workshop at which the overall goals and process were agreed upon. APSH, BP, CM, PW, CW and NdW drafted statements. BP, PW, LA, PF, CL, JM, J-MPdF, GR, NdW and Bohumil Seifert voted on the statements. Paul Sinclair provided support and guidance on the Delphi voting process. CM, CW and Jane Mason prepared an outline and revised subsequent drafts of the paper. APSH, BP, PW, LA, PF, CL, JM, J-MPdF, GR and NdW revised the outline and subsequent drafts of the paper. All authors have approved the final version of the article, including the authorship list.
Acknowledgments
Declaration of personal interests: APSH, LA, PF, CL, JM, GR and NdW are committee members of the ESPCG.
PH has received financial support for meeting travel and literature review and consensus development activities from Danone, and medical writing assistance from Oxford PharmaGenesis Ltd funded by Danone, for the submitted study. PH has also received payment for board membership and consultancy from Almirall and Shire, for expert testimony from Novartis, for lectures from Reckitt Benckiser and Shire and for development of educational presentations from Almirall and Shire. CM is an employee of Oxford PharmaGenesis Ltd, which has received project funding from Danone (for the submitted study), AstraZeneca, Shire and the Canadian Association for Gastroenterology, and payment for the development of educational presentations from Shire. BP has received financial support for meeting travel and literature review activities from Danone for the submitted study. BP has also received payment from Danone for developing educational presentations. He has acted as a consultant for Merck, Lallemand, Danone and GAP, has received payment for delivering lectures and presentations from Metagenics and Yakult and has received expenses for meetings from PRI. BP's institution has received research grants/service contracts from BioProx, Danisco, Danone, Kemin, Lesaffre, Roquette and Vesale Pharma. PW has received financial support for meeting travel and literature review activities from Danone for the submitted study. PW has also received consultancy payments from Norgine, Danone, Shire and Almirall and delivered lectures funded by Abbott, Danone and Shire. PW's institution has received grant support from Danone. LA is an employee of the Karolinska Institute. PF has received financial support for meeting travel from Danone for the submitted study. CL has received financial support for meeting travel from Danone for the submitted study. CL has also received payment for consultancy from GlaxoSmithKline AEBE, payment for participation in an expert panel from Pfizer International Operations, S.A.S., and payment for a lecture from Pfizer Hellas A.E./Pfizer Inc. CL's institution has received payment for his participation in an advisory board meeting from Medtronic Inc., grants from the European Commission and payment for his participation in a satellite symposium from Boehringer Ingelheim Pharma GmbH & Co. KG. JM has received financial support for meeting travel from Danone for the submitted study. J-MPdF has received financial support for meeting travel from Danone for the submitted study. J-MPdF has also received payment for consultancy and development of educational presentations from Metagenics. GR has received financial support for meeting travel and literature review activities from Danone for the submitted study. GR has also received payment for consultancy and development of educational presentations from Almirall. CW has shares in AstraZeneca, and is an employee of Oxford PharmaGenesis Ltd, which has received project funding from Danone (for the submitted study), AstraZeneca, Shire and the Canadian Association for Gastroenterology, and payment for the development of educational presentations from Shire. NdW's institution has received a grant from the ESPCG for the submitted study. NdW has received payment for developing E-learning modules for UEGF and book royalties from Elsevier and Bohn Stafleu. His institution holds several research grants from ZON MW, the Dutch Research Institute.
We thank ECD Solutions, Atlanta, GA, USA, for providing the online voting platform, and Paul Sinclair of INSINC Consulting Inc., Guelph, ON, Canada, for support and guidance on the Delphi process. We also thank Professor Bohumil Seifert (Prague, Czech Republic) for his participation in the workshop and for voting on the statements.
Declaration of funding interests: This consensus was supported and facilitated by the ESPCG, which received an unrestricted grant from Danone (Paris, France). At the request of the ESPCG, support for the consensus was provided by Dr Claire Mulligan, Jane Mason MPharm and Dr Chris Winchester (Research Evaluation Unit, Oxford PharmaGenesis Ltd, Oxford, UK). The systematic literature searches and initial title/abstract screening were conducted by Lamya Moulay (iNTUiTiO, Paris, France). iNTUiTiO and Oxford PharmaGenesis Ltd received project funding from Danone. Danone also provided logistical and financial support for the workshop.
The funding body had no input into the screening or analysis of retrieved references, the content or conduct of the workshop, the grading of the evidence, voting on the statements or the decision to submit the manuscript for publication.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article
Probiotic availability by country in Europe, the USA and China.
References
- McCormick A, Fleming D, Charlton J. Morbidity Statistics from General Practice: Fourth National Study 1991–1992. London: Office of Population Censuses and Surveys; 1995. Available at: http://www.statistics.gov.uk/downloads/theme_health/MB5No3.pdf. Accessed February 28, 2005. [Google Scholar]
- Quigley E, Fried M, Gwee KA, et al. 2009. Irritable bowel syndrome: a global perspective. Available at: http://www.worldgastroenterology.org/assets/downloads/en/pdf/guidelines/20_irritable_bowel_syndrome.pdf. Accessed December 2012.
- Birtwhistle RV. Irritable bowel syndrome: are complementary and alternative medicine treatments useful? Can Fam Physician. 2009;55:8–9. 126–7. [PMC free article] [PubMed] [Google Scholar]
- Moynihan NT, Callahan MJ, Kalsmith B, Moses PL. How do you spell relief for irritable bowel syndrome? J Fam Pract. 2008;57:100–8. [PubMed] [Google Scholar]
- Simrén M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–76. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: the incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:535–44. doi: 10.1111/j.1365-2036.2007.03399.x. [DOI] [PubMed] [Google Scholar]
- Porter CK, Gormley R, Tribble DR, Cash BD, Riddle MS. The incidence and gastrointestinal infectious risk of functional gastrointestinal disorders in a healthy US adult population. Am J Gastroenterol. 2011;106:130–8. doi: 10.1038/ajg.2010.371. [DOI] [PubMed] [Google Scholar]
- Whelan K. Probiotics and prebiotics in the management of irritable bowel syndrome: a review of recent clinical trials and systematic reviews. Curr Opin Clin Nutr Metab Care. 2011;14:581–7. doi: 10.1097/MCO.0b013e32834b8082. [DOI] [PubMed] [Google Scholar]
- FAO/WHO Working Group. London, Ontario, Canada: 2002. Guidelines for the evaluation of probiotics in food. Report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food. Available at: ftp://ftp.fao.org/es/esn/food/wgreport2.pdf. Accessed 21 February 2013. [Google Scholar]
- Guarner F, Khan AG, Garisch J, et al. Probiotics and prebiotics. 2011. Available at: http://www.worldgastroenterology.org/probiotics-prebiotics.html. Accessed March 6, 2013.
- Córdoba, Argentina: 2001. FAO/WHO Expert Consultation Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria,. Available at: http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf. Accessed July 11, 2012. [Google Scholar]
- Gardiner GE, O'Sullivan E, Kelly J, et al. Comparative survival rates of human-derived probiotic Lactobacillus paracasei and L. salivarius strains during heat treatment and spray drying. Appl Environ Microbiol. 2000;66:2605–12. doi: 10.1128/aem.66.6.2605-2612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran BM, Ross RP, Fitzgerald GF, Stanton C. Comparative survival of probiotic lactobacilli spray-dried in the presence of prebiotic substances. J Appl Microbiol. 2004;96:1024–39. doi: 10.1111/j.1365-2672.2004.02219.x. [DOI] [PubMed] [Google Scholar]
- Hopkins MJ, Sharp R, Macfarlane GT. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut. 2001;48:198–205. doi: 10.1136/gut.48.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders ME. How do we know when something called “probiotic” is really a probiotic? A guideline for consumers and health care professionals. Funct Food Rev. 2009;1:3–12. [Google Scholar]
- Williams MD, Ha CY, Ciorba MA. Probiotics as therapy in gastroenterology: a study of physician opinions and recommendations. J Clin Gastroenterol. 2010;44:631–6. doi: 10.1097/MCG.0b013e3181d47f5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordina C, Shaikh I, Shrestha S, Camilleri-Brennan J. Probiotics in the management of gastrointestinal disease: analysis of the attitudes and prescribing practices of gastroenterologists and surgeons. J Dig Dis. 2011;12:489–96. doi: 10.1111/j.1751-2980.2011.00534.x. [DOI] [PubMed] [Google Scholar]
- Sharp RR, Achkar JP, Brinich MA, Farrell RM. Helping patients make informed choices about probiotics: a need for research. Am J Gastroenterol. 2009;104:809–13. doi: 10.1038/ajg.2008.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz M, Baranchi A, Thurston L, et al. Consumer demographics and expectations of probiotic therapy in New Zealand: results of a large telephone survey. N Z Med J. 2011;124:36–43. [PubMed] [Google Scholar]
- Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–90. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:E839–42. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M. Considering the implication of variations within Delphi research. Fam Pract. 2009;26:420–4. doi: 10.1093/fampra/cmp051. [DOI] [PubMed] [Google Scholar]
- Linstone HA, Turoff M. The Delphi method: techniques and applications. 2002. Available at: http://is.njit.edu/pubs/delphibook/. Accessed December 2012.
- Dalkey N. An experimental study of group opinion: the Delphi method. Futures. 1969;1:408–26. [Google Scholar]
- Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouault-Holowacz S, Bieuvelet S, Burckel A, Cazaubiel M, Dray X, Marteau P. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2008;32:147–52. doi: 10.1016/j.gcb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Enck P, Zimmermann K, Menke G, Klosterhalfen S. Randomized controlled treatment trial of irritable bowel syndrome with a probiotic E. coli preparation (DSM17252) compared to placebo. Z Gastroenterol. 2009;47:209–14. doi: 10.1055/s-2008-1027702. [DOI] [PubMed] [Google Scholar]
- Kajander K, Myllyluoma E, Rajilic-Stojanovic M, et al. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27:48–57. doi: 10.1111/j.1365-2036.2007.03542.x. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17:687–96. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- Sen S, Mullan MM, Parker TJ, Woolner JT, Tarry SA, Hunter JO. Effect of Lactobacillus plantarum 299v on colonic fermentation and symptoms of irritable bowel syndrome. Dig Dis Sci. 2002;47:2615–20. doi: 10.1023/a:1020597001460. [DOI] [PubMed] [Google Scholar]
- Simrén M, Ohman L, Olsson J, et al. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome – a randomized, double-blind, controlled study. Aliment Pharmacol Ther. 2010;31:218–27. doi: 10.1111/j.1365-2036.2009.04183.x. [DOI] [PubMed] [Google Scholar]
- Sondergaard B, Olsson J, Ohlson K, Svensson U, Bytzer P, Ekesbo R. Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: a randomized, placebo-controlled trial. Scand J Gastroenterol. 2011;46:663–72. doi: 10.3109/00365521.2011.565066. [DOI] [PubMed] [Google Scholar]
- Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–90. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- Williams EA, Stimpson J, Wang D, et al. Clinical trial: a multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2009;29:97–103. doi: 10.1111/j.1365-2036.2008.03848.x. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Houghton LA, Morris J, et al. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2009;29:104–14. doi: 10.1111/j.1365-2036.2008.03853.x. [DOI] [PubMed] [Google Scholar]
- Guyonnet D, Chassany O, Ducrotte P, et al. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. 2007;26:475–86. doi: 10.1111/j.1365-2036.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- Ki Cha B, Mun Jung S, Hwan Choi C, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46:220–7. doi: 10.1097/MCG.0b013e31823712b1. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- Zeng J, Li YQ, Zuo XL, Zhen YB, Yang J, Liu CH. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:994–1002. doi: 10.1111/j.1365-2036.2008.03818.x. [DOI] [PubMed] [Google Scholar]
- Beausoleil M, Fortier N, Guenette S, et al. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterol. 2007;21:732–6. doi: 10.1155/2007/720205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105:1636–41. doi: 10.1038/ajg.2010.11. [DOI] [PubMed] [Google Scholar]
- Sampalis J, Psaradellis E, Rampakakis E. Efficacy of BIO K+ CL1285 in the reduction of antibiotic-associated diarrhea – a placebo controlled double-blind randomized, multi-center study. Arch Med Sci. 2010;6:56–64. doi: 10.5114/aoms.2010.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson M, D'Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80. doi: 10.1136/bmj.39231.599815.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer S, Weaver MA, Harris JC, Dee P, Hunter J. Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of C. difficile diarrhoea. Int Microbiol. 2004;7:59–62. [PubMed] [Google Scholar]
- Song HJ, Kim JY, Jung SA, et al. Effect of probiotic Lactobacillus (Lacidofil® cap) for the prevention of antibiotic-associated diarrhea: a prospective, randomized, double-blind, multicenter study. J Korean Med Sci. 2010;25:1784–91. doi: 10.3346/jkms.2010.25.12.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cindoruk M, Erkan G, Karakan T, Dursun A, Unal S. Efficacy and safety of Saccharomyces boulardii in the 14-day triple anti-Helicobacter pylori therapy: a prospective randomized placebo-controlled double-blind study. Helicobacter. 2007;12:309–16. doi: 10.1111/j.1523-5378.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- Cremonini F, Di Caro S, Covino M, et al. Effect of different probiotic preparations on anti-Helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol. 2002;97:2744–9. doi: 10.1111/j.1572-0241.2002.07063.x. [DOI] [PubMed] [Google Scholar]
- de Vrese M, Kristen H, Rautenberg P, Laue C, Schrezenmeir J. Probiotic lactobacilli and bifidobacteria in a fermented milk product with added fruit preparation reduce antibiotic associated diarrhea and Helicobacter pylori activity. J Dairy Res. 2011;78:396–403. doi: 10.1017/S002202991100063X. [DOI] [PubMed] [Google Scholar]
- Nista EC, Candelli M, Cremonini F, et al. Bacillus clausii therapy to reduce side-effects of anti-Helicobacter pylori treatment: randomized, double-blind, placebo controlled trial. Aliment Pharmacol Ther. 2004;20:1181–8. doi: 10.1111/j.1365-2036.2004.02274.x. [DOI] [PubMed] [Google Scholar]
- Waller PA, Gopal PK, Leyer GJ, et al. Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand J Gastroenterol. 2011;46:1057–64. doi: 10.3109/00365521.2011.584895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyonnet D, Schlumberger A, Mhamdi L, Jakob S, Chassany O. Fermented milk containing Bifidobacterium lactis DN-173 010 improves gastrointestinal well-being and digestive symptoms in women reporting minor digestive symptoms: a randomised, double-blind, parallel, controlled study. Br J Nutr. 2009;102:1654–62. doi: 10.1017/S0007114509990882. [DOI] [PubMed] [Google Scholar]
- Kalman DS, Schwartz HI, Alvarez P, Feldman S, Pezzullo JC, Krieger DR. A prospective, randomized, double-blind, placebo-controlled parallel-group dual site trial to evaluate the effects of a Bacillus coagulans-based product on functional intestinal gas symptoms. BMC Gastroenterol. 2009;9:85. doi: 10.1186/1471-230X-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojetti V, Gigante G, Gabrielli M, et al. The effect of oral supplementation with Lactobacillus reuteri or tilactase in lactose intolerant patients: randomized trial. Eur Rev Med Pharmacol Sci. 2010;14:163–70. [PubMed] [Google Scholar]
- Pitkala KH, Strandberg TE, Finne Soveri UH, Ouwehand AC, Poussa T, Salminen S. Fermented cereal with specific bifidobacteria normalizes bowel movements in elderly nursing home residents. A randomized, controlled trial. J Nutr Health Aging. 2007;11:305–11. [PubMed] [Google Scholar]
- West NP, Pyne DB, Cripps AW, et al. Lactobacillus fermentum (PCC®) supplementation and gastrointestinal and respiratory-tract illness symptoms: a randomised control trial in athletes. Nutr J. 2011;10:30. doi: 10.1186/1475-2891-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life – a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123–32. doi: 10.1111/j.1365-2036.2011.04633.x. [DOI] [PubMed] [Google Scholar]
- Hong KS, Kang HW, Im JP, et al. Effect of probiotics on symptoms in Korean adults with irritable bowel syndrome. Gut Liver. 2009;3:101–7. doi: 10.5009/gnl.2009.3.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajander K, Hatakka K, Poussa T, Farkkila M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment Pharmacol Ther. 2005;22:387–94. doi: 10.1111/j.1365-2036.2005.02579.x. [DOI] [PubMed] [Google Scholar]
- Ligaarden SC, Axelsson L, Naterstad K, Lydersen S, Farup PG. A candidate probiotic with unfavourable effects in subjects with irritable bowel syndrome: a randomised controlled trial. BMC Gastroenterol. 2010;10:16. doi: 10.1186/1471-230X-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn DH, Song JH, Kim HJ, et al. Therapeutic effect of Lactobacillus acidophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Dig Dis Sci. 2008;53:2714–8. doi: 10.1007/s10620-007-0196-4. [DOI] [PubMed] [Google Scholar]
- Kim LS, Hilli L, Orlowski J, Kupperman JL, Baral M, Waters RF. Efficacy of probiotics and nutrients in functional gastrointestinal disorders: a preliminary clinical trial. Dig Dis Sci. 2006;51:2134–44. doi: 10.1007/s10620-006-9297-8. [DOI] [PubMed] [Google Scholar]
- Larsen CN, Nielsen S, Kaestel P, et al. Dose-response study of probiotic bacteria Bifidobacterium animalis subsp lactis BB-12 and Lactobacillus paracasei subsp paracasei CRL-341 in healthy young adults. Eur J Clin Nutr. 2006;60:1284–93. doi: 10.1038/sj.ejcn.1602450. [DOI] [PubMed] [Google Scholar]
- D'Souza AL, Rajkumar C, Cooke J, Bulpitt CJ. Probiotics in prevention of antibiotic associated diarrhoea: meta-analysis. BMJ. 2002;324:1361. doi: 10.1136/bmj.324.7350.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremonini F, Di Caro S, Nista EC, et al. Meta-analysis: the effect of probiotic administration on antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2002;16:1461–7. doi: 10.1046/j.1365-2036.2002.01318.x. [DOI] [PubMed] [Google Scholar]
- Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959–69. doi: 10.1001/jama.2012.3507. [DOI] [PubMed] [Google Scholar]
- McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101:812–22. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- Tong JL, Ran ZH, Shen J, Zhang CX, Xiao SD. Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2007;25:155–68. doi: 10.1111/j.1365-2036.2006.03179.x. [DOI] [PubMed] [Google Scholar]
- Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection – the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646–64. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- Hickson M. Probiotics in the prevention of antibiotic-associated diarrhoea and Clostridium difficile infection. Therap Adv Gastroenterol. 2011;4:185–97. doi: 10.1177/1756283X11399115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland LV. Meta-analysis of probiotics for the prevention of traveler's diarrhea. Travel Med Infect Dis. 2007;5:97–105. doi: 10.1016/j.tmaid.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sazawal S, Hiremath G, Dhingra U, Malik P, Deb S, Black RE. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006;6:374–82. doi: 10.1016/S1473-3099(06)70495-9. [DOI] [PubMed] [Google Scholar]
- Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325–32. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- Sanders ME, Akkermans LM, Haller D, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164–85. doi: 10.4161/gmic.1.3.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen MK, Rautelin H, Tynkkynen S, et al. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GG. Clin Infect Dis. 2004;38:62–9. doi: 10.1086/380455. [DOI] [PubMed] [Google Scholar]
- Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83:1256–64. doi: 10.1093/ajcn/83.6.1256. [DOI] [PubMed] [Google Scholar]
- Hempel S, Newberry S, Ruelaz A, et al. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Safety of probiotics to reduce risk and prevent or treat disease. Evidence Report/Technology Assessment No. 200. (Prepared by the Southern California Evidence-based Practice Center under Contract No. 290-2007-10062-I.) AHRQ Publication No. 11-E007.. Available at: http://www.ahrq.gov/clinic/tp/probiotictp.htm. Accessed July 29, 2013. [Google Scholar]
- Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2009;104:1033–49. doi: 10.1038/ajg.2009.25. [DOI] [PubMed] [Google Scholar]
- Mercer M, Brinich MA, Geller G, et al. How patients view probiotics: findings from a multicenter study of patients with inflammatory bowel disease and irritable bowel syndrome. J Clin Gastroenterol. 2012;46:138–44. doi: 10.1097/MCG.0b013e318225f545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siro I, Kapolna E, Kapolna B, Lugasi A. Functional food. Product development, marketing and consumer acceptance – a review. Appetite. 2008;51:456–67. doi: 10.1016/j.appet.2008.05.060. [DOI] [PubMed] [Google Scholar]
- 2009. International Scientific Association for Probiotics and Prebiotics. Probiotics: a consumer guide for making smart choices, 11 March. Available at: http://www.isapp.net/docs/Consumer_Guidelines-probiotic.pdf. Accessed September 26, 2012.
- O'Connell Motherway M, Zomer A, Leahy SC, et al. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc Natl Acad Sci U S A. 2011;108:11217–22. doi: 10.1073/pnas.1105380108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankainen M, Paulin L, Tynkkynen S, et al. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc Natl Acad Sci U S A. 2009;106:17193–8. doi: 10.1073/pnas.0908876106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie DA, Tailford LE, Hemmings AM, Juge N. Crystal structure of a mucus-binding protein repeat reveals an unexpected functional immunoglobulin binding activity. J Biol Chem. 2009;284:32444–53. doi: 10.1074/jbc.M109.040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Probiotic availability by country in Europe, the USA and China.