Significance
Early detection of new or novel variants of nosocomial pathogens (such as hospital-acquired methicillin-resistant Staphylococcus aureus) is a public health priority. However, surveillance effort is often limited by financial and practical constraints. Although this is widely recognized, no good evidence base exists to inform the design of efficient hospital-based surveillance systems. We address the key questions of how many and which hospitals should be included in such a surveillance system. Using hospital admissions data from England and The Netherlands, we model the spread of a pathogen among the network of hospitals connected by the movement of patients between them. We show how it is possible to design hospital-based surveillance systems that deliver earlier detection times for reduced effort.
Keywords: patient referrals, network
Abstract
Early detection of new or novel variants of nosocomial pathogens is a public health priority. We show that, for healthcare-associated infections that spread between hospitals as a result of patient movements, it is possible to design an effective surveillance system based on a relatively small number of sentinel hospitals. We apply recently developed mathematical models to patient admission data from the national healthcare systems of England and The Netherlands. Relatively short detection times are achieved once 10–20% hospitals are recruited as sentinels and only modest reductions are seen as more hospitals are recruited thereafter. Using a heuristic optimization approach to sentinel selection, the same expected time to detection can be achieved by recruiting approximately half as many hospitals. Our study provides a robust evidence base to underpin the design of an efficient sentinel hospital surveillance system for novel nosocomial pathogens, delivering early detection times for reduced expenditure and effort.
There is a worldwide concern about the recent emergence, and rapid widespread dissemination, of novel strains of existing nosocomial pathogens as well as of new genetic determinants of virulence and resistance (1–5). Local and national surveillance is considered an important component of the strategy to control these strains (6–8). However, surveillance is costly in monetary terms, effort, and facilities and it is important to consider ways in which surveillance systems can be made more efficient both at the hospital and the national level. Although this is widely recognized (9, 10), there is still no good evidence base to inform the design of efficient surveillance systems at the national level. A key question is how many hospitals should be included in enhanced surveillance programs.
Reflecting this, existing surveillance programs are markedly diverse. For example, in The Netherlands the national antibiotic resistance surveillance system (11) consists of 30 participating laboratories serving ∼50% of hospitals beds in the country. In Britain, the most prominent surveillance schemes include the voluntary reporting of all bacteraemias (90% of clinical laboratories in England, Wales, and Northern Ireland), mandatory bacteraemia surveillance (all acute health trusts in England), and the British Society for Antimicrobial Chemotherapy Resistance Surveillance Project (20–25 collecting laboratories covering the United Kingdom and Ireland) (12). Here, we consider a single, simple, generic approach to this problem that is applicable to a range of nosocomial pathogens including, importantly, novel pathogens or variants whose epidemiology is, by definition, unknown. The only condition is that the major transmission route is the movement of patients between hospitals.
The movement of patients between hospitals in a national healthcare system plays an important role in the spread of healthcare-associated infections (HCAIs) (13–17). Patient movements have also been suggested as an important factor in the spread of antimicrobial resistance between healthcare institutions (18, 19). Mathematical models have confirmed the importance of patient movements in the propagation of nosocomial pathogens (20–25). Further support for the important role of interhospital transmission of HCAIs has been gained, in the case of MRSA, through population genetics and phylogenetic approaches (26, 27).
Although this extensive body of work highlights the role of patient movements in the spread of nosocomial infections, the impact of between-hospital connectivity on the performance of transmission control strategies, such as surveillance programs and eradication, has not been addressed. In the context of individual contact networks, there is an increasing body of research on the early detection and control of outbreaks by careful selection of a small fraction of the population (28, 29). There has also been one study (21) that evaluated methods of selectively targeting hospitals to more efficiently control the dissemination of highly resistant hospital-acquired microorganisms in the specific context of critical care transfers. Here, however, we consider the entire patient population.
We build on previous work to quantify the expected gains in efficiency of carefully targeted, national scale, sentinel surveillance systems for novel nosocomial pathogens. In the context of established healthcare-associated pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA) and Vancomycin-resistant Enterococci (VRE), we use the same analytical framework to consider a closely related question: How rapidly do hospitals become reaffected following successful infection control programs?
To study the dissemination of novel nosocomial pathogens, we use a hospital-based susceptible-infected (SI) epidemic model. Each patient discharged from an affected hospital is associated with a probability β of successfully introducing the pathogen in the hospital of most recent admission. We apply our model to patient admission data from England (n = 146 acute trusts) and The Netherlands (n = 98 acute hospitals). Sentinel surveillance systems are modeled by building a hospital priority list {H1, H2,…,HN} from which we recruit the first k hospitals when k sentinels are required. We propose a gold standard for sentinel selection, which involves a heuristic optimization approach based on minimization of two different public health measures: time to detection, and number of affected hospitals. We also explored hospital prioritization based on a set of six standard metrics, which quantify hospital connectivity with other hospitals in the country. These methods were compared with random orderings of hospitals.
To simulate the effect of hospital infection control measures, we use a susceptible-infected-susceptible (SIS) epidemic model, in which hospitals, after becoming affected by the novel pathogen, recover an unaffected status at an average rate γ (elimination rate). This results in an endemic regime in which we measure mean pathogen-free time, i.e., the average time a hospital remains unaffected after recovery, before newly admitted patients successfully reintroduce the nosocomial pathogen.
We use a baseline configuration (β = 0.001, γ = 0) that yields, for the English data, 146 affected hospitals over a period of 5–6 y. This is comparable with the observed increase in hospital-level prevalence of the MRSA strain EMRSA-15 in England and Wales between the years 1992 and 1997 (30). We later repeat our analysis for different values of β and, where appropriate, γ.
Results
We determined, during a 1-y period, the number of movements of patients between all pairs of hospitals in both England and The Netherlands. We consider as movements direct interfacility patient transfers, as well as indirect transfers, i.e., patients being discharged from hospital into the community, and being later admitted to a different hospital.
There were 531,977 patient movements between the 146 English acute trusts during the 1-y period 2006–2007, which realized 73% of all possible connections between them (15,514 out of 21,170). The median time between discharge and subsequent admission was 17 d (90th percentile: 134; the corresponding probability distribution is shown in SI Appendix, Fig. S1). On average, there were 3,643 patient movements per trust, and there were 34 movements along each of the existing connections (median: 3, range: 1–6,619). Analogously, there were 129,620 movements among the 98 Dutch acute hospitals during the year 2004, which realized 58% of all possible connection (5,501 out of 9,506). The median time between discharge and subsequent admission was 25 d (90th percentile: 145; the corresponding probability distribution is shown in SI Appendix, Fig. S1). On average, there were 1,323 patient movements per hospital, and each existing connection supported 24 movements (median: 2, range: 1–1199). In addition, the healthcare networks of both countries are strongly connected, and any two hospitals can be epidemiologically linked following the path of patient movements. Additional properties of these movement networks are listed in refs. 20, 25.
The simulation model predicts that for our choice of transmission probability and no infection control measures (β = 0.001, γ = 0), a single introduction in a randomly selected hospital will result in the totality of the English hospitals becoming affected by the pathogen, on average, after a period of 5.7 y. In 90% of the simulated epidemics the pathogen reaches all hospitals after 4.1–7.7 y. In The Netherlands, the model predicts that all hospitals will become affected, on average, after a period of 25.5 y, with the pathogen reaching all hospitals after 11.1–55.9 y in 90% of the simulated epidemics.
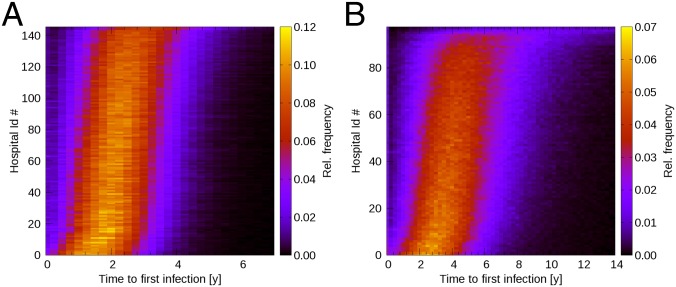
For each hospital we estimated, through time, the probability of becoming affected following single introduction in a randomly selected hospital at time t = 0. In mathematical terms, we obtained the probability density function of time to first infection. The results, displayed in Fig. 1, show considerable variation between hospitals. In England, the mean time to first successful introduction ranges, among the different hospitals, from 1.73 y [90% confidence interval (C.I.) 0.29–3.45] up to 3.44 y (90% C.I. 1.28–6.05). Analogously, the time to first successful introduction in Dutch hospitals ranges, on average, from 3.26 y (90% C.I. 0.55–6.73) up to 20.75 y (90% C.I. 3.49–55.24). These results suggest that a careful selection of sentinel hospitals could lower detection times in hospital-based surveillance programs, improving their performance.
Fig. 1.
Probability, estimated as relative frequency through time, of each individual hospital in England (A) and The Netherlands (B) becoming affected by a novel nosocomial pathogen, following single introduction in a randomly selected hospital at time t = 0. Results obtained in the baseline scenario (β = 0.001 and γ = 0). Hospitals have been sorted along the y axis according to increasing value of median time to first infection.
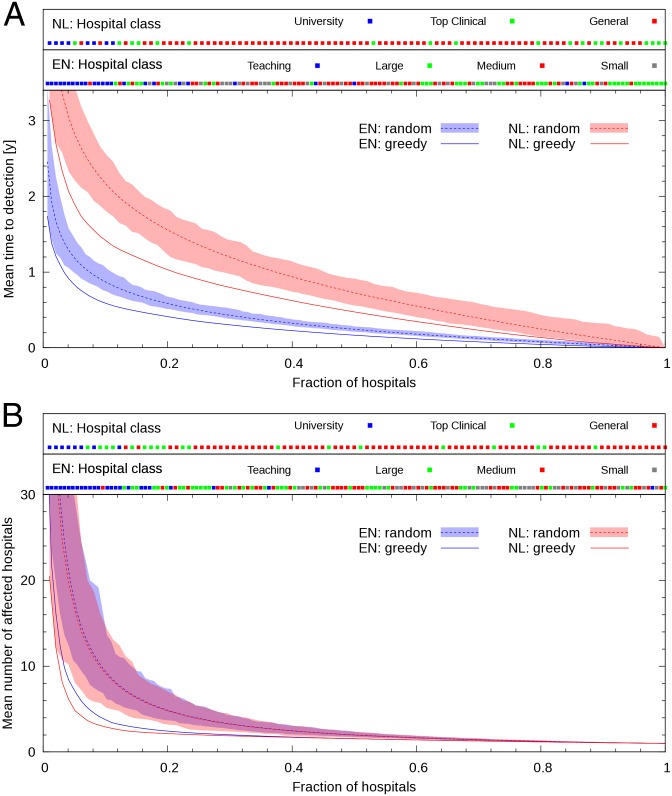
Fig. 2 shows, in the baseline configuration (β = 0.001, γ = 0), detection time (Fig. 2A), and number of affected hospitals at detection time (Fig. 2B), versus the fraction of hospitals used as sentinels (k/N), obtained with the gold-standard and random selection schemes. Results in Fig. 2A were obtained by minimizing detection time, whereas results in Fig. 2B were obtained by minimizing the number of affected hospitals at detection time. Mean detection times, as well as 90th percentiles, when 20% of hospitals are recruited as sentinels according to the different selection schemes, are shown in SI Appendix, Table S1. It is clear from Fig. 2 that, with both selection schemes, detection time follows a law of diminishing returns. As the number of sentinels increases, the contribution of each additional hospital to the improvement in detection time becomes smaller. The fraction of English hospitals required as sentinels to detect a novel circulating pathogen within 1 y yields, with the greedy algorithm and detection time minimization, 4%. This compares favorably with the required fraction when hospitals are chosen randomly, which is 8%. In The Netherlands, detecting a novel circulating pathogen within 1 y requires selecting, with the greedy algorithm, 21% of all hospitals, whereas the required fraction when hospitals are chosen randomly is 37%. Another metric of performance is the number of sentinels that are required to obtain detection times comparable to those obtained with the gold-standard method and 20% of hospitals acting as sentinels. In this case, an emergent pathogen is detected, on average, after 0.41 and 1.02 y in England and The Netherlands, respectively (compare SI Appendix, Table S1). With random selection, comparable detection times can be achieved, in both countries, only with a fraction of sentinel hospitals of 30–40%. In other words, considering as a measure of efficiency the fraction of hospitals required as sentinels, these results suggest that targeted surveillance can be up to twice as efficient as random selection of hospitals.
Fig. 2.
Mean detection time of a novel nosocomial pathogen (A), and mean number of affected hospitals at detection time (B), following emergence in a single, randomly selected hospital, versus fraction of hospitals participating in a sentinel surveillance program. The continuous lines correspond to results obtained using the greedy algorithm with the English (EN: greedy) and Dutch (NL: greedy) data sets. The optimization metric was time to detection (A) and number of affected hospitals at detection (B). The shaded region and the dashed lines (EN, random, and NL, random) correspond to 1,000 random selections of sentinel hospitals and their mean, respectively. (A and B, Upper) Information on hospital category for England (EN) and The Netherlands (NL): the symbol corresponding to the ith element in the priority list obtained with the greedy algorithm is displayed at position i/N along the x axis, with N the total number of hospitals in the country. All curves obtained in the baseline scenario (β = 0.001 and γ = 0).
This improvement in efficiency is also observed when the priority list is obtained by minimizing the number of affected hospitals (Fig. 2B; SI Appendix, Table S2). Twenty percent of hospitals selected as sentinels with the greedy algorithm are able to detect the emerging outbreak when, on average, 2–3 hospitals are affected. Conversely, to obtain a similar performance using the random selection scheme would require 40–45% of hospitals acting as sentinels.
Along the top of Fig. 2 we include information on hospital category. According to increasing hospital size and complexity, English acute trusts are classified into small acute trusts, medium acute trusts, large acute trusts, and teaching trusts (31); Dutch hospitals are classified into general hospitals, top clinical hospitals, and university medical centers (32). Here, the color of the symbol at position k/N corresponds to the type of the kth hospital (Hk) in the priority list built with the greedy algorithm (see SI Appendix, Fig. S2 for a version including, in addition to hospital class, all network metrics). In both the English and Dutch healthcare networks, teaching trusts and university medical centers, respectively, are placed high in the priority list. We can compare the performance of these hospitals used as unique sentinels with that of the same number of hospitals selected according to the greedy algorithm. However, detection times obtained in England and The Netherlands are not to be compared between them, because the number of tertiary hospitals is different in both countries (25 and 8, respectively). Selecting the 25 teaching trusts as sentinels in England yields an average detection time of 0.51 y. This value is comparable with the result obtained with the first 25 hospitals from the empirically built priority list (0.45 y). Similarly, average detection time in The Netherlands with the eight university medical centers used as sentinels is 1.70 y, comparable with using the first eight hospitals from the empirically built priority list (1.57 y). Moreover, the 25 English tertiary hospitals are able to detect the emerging outbreak when, on average, 2–3 hospitals have been affected. To achieve this detection efficiency with randomly selected sentinels, an average of 50 hospitals would be required. The eight Dutch university medical centers are able to detect the emerging outbreak when, on average, 3–4 hospitals have been affected. A similar outcome can be achieved by randomly selecting, on average, 27 hospitals.
In both England and The Netherlands, the greedy algorithm is always the best-performing one, for both time to detection and number of hospitals affected, although the metrics in-flux, in-degree, and h index also improved efficiency compared with random selection in some scenarios (compare SI Appendix, Fig. S3, Tables S1 and S2).
For surveillance programs which require a fixed effort per hospital, the y axes in Fig. 2 also represent the total effort (and so the total cost of the program). If the effort invested per hospital is a function of hospital size, then an approximate estimate of the costs could be obtained by measuring the number of beds under surveillance. In this situation, the greedy algorithm may not be the least costly (SI Appendix, Table S1), and the increased costs of targeted surveillance would need to be weighed against the benefits of earlier detection.
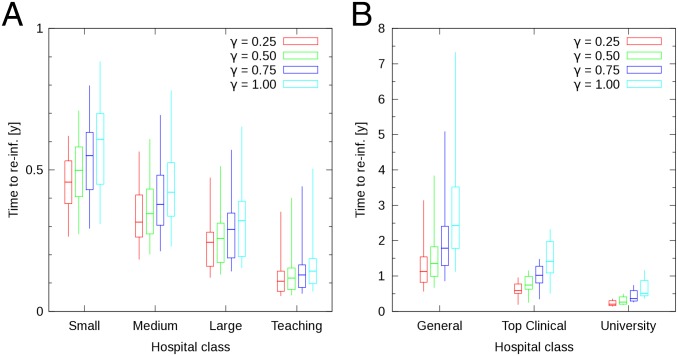
Using the SIS model for established nosocomial pathogens, we measure the average time for a hospital to become reaffected by a pathogen after successful implementation of infection control measures. We have estimated the infection pressure on each individual hospital by measuring the median HCAI-free time after successful pathogen elimination. We first calculated, in the endemic regime, the median time each hospital remains free of the pathogen. Next we grouped, according to hospital category, the 146 values obtained with the English healthcare network into 4 subsets, and the 98 values obtained with the Dutch network into 3 subsets. For each of these subsets, we calculated the 5th, 25th, 50th, 75th, and 95th percentiles, and display these results for γ = 0.25, 0.50, 0.75, and 1.00 y−1 with box plots in Fig. 3.
Fig. 3.
Box plots of median HCAI-free time in the endemic regime in England (A) and The Netherlands (B). Results obtained with the baseline transmission probability β = 0.001. Median times have been grouped according to type of hospital. Different colors correspond to different values of γ. Box bottom and top represent the lower and upper quartiles, respectively. Lower and upper line ends represent 5th and 95th percentiles, respectively. The horizontal bar corresponds to the median. Elimination rates (γ) are expressed in units of y−1.
Teaching trusts and university medical centers remain free of infection for a shorter period than small acute trusts and general hospitals, respectively. Median reinfection times differ between these types of hospitals, for the chosen elimination rates, by approximately a factor of 4–6. This shows that the need for effective infection control against endemically established HCAIs may be substantially greater for tertiary hospitals than for the other hospital categories.
The effects of varying model parameters and assumptions are reported in SI Appendix, Supporting Methods. Although, as expected, the rate of spread (and, consequently, the speed of detection) increases with increasing β (and with increasing discharge–admission interval cutoff), the main comparisons between different prioritizations of hospitals for targeted surveillance are essentially unchanged. We observe a similar outcome in model configurations with larger β associated with patient movements from/to tertiary hospitals. All this implies that our key results are robust for a wide range of pathogens and epidemiological scenarios.
Discussion
A variety of nosocomial pathogens––such as newly emergent variants of MRSA or Klebsiella pneumonia––can spread between hospitals as a consequence of patient movements. Hospitals occupy different positions in the network of movements and this translates into differences in the risk of being affected or reaffected by such pathogens (13–17, 20–25). Here we have shown that these differences also provide an opportunity to design more efficient surveillance programs based on a relatively small number of hospitals acting as sentinels. This addresses the current lack of evidence available to policy makers aiming to design efficient surveillance systems at a national level.
Three key results emerge from our analyses. First, there is a marked effect of diminishing returns: Detection time or number of affected hospitals both decline rapidly as up to 20% of hospitals are recruited in the surveillance system, but much less rapidly thereafter. Second, the surveillance system can be made considerably more efficient if, instead of randomly targeting a set of hospitals, sentinels are selected on the basis of their position in the movement network; to a good approximation, an equivalent expected time to first detection of a novel pathogen can be achieved by selecting half as many hospitals. Third, although hospitals to be targeted can be identified using the computational methods described here, a near-optimal solution is to prioritize hospitals simply on the basis of the number of admitted patients that have previously been discharged from a different hospital. This corresponds well to prioritizing tertiary hospitals.
Tertiary hospitals have previously been suggested as potential sentinels based on arguments of feasibility and adequate patient volume (33, 34). Here we provide evidence that supports this choice from the perspective of HCAI transmission dynamics. If the healthcare network cannot be reconstructed, targeting these hospitals still yields a considerable improvement in detection times, becoming a useful alternative to the gold-standard method.
For endemically established nosocomial pathogens, tertiary hospitals become reaffected, after successful implementation of infection control measures, 4–6× faster than small acute trust and general hospitals. Consequently, tertiary hospitals must implement control measures more frequently, and incur higher yearly associated costs, to remain free from the HCAI. This further supports the need to target resources in these hospitals.
We obtained essentially the same results for a wide range of parameter values, confirming that our findings should be relevant not only to MRSA but to other nosocomial pathogens as well. There are, however, some factors which we have not been able to fully include in our model and that may, a priori, impact the results of our analysis. These are the correlation between probability of transmission and type of hospital, the impact of single-hospital random seeding, transmission in the community and other nonacute care facilities, data censoring, administrative scale, and the overall optimality of the greedy algorithm.
Probability of transmission could be positively correlated with hospital type (i.e., higher β for movements associated with tertiary hospitals). Patients admitted to tertiary hospitals after discharge from other hospitals are more at risk for carrying a hospital-acquired infection (20, 25). Moreover, admission data show that patients discharged from tertiary hospitals have a higher probability of readmission to a different hospital within comparatively shorter times. Increasing transmission probabilities associated with patient movements to/from tertiary hospitals yields, for England, an increase in the efficiency of our approach compared with random selection. In other words, the benefit of our method would be even greater if the prioritized hospitals also represented the most at-risk patients [as is the case for hospital-acquired MRSA (25)].
We have assumed that a novel pathogen originates in a single, randomly chosen hospital. Other scenarios are possible; for example, the probability of emergence in a particular hospital could depend on its size or other attributes. This would influence our results insofar as this dependence was positively (or, conceivably, negatively) correlated with the risk associated with network properties, potentially amplifying (or reducing) heterogeneities in time to first infection.
We assumed that no transmission occurs outside the hospital setting. The impact of community transmission on our results would be twofold. Firstly, per-patient probability of transmission would increase with increasing time spent in the community between successive hospital admissions. We expect this effect to be small, as patients that spend longer periods of time in the community are also the ones with low numbers of readmissions (20). Secondly, first-time admissions, i.e., admission of patients that have never been admitted to hospital before, will also result in a probability of pathogen introduction. The impact of the latter will depend on the relative rates of first admissions of different types of hospitals. We do not model the effect of nursing homes and other long-term care facilities, which are recognized as important community reservoirs of nosocomial pathogens (35, 36). The impact of excluding these care facilities in our analysis would depend on their relative position in the movement structure (37).
Our use of patient movement data from a single calendar year means that these data are right censored and true movement rates are underestimated. Because the median intervals between discharge and readmission were short (and longer intervals may correspond to reduced risk) we expect the effects of this to be small.
There are differences in the administrative scale of the reconstructed English and Dutch healthcare networks. Whereas the most fundamental element in the Dutch network is a hospital, the corresponding element in England is a hospital trust (i.e., a group of hospitals under the same management). Most English acute trusts are dominated by a single large hospital, responsible for most patient movements between its trust and other trusts in the country (although a few trusts consist of two or more dominant hospitals; see ref. 38). Therefore, we do not regard these systems as directly comparable, but our aim is to demonstrate the general applicability of our approach.
These issues notwithstanding, we suggest that our key findings will be both robust and likely to be widely applicable. This is because the crucial component is the network of patient movements between hospitals, which is well-characterized for both England and The Netherlands. However, there is clearly scope for further research on the contributions of heterogeneities in transmission rate and seeding of infection, the gain and loss of infection outside hospitals, or fine structure of the movement network. More detailed models incorporating these features could be parameterized for specific pathogens, not only in terms of epidemiological variables but also the costs and constraints of surveillance.
We should also mention a more general issue worthy of further research: the optimality of the greedy algorithm. Although in our analysis, of all of the considered alternatives, the greedy algorithm was the best-performing selection scheme, the corresponding priority list may not be the true optimal one (i.e., the one yielding the absolute minimum detection time among all possible alternatives). This is a general feature of greedy algorithms, which make a locally optimal choice that may not result in a globally optimal solution (39). We note that although a better priority list may exist, sentinel selection according to the greedy algorithm has already allowed us to design a more efficient surveillance strategy.
In conclusion, based on data from two countries, we have shown that efficient hospital-based, sentinel surveillance systems for novel nosocomial pathogens transmitted by means of patient movements are a practical proposition. Relatively rapid detection can be achieved by prioritizing a small fraction of hospitals, and this fraction can be further reduced by targeting surveillance at specific hospitals or categories of hospital. We believe that this kind of evidence-based approach to the design of surveillance systems can both decrease detection times and/or decrease costs to national governments, facilitating reduction of the substantial public health burden imposed by nosocomial pathogens.
Materials and Methods
Patient-Movement Data.
To quantify the amount of contact between hospitals we use patient-movement rates. We consider as movements direct interfacility patient transfers, as well as indirect transfers, i.e., the instance of a patient being discharged from hospital into the community, and being later admitted to a different hospital. For every pair of hospitals (i, j) in England and The Netherlands, we calculated the annual patient-movement rate wij from hospital j to hospital i by counting the number of patients that were admitted to hospital i, following discharge from hospital j, during a period of 1 y (with or without an intermediate stay in the community). This procedure yields an N × N movement matrix, where N is the number of hospital in the chosen country.
The English movement matrix was obtained from patient admission data covering the 1-y period April 1, 2006–March 31, 2007, provided by the National Health Service Hospital Episode Statistics. We consider the n = 146 acute trusts in the English National Health Service. An acute trust is defined as a group of hospitals under the same management with 85% or more of its expenditure in acute specialties [medicine, surgery, accident and emergency (A&E), and maternity], an A&E department, and all core acute specialties. These trusts are classified into 4 categories: small acute trusts (n = 29), medium acute trusts (n = 50), large acute trusts (n = 42), and teaching trusts (n = 25), corresponding to increasing hospital size and complexity (31).
The Dutch movement matrix was obtained from patient admission data covering the 1-y period January 1, 2004–December 31, 2004, provided by the Dutch National Medical Register (Landelijke Medische Registratie; Prismant). We consider the n = 98 hospitals consisting, in increasing hospital size and complexity, of all general hospitals (n = 71), all top clinical hospitals (n = 19), and all university medical centers (n = 8) (32).
Teaching trusts and university medical centers are associated with a university, and usually act as top referral centers. They correspond to “tertiary hospitals” according to the hospital type definition used by the European Centre for Disease Prevention and Control (e.g., ref. 40).
Further details on the raw patient admission data, and their use to obtain annual movement rates, can be found in refs. 20, 25.
Hospital Size Data.
The number of available beds in each English acute trust during fiscal year 2006–07 was retrieved from the Estates Return Information Collection, hosted at the National Health Service Hospital Estates and Facilities Statistics website (www.hefs.ic.nhs.uk/).
Mathematical Model.
We use a hospital-based SI compartmental model in which each hospital can be in one of two possible states: susceptible to, and free of, the pathogen (S), or affected by it (I). Affected hospitals harbor one or more colonized or infected patients, and/or an environmental reservoir of the pathogen. Susceptible hospitals become affected when they admit infected or colonized patients that shed the pathogen in the environment, or that transmit the pathogen to other susceptible patients in the hospital. Interhospital transmission is a complex process that involves a patient being infected or colonized when discharged from hospital, remaining infectious until the next admission, and successfully introducing the pathogen in the admitting hospital. We combine the effect of this sequence of events into a single probability of transmission (β).
Reports of interhospital outbreaks (2, 41) have shown that after a period of rapid intrahospital spread (typically 1 or 2 mo), pathogens spread to other hospitals on time scales under 1 y. Moreover, mathematical models including within-hospital dynamics show that colonized patients are able to spread the pathogen to other hospitals within days (20, 24). We therefore assume that once a hospital becomes affected all patients who are subsequently admitted to a different hospital (which in 50% of cases occurs within 17–25 d) have a fixed, low probability of introducing infection into the second hospital. In the case of England, we make a similar assumption at the trust level: Transmission within all hospitals in each acute trust occurs faster than between hospitals belonging to different trusts.
To simulate the introduction of infection control measures we use an SIS model. We assume that once a hospital becomes affected by the pathogen it will recover a susceptible status, following complete pathogen eradication, after an average time 1/γ, with γ the average elimination rate.
The two models are stochastic, and progress in discrete time steps of length δt = 1 d. The probability of a susceptible hospital becoming affected by the emergent pathogen during one time step [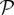 S→I(t)] is a function of the per-patient transmission probability (β), and the rates at which other affected hospitals move patients onto it. In the SIS model, the probability of an affected hospital recovering a susceptible status during one time step [
S→I(t)] is a function of the per-patient transmission probability (β), and the rates at which other affected hospitals move patients onto it. In the SIS model, the probability of an affected hospital recovering a susceptible status during one time step [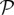 I→S(t)] is a function of the elimination rate (γ). Mathematical formulae defining these probabilities are listed in SI Appendix, Supporting Equations.
I→S(t)] is a function of the elimination rate (γ). Mathematical formulae defining these probabilities are listed in SI Appendix, Supporting Equations.
Hospital-Based Surveillance.
To simulate the implementation of a sentinel surveillance system, a method for selecting the hospitals that will act as sentinels is needed. A selection scheme involves building a hospital priority list {H1, H2,…,HN} from which we use the first k hospitals when k sentinels are required.
We compare the performance of eight different selection schemes. In addition to random selection (i.e., the priority list is just a random ordering of all of the hospitals in the country), we prioritize hospitals according to values for a set of six standard metrics which quantify their connectivity with other hospitals in the network. These are the number of hospitals from which a sentinel admits moved patients in a 1-y period (i.e., in-degree), the number of patients admitted following discharge from other hospital (i.e., in-flux), hospital h index (42), and three measures of hospital network prominence: betweenness, closeness, and eigenvector centrality (43).
We also propose a gold standard for sentinel selection, which involves the following procedure (known as a “greedy” algorithm). The first hospital in the list (H1) is selected such that, on average, it is the earliest affected hospital following emergence of the novel pathogen in an arbitrary hospital. The second hospital (H2) is chosen such that, together with the already selected sentinel, they minimize detection time, i.e., the earliest time at which any of the sentinels becomes affected by the pathogen. This procedure is repeated, increasing one by one the number of sentinels, yielding the required hospital priority list. Alternatively, instead of building the priority list by minimizing detection time, other quantities of interest may be optimized. We illustrate this by using the greedy algorithm to minimize the number of hospitals affected by the pathogen at detection time.
Pathogen Reintroduction.
The SIS model variant is suitable for describing endemically established pathogens, such as MRSA and VRE. In this case, the simulation model yields, with a probability that depends on the values of the model parameters, an endemic regime in which the prevalence of affected hospitals is, in general, lower than 100%.
In the endemic regime, when affected hospitals regain a susceptible status, they remain HCAI-free for a period of time before becoming reaffected by the pathogen. We estimate the infection pressure on each individual hospital by measuring the median HCAI-free time after successful pathogen elimination. We then compare, for a range of elimination rates, the reinfection times associated with different categories of hospital. We stress that this analysis is only relevant for endemically established pathogens, such that a stable, nonzero hospital-level prevalence has already been reached. Only in this regime can the impact on our results of the order in which hospitals become affected by the pathogen be ignored.
Model Parameters and Simulation Configurations.
We initially focus on the effect of patient movements, assuming that β is constant through time and does not depend on hospital or individual patient characteristics. We later repeat our analysis relaxing this homogeneity assumption.
We present results corresponding to a baseline scenario defined by β = β0 ≡ 0.001 and, in the SIS model, a range of elimination rates γ = 0.25, 0.50, 0.75, and 1.00 y−1. For β = β0 our model predicts that, with unconstrained transmission (γ = 0), all English hospitals become affected by a novel nosocomial pathogen in an average period of 5.7 y (4.1–7.7 y in 90% of simulated outbreaks), after single introduction in a randomly selected hospital. This result is comparable with the observed increase in hospital-level prevalence of EMRSA-15 in England and Wales between the years 1992 and 1997 (30).
To explore the generality of the results for pathogens other than MRSA we repeat our analyses for different values of β and, where appropriate, γ. We consider values of β in the range 0.1×–10× of the baseline value. Higher β-values correspond to higher rates of spread between hospitals, whereas lower β-values are associated with lower dissemination rates. For γ, we consider values ranging from 0.025 to 10.0 per y. We also introduce dependence of the per-patient transmission probability on the time a patient remains in the community before being readmitted to hospital. This allows us to consider pathogens that affect patients only in a transient manner. We repeat our analyses assuming patients can only transmit the pathogen if the time between discharge and subsequent admission does not exceed a cutoff value chosen in the range 30–180 d.
All simulations were started by randomly selecting one single affected hospital. The model was run for a period of at least 30 y, enough for the median prevalence of affected hospitals to reach 100% in the baseline configuration, or for the system to reach a quasistationary endemic regime when control measures are implemented. Results were obtained with at least 10,000 simulation replicates.
Supplementary Material
Acknowledgments
We are grateful to Dr. Ian Laurenson, Royal Edinburgh Infirmary, for helpful discussions, and to two anonymous reviewers for helpful suggestions. The research leading to these results has received funding from the European Union (EU) FP7-Health Program CONCORD (222718) and the EU FP7-Health Program EvoTAR (282004). M.C., T.D., and H.G. receive grant funding from the Health Protection Agency, and from the UK Clinical Research Collaboration Translational Infection Research Initiative supported by the Medical Research Council (Grant G1000803).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.T.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308062111/-/DCSupplemental.
References
- 1.Yigit H, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45(4):1151–1161. doi: 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Won SY, et al. Centers for Disease Control and Prevention Epicenter Program Emergence and rapid regional spread of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis. 2011;53(6):532–540. doi: 10.1093/cid/cir482. [DOI] [PubMed] [Google Scholar]
- 3.Li M, et al. MRSA epidemic linked to a quickly spreading colonization and virulence determinant. Nat Med. 2012;18(5):816–819. doi: 10.1038/nm.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García-Álvarez L, et al. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: A descriptive study. Lancet Infect Dis. 2011;11(8):595–603. doi: 10.1016/S1473-3099(11)70126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson ME, Chen LH. NDM-1 and the role of travel in its dissemination. Curr Infect Dis Rep. 2012;14(3):213–226. doi: 10.1007/s11908-012-0252-x. [DOI] [PubMed] [Google Scholar]
- 6.ECDC . Risk Assessment on the Spread of Carbapenemase-Producing Enterobacteriaceae (CPE) Through Patient Transfer Between Healthcare Facilities, with Special Emphasis on Cross-Border Transfer. Stockholm: ECDC; 2011. [Google Scholar]
- 7.Struelens MJ, Monnet DL, Magiorakos AP, Santos O’Connor F, Giesecke J. European NDM-1 Survey Participants New Delhi metallo-beta-lactamase 1-producing Enterobacteriaceae: Emergence and response in Europe. Euro Surveill. 2010;15(46):19716. doi: 10.2807/ese.15.46.19716-en. [DOI] [PubMed] [Google Scholar]
- 8.Davies SC. Annual Report of the Chief Medical Officer, Volume Two, 2011, Infections and the Rise of Antimicrobial Resistance. London: Department of Health; 2013. [DOI] [PubMed] [Google Scholar]
- 9.Ducel G, Fabry J, Nicolle L. Prevention of Hospital-Acquired Infections. A Practical Guide. Geneva: World Health Organization; 2002. [Google Scholar]
- 10.WHO . Report on the Burden of Endemic Health Care-Associated Infection Worldwide. Geneva: World Health Organization; 2011. [Google Scholar]
- 11.Van de Sande N, Thijsen S, Leverstein-van Hall M. Antibioticaresistentiesurveillance in Nederland: ISIS-AR en ISISweb. Ned Tijdschr Med Microbiol. 2010;18(4):10–18. Dutch. [Google Scholar]
- 12.Reynolds R. Antimicrobial resistance in the UK and Ireland. J Antimicrob Chemother. 2009;64(Suppl 1):i19–i23. doi: 10.1093/jac/dkp257. [DOI] [PubMed] [Google Scholar]
- 13.Schaefler S, et al. Methicillin-resistant Staphylococcus aureus strains in New York City hospitals: Inter-hospital spread of resistant strains of type 88. J Clin Microbiol. 1984;20(3):536–538. doi: 10.1128/jcm.20.3.536-538.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eveillard M, Quenon JL, Rufat P, Mangeol A, Fauvelle F. Association between hospital-acquired infections and patients’ transfers. Infect Control Hosp Epidemiol. 2001;22(11):693–696. doi: 10.1086/501847. [DOI] [PubMed] [Google Scholar]
- 15.Cordeiro JC, Silbert S, Reis AO, Sader HS. Inter-hospital dissemination of glycopeptide-resistant Enterococcus faecalis in Brazil. Clin Microbiol Infect. 2004;10(3):260–262. doi: 10.1111/j.1198-743x.2004.00866.x. [DOI] [PubMed] [Google Scholar]
- 16.Widerström M, Monsen T, Karlsson C, Wiström J. Molecular epidemiology of meticillin-resistant coagulase-negative staphylococci in a Swedish county hospital: Evidence of intra- and interhospital clonal spread. J Hosp Infect. 2006;64(2):177–183. doi: 10.1016/j.jhin.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Robotham JV, Scarff CA, Jenkins DR, Medley GF. Meticillin-resistant Staphylococcus aureus (MRSA) in hospitals and the community: Model predictions based on the UK situation. J Hosp Infect. 2007;65(Suppl 2):93–99. doi: 10.1016/S0195-6701(07)60023-1. [DOI] [PubMed] [Google Scholar]
- 18.Smith DL, Dushoff J, Perencevich EN, Harris AD, Levin SA. Persistent colonization and the spread of antibiotic resistance in nosocomial pathogens: Resistance is a regional problem. Proc Natl Acad Sci USA. 2004;101(10):3709–3714. doi: 10.1073/pnas.0400456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith DL, Levin SA, Laxminarayan R. Strategic interactions in multi-institutional epidemics of antibiotic resistance. Proc Natl Acad Sci USA. 2005;102(8):3153–3158. doi: 10.1073/pnas.0409523102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donker T, Wallinga J, Grundmann H. Patient referral patterns and the spread of hospital-acquired infections through national health care networks. PLOS Comput Biol. 2010;6(3):e1000715. doi: 10.1371/journal.pcbi.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karkada UH, Adamic LA, Kahn JM, Iwashyna TJ. Limiting the spread of highly resistant hospital-acquired microorganisms via critical care transfers: A simulation study. Intensive Care Med. 2011;37(10):1633–1640. doi: 10.1007/s00134-011-2341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin DJ, Anderson RM. Transmission dynamics of epidemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci in England and Wales. J Infect Dis. 1999;179(4):883–891. doi: 10.1086/314682. [DOI] [PubMed] [Google Scholar]
- 23.Lesosky M, et al. Effect of patterns of transferring patients among healthcare institutions on rates of nosocomial methicillin-resistant Staphylococcus aureus transmission: A Monte Carlo simulation. Infect Control Hosp Epidemiol. 2011;32(2):136–147. doi: 10.1086/657945. [DOI] [PubMed] [Google Scholar]
- 24.Lee BY, et al. Modeling the spread of methicillin-resistant Staphylococcus aureus (MRSA) outbreaks throughout the hospitals in Orange County, California. Infect Control Hosp Epidemiol. 2011;32(6):562–572. doi: 10.1086/660014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donker T, Wallinga J, Slack R, Grundmann H. Hospital networks and the dispersal of hospital-acquired pathogens by patient transfer. PLoS ONE. 2012;7(4):e35002. doi: 10.1371/journal.pone.0035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAdam PR, et al. Molecular tracing of the emergence, adaptation, and transmission of hospital-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci USA. 2012;109(23):9107–9112. doi: 10.1073/pnas.1202869109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ke W, et al. Patient sharing and population genetic structure of methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci USA. 2012;109(17):6763–6768. doi: 10.1073/pnas.1113578109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christakis NA, Fowler JH. Social network sensors for early detection of contagious outbreaks. PLoS ONE. 2010;5(9):e12948. doi: 10.1371/journal.pone.0012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smieszek T, Salathé M. A low-cost method to assess the epidemiological importance of individuals in controlling infectious disease outbreaks. BMC Med. 2013;11:35. doi: 10.1186/1741-7015-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Public Health Laboratory Service (1997) Epidemic Methicillin-resistant Staphylococcus aureus. Commun Dis Rep CDR Wkly 7:191. Available at www.hpa.org.uk/cdr/archives/1997/cdr2297.pdf. Accessed September 19, 2013.
- 31.Health Protection Agency . Surveillance of Healthcare Associated Infections Report: 2008. London: Health Protection Agency; 2008. [Google Scholar]
- 32.Boot JM, Knapen MHJM. De Nederlandse Gezondheidszorg. Houten, The Netherlands: Bohn Stafleu van Loghum; 2005. [Google Scholar]
- 33. Center for Disease Prevention and Control (2003) Drug-Resistant Streptococcus pneumoniae Surveillance Manual (CDC, Atlanta). Available at www.cdc.gov/abcs/reports-findings/surv-manual.html. Accessed January 6, 2014.
- 34.WHO . WHO Regional Office for Europe Guidance for Sentinel Influenza Surveillance in Humans. Updated May 2011. Copenhagen: WHO; 2010. [Google Scholar]
- 35.Hsu CC, Macaluso CP, Special L, Hubble RH. High rate of methicillin resistance of Staphylococcus aureus isolated from hospitalized nursing home patients. Arch Intern Med. 1988;148(3):569–570. [PubMed] [Google Scholar]
- 36.Goettsch W, et al. MRSA in nursing homes in the Netherlands 1989 to 1998: A developing reservoir? Euro Surveill. 2000;5(3):28–31. doi: 10.2807/esm.05.03.00022-en. [DOI] [PubMed] [Google Scholar]
- 37.Lee BY, et al. Long-term care facilities: Important participants of the acute care facility social network? PLoS ONE. 2011;6(12):e29342. doi: 10.1371/journal.pone.0029342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. NHS Choices web site Authorities and Trusts. Available at www.nhs.uk/ServiceDirectories/Pages/AcuteTrustListing.aspx. Accessed March 28, 2013.
- 39.Cormen TH, Leiserson CE, Rivest RL, Stein C. Introduction to Algorithms. Cambridge, MA: MIT Press; 2009. [Google Scholar]
- 40.ECDC . Point Prevalence Survey of HCAI and Antimicrobial Use in European Acute Care Hospitals - Protocol version 4.3. Stockholm: ECDC; 2012. [Google Scholar]
- 41.Pearman JW. 2004 Lowbury Lecture: The Western Australian experience with vancomycin-resistant enterococci - from disaster to ongoing control. J Hosp Infect. 2006;63(1):14–26. doi: 10.1016/j.jhin.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 42. Campiteli MG, Holanda AJ, Soles PRC, Soares LHD, Kinouchi O (2010) Hirsch index as a network centrality measure. arXiv:1005.4803.
- 43.Borgatti SP. Centrality and network flow. Soc Networks. 2005;27(1):55–71. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.