Significance
Many higher cognitive functions involve working memory (WM), the storage and manipulation of information across limited time intervals. Comparing the WM capacity of different species is a key step toward understanding the underlying brain mechanisms. This study uncovers previously unknown sensory WM abilities in rats. They received two vibratory stimuli on their whiskers, separated by a variable delay, and had to compare vibration features. In analogous experiments, human subjects compared two stimuli applied to the fingertip. The acuity shown by rats in judging stimulus differences and their WM proficiency (across delays of 8 s, the longest tested) overlapped those of humans. Sensory WM now joins other cognitive functions within the rodent repertoire, setting the stage for exploration of its neuronal coding.
Keywords: psychophysics, somatosensory, decision making, delayed comparison, vibrissa
Abstract
Primates can store sensory stimulus parameters in working memory for subsequent manipulation, but until now, there has been no demonstration of this capacity in rodents. Here we report tactile working memory in rats. Each stimulus is a vibration, generated as a series of velocity values sampled from a normal distribution. To perform the task, the rat positions its whiskers to receive two such stimuli, “base” and “comparison,” separated by a variable delay. It then judges which stimulus had greater velocity SD. In analogous experiments, humans compare two vibratory stimuli on the fingertip. We demonstrate that the ability of rats to hold base stimulus information (for up to 8 s) and their acuity in assessing stimulus differences overlap the performance demonstrated by humans. This experiment highlights the ability of rats to perceive the statistical structure of vibrations and reveals their previously unknown capacity to store sensory information in working memory.
Advances in understanding the neuronal mechanisms of cognition often occur when investigators examine in simpler mammals a behavioral capacity known to be part of the primate repertoire. An example is the perception of space, where the inquiry into the fundamental neuronal mechanisms in rats (1–4) has informed research in humans (5, 6).
Working memory (WM), the storage and manipulation of information across a limited time interval, has been explored in humans and monkeys in many experimental paradigms (7). However, “remarkably, given its central importance in human life, there has been very little comparative investigation of WM abilities across species” (ref. 8, p. 10371). In rats, WM has been examined in the framework of match- or nonmatch-to-sample tasks that involve the comparison of stimuli that differ by their quality and identity (9, 10); WM has also been examined in navigation tasks that involve the storage of multimodal sensory inputs (e.g., combined visual cues and path integration) (11, 12).
In contrast, the experiment reported here is a delayed comparison between stimuli that reside within a single, defined sensory domain and differ only by the value of one parameter, the velocity SD of the vibration. Rats compare two vibrations delivered sequentially to their whiskers, whereas humans compare two vibrations on the fingertip. This task requires several operations: (i) encoding the first stimulus and extracting the relevant parameter; (ii) storing the parameter value in memory; (iii) encoding the second stimulus and extracting the relevant parameter; (iv) comparing the second parameter value to the memory of the first; and (v) from the outcome of the comparison, applying the decision rule. The task was designed to open the way to the study of how neuronal circuits in the rat encode and store stimulus parameters.
Results
Experimental Design.
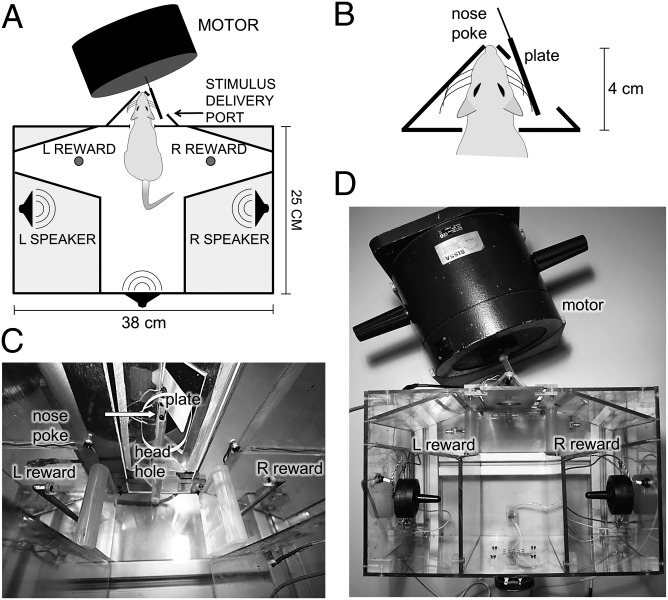
The main chamber of the behavioral apparatus (Fig. 1) was a Plexiglas compartment. The rat received whisker stimulation by extending its head from the main chamber into the stimulus delivery port.
Fig. 1.
Apparatus for rats. (A) Dark boundaries represent Plexiglas walls. The rat is sketched with snout extended into the stimulus delivery port. Left (L) and right (R) reward ports are indicated. (B) Magnified sketch of the stimulus delivery port. The rat extended through the head hole and placed its whiskers in contact with the plate. The plate’s surface is approximately vertical and is seen as a line segment from above. (C) Photograph from within the apparatus. Reward spouts are visible laterally. In front, the head hole opens to the stimulus delivery port, which houses the vibrator plate. Arrow points to nose poke hole. (D) Photograph of the apparatus from above. The configuration mirrors the sketch in A.
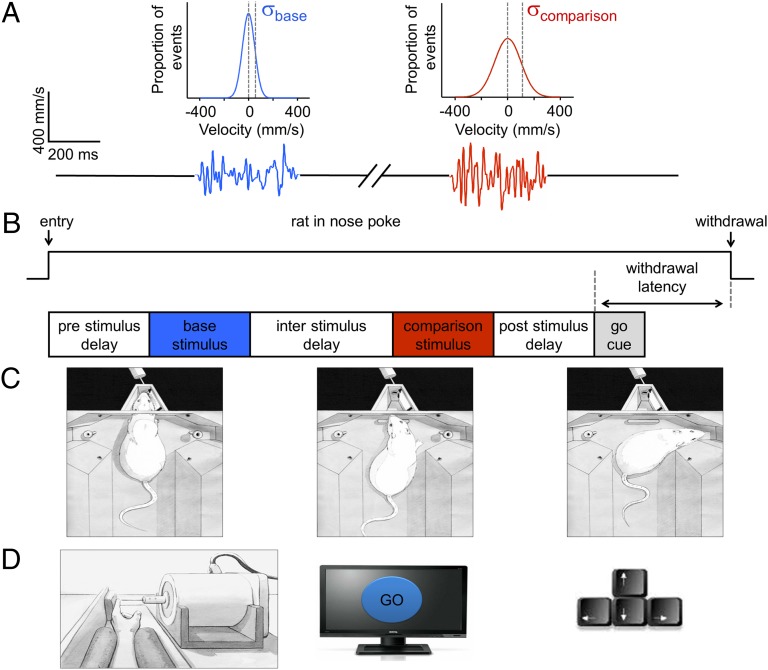
Stimuli were irregular “noisy” vibrations, consisting of changes in the plate position in the rostral/caudal direction. The sequence of velocity values was taken from a normal distribution with 0 mean, and SD denoted by σ. Velocity distributions and time series for two example stimuli are illustrated in Fig. 2A.
Fig. 2.
Structure of a single trial. (A) Stimuli were composed of a series of velocity values where the sampling probability of a given velocity value was given by a normal distribution with mean = 0 and SD = σ. Example base and comparison stimuli are illustrated, resulting from the sampling of the distribution shown above each stimulus, with σ = 55 mm/s (blue) and σ = 110 mm/s (red) and duration 400 ms. (B) Upper trace indicates at far left the time of entry of the rat in the nose poke and at far right the time of withdrawal. Below, key events of the trial are given. Withdrawal latency was measured as elapsed time between the onset of the go cue and withdrawal from the nose poke. (C) Sketches depicting one trial. (Left) The rat places its snout in the stimulus delivery port to initiate the trial and receive stimuli. (Center) Upon hearing the go cue, the rat withdraws. (Right) The rat selects the right reward port. (D) Human participants performed the same discrimination task as rats, holding their fingertip in contact with the tip of a rod attached to the motor (Left). After the base and comparison stimuli were delivered, a go cue appeared on the monitor (Center), and the subject responded by pressing left or right arrow keys on a standard keyboard (Right).
Fig. 2B shows the task structure. When the rat positioned its snout in the nose poke, the trial began with the prestimulus delay. At the conclusion of the delay, the base stimulus was presented, characterized by σbase. After the interstimulus delay, the comparison stimulus was presented, characterized by σcomparison. The rat had to remain in the nose poke for the entire trial, including the poststimulus delay. When the “go” cue sounded, the rat withdrew and selected the left or right spout according to the relative values of σcomparison and σbase. An advantage of the delayed comparison paradigm is that it allows a more accurate estimate of acuity. Thresholds in discriminating stimulus difference are lower in tasks where subjects compare two sequential stimuli than in tasks where they compare single stimuli to reference memory (13, 14).
As for any discrimination task, difficulty increased as the stimulus difference decreased. Difficulty depended on the difference between σbase and σcomparison, quantified by the SD index (SDI):
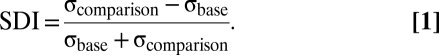 |
On a typical trial, a well-trained rat (Fig. 2C) placed its snout in the stimulus delivery port to initiate the trial and receive stimuli (Fig. 2C, Left, and Movie S1); it withdrew from the nose poke after the go cue (Fig. 2C, Center) and turned to one of the two reward ports (Fig. 2C, Right). Fig. S1 confirms that rats remained in the nose poke to attend to both stimuli and the go cue, excluding the possibility that they adhered to some stereotyped timing routine (15). In well-trained rats, the self-generated motion known as “whisking” was suppressed throughout the trial (Movie S2), indicating that the sensorimotor system entered a “receptive sensing” mode of operation (16, 17).
Experiments with human subjects (Fig. 2D) used corresponding stimuli delivered to the index finger (see SI Text for details).
Stimulus Generalization Matrix.
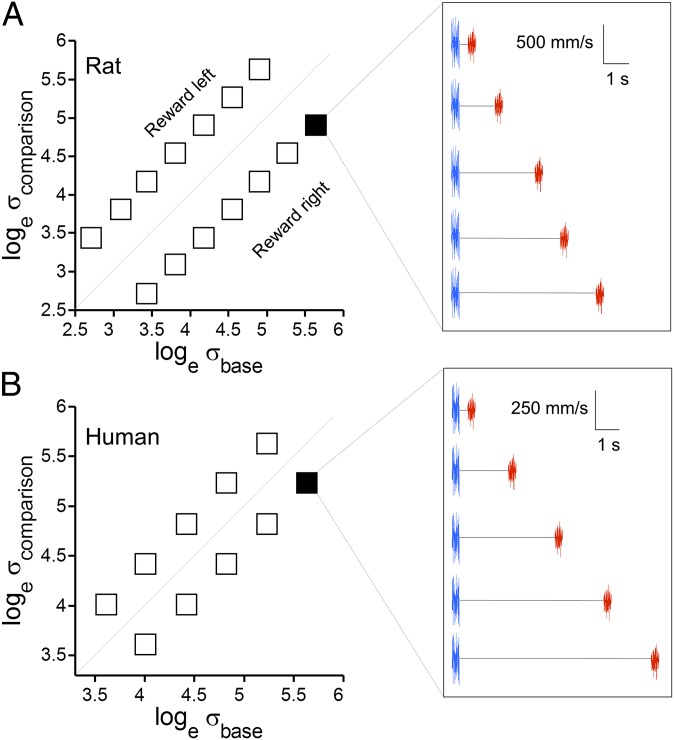
The first result involves training rats to generalize the comparison rule across the entire stimulus dimension (see SI Text and Table S1 for details). If the base stimulus were fixed across all trials and only the comparison stimulus shifted, the rat might solve the task by ignoring the base stimulus and applying a constant threshold to the comparison stimulus. Likewise, if the comparison stimulus were fixed across all trials, the rat might simply apply a constant threshold to the base stimulus. To avoid such shortcut strategies, we used the stimulus generalization matrix (SGM). The SGM, adapted from Romo and coworkers (13, 18), consisted of stimuli spanning a wide range of σ values (Fig. 3). Neither the base stimulus nor the comparison stimulus, taken alone, contained sufficient information to solve the task, so the rat was required to execute a direct comparison between the two stimuli on every trial. Fig. S2 shows that during training, rats learned to weigh both stimuli.
Fig. 3.
Stimulus generalization matrix. (A) Stimulus set for rats. The [σbase, σcomparison] pair for each trial was selected randomly from among those represented by the boxes. Base stimulus values are distributed along the abscissa, and comparison stimulus values are distributed along the ordinate; note logarithmic scales. Diagonal line separates σcomparison > σbase (reward left) from σcomparison < σbase (reward right) stimulus pairs. (Right) For one stimulus pair, varying interstimulus delay intervals are illustrated. (B) Stimulus set for humans. As in A, for one stimulus pair, varying interstimulus delay intervals are illustrated.
In the final stage of training (SI Text), rats proceeded to an SGM with 10–14 [σbase, σcomparison] stimulus pairs (Fig. 3A, Left). The σ values were evenly distributed in a logarithmic scale. The diagonal line represents σbase = σcomparison; all stimulus pairs on one side of the diagonal were associated with the same action.
When rats showed stable performance across sessions, they were assigned to (i) a protocol to test tactile working memory proficiency or (ii) a protocol to measure acuity in judging σ differences. The working memory protocol (Fig. 3A, Right) involved a fixed SDI (absolute value of 0.35) with systematic modulation of the delay between base and comparison stimuli. The values of delay were taken from the set 0.2, 2, 4, 6, and 8 s; trials with different delays were randomly interleaved. For humans, the SGM and working memory protocol are illustrated in Fig. 3B. Typically, the SGM included 10 stimulus pairs and SDI of 0.25. Delays were 0.5, 3, 6, 9, and 12 s, randomly interleaved.
Performance.
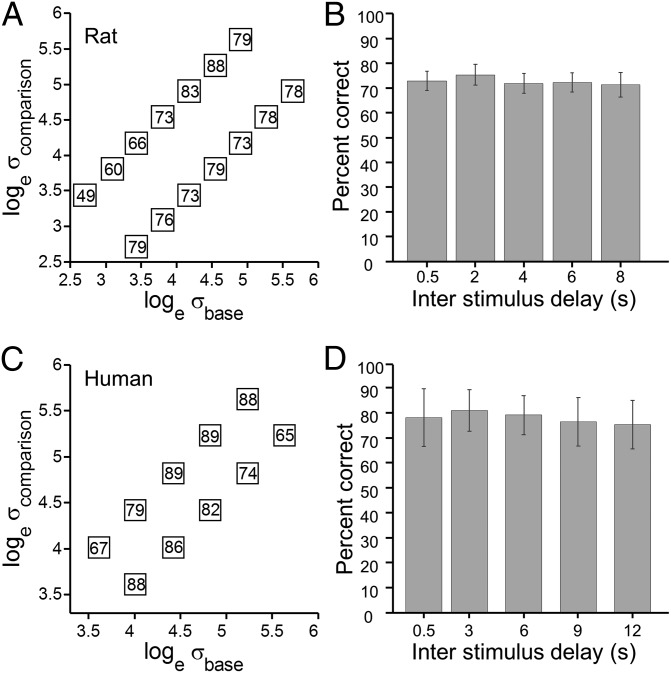
Four rats participated in the working memory protocol. Fig. 4A shows mean performance for each stimulus pair, averaged across rats and across delay durations. Performance was good for all pairs except [2.8, 3.4]; a potential explanation is given in Discussion.
Fig. 4.
Working memory performance. (A) For rats, stimuli had duration of 400 ms, and SDI was held constant at 0.35; interstimulus delay varied randomly from 0.5 s to 8 s. Data from four rats are separated by [σbase, σcomparison] pair but averaged across rats and over different delay durations. (B) Performance, averaged across rats and across all stimuli, as a function of delay duration. (C) For humans, stimuli had duration of 400 ms, and SDI was held constant at 0.25; the interstimulus delay varied randomly from 0.5 s to 12 s. Data from 19 subjects are shown. (D) Performance, averaged across subjects and across all stimuli, as a function of delay duration.
Fig. 4B illustrates the same data but now sorted by the interstimulus delay, with all stimulus pairs merged. Rats achieved just above 70% correct and did not present any decrement with interstimulus delay up to 8 s. Data from 19 human subjects, under analogous experimental conditions, are shown in Figs. 4 C and D. It is possible that faced with more difficult stimulus comparisons, both rats and humans would show a performance decrease in relation to the duration of the delay interval, as found in other tasks (19).
The sensory acuity protocol entailed the fine-grain modulation of trial difficulty within a session. In one group of stimulus pairs, σbase was fixed, whereas σcomparison varied, yielding a graded set of SDI values. In a second group, σcomparison was fixed, whereas σbase varied, yielding another graded set of SDI values. Both stimuli had a duration of 400 ms; interstimulus interval was 800 ms. To ensure that subjects did not shift to a strategy of merely applying a threshold to the base or comparison stimulus, at least 30% of trials adhered to the SGM stimulus set. Rats and humans performed well on SGM trials, implying that they solved the trials in the acuity test using the intended stimulus comparison strategy.
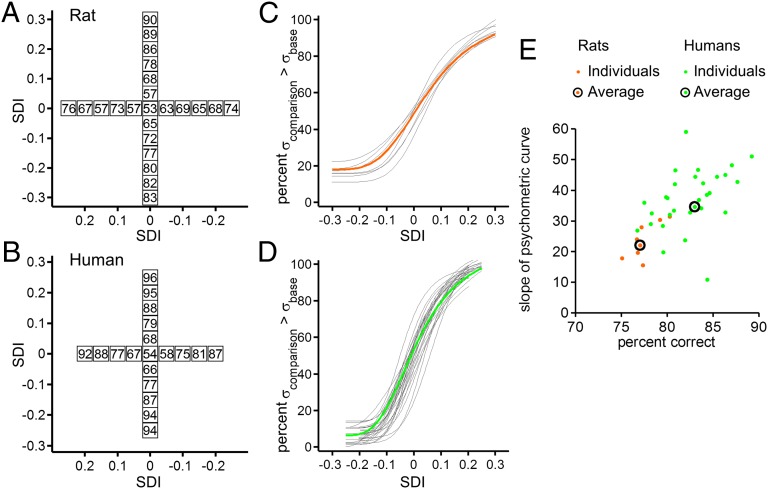
Seven rats participated in the tactile acuity protocol. On the fixed σbase stimulus set (Fig. 5A, vertically arranged stimulus pairs) they performed well, showing accuracy close to or above 70% when the SDI absolute value was equal to or greater than 0.1. On the fixed σcomparison stimulus set (Fig. 5A, horizontally arranged stimulus pairs), performance was slightly lower. Data from 29 human subjects are shown in Fig. 5B. Like the rats, the humans performed worse on the variable-base stimulus set, a stimulus configuration known to be more difficult in monkeys as well (20).
Fig. 5.
Tactile acuity. (A) Data averaged across seven rats. To generate the vertical set of stimulus pairs, σbase was fixed (80 mm/s), whereas σcomparison varied in small steps. To generate the horizontal set of stimulus pairs, σbase varied in small steps, whereas σcomparison was fixed (80 mm/s). Both stimulus sets were embedded within the full SGM. (B) Analogous data from 29 humans. (C) Individual psychometric curves for seven rats; orange trace is the average. (D) Individual psychometric curves for 29 humans; green trace is the average. (E) Two measures of performance are plotted for individual rats (orange) and humans (green). Average values are indicated by black circles.
To quantify the effect of comparison difficulty on accuracy, for the vertically arranged stimulus pairs of Fig. 5 A and B we computed the percent of trials, averaged across sessions, in which each subject judged σcomparison > σbase as a function of SDI. We fit the resulting data with a four-parameter logistic function (SI Text) to generate psychometric curves. If performance were perfect, subjects would report σcomparison > σbase on 0% of trials with negative SDI and on 100% of trials with positive SDI; this would give rise to a step function, going from 0% to 100% at SDI = 0. Because performance is never perfect, psychometric curves assume a sigmoid (S-shape) function. Fig. 5C shows the psychometric curves for seven rats (gray traces) and their average (orange). Fig. 5D shows psychometric curves for 29 humans (average in green). Humans on average exhibited a steeper psychometric function and lower error rates on easy stimulus comparisons.
To directly compare rats and humans, for each subject’s curve we calculated the maximum slope (SI Text); we also calculated the subject’s accuracy over all pairs. Fig. 5E illustrates both values together as a scatter plot. The two performance measures are correlated, as expected. Although the average performance (circled points) of humans is better than that of rats, there is overlap between the rat cloud and the human cloud. We conclude that although a typical human is better than a typical rat, nevertheless, a well-performing rat is better than a poorly performing human and approaches the average human performance.
Statistical Evidence for Delayed Comparison.
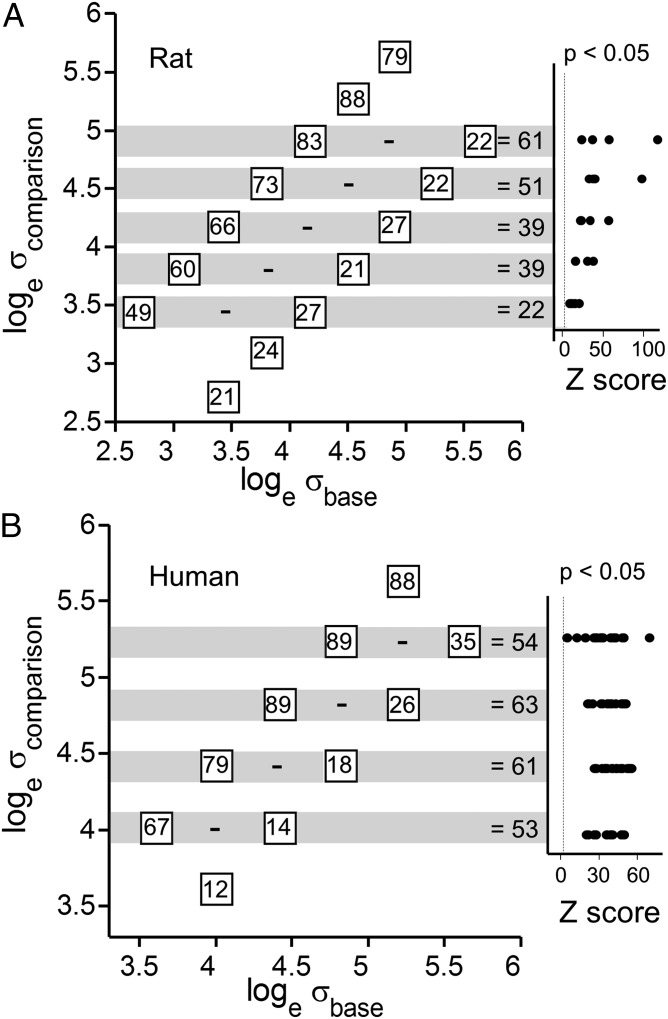
The final step in demonstrating sensory working memory in rats is to prove that they attended to and stored the base stimulus. To do so, we applied statistical tests to assess to what extent their choices depended on the value of σbase. We computed the percent of trials judged as σcomparison > σbase for each value of σcomparison. Each value of σcomparison could be preceded by one of two values of σbase. The results, averaged across rats, are given in the boxes in Fig. 6A. If the values in the paired boxes along a gray iso-σcomparison band were equal, we would conclude that choice was unaffected by the value of σbase. Instead, the large differences (right side of the gray bands) indicate that choices depended on σbase.
Fig. 6.
Statistical analysis of effect of σbase. (A) Values in the boxes give the percent of trials in which rats, on average, judged σcomparison > σbase. The difference between paired boxes in a gray band represents the dependence of choice on whether σcomparison was preceded by smaller or larger σbase. (Right) The statistical significance (see Statistical Evidence for Delayed Comparison) of the choice for all single rats is given as a Z-score. (B) Same analysis carried out on data from humans.
By a resampling procedure, we obtained Z-scores to estimate whether the apparent dependence of rats’ choices on σbase could be explained by chance (SI Text). In Fig. 6A, Right, the Z-scores of individual rats are shown as black points; they commonly exceeded 10 SDs, where a value of 2 may be considered significant. Fig. 6B reports the same analysis carried out on data from humans.
We set up statistical tests using the algorithm described above to prove that rats and humans attended not only to the base stimulus but also to the comparison stimulus. Results are given in SI Text and Fig. S3.
To summarize, Fig. 6 demonstrates that rats and humans encoded the base stimulus and therefore executed the task as a true delayed comparison. One apparent species difference is that Z-scores were more dispersed in the rats, suggesting more pronounced individual differences.
Discussion
Although there can be no doubt that rodents store short-term memories, it was unclear before now what form of information they could hold in working memory. Rodents express spatial working memory, but navigation tasks do not constrain the modality or entity of stored information; choices could even be held in memory through body posture and other nonneuronal mechanisms (8, 21). Can rodents perform parametric working memory; that is, can they store a stimulus not according to its identity or quality (22) but only by its position along the scale of a single sensory dimension? One earlier study showed that rats could compare two sequential odorant mixtures (23). However, because shifts in the proportion of odorants in a mixture lead to qualitatively different odor percepts (24), it is not clear that the odor mixtures are sensed as steps in a single parameter or else as discrete percepts.
Delayed comparison tasks have been an effective means for studying working memory for over 30 y (25). The present report demonstrates that the performance of rats in a tactile delayed comparison task resembles, to a first approximation, that of humans (Fig. 5). Our study is notable for its parallels to studies of tactile delayed comparison in monkeys by Romo and Salinas (26). In common with our task, the monkey receives two vibrations separated by a variable delay; it then makes a choice according to the difference between the vibrations (26, 27). There are several distinctions in experimental design. In our task, rather than applying stimuli to the fingertip, we selected the whisker sensory system due to its behavioral importance in rats (16, 17, 28–34). Another distinction is the structure of the vibration. Although the studies in monkeys typically use regular, periodic skin deflections in the form of either a sinusoid or a pulse train (35), we opted for a stochastic stimulus composed of filtered noise (36). The choice was motivated by several factors. First, in pilot studies, rats attended to noisy stimuli better than to periodic stimuli and were more likely await the go cue before withdrawing. Second, noisy vibrissal stimuli evoke a more robust cortical response (37, 38), an advantage for future neurophysiological studies. Third, the structure of the noise stimulus is well suited to reverse correlation methods (39) and will provide rich data for studying the kinematic features extracted by sensory neurons (40, 41).
We implemented unique strategies to uncover rats’ perceptual capacities. We trained them to remain immobile in the nose poke for variable times, as short as 100 ms and as long as 5 s (stage 3 of training; SI Text). At this point, we were able to introduce two whisker stimuli on each trial with the rat constrained to receive both stimuli (stage 4). We could then allow the rat to discover the rule that related the tactile stimuli to the reward location (stages 4 and 5). Moreover, with the rat immobile for extended periods, we could vary the interstimulus delay duration to study working memory proficiency (stages 6 and 7).
In addition, we introduced the SGM (Fig. 3) to ensure that subjects attended to both the base and comparison stimuli. Neither stimulus, taken alone, contained sufficient information to solve the task. Thus, the simpler strategy of ignoring one stimulus and attending to the other would lead to performance close to chance (13).
Performance in rats faltered for the stimulus pairs [2.8, 3.4] and [3.2, 3.8], where σbase assumed low values (Fig. 4A). Poor performance might be explained by “contraction bias,” which posits that during the interstimulus delay, the neuronal representation of σbase drifts toward the expected value, or “prior,” of all base stimuli presented in recent history (42, 43).† By this account, on low-σbase trials, the representation of σbase shifted in the upward direction, toward the mean σbase of the complete SGM. As a consequence, σcomparison, whose value was greater than that of σbase, was matched against a memory of σbase that had grown during the delay. The outcome was a reduction in likelihood that σcomparison was correctly judged to be greater than σbase.
For human subjects (Fig. 4C), performance also was poor (67% correct) for the stimulus pair where σbase assumed its lowest value. This finding of contraction bias suggests that there may be shared mechanisms for working memory across rats and humans. However, one species difference emerged: human subjects—but not rats—showed a contraction bias (65%) for the stimulus pair with highest σbase value, [5.6, 5.2]. At present, we have no explanation for the symmetric high/low contraction bias in humans versus the asymmetric bias in rats.
Until a few years ago, many neuroscientists attributed a wide range of perceptual functions to primates but not to rodents. The capacities of rats might have been overlooked because training regimes were not effectively adapted to their natural deportment. With improving behavioral methodologies, rodents have been found to express surprising abilities. For instance, rats spontaneously recognize views of an object that differ by angle, size, and position (44, 45); such generalization is a hallmark of true visual perception and was once believed to belong only to primates. With regard to more abstract computations, rodents weigh sensory evidence (46), assess reward statistics (47), integrate multimodal sensory inputs (48), accumulate evidence for optimal decision-making (49), express certainty in the outcome of their choices (50), and even generalize rules (51). In sum, mice and rats are becoming increasingly important for the study of perception (52). From the present effort, parametric working memory joins other cognitive functions within the repertoire of rodent capacities. In humans and primates, parametric working memory has been associated with a network of prefrontal and parietal cortical regions (7, 18, 25, 53–55); the analogous networks have yet to be systematically explored in rodents.
Materials and Methods
Subjects.
Eleven male Wistar rats (Harlan Laboratories) were housed individually or with one cage mate and kept on a 14/10 light/dark cycle. They were examined weekly by a veterinarian. At the start of the experiment they were 6–8 wk old and weighed 225–250 g; they gained weight steadily throughout the study. Protocols conformed to international norms and were approved by the Italian Health Ministry and the Ethics Committee of the International School for Advanced Studies.
Forty-four human subjects (16 males and 28 females, ages 22–35) were tested. Protocols conformed to international norms and were approved by the Ethics Committee of the International School for Advanced Studies. Subjects signed informed consent.
Materials and Instrumentation.
The animal apparatus (Fig. 1) consisted of a Plexiglas compartment measuring 25 × 25 × 38 cm (height × width × length). In the front wall, a 3.8 cm (width) by 5 cm (height) head hole opened to the stimulus delivery port. Within the stimulus delivery port a 0.85-cm-diameter nose poke was centered in front; the nose poke contained an optic sensor illuminated by an infrared photo beam to detect the rat’s snout. Above the nose poke, a blue LED was fixed. LED illumination signaled to the rat that the next trial may begin.
A shaker motor (type 4808; Bruel and Kjaer), with 12.7 mm peak-to-peak displacement, was used to generate stimuli. The motor was placed on its flank to produce motion in the horizontal dimension (Fig. 1). A 20 × 30 mm plate was attached to the diaphragm of the shaker. Once trained, the rat received the stimulus by placing its whiskers on the plate. Double-sided adhesive was fixed to the plate to keep the whiskers in contact and thus to follow the motor’s motion.
Both rat and human experiments were controlled using LabVIEW software (National Instruments).
Generation of Vibrations and Their Statistical Structure.
We generated the velocity time series as follows. First, we constructed in LabVIEW a unitless normal distribution centered at 0 and sampled it 10,000 times per s; then, we applied a Butterworth filter with 150 Hz cutoff to yield low-pass filtered noise. This time series was amplified (type 2719; Bruel and Kjaer) and transmitted as voltage values to the motor. Thus, the velocity distribution delivered to the whiskers had SD proportional to the SD of the original unitless normal distribution. The velocity time series for a given trial was taken randomly from among 50 seeds.
Because the motor was constructed to keep acceleration constant across a frequency range from 5 Hz to 10 KHz, it follows that if peak-to-peak input voltage was held constant, displacement diminished as frequency increased. Due to this built-in displacement clamp, a theoretical derivation of motion was complex: motion must be assessed empirically. We tested the motor before installing it in the apparatus by fixing a position transducer (LD 310-25; OMEGA Engineering) to a rod extending from the diaphragm and then executing the entire stimulus library while recording 1,000 frames per s video clips (Optronis CamRecord 450). We computed plate motion with a custom-made video tracking script in MATLAB (MathWorks) and used the tracked video to calibrate the transducer. Finally, we compared tracked videos of plate motion under the two conditions—position transducer attached and removed—and confirmed that the transducer did not measurably affect motion. At this point, we could install the motor in the behavioral apparatus with full knowledge of its output. The position transducer provided an online signal to check the operation of the motor. Descriptions of the stimulus are based on the true measured output of the motor.
The same stimuli used in rats were delivered to the subject’s fingertip except that the range of velocity distribution width was limited to a maximum of 270 mm/s. Subjects viewed a computer monitor and wore headphones that presented acoustic noise and eliminated ambient sounds. They received feedback (correct/incorrect) on each trial through the monitor.
Exclusion of Nontactile Signals.
Test sessions on rats were run under dim ambient or red light that did not allow visualization of the stimulator motion. No potential olfactory or gustatory cues about the vibrations were available. However, the motor generated acoustic signals (easily heard by humans), and precautions were taken to ensure that rats did not use such signals to judge the stimuli, as described in SI Text and Figs. S4 and S5.
Supplementary Material
Acknowledgments
The authors thank Armin Lak for participation in pilot experiments, Fabrizio Manzino and Marco Gigante for technical contributions, and Francesca Pulecchi for animal care and training. Marco Gigante made the drawings in Fig. 2. This work was supported by the Human Frontier Science Program (Project RG0041/2009-C), European Research Council Advanced Grant Construction of Perception from Touch Signals (CONCEPT) (Project 294498), European Union Future and Emerging Technologies (FET) Grant Choreographing Neural Networks: Coupling Activity Dynamics Across Biomimetic Brain Interfaces with Neuromorphic VLSI (CORONET) (Project 269459), and the Compagnia San Paolo.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315171111/-/DCSupplemental.
†Akrami A, Fassihi A, Esmaeili V, Diamond ME (2013) Tactile working memory in rat and human: Prior competes with recent evidence. Cosyne Abstracts 2013 Salt Lake City USA.
References
- 1.O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 2.Moser EI, Kropff E, Moser M-B. Place cells, grid cells, and the brain’s spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- 3.McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Brain Res Rev. 1993;18(1):33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- 4.Itskov PM, Vinnik E, Diamond ME. Hippocampal representation of touch-guided behavior in rats: Persistent and independent traces of stimulus and reward location. PLoS ONE. 2011;6(1):e16462. doi: 10.1371/journal.pone.0016462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 6.Doeller CF, Barry C, Burgess N. Evidence for grid cells in a human memory network. Nature. 2010;463(7281):657–661. doi: 10.1038/nature08704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasternak T, Greenlee MW. Working memory in primate sensory systems. Nat Rev Neurosci. 2005;6(2):97–107. doi: 10.1038/nrn1603. [DOI] [PubMed] [Google Scholar]
- 8.Carruthers P. Evolution of working memory. Proc Natl Acad Sci USA. 2013;110(Suppl 2):10371–10378. doi: 10.1073/pnas.1301195110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grobe C, Spector A (2006) Rats can learn a “Delayed Match/Delayed Non-Match to Sample” task using only taste stimuli. FASEB J 20(4):A381 (abstr)
- 10.Peña T, Pitts RC, Galizio M. Identity matching-to-sample with olfactory stimuli in rats. J Exp Anal Behav. 2006;85(2):203–221. doi: 10.1901/jeab.2006.111-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336(6087):1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris RG. Developments of a water maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 13.Hernández A, Salinas E, García R, Romo R. Discrimination in the sense of flutter: New psychophysical measurements in monkeys. J Neurosci. 1997;17(16):6391–6400. doi: 10.1523/JNEUROSCI.17-16-06391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nahum M, Daikhin L, Lubin Y, Cohen Y, Ahissar M. From comparison to classification: A cortical tool for boosting perception. J Neurosci. 2010;30(3):1128–1136. doi: 10.1523/JNEUROSCI.1781-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ölveczky BP. Motoring ahead with rodents. Curr Opin Neurobiol. 2011;21(4):571–578. doi: 10.1016/j.conb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Diamond ME, Arabzadeh E. Whisker sensory system - from receptor to decision. Prog Neurobiol. 2013;103:28–40. doi: 10.1016/j.pneurobio.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Prescott TJ, Diamond ME, Wing AM. Active touch sensing. Philos Trans R Soc Lond B Biol Sci. 2011;366(1581):2989–2995. doi: 10.1098/rstb.2011.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romo R, Brody CD, Hernández A, Lemus L. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature. 1999;399(6735):470–473. doi: 10.1038/20939. [DOI] [PubMed] [Google Scholar]
- 19.Müller U, von Cramon DY, Pollmann S. D1- versus D2-receptor modulation of visuospatial working memory in humans. J Neurosci. 1998;18(7):2720–2728. doi: 10.1523/JNEUROSCI.18-07-02720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernández A, et al. Decoding a perceptual decision process across cortex. Neuron. 2010;66(2):300–314. doi: 10.1016/j.neuron.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 21.Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev. 2004;28(7):699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Ennaceur A. One-trial object recognition in rats and mice: Methodological and theoretical issues. Behav Brain Res. 2010;215(2):244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 23.Perry C, Felsen G. Rats can make relative perceptual judgments about sequential stimuli. Anim Cogn. 2012;15(4):473–481. doi: 10.1007/s10071-012-0471-4. [DOI] [PubMed] [Google Scholar]
- 24.Barkat S, Le Berre E, Coureaud G, Sicard G, Thomas-Danguin T. Perceptual blending in odor mixtures depends on the nature of odorants and human olfactory expertise. Chem Senses. 2012;37(2):159–166. doi: 10.1093/chemse/bjr086. [DOI] [PubMed] [Google Scholar]
- 25.Kojima S, Goldman-Rakic PS. Delay-related activity of prefrontal neurons in rhesus monkeys performing delayed response. Brain Res. 1982;248(1):43–49. doi: 10.1016/0006-8993(82)91145-3. [DOI] [PubMed] [Google Scholar]
- 26.Romo R, Salinas E. Flutter discrimination: Neural codes, perception, memory and decision making. Nat Rev Neurosci. 2003;4(3):203–218. doi: 10.1038/nrn1058. [DOI] [PubMed] [Google Scholar]
- 27.Mountcastle VB, Steinmetz MA, Romo R. Frequency discrimination in the sense of flutter: Psychophysical measurements correlated with postcentral events in behaving monkeys. J Neurosci. 1990;10(9):3032–3044. doi: 10.1523/JNEUROSCI.10-09-03032.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diamond ME, von Heimendahl M, Knutsen PM, Kleinfeld D, Ahissar E. ‘Where’ and ‘what’ in the whisker sensorimotor system. Nat Rev Neurosci. 2008;9(8):601–612. doi: 10.1038/nrn2411. [DOI] [PubMed] [Google Scholar]
- 29.Diamond ME. Texture sensation through the fingertips and the whiskers. Curr Opin Neurobiol. 2010;20(3):319–327. doi: 10.1016/j.conb.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Diamond ME, von Heimendahl M, Arabzadeh E. Whisker-mediated texture discrimination. PLoS Biol. 2008;6(8):e220. doi: 10.1371/journal.pbio.0060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brecht M. Barrel cortex and whisker-mediated behaviors. Curr Opin Neurobiol. 2007;17(4):408–416. doi: 10.1016/j.conb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Petersen CC. The functional organization of the barrel cortex. Neuron. 2007;56(2):339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Adibi M, Arabzadeh E. A comparison of neuronal and behavioral detection and discrimination performances in rat whisker system. J Neurophysiol. 2011;105(1):356–365. doi: 10.1152/jn.00794.2010. [DOI] [PubMed] [Google Scholar]
- 34.Harris JA, Petersen RS, Diamond ME. Distribution of tactile learning and its neural basis. Proc Natl Acad Sci USA. 1999;96(13):7587–7591. doi: 10.1073/pnas.96.13.7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernández A, Zainos A, Romo R. Neuronal correlates of sensory discrimination in the somatosensory cortex. Proc Natl Acad Sci USA. 2000;97(11):6191–6196. doi: 10.1073/pnas.120018597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maravall M, Petersen RS, Fairhall AL, Arabzadeh E, Diamond ME. Shifts in coding properties and maintenance of information transmission during adaptation in barrel cortex. PLoS Biol. 2007;5(2):e19. doi: 10.1371/journal.pbio.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lak A, Arabzadeh E, Diamond ME. Enhanced response of neurons in rat somatosensory cortex to stimuli containing temporal noise. Cereb Cortex. 2008;18(5):1085–1093. doi: 10.1093/cercor/bhm144. [DOI] [PubMed] [Google Scholar]
- 38.Lak A, Arabzadeh E, Harris JA, Diamond ME. Correlated physiological and perceptual effects of noise in a tactile stimulus. Proc Natl Acad Sci USA. 2010;107(17):7981–7986. doi: 10.1073/pnas.0914750107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ringach D, Shapley R. Reverse correlation in neurophysiology. Cogn Sci. 2004;28(2):147–166. [Google Scholar]
- 40.Arabzadeh E, Panzeri S, Diamond ME. Whisker vibration information carried by rat barrel cortex neurons. J Neurosci. 2004;24(26):6011–6020. doi: 10.1523/JNEUROSCI.1389-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lottem E, Azouz R. A unifying framework underlying mechanotransduction in the somatosensory system. J Neurosci. 2011;31(23):8520–8532. doi: 10.1523/JNEUROSCI.6695-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashourian P, Loewenstein Y. Bayesian inference underlies the contraction bias in delayed comparison tasks. PLoS ONE. 2011;6(5):e19551. doi: 10.1371/journal.pone.0019551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hollingworth HL. The central tendency of judgment. The Journal of Philosophy. J Philos Psychol Sci Methods. 1910;7(17):461–469. [Google Scholar]
- 44.Tafazoli S, Di Filippo A, Zoccolan D. Transformation-tolerant object recognition in rats revealed by visual priming. J Neurosci. 2012;32(1):21–34. doi: 10.1523/JNEUROSCI.3932-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zoccolan D, Oertelt N, DiCarlo JJ, Cox DD. A rodent model for the study of invariant visual object recognition. Proc Natl Acad Sci USA. 2009;106(21):8748–8753. doi: 10.1073/pnas.0811583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kepecs A, Uchida N, Zariwala HA, Mainen ZF. Neural correlates, computation and behavioural impact of decision confidence. Nature. 2008;455(7210):227–231. doi: 10.1038/nature07200. [DOI] [PubMed] [Google Scholar]
- 47.Karlsson MP, Tervo DG, Karpova AY. Network resets in medial prefrontal cortex mark the onset of behavioral uncertainty. Science. 2012;338(6103):135–139. doi: 10.1126/science.1226518. [DOI] [PubMed] [Google Scholar]
- 48.Raposo D, Sheppard JP, Schrater PR, Churchland AK. Multisensory decision-making in rats and humans. J Neurosci. 2012;32(11):3726–3735. doi: 10.1523/JNEUROSCI.4998-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brunton BW, Botvinick MM, Brody CD. Rats and humans can optimally accumulate evidence for decision-making. Science. 2013;340(6128):95–98. doi: 10.1126/science.1233912. [DOI] [PubMed] [Google Scholar]
- 50.Lavan D, McDonald JS, Westbrook RF, Arabzadeh E. Behavioural correlate of choice confidence in a discrete trial paradigm. PLoS ONE. 2011;6(10):e26863. doi: 10.1371/journal.pone.0026863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy RA, Mondragón E, Murphy VA. Rule learning by rats. Science. 2008;319(5871):1849–1851. doi: 10.1126/science.1151564. [DOI] [PubMed] [Google Scholar]
- 52.Carandini M, Churchland AK. Probing perceptual decisions in rodents. Nat Neurosci. 2013;16(7):824–831. doi: 10.1038/nn.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283(5408):1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 54.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24(1):167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 55.McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11(1):103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.