Significance
The six-layered cerebral cortex forms through tightly regulated steps including neuronal proliferation, migration, and circuitry formation. Alterations of cortical development are associated with several neurological conditions, but the underlying pathogenetic mechanisms remain largely unknown. Here, we describe the role of TBC1 domain family member 24 (TBC1D24), a gene associated with syndromes combining epilepsy and cognitive deficits, in cortical development. Using an in vivo approach, we found that TBC1D24 regulates neuronal polarity, thus promoting neuronal migration and maturation. We further show that TBC1D24 exerts its function through the modulation of the activity of ADP ribosylation factor 6, a GTPase involved in membrane trafficking. Collectively, our data disclose a previously uncharacterized molecular mechanism involved in cortical development and underline how appropriate and timely neuronal positioning is essential for brain function.
Keywords: dendritogenesis, synaptogenesis, epileptic encephalopathies, gene, RNA interference
Abstract
Alterations in the formation of brain networks are associated with several neurodevelopmental disorders. Mutations in TBC1 domain family member 24 (TBC1D24) are responsible for syndromes that combine cortical malformations, intellectual disability, and epilepsy, but the function of TBC1D24 in the brain remains unknown. We report here that in utero TBC1D24 knockdown in the rat developing neocortex affects the multipolar-bipolar transition of neurons leading to delayed radial migration. Furthermore, we find that TBC1D24-knockdown neurons display an abnormal maturation and retain immature morphofunctional properties. TBC1D24 interacts with ADP ribosylation factor (ARF)6, a small GTPase crucial for membrane trafficking. We show that in vivo, overexpression of the dominant-negative form of ARF6 rescues the neuronal migration and dendritic outgrowth defects induced by TBC1D24 knockdown, suggesting that TBC1D24 prevents ARF6 activation. Overall, our findings demonstrate an essential role of TBC1D24 in neuronal migration and maturation and highlight the physiological relevance of the ARF6-dependent membrane-trafficking pathway in brain development.
The mammalian cerebral cortex is a multilayered structure derived from cells of the neural tube (1). Cortical projection neurons are generated from proliferating progenitor cells located in the ventricular zone (VZ) adjacent to the lateral ventricle (2). After their final mitotic division, the majority of neurons migrate radially, along radial glia fibers, from the VZ toward the pial surface, where each successive generation passes one another and settles in an inside-out pattern within the cortical plate (CP) (3, 4). When neurons reach their final destination, they stop migrating and order themselves into specific “architectonic” patterns, according to a complex signaling pathway, guiding cells to the correct location in the brain (5). Overall, these steps lead to the formation of a six-layered cortex that plays a key role in cognitive processes. It is therefore not surprising that disruption of early cortical development causes severe neurological conditions usually featuring intellectual disabilities (IDs) and epilepsy, which may be associated with brain malformations (6).
Over the past decades, advances in our understanding of the genetics of ID and epilepsy have revolutionized the diagnostic and the clinical approach to these disorders, and an increasing number of pathogenic gene mutations have been identified. TBC1D24 is a novel epilepsy-related gene mutated in different autosomal recessive forms of early-onset epilepsy, which can be accompanied by variable degrees of ID (7–11). The TBC1D24 gene encodes a protein of 553 aa that contains a Tre2/Bub2/Cdc16 (TBC) domain, shared by Rab GTPase-activating proteins (Rab-GAPs) and a TLDc domain of unknown function (8). The TBC1D24 protein is expressed in the brain and interacts with the ADP ribosylation factor (ARF) 6, a small GTP-binding protein implicated in membrane exchange between the plasma membrane and the endocytic compartments (8, 12). In the brain, ARF6 is known to play various roles including regulation of neurite outgrowth (13, 14) and maintenance of apical adhesion of neural progenitors in the developing neocortex (15). Interestingly, pathogenic mutations in TBC1D24 affect its binding to ARF6 and result in a severe impairment of neuronal development (7, 8, 10). Conversely, overexpression of TBC1D24 increases neuronal differentiation in vitro (7, 8). These findings, along with the subtle cortical malformation observed in some patients carrying a TBC1D24 mutation (7, 16), suggest that TBC1D24 is likely to be involved in brain development and maturation.
To investigate TBC1D24 function in the developing neocortex, we down-regulated its expression in neuronal progenitor cells of the embryonic rat brain by in utero electroporation (IUE). We found that silencing of TBC1D24 delays radial migration and morphological maturation of pyramidal neurons. In addition, we showed that concomitant expression of an inactive form of ARF6, ARF6T27N, was able to rescue the TBC1D24-knockdown phenotype in vivo. Furthermore, overexpression of the constitutively active ARF6Q67L mutant results in a significant delay of neuronal migration. Overall, our findings suggest that TBC1D24, by regulating ARF6 activity in postmitotic neurons, controls radial migration and neurite outgrowth during cortical development.
Results
TBC1D24 Is Involved in Radial Migration of Cortical Neurons.
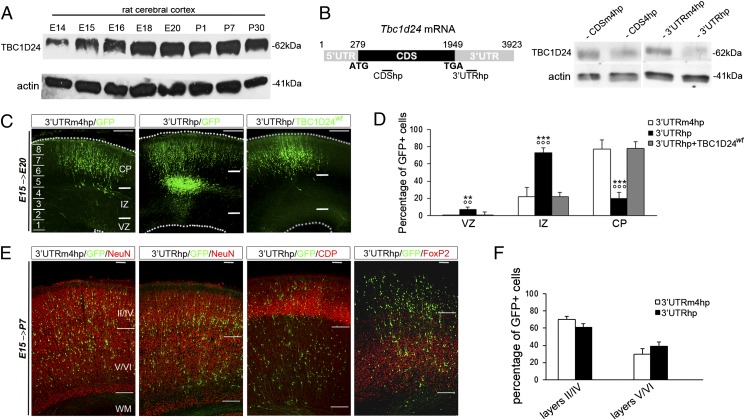
To investigate the role of TBC1D24 in cerebral cortex development in vivo, we used the in utero RNAi-mediated knockdown approach (17) to silence its expression in rat embryonic neural progenitors. We first examined the temporal expression profile of TBC1D24 in the developing rat neocortex from embryonic day (E) 14 to postnatal day (P) 30 by Western blot (WB). TBC1D24 expression increased from E14 to E18 and remained stable at perinatal and postnatal stages (E20 to P30) (Fig. 1A). We next generated two small hairpin (sh) RNAs targeting either the coding sequence (CDShp) or the 3′-untranslated region (3′UTRhp) of rat Tbc1d24 mRNA (Fig. 1B) and two corresponding negative control shRNAs with four point mutations (CDSm4hp and 3′UTRm4hp). These shRNAs were transiently transfected into primary cortical neurons prepared from E18 rat embryos, to evaluate their ability to suppress endogenous TBC1D24 expression. We found that CDShp and 3′UTRhp, but not the two negative control shRNAs, resulted in a significant and long-lasting reduction of TBC1D24 protein expression, as assessed by WB analysis performed 12 d after transfection (Fig. 1B). The 3′UTRhp or 3′UTRm4hp were then introduced in combination with a green fluorescent protein (GFP)-encoding reporter construct into neural progenitor cells by IUE at E15 and the distribution of GFP-positive cells was analyzed 2 and 5 d later. At E17, in both 3′UTRhp and 3′UTRm4hp brains, the majority of GFP-positive cells were found in the intermediate zone (IZ), suggesting that early silencing of TBC1D24 did not prevent radial migration out of the VZ and subventricular zone (SVZ) (Fig. S1 A and B). We also found that TBC1D24 knockdown did not affect the polarized organization (Fig. S1 C and D), the cell cycle progression, or survival of UTRhp-transfected neural progenitors (Fig. S2). In contrast, at E20, we found that 3′UTRhp led to a significant arrest of transfected cells in the IZ with only 20.1 ± 5.7% (n = 12 embryos; P < 0.0001) of GFP-positive cells reaching the CP, whereas the majority of 3′UTRm4hp-transfected cells (77.3 ± 10.5%; n = 10 embryos) correctly reached the CP (Fig. 1 C and D). In addition, we found no cell death induced by TBC1D24 knockdown at E20 or P0 (Fig. S3). To further validate the specificity of our results, we conducted in utero knockdown experiments with the CDShp construct and found that it led to the same migration defect, with only 31.3 ± 11.9% (n = 8 embryos) of GFP-positive cells reaching the CP (Fig. S4). Next, we performed a rescue experiment in which we cotransfected the 3′UTRhp along with a TBC1D24-IRES-GFP construct (TBC1D24wt) driving the expression of the full-length human TBC1D24 coding sequence (GenBank accession no. NM_001199107.1) and GFP as a reporter. Although the overexpression of this construct per se did not impair radial migration (Fig. S5), TBC1D24wt in combination with 3′UTRhp significantly reduced the migration arrest of transfected cells in the IZ at E20 (Fig. 1 C and D), demonstrating the target specificity of the shRNA effect. To determine whether silencing of TBC1D24 caused a permanent migration arrest in the IZ or a transient delay in migration, we examined the positions of 3′UTRhp-transfected neurons at later developmental stages. At P7, the majority of TBC1D24-knockdown neurons were able to reach the CP and extend apical dendrites toward the pial surface. These observations were confirmed by immunohistochemical analysis using a neuronal marker (NeuN), a marker of cortical upper layers (II/III; CDP) and a marker of the deep layers (V/VI; FoxP2), none of which revealed any obvious alterations in the cortical layering of 3′UTRhp-transfected brains (n = 7) compared with controls (n = 7; Fig. 1 E and F). Overall, our findings establish an important role for TBC1D24 in radial migration toward the CP.
Fig. 1.
Neuronal migration following embryonic TBC1D24 knockdown. (A) WB analysis of TBC1D24 protein expression from rat cortical lysates during development. (B, Left) Diagram showing the position of shRNAs targeting the coding sequence (CDShp) and the 3′UTR (3′UTRhp) of rat Tbc1d24 mRNA. (B, Right) TBC1D24 protein expression in rat primary neurons transfected at the day of plating with the indicated plasmids and analyzed after 12 d by WB. (C) Representative neocortical coronal sections showing migration of transfected cells 5 d after electroporation at E15 with pCAGGs-IRES-EGFP (GFP) construct combined with the indicated plasmids (3′UTRm4hp, n = 10; 3′UTRhp, n = 12; 3′UTRhp + TBC1D24wt, n = 9). (Scale bar: 200 μm.) (D) Quantification of GFP-positive cell distribution 5 d after transfection at E15 expressed as a percentage of total transfected cells. Data, expressed as means ± SEM, were compared via one-way ANOVA for repeated measures, followed by the Bonferroni’s multiple comparison test. ***P < 0.0001 and **P < 0.005 vs. 3′UTRm4hp; °°°P < 0.0001 and °°P < 0.005 vs. 3′UTRhp + TBC1D24wt. (E) Representative P7 neocortical sections showing the laminar position of GFP cells in rats electroporated at E15 with the indicated plasmids and immunostained with the neuronal marker NeuN, cortical II–IV layers marker CDP and the V–VI layers marker FoxP2. (Scale bar: 500 μm.) (F) Quantification of GFP cells in cortical upper layers (II–IV) and the cortical deep layers (V–VI) and expressed as a percentage of the total number of transfected cells (n = 7 for each condition).
Multipolar–Bipolar Transition Is Impaired in a TBC1D24-Knockdown Model.
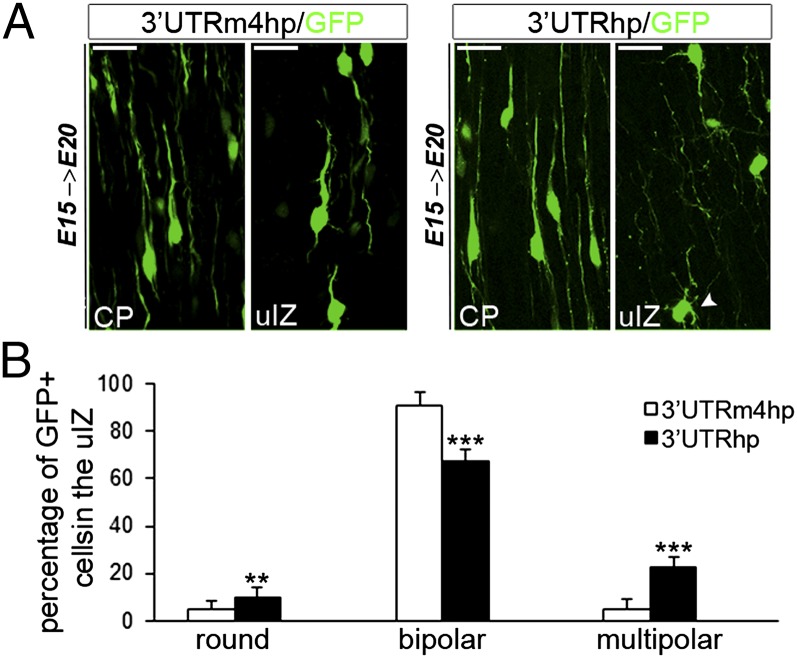
During the process of neuronal migration, future pyramidal cells initially move radially from the VZ/SVZ into the lower part of the IZ, where they pause and acquire a multipolar morphology (18, 19). At this stage, neurons rapidly extend and retract processes until they acquire the bipolar shape in the upper part of the IZ (uIZ), which allows them to undergo glia-guided locomotion toward the CP (20). To determine whether the persistence of the multipolar phase in TBC1D24-knockdown neurons underlie their transient arrest in migration, we analyzed the morphology of 3′UTRhp- and 3′UTRm4hp-transfected cells in the uIZ at E20. In 3′UTRhp brains (n = 9), a significant percentage of IZ-trapped neurons (22.9 ± 4.3%) exhibited a multipolar morphology characterized by multiple minor processes or showing a rounded morphology with no evident processes, whereas the small portion of TBC1D24-knockdown neurons that could reach the CP displayed a normal bipolar morphology with a leading process extended normally toward the pial surface (Fig. 2). By comparison, in age-matched control brains (n = 7), only a few neurons were still present in the uIZ, and those cells displayed the typical unipolar or bipolar shape with one prominent leading process orienting toward the marginal zone (Fig. 2). These data indicate that cortical neurons require TBC1D24 for a timely multipolar-to-bipolar transition in the uIZ, because loss of TBC1D24 leads to a delay in both acquisition of bipolar morphology and radial migration.
Fig. 2.
TBC1D24 knockdown impairs multipolar/bipolar transition in uIZ. (A) E20 rat cortices electroporated at E15 with 3′UTRhp or 3′UTRm4hp in the indicated regions. (Scale bar: 20 μm.) In the TBC1D24-knockdown model, a high percentage of migrating neurons persisted at the multipolar stage (arrowhead). (B) Histograms showing the average percentages (means ± SEM) of the polarity in the uIZ from similar sections as in A (3′UTRm4hp, n = 7; 3′UTRhp, n = 9). One-way ANOVA for repeated measures: ***P < 0.0001; **P < 0.001.
TBC1D24 Knockdown Alters Dendritic Arborization and Glutamatergic Synapse Formation.
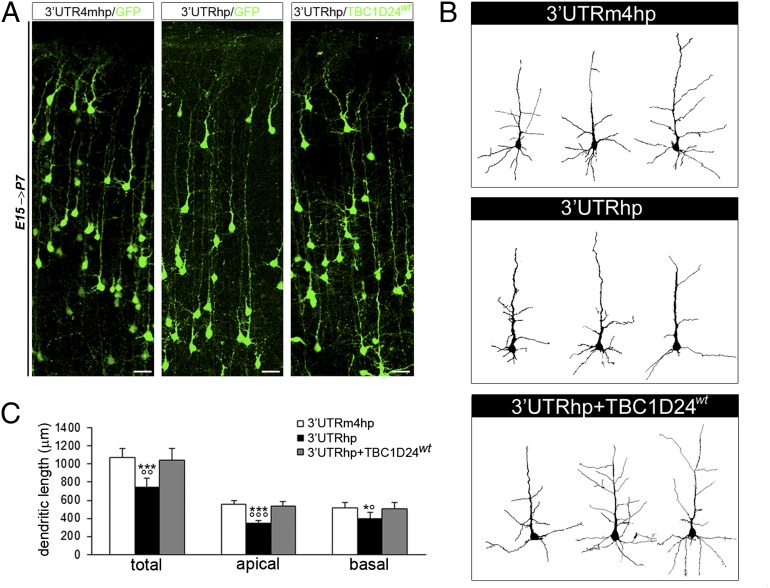
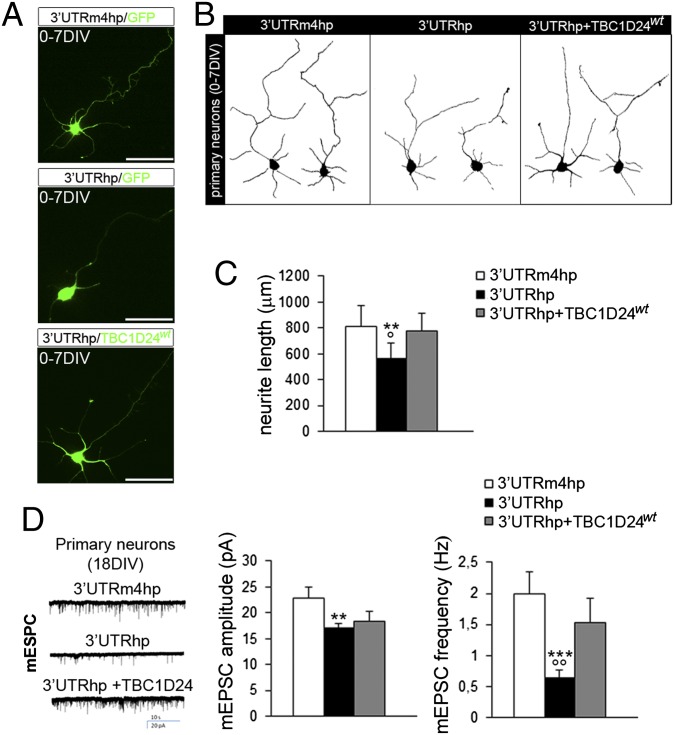
It has been shown that the inability of cortical neurons to exit from the multipolar phase of radial migration results in abnormal dendritic development of pyramidal neurons in the postnatal cerebral cortex (21, 22). Interestingly, previous studies revealed a role for TBC1D24 in neurite outgrowth in vitro (7, 8, 10), and we found that TBC1D24 expression also increased during postnatal development (Fig. 1A). These observations led us to investigate whether TBC1D24 also plays a role in the morphological maturation of pyramidal neurons, in addition to its function in radial migration. We found that in utero silencing of TBC1D24 significantly impairs the formation of apical and basal dendrites at P7 (Fig. 3). Remarkably, coexpression of TBC1D24wt in combination with 3′UTRhp restored dendritic outgrowth (Fig. 3). A similar alteration in neurite formation was also observed in TBC1D24-knockdown cortical primary neurons in vitro (Fig. 4 A–C), consistent with a cell-autonomous function of TBC1D24 in regulating neurite length. We therefore investigated whether this protein was also involved in the maturation/function of excitatory synapses at the single-cell level. Using rat primary cortical neurons transfected at 14 d in vitro (DIV), a stage at which neuronal maturation and the initial wave of synaptogenesis has occurred, we analyzed spontaneous excitatory transmission in TBC1D24-knockdown neurons by recording miniature excitatory postsynaptic currents (mEPSCs) (Fig. 4D). Loss of TBC1D24 strongly decreased the frequency and, to a lesser extent, the amplitude of mEPSCs. The effect on the mEPSC frequency was virtually restored in TBC1D24-knockdown neurons by the concomitant expression of a TBC1D24wt construct (Fig. 4D). Changes in mEPSC frequency can be attributable to a change in the probability of spontaneous synaptic vesicle fusion at presynaptic sites or to a change in the number of synaptic contacts onto the patched neurons. Importantly, because of the low efficiency of the transfection (less than 2% of neurons were transfected per coverslip), we recorded TBC1D24-knockdown neurons receiving inputs from nontransfected neurons expressing physiological levels of TBC1D24, ruling out a defect in the presynaptic release machinery. In contrast, the reduction in the frequency and amplitude of the mEPSC could be mainly related to a reduction in the excitatory synaptic contacts attributable to the impaired maturation of postsynaptic TBC1D24-knockdown neurons and a decreased density/function of glutamatergic synapses. Indeed, we showed that the density of VGLUT1-positive puncta identifying axodendritic and axosomatic excitatory presynaptic boutons was significantly lower in 3′UTRhp-transfected neurons than in control neurons (Fig. S6). Altogether, these findings suggest that TBC1D24 is crucial for the morphofunctional maturation of developing glutamatergic neurons in a cell-autonomous manner.
Fig. 3.
TBC1D24 is required for dendritic arborization in vivo. (A) Representative coronal sections of P7 rat brains transfected with the indicated constructs by IUE at E15. (Scale bar, 50 μm.) (B) Representative reconstructed layers II/III pyramidal neurons showing dendritic arborization patterns from the same experimental conditions shown in A. (C) Quantitative analysis (means ± SEM) of total dendritic length revealed that TBC1D24 knockdown significantly impairs neuronal morphological maturation affecting both apical and basal dendrites (n = 130–140 in each group). Two-tailed t test: ***P < 0.0005 and *P < 0.05 vs. 3′UTRm4hp; °°°P < 0.0005, °°P < 0.005, and °P < 0.05 vs. 3′UTRhp + TBC1D24wt.
Fig. 4.
TBC1D24 regulates neuronal morphogenesis and maturation in vitro. (A) Representative images of 7 DIV cultured cortical neurons transfected at the time of plating with the indicated plasmids. (Scale bar: 50 μm.) (B) Representative traces of 7 DIV cortical neurons treated as in A. (C) Histogram showing quantitative analysis of total neurite length (three independent preparations; 90–100 cells for each group). Data are means ± SEM and were analyzed by paired two-tailed t test. **P < 0.001 vs. 3′UTRm4hp; °P < 0.05 vs. 3′UTRhp + TBC1D24wt. (D, Left) Representative traces of mEPSCs recorded at −70mV in 18 DIV cortical neurons transfected at 13 DIV with the indicated constructs. (D, Right) Histograms showing average amplitude and frequency of mEPSCs. Data are expressed as means ± SEM from 30 to 35 neurons per experimental condition obtained from three independent preparations. Two-tailed t test: **P < 0.01 and ***P < 0.0005 vs. 3′UTRm4hp; °°P < 0.01 vs. 3′UTRhp + TBC1D24wt.
TBC1D24 Regulates Neuronal Migration and Maturation Through the ARF6 Pathway.
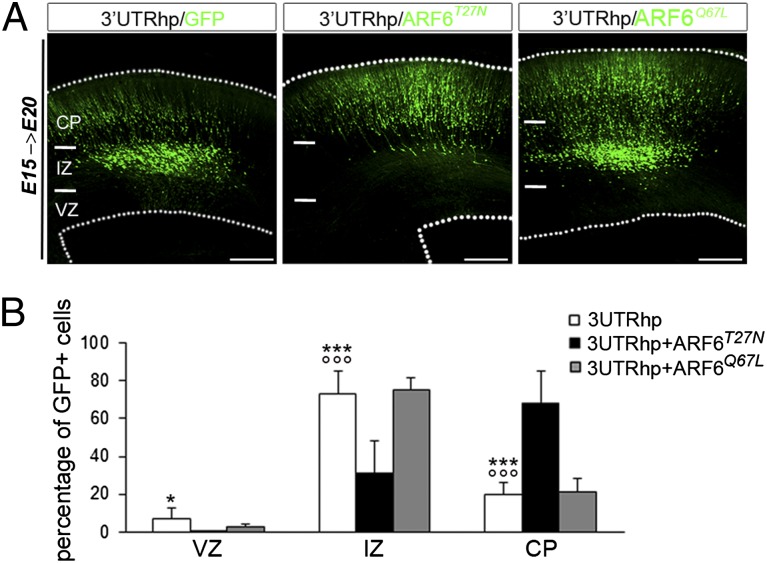
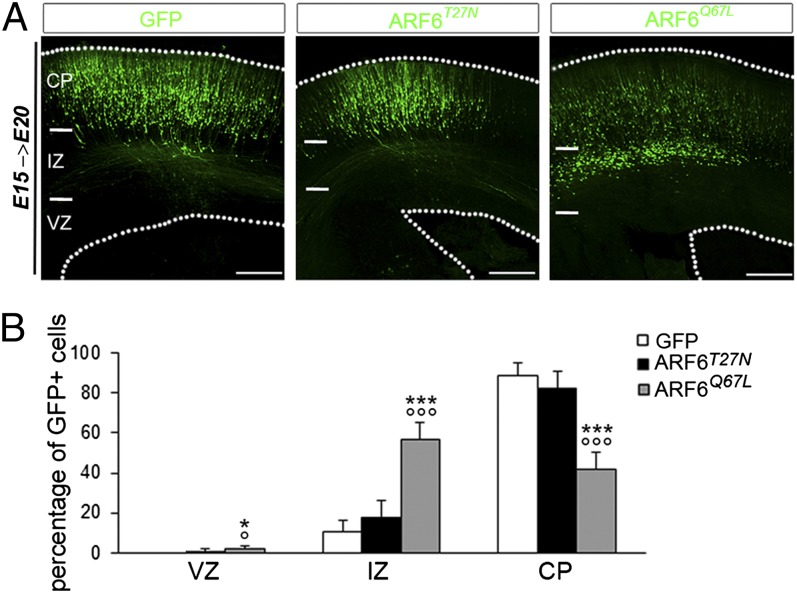
Most reported TBC1D24 pathogenic variants identified in patients are located in the ARF6-binding domain (7, 8, 10), suggesting that TBC1D24 function is dependent on its interaction with ARF6, a key regulator in cell motility. To investigate whether the TBC1D24-ARF6 association is critical for normal cortical development, we tested in vivo the ability of two distinct ARF6-binding defective mutants (TBC1D24F251L and TBC1D24D147H; Fig. S7 A–C) to rescue the radial migration defect caused by TBC1D24 knockdown. We electroporated the TBC1D24F251L or TBC1D24D147H constructs either alone or in combination with 3′UTRhp in E15.5 rat brains and analyzed the position of GFP-positive cells within the cortex 5 d later. We found that expression of either the TBC1D24F251L or the TBC1D24D147H constructs in 3′UTRhp-transfected neurons failed to rescue the migration delay (Fig. S7 D and E), whereas neural progenitors transfected with either mutant reached the CP as expected (Fig. S7F). These results support the hypothesis that TBC1D24 regulates neuronal migration through its interaction with ARF6, and so we would expect that ARF6 would rescue the TBC1D24-knockdown phenotype. Exogenous expression of wild-type ARF6 (in the absence of either GDP or GTP) has no discernible effects on cells in most cases (23). ARF6 exists in two forms in vivo: an inactive GDP-bound form and an active GTP-bound form (24). Hence, to investigate this issue, we used two constructs that mimic either the ARF6-GDP inactive form (ARF6T27N) or ARF6-GTP active form (ARFQ67L) (24). We found that overexpression of ARF6T27N rescues the effect of TBC1D24 knockdown on radial migration, whereas coexpression of ARF6Q67L and 3′UTRhp was not able to restore the migratory phenotype (Fig. 5 A and B). As previously reported, expression of dominant negative ARF6T27N resulted in enhanced axodendritic outgrowth in vitro (13, 14). This led us to test whether increased ARF6-GDP levels might also restore the dendritic outgrowth defects observed in TBC1D24-knockdown neurons at P7. Indeed, we found that in vivo overexpression of ARF6T27N restored both apical and basal dendrite development in TBC1D24-knockdown cerebral cortex (Fig. 6). Altogether, these findings suggest that TBC1D24 function relies on its ability to prevent ARF6 activation (ARF6-GTP) and that this regulation is critical in cortical development. In agreement with this hypothesis, we found that numerous cortical progenitor cells transfected at E15 with ARF6Q67L were located in the IZ at E20, phenocopying the migratory phenotype observed in TBC1D24-knockdown brains at the same developmental stage (Fig. 7). Furthermore, IZ-trapped ARF6Q67L-overexpressing neurons displayed a multipolar morphology, highly reminiscent of that observed in TBC1D24-silenced neurons (Fig. S8). In contrast, in age-matched brains, the majority of neurons expressing the constitutively inactive ARF6 form (ARF6T27N) reached the CP (Fig. 7). Moreover, biochemical investigations showed that TBC1D24 inhibits the formation of ARF6-GTP in HeLa cells (Fig. S9). Overall, these data indicate that TBC1D24 regulates ARF6 activity and suggest that ARF6-GTP levels could be a critical effector of TBC1D24 in cortical network formation in vivo.
Fig. 5.
TBC1D24 regulates neuronal migration through ARF6. (A) Representative coronal sections of E20 rat brains 5 d after electroporation with indicated plasmids. (Scale bar: 200 μm.) (B) Quantification of GFP-positive cell distribution in the cortex expressed as a percentage of total transfected cells (3′UTRhp, n = 12; 3′UTRhp + ARF6T27N, n = 7; 3′UTRhp + ARF6Q67L, n = 4). Data, expressed as means ± SEM, were compared using one-way ANOVA for repeated measure, followed by the Bonferroni’s multiple comparison test. ***P < 0.0001 and *P < 0.05 vs. 3′UTRhp + ARF6T27N; °°°P < 0.0001 vs. 3′UTRhp + ARF6Q67L.
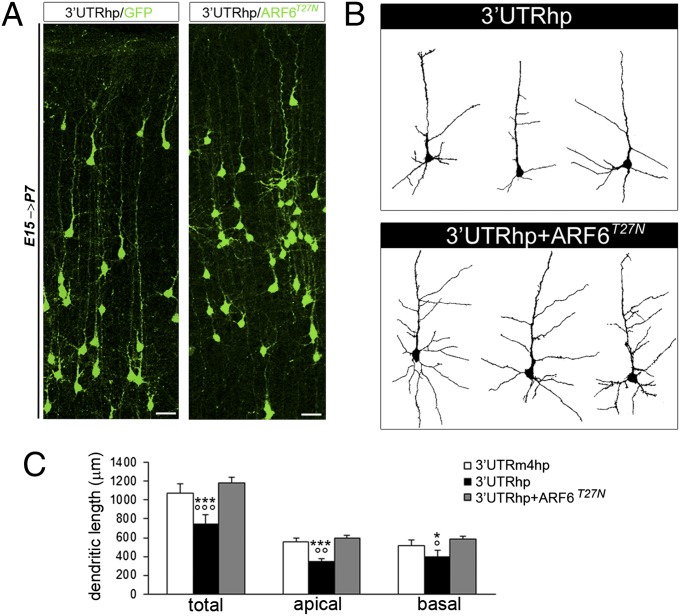
Fig. 6.
In vivo down-regulation of ARF6 restores dendritic outgrowth in TBC1D24-knockdown model. (A) Representative images of coronal slices of P7 rat brains transfected with indicated constructs by IUE at E15. (Scale bar: 50 μm.) (B) Representative dendritic arborization patterns in cortical neurons from the same experimental conditions shown in A. (C) Concomitant expression of ARF6T27N in TBC1D24-knockdown neurons restores both apical and basal dendritic outgrowth. Data are expressed as means ± SEM and analyzed by two-tailed t test. ***P < 0.0001 and *P < 0.05 vs. 3′UTRm4hp; °°°P < 0.0001, °°P < 0.0005, and °P < 0.005 vs. 3′UTRhp + ARF6T27N (3′UTRm4hp, n = 130 from six rats; 3′UTRhp, n = 140 from six rats; 3′UTRhp + ARF6T27N, n = 81 from four rats).
Fig. 7.
Dysregulation of ARF6 activity impairs neuronal migration. (A) Representative coronal sections of E20 rat brains 5 d after electroporation with indicated plasmids. (Scale bar: 200 μm.) (B) Quantification of GFP-positive cell distribution in the cortex expressed as a percentage of the total (GFP, n = 10; ARF6T27N, n = 6; ARF6Q67L, n = 7). Data, expressed as means ± SEM, were compared via one-way ANOVA for repeated measure, followed by the Bonferroni’s multiple comparison test. ***P < 0.0001 and *P < 0.05 vs. GFP; °°°P < 0.0001 and °P < 0.05 vs. ARF6T27N.
Discussion
The formation of the six-layered cerebral cortex requires a complex spatiotemporal coordination of different cellular and intracellular processes, such as neuronal proliferation, migration, differentiation, and circuitry formation (25, 26). Newborn neurons must reach their final laminar positions at the established time to undergo morphofunctional changes enabling them to integrate intracortical inputs (27). The morphological transition from a multipolar to a bipolar shape represents a critical event allowing immature neurons to migrate from the VZ to the CP by glial-guided locomotion (18, 19, 28). Although this transitional phase requires a reorganization of the cytoskeleton, the molecular mechanism regulating this process has just begun to be dissected. Several microtubule regulators, such as LIS1, DCX, and CDK5, are essential for the exit of neurons from the multipolar stage (17, 22, 29), and accumulating evidence now indicates that membrane trafficking can also play a role in the morphological changes underlying radial migration of newborn neurons (30). Notably, Rab5- and Rab11-dependent endocytic pathways control multipolar transition and final CP positioning by regulating the surface expression of N-Cadherin in migrating neurons (30). Our findings also support the physiological importance of monomeric G proteins regulating endocytic pathways in neuronal migration. Indeed, using IUE, we demonstrate that TBC1D24 promotes the multipolar-to-bipolar transition occurring during glial-dependent migration in the rat neocortex through its functional interaction with ARF6. ARF6 is involved in membrane trafficking, actin remodeling, and plasma membrane endocytosis (12). In neurons, the ARF6 protein has been reported to act directly through PIP5KI (14) or indirectly through Rac1, a member of the Rho-GTPase family, to control dendritic dynamics and growth (13). PIP5KI catalyzes the formation of phosphatidylinositol 4,5-bisphosphate, which acts as a negative regulator of neurite elongation by promoting microtubule depolymerization (31). Rac1 plays a crucial role in several aspects of neural development including neuronal migration, axon formation, and neural progenitor proliferation and survival by coordinating both actin and microtubule remodeling (32, 33). However, although a direct role for ARF6 in neuronal migration and cortical layering has not yet been reported, we find that the fine-tuning of ARF6 activity by TBC1D24 is important for radial migration. Indeed, increasing levels of active GTP-bound ARF6 phenocopies the neuronal polarity and migration defects caused by in utero silencing of TBC1D24, whereas inactive GDP-bound ARF6 rescues the TBC1D24 knockdown-induced migration phenotype. These observations suggest that TBC1D24 regulates radial neuronal migration by maintaining ARF6 in the inactive GDP-bound state. In fact, a recent report showed that low ARF6-GTP levels are also necessary in the early stages of corticogenesis to maintain the apical localization of integrin β1 receptor in neuronal progenitors, a process crucial for apical adhesion and, therefore, for neuroepithelium integrity (15). Furthermore, the combination of TBC1D24 knockdown and overexpression of the constitutively active form of ARF6 displayed no synergistic effect on neuronal migration, leading us to hypothesize that TBC1D24 may act upstream to ARF6 pathway to control the turnover of adhesion molecules and/or the remodeling of the cytoskeleton.
Besides this important role in neuronal migration, our data strongly indicate that TBC1D24 also promotes cortical maturation. The transition of migrating neurons into a bipolar shape is emerging as a fundamental requirement for pyramidal neurons to obtain their correct morphology (18, 34). The inability to acquire a bipolar morphology during migration, resulting in abnormal dendrite development in TBC1D24-knockdown pyramidal neurons, provides additional evidence that supports this hypothesis. Moreover, we found that these defects in neuronal branching were not associated with abnormal positioning of TBC1D24-silenced neurons in the neocortex consistent with a cell-autonomous effect on dendrite development. Indeed, the migration delay induced by in utero knockdown of TBC1D24 is temporary and no longer visible in rat brains at P7. Interestingly, these morphological alterations are rescued by the in vivo expression of dominant negative ARF6, further supporting the idea that the modulation of ARF6 activity by TBC1D24 is critical for neurite outgrowth. We propose that the control of ARF6-GTP levels by TBC1D24 may be responsible for the precise developmental control of ARF6 downstream effectors in neurons. This developmental regulation could influence the synaptic integration and information processing of neurons in the cortex and, when absent, result in abnormal excitability and/or impaired cognitive function. Further studies will be necessary to investigate whether downstream effectors are involved in the molecular mechanism underlying TBC1D24-dependent ARF6 regulation.
In summary, we show that TBC1D24 regulates cortical development because its silencing in neural progenitors results in delays in radial migration and defective terminal branching of projection neurons. We also establish a functional link between TBC1D24 and the ARF6-dependent endocytic pathway and demonstrate that the modulation of ARF6 activity by TBC1D24 is critical for appropriate corticogenesis (Fig. S10). Altogether, these findings provide further evidence that membrane trafficking and intercellular signaling play an important role in cortical network formation. In this context, further studies on the molecular and cellular events mediated by TBC1D24 may provide insights into the pathogenesis of brain disorders linked to mutations of this gene.
Materials and Methods
Plasmids, antibodies, WB, and immunostaining procedures used in this study are described in the SI Materials and Methods.
Rats.
Breeding and experimental procedures were carried out in accordance with European and the local ethical committee guidelines for animal research. IUE and neuronal migration analysis were performed as described previously (35). For more details, see SI Materials and Methods.
Morphological Analysis.
Morphology of migrating neurons was analyzed on cortical slices at E20. Dendritic length analysis was performed after neuronal reconstructions with the ImageJ plug-in NeuronJ 1.2 software. See SI Materials and Methods for details.
Neuronal Cultures and Excitatory Synapse Analysis.
Rat primary cortical neurons were prepared as described (36). Neurons were transfected at the time of plating by Nucleofection (Amaxa) and for later transfection using Lipofectamine 2000 (Life Technologies). To measure excitatory synapses, neurons transfected at 13 DIV and fixed 48–72 h posttransfection were labeled for VGLUT1. See SI Materials and Methods for details
Electrophysiological Recordings.
mEPSC recordings were performed on 17–18 DIV neurons. Details are provided in SI Materials and Methods.
Statistical Analysis.
Data are given as means ± SEM for n sample size. The normal distribution of experimental data were assessed using D’Agostino–Pearson’s normality test. To compare two normally distributed sample groups, the two-tailed Student’s t test was used. To compare more than two normally distributed sample groups, one-way ANOVA, followed by the Bonferroni’s post hoc test, was used. When more than one parameter was analyzed per group, ANOVA for repeated measures was performed. Analysis was carried out by using SPSS software.
Supplementary Material
Acknowledgments
We thank Prof. K. Dudley, Drs. S. Muldoon, and J. B. Manent for critical reading of our manuscript and helpful discussions. We thank Dr. S. Casagrande, Dr. F. Michel, and F. Schaller for technical help. This work was supported by the Italian Ministry of Health Progetto Giovani (A. Fassio and F.Z.); Fondazione Mariani (A. Fassio); Institut National de la Santé et de la Recherche Médicale (E.B., F.W., A.R., C.C., and E.P.-P.); European Union Sixth Framework Thematic Priority Life Sciences, Genomics and Biotechnology for Health EPICURE (Functional Genomics and Neurobiology of Epilepsy: A Basis for New Therapeutic Strategies) project Contract 2006-037315 (to A. Falace, F.Z., C.C., E.P.-P., and A.R.); and Else Kröner-Fresenius Foundation Project 2010_A145 (to C.C. and E.P.-P.). A. Falace was supported by Consorzio Italiano Biotecnologie, a European Molecular Biology Organization short-term fellowship, and a Boehringer travel grant.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316294111/-/DCSupplemental.
References
- 1.McConnell SK. Strategies for the generation of neuronal diversity in the developing central nervous system. J Neurosci. 1995;15(11):6987–6998. doi: 10.1523/JNEUROSCI.15-11-06987.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rakic P. Evolution of the neocortex: A perspective from developmental biology. Nat Rev Neurosci. 2009;10(10):724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayala R, Shu T, Tsai LH. Trekking across the brain: The journey of neuronal migration. Cell. 2007;128(1):29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 5.Rakic P, Ayoub AE, Breunig JJ, Dominguez MH. Decision by division: Making cortical maps. Trends Neurosci. 2009;32(5):291–301. doi: 10.1016/j.tins.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermann BP, et al. Growing up with epilepsy: A two-year investigation of cognitive development in children with new onset epilepsy. Epilepsia. 2008;49(11):1847–1858. doi: 10.1111/j.1528-1167.2008.01735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbett MA, et al. A focal epilepsy and intellectual disability syndrome is due to a mutation in TBC1D24. Am J Hum Genet. 2010;87(3):371–375. doi: 10.1016/j.ajhg.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falace A, et al. TBC1D24, an ARF6-interacting protein, is mutated in familial infantile myoclonic epilepsy. Am J Hum Genet. 2010;87(3):365–370. doi: 10.1016/j.ajhg.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guven A, Tolun A. TBC1D24 truncating mutation resulting in severe neurodegeneration. J Med Genet. 2013;50(3):199–202. doi: 10.1136/jmedgenet-2012-101313. [DOI] [PubMed] [Google Scholar]
- 10.Milh M, et al. Novel compound heterozygous mutations in TBC1D24 cause familial malignant migrating partial seizures of infancy. Hum Mutat. 2013;34(6):869–872. doi: 10.1002/humu.22318. [DOI] [PubMed] [Google Scholar]
- 11.Campeau PM, et al. The genetic basis of DOORS syndrome: An exome-sequencing study. Lancet Neurol. 2014;13(1):44–58. doi: 10.1016/S1474-4422(13)70265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Souza-Schorey C, Chavrier P. ARF proteins: Roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7(5):347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 13.Hernández-Deviez DJ, Casanova JE, Wilson JM. Regulation of dendritic development by the ARF exchange factor ARNO. Nat Neurosci. 2002;5(7):623–624. doi: 10.1038/nn865. [DOI] [PubMed] [Google Scholar]
- 14.Hernández-Deviez DJ, Roth MG, Casanova JE, Wilson JM. ARNO and ARF6 regulate axonal elongation and branching through downstream activation of phosphatidylinositol 4-phosphate 5-kinase alpha. Mol Biol Cell. 2004;15(1):111–120. doi: 10.1091/mbc.E03-06-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arvanitis DN, et al. Ephrin B1 maintains apical adhesion of neural progenitors. Development. 2013;140(10):2082–2092. doi: 10.1242/dev.088203. [DOI] [PubMed] [Google Scholar]
- 16.Afawi Z, et al. TBC1D24 mutation associated with focal epilepsy, cognitive impairment and a distinctive cerebro-cerebellar malformation. Epilepsy Res. 2013;105(1-2):240–244. doi: 10.1016/j.eplepsyres.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Bai J, et al. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;6(12):1277–1283. doi: 10.1038/nn1153. [DOI] [PubMed] [Google Scholar]
- 18.Noctor SC, Martínez-Cerdeño V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7(2):136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 19.LoTurco JJ, Bai J. The multipolar stage and disruptions in neuronal migration. Trends Neurosci. 2006;29(7):407–413. doi: 10.1016/j.tins.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145(1):61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- 21.Bellenchi GC, et al. N-cofilin is associated with neuronal migration disorders and cell cycle control in the cerebral cortex. Genes Dev. 2007;21(18):2347–2357. doi: 10.1101/gad.434307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohshima T, et al. Cdk5 is required for multipolar-to-bipolar transition during radial neuronal migration and proper dendrite development of pyramidal neurons in the cerebral cortex. Development. 2007;134(12):2273–2282. doi: 10.1242/dev.02854. [DOI] [PubMed] [Google Scholar]
- 23.Song J, Khachikian Z, Radhakrishna H, Donaldson JG. Localization of endogenous ARF6 to sites of cortical actin rearrangement and involvement of ARF6 in cell spreading. J Cell Sci. 1998;111(Pt 15):2257–2267. doi: 10.1242/jcs.111.15.2257. [DOI] [PubMed] [Google Scholar]
- 24.Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139(1):49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6(10):777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 26.Clowry G, Molnár Z, Rakic P. Renewed focus on the developing human neocortex. J Anat. 2010;217(4):276–288. doi: 10.1111/j.1469-7580.2010.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4(2):143–150. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- 28.Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27(7):392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Tsai JW, Chen Y, Kriegstein AR, Vallee RB. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J Cell Biol. 2005;170(6):935–945. doi: 10.1083/jcb.200505166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawauchi T, et al. Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. Neuron. 2010;67(4):588–602. doi: 10.1016/j.neuron.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Noda Y, et al. Phosphatidylinositol 4-phosphate 5-kinase alpha (PIPKα) regulates neuronal microtubule depolymerase kinesin, KIF2A and suppresses elongation of axon branches. Proc Natl Acad Sci USA. 2012;109(5):1725–1730. doi: 10.1073/pnas.1107808109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawauchi T, Chihama K, Nabeshima Y, Hoshino M. The in vivo roles of STEF/Tiam1, Rac1 and JNK in cortical neuronal migration. EMBO J. 2003;22(16):4190–4201. doi: 10.1093/emboj/cdg413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Curtis I. Functions of Rac GTPases during neuronal development. Dev Neurosci. 2008;30(1-3):47–58. doi: 10.1159/000109851. [DOI] [PubMed] [Google Scholar]
- 34.Hatanaka Y, Murakami F. In vitro analysis of the origin, migratory behavior, and maturation of cortical pyramidal cells. J Comp Neurol. 2002;454(1):1–14. doi: 10.1002/cne.10421. [DOI] [PubMed] [Google Scholar]
- 35.Carabalona A, et al. A glial origin for periventricular nodular heterotopia caused by impaired expression of Filamin-A. Hum Mol Genet. 2012;21(5):1004–1017. doi: 10.1093/hmg/ddr531. [DOI] [PubMed] [Google Scholar]
- 36.Banker G, Goslin K. Culturing Nerve Cells. Cambridge, Mass: MIT Press; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.