Abstract
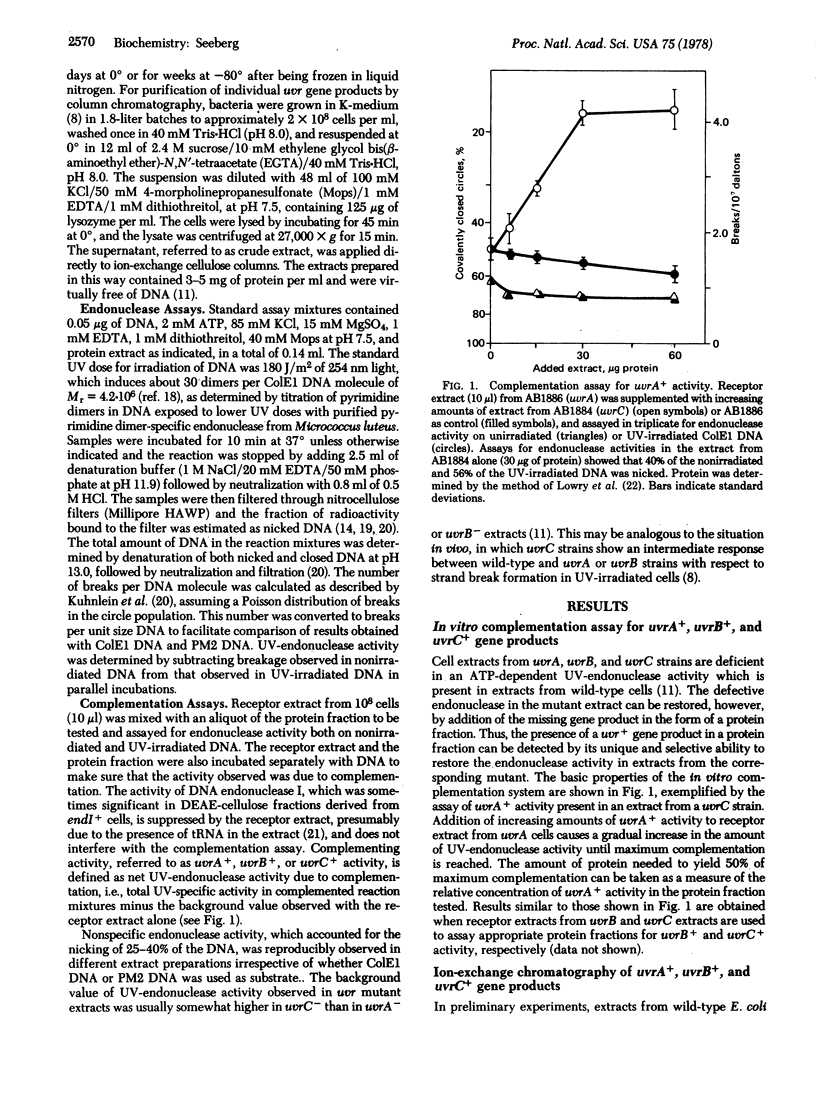
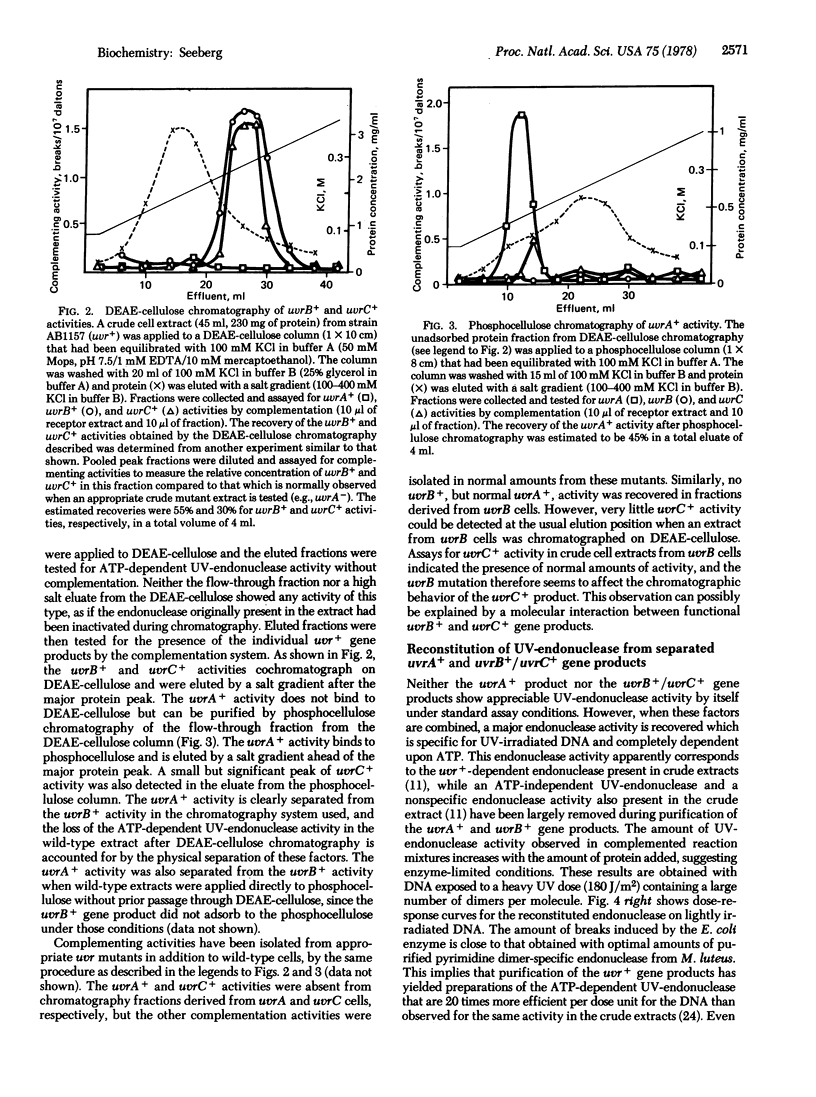
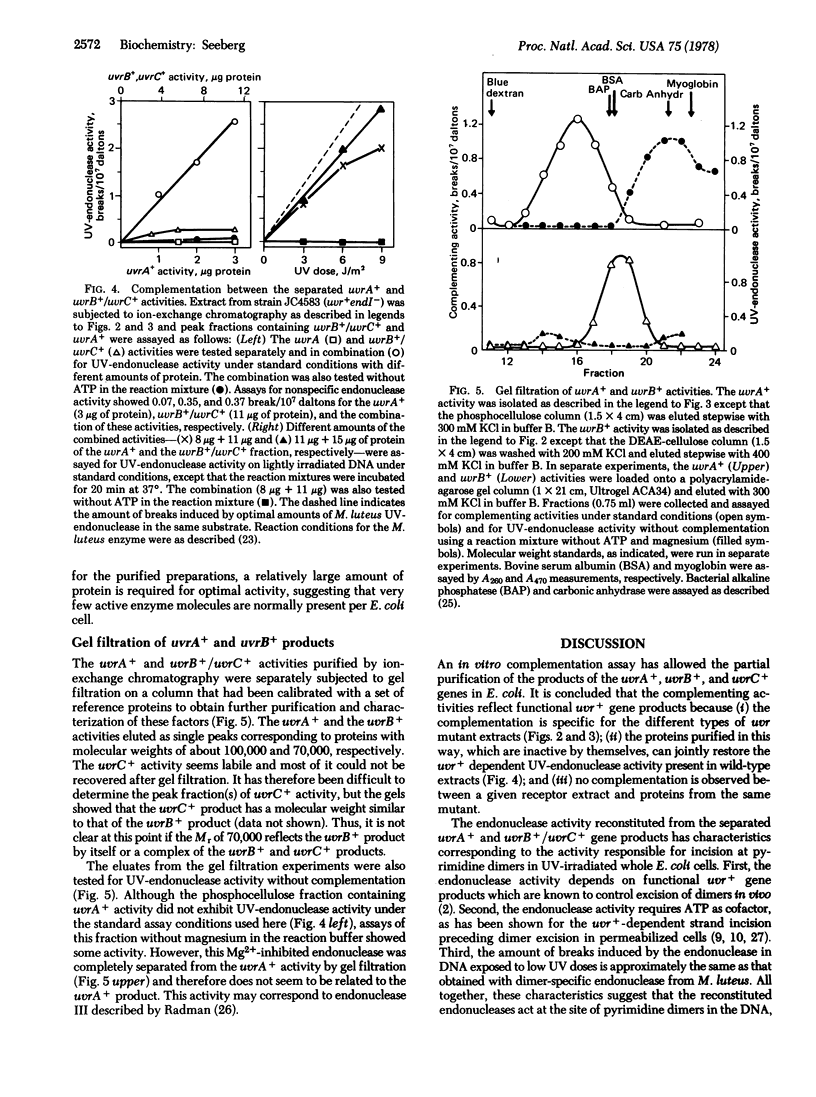
An in vitro complementation assay has been used for partial purification of uvrA+, uvrB+, and uvrC+ gene products from Escherichia coli. The uvrB+ and uvrC+ products cochromatograph on DEAE-cellulose and are completely resolved from the uvrA+ product, which has been further purified by phosphocellulose chromatography of the nonadsorbed protein fraction from the DEAE-cellulose. Neither the uvrB+/uvrC+ nor the uvrA+ product shows appreciable endonuclease activity on UV-irradiated DNA when tested separately. However, these factors complement each other to yield and ATP-dependent endonuclease activity specific for UV-irradiated DNA. Gel filtration experiments with the partially purified proteins indicate that the functional uvrA+ gene product has a molecular weight of 100,000. The uvrB+ gene product has an apparent molecular weight of 70,000, but it is presently unclear if this is the size of the uvrB+ product alone or the size of a complex of the uvrB+ and uvrC+ gene products.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Monem M., Dürwald H., Hoffmann-Berling H. Enzymic unwinding of DNA. 2. Chain separation by an ATP-dependent DNA unwinding enzyme. Eur J Biochem. 1976 Jun 1;65(2):441–449. doi: 10.1111/j.1432-1033.1976.tb10359.x. [DOI] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D. G., Sherratt D. J., Clewell D. B., Helinski D. R. Isolation of supercoiled colicinogenic factor E 1 DNA sensitive to ribonuclease and alkali. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2518–2522. doi: 10.1073/pnas.69.9.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A. G., Radman M., Grossman L. Enzymatic repair of DNA: SITES OF HYDROLYSIS BY THE Escherichia coli endonuclease specific for pyrimidine dimers (correndonuclease II). Biochemistry. 1976 Sep 7;15(18):4116–4120. doi: 10.1021/bi00663a031. [DOI] [PubMed] [Google Scholar]
- Braun A., Grossman L. An endonuclease from Escherichia coli that acts preferentially on UV-irradiated DNA and is absent from the uvrA and uvrB mutants. Proc Natl Acad Sci U S A. 1974 May;71(5):1838–1842. doi: 10.1073/pnas.71.5.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center M. S., Studier F. W., Richardson C. C. The structural gene for a T7 endonuclease essential for phage DNA synthesis. Proc Natl Acad Sci U S A. 1970 Jan;65(1):242–248. doi: 10.1073/pnas.65.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch W. A., Dorson J. W., Moses R. E. Excision of pyrimidine dimers in toluene-treated Escherichia coli. J Bacteriol. 1976 Jan;125(1):220–224. doi: 10.1128/jb.125.1.220-224.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Dark repair of ultraviolet-irradiated deoxyribonucleic acid by bacteriophage T4: purification and characterization of a dimer-specific phage-induced endonuclease. J Bacteriol. 1971 May;106(2):500–507. doi: 10.1128/jb.106.2.500-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman L., Braun A., Feldberg R., Mahler I. Enzymatic repair of DNA. Annu Rev Biochem. 1975;44:19–43. doi: 10.1146/annurev.bi.44.070175.000315. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnlein U., Penhoet E. E., Linn S. An altered apurinic DNA endonuclease activity in group A and group D xeroderma pigmentosum fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1169–1173. doi: 10.1073/pnas.73.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHMAN I. R., ROUSSOS G. G., PRATT E. A. The deoxyribo-nucleases of Escherichia coli. III. Studies on the nature of the inhibition of endonuclease by ribonucleic acid. J Biol Chem. 1962 Mar;237:829–833. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ljungquist S., Lindahl T. A mammalian endonuclease specific for apurinic sites in double-stranded deoxyribonucleic acid. I. Purification and general properties. J Biol Chem. 1974 Mar 10;249(5):1530–1535. [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman M. An endonuclease from Escherichia coli that introduces single polynucleotide chain scissions in ultraviolet-irradiated DNA. J Biol Chem. 1976 Mar 10;251(5):1438–1445. [PubMed] [Google Scholar]
- Riazuddin S., Grossman L. Micrococcus luteus correndonucleases. I. resolution and purification of two endonucleases specific for DNA containing pyrimidine dimers. J Biol Chem. 1977 Sep 25;252(18):6280–6286. [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R., Weiner J. H., Weiner A., Kornberg A. Ten proteins required for conversion of phiX174 single-stranded DNA to duplex form in vitro. Resolution and reconstitution. J Biol Chem. 1975 Aug 10;250(15):5859–5865. [PubMed] [Google Scholar]
- Scott J. F., Eisenberg S., Bertsch L. L., Kornberg A. A mechanism of duplex DNA replication revealed by enzymatic studies of phage phi X174: catalytic strand separation in advance of replication. Proc Natl Acad Sci U S A. 1977 Jan;74(1):193–197. doi: 10.1073/pnas.74.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E., Johansen I. Incisions in ultraviolet irradiated circular bacteriophage lambda DNA molecules in excision proficient and deficient lysogens of E. coli. Mol Gen Genet. 1973;123(2):173–184. doi: 10.1007/BF00267333. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Nissen-Meyer J., Strike P. Incision of ultraviolet-irradiated DNA by extracts of E. coli requires three different gene products. Nature. 1976 Oct 7;263(5577):524–526. doi: 10.1038/263524a0. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Strike P. Excision repair of ultraviolet-irradiated deoxyribonucleic acid in plasmolyzed cells of Escherichia coli. J Bacteriol. 1976 Mar;125(3):787–795. doi: 10.1128/jb.125.3.787-795.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Ogawa H., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. II. Breakage and repair of ultraviolet irradiated intracellular DNA of phage lambda. Mol Gen Genet. 1968 May 3;101(3):245–256. doi: 10.1007/BF00271626. [DOI] [PubMed] [Google Scholar]
- Tomizawa J. I., Sakakibara Y., Kakefuda T. Replication of colicin E1 plasmid DNA added to cell extracts. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1050–1054. doi: 10.1073/pnas.72.3.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstein E. A., Sharon R., Ben-Ishai R. Role of ATP in excision repair of ultraviolet radiation damage in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2651–2654. doi: 10.1073/pnas.71.7.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Hurwitz J. DNA replication in Escherichia coli made permeable by treatment with high sucrose. Biochem Biophys Res Commun. 1972 Apr 14;47(1):202–211. doi: 10.1016/s0006-291x(72)80029-9. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Wright M., Wickner S., Hurwitz J. Conversion of phiX174 and fd single-stranded DNA to replicative forms in extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3233–3237. doi: 10.1073/pnas.69.11.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Interaction of Escherichia coli dnaB and dnaC(D) gene products in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):921–925. doi: 10.1073/pnas.72.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. Mechanism of repair of DNA in bacteriophage. II. Inability of ultraviolet-sensitive strains of bacteriophage in inducing an enzyme activity to excise pyrimidine dimers. J Mol Biol. 1970 Jan 28;47(2):243–255. doi: 10.1016/0022-2836(70)90343-8. [DOI] [PubMed] [Google Scholar]
- van de Putte P., van Sluis C. A., van Dillewijn J., Rörsch A. The location of genes controlling radiation sensitivity in Escherichia coli. Mutat Res. 1965 Apr;2(2):97–110. doi: 10.1016/0027-5107(65)90041-2. [DOI] [PubMed] [Google Scholar]



