Significance
Small-fiber neuropathy (SFN) is a disorder of peripheral nerves that affects millions of people around the world. Currently, there are no treatments for SFN, but target-derived neurotrophic factors have been considered potential therapeutics. Here, we show that topical application of XIB4035, a small molecule that enhances signaling by the glial-derived neurotrophic factor family of molecules, ameliorates SFN pathologies and symptoms in two mouse models. These findings imply that XIB4035 or related molecules could provide a new strategy for SFN treatment with potentially lower risk for side effects because of the topical delivery route and their limited signaling effects.
Keywords: topical drug, pain, thermonociception, sensory, trophic factor
Abstract
Small-fiber neuropathy (SFN) is a disorder of peripheral nerves commonly found in patients with diabetes mellitus, HIV infection, and those receiving chemotherapy. The complexity of disease etiology has led to a scarcity of effective treatments. Using two models of progressive SFN, we show that overexpression of glial cell line-derived neurotrophic factor (GDNF) in skin keratinocytes or topical application of XIB4035, a reported nonpeptidyl agonist of GDNF receptor α1 (GFRα1), are effective treatments for SFN. We also demonstrate that XIB4035 is not a GFRα1 agonist, but rather it enhances GFRα family receptor signaling in conjunction with ligand stimulation. Taken together, our results indicate that topical application of GFRα/RET receptor signaling modulators may be a unique therapy for SFN, and we have identified XIB4035 as a candidate therapeutic agent.
Small-fiber neuropathy (SFN), i.e., damage or dysfunction of small-diameter axons (C fibers) in peripheral nerves, is a prevalent neurological disorder that afflicts an estimated 1.5% of the world’s population and millions of people in the United States (1–3). SFN is associated with diabetes, HIV infection, and chemotherapy treatment, but many patients suffer from idiopathic SFN (3–5). SFN patients can exhibit a large variety of symptoms, including loss of sensation and chronic pain. Despite the pervasiveness of SFN, its etiology is poorly understood, resulting in a lack of disease-modifying treatments.
Initial stages of SFN commonly involve nerve terminal degeneration before sensory neuron death (6), and reduction in target-derived trophic factor expression has been observed in multiple models of peripheral neuropathy (7, 8). Therefore, replenishing or replacing neurotrophic factors promptly after disease onset could potentially be used as a treatment to promote the survival and restore the function of C-fiber neurons (9, 10). One group of trophic factors necessary for development and survival of C fibers is the glial cell line-derived neurotrophic factor (GDNF) family ligands (GFLs) (10, 11). Each family member interacts selectively with a different high affinity receptor: GDNF with GDNF receptor α1 (GFRα1), neurturin (NRTN) with GFRα2, artemin (ARTN) with GFRα3, and persephin (PSPN) with GFRα4, although GDNF and NRTN have the capacity to bind and activate GFRα2 and GFRα1, respectively (12). Each ligand/receptor pair forms a complex with the RET receptor tyrosine kinase, resulting in downstream effects that include tyrosine hydroxylase (TH) gene transcription (13, 14). GFLs not only play a pivotal role in sensory neuron development, but also appear to be beneficial in the context of peripheral nerve injury. For example, systemic, transgenic, or viral delivery of GFLs has been shown to attenuate neuropathic symptoms in mouse models of nerve injury (15–19). Thus, we hypothesized that topical delivery of GDNF receptor agonists to the skin would be an effective, noninvasive therapeutic approach for treating progressive SFN.
In the present study, we tested whether GDNF skin overexpression or topical application of XIB4035, a reported nonpeptidyl small-molecule agonist for GFRα1 (20), could be used to treat two mouse models of progressive SFN. We found that GDNF skin overexpression and topical XIB4035 are equally effective in treating SFN caused by dysfunction of nonmyelinating Schwann cells and that topical XIB4035 treatment also ameliorates SFN symptoms in a mouse model of diabetic SFN. Furthermore, we establish that XIB4035 is not a GDNF mimetic as previously reported, but rather a positive modulator that enhances ligand-induced GFL receptor function. Together, our results suggest that modulation of GFL signaling is an effective, disease-modifying treatment for SFN, and we identify XIB4035 as a potentially therapeutic molecule.
Results
Overexpression of GDNF by Keratinocytes Prevents Progressive SFN.
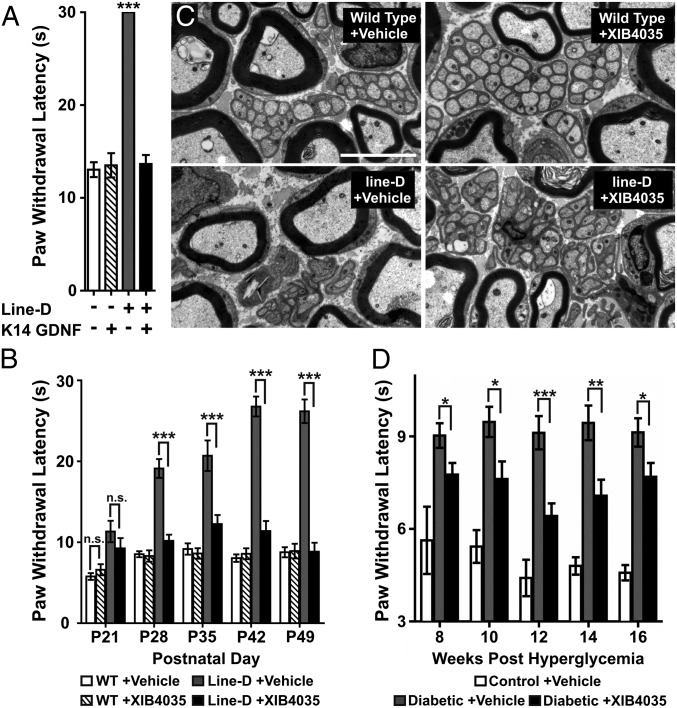
We previously characterized a transgenic mouse line (line D) expressing a dominant-negative ErbB4 receptor in nonmyelinating Schwann cells (NMSCs) (7). The truncated ErbB4 receptor forms nonfunctional dimers with wild-type ErbB2 and ErbB3 expressed by these glia, rendering them nonfunctional (7, 21). By the age of 21 d [postnatal day 21 (P21)] line-D mice present mild SFN symptoms, which worsen rapidly (7). The SFN in this model involves loss of thermal nociception, breakdown of Remak bundles (unmyelinated axons surrounded by a NMSC), and degeneration of C-fiber axons (7). Furthermore, analysis of the glabrous skin of the footpad using the panneuronal marker protein gene product 9.5 (PGP9.5) showed these mice also have progressive loss of intraepidermal nerve fiber (IENF) density (Fig. S1), a parameter considered a clinical indicator of neuropathy severity in humans (22) and in animal models of SFN (23). Interestingly, line-D SFN correlates with a significant reduction of GDNF protein in peripheral nerves (7). We hypothesized that the reduced levels of GDNF contributes to the pathogenesis of the SFN, and that increasing the availability of target (i.e., skin)-derived GDNF could modify the SFN in line-D mice. To test this hypothesis, we crossed line-D mice with a transgenic line that overexpresses GDNF in the skin under the control of the keratin 14 promoter (K14-GDNF) (11). Importantly, K14-GDNF mice have normal thermonociception despite having increased IENF density (11). Behavioral analysis demonstrated that, as shown previously (7), line-D mice exhibit a dramatic loss in thermal nociception at 6 wk of age (Fig. 1A). In contrast, thermonociception in double-transgenic mice (line-D::K14-GDNF) is indistinguishable from that in wild-type and K14-GDNF mice (Fig. 1A). Microscopy showed that line-D::K14-GDNF mice have normal sciatic nerve Remak bundles (Fig. S2A) and retain intraepidermal innervation (Fig. S2 B and C). IENF density in double-transgenic mice is lower than in K14-GDNF mice, but the magnitude of the reduction (40%) is much lower than in line-D mice compared with wild type (81%).
Fig. 1.
Overexpression of GDNF in the skin or topically applied XIB4035 curtails progression of SFN in two animal models. (A) Hot-plate (54 °C) test shows that the loss of thermal nociception in line-D mice is prevented by GDNF overexpression in keratinocytes (K14-GDNF). Only responses of line-D differ from those observed in the other three genotypes (one-way ANOVA with Newman–Keuls post hoc test, n ≥ 3; ***P < 0.001; error bars indicate SEM). (B) Hot-plate (54 °C) test shows that XIB4035 treatment starting at P21 prevents loss of thermal nociception in line-D mice. Wild-type (WT) and line-D mice were randomly divided into two groups at P21, a stage at which line-D mice had a small but significant increase in paw withdrawal latency compared with WT animals (Student t test, P = 0.0002). No difference existed within the groups of each genotype (Student t test, n ≥ 6; WT +vehicle vs. WT +XIB4035, P = 0.3067; line-D +vehicle vs. line-D +XIB4035, P = 0.285). Line-D mice treated with vehicle had progressively longer withdrawal latencies over the duration of the treatment. In contrast, WT mice treated with either vehicle or XIB4035, and XIB4035-treated line-D mice had similar latencies throughout the duration of the treatment. For P28, P35, P42, and P49: one-way ANOVA with Newman–Keuls post hoc test, n ≥ 6; WT +vehicle vs. line-D +vehicle (P < 0.001), WT +vehicle vs. line-D +XIB4035, n.s., line-D +vehicle vs. line-D +XIB4035 (P < 0.001); error bars indicate SEM. (C) Electron micrographs of transverse sections from the sciatic nerve of line-D mice treated for 4 wk (P49) show that Remak bundle structure is lost in mutants treated with vehicle but preserved in line-D mice treated with XIB4035. Remak bundle structure in wild types is not changed by the treatment. (Scale bar: 4 μm.) (D) Modified Hargreaves test show that XIB4035 treatment improves thermal nociception in diabetic mice. STZ-injected mice were treated with either XIB4035-containing cream or vehicle from onset of hyperglycemia. Hindpaw thermal response latency was measured every other week from week 8 posthyperglycemia onward. At week 8, vehicle-treated diabetic (diabetic +vehicle) mice showed thermal hypoalgesia (P < 0.01 vs. control +vehicle by ANOVA with Newman–Keuls post hoc test) that was attenuated by XIB4035 treatment (*P < 0.05 for diabetic +vehicle vs. diabetic +XIB4035; n ≥ 7; error bars indicate SEM) but not completely normalized (P < 0.05 for control +vehicle vs. diabetic +XIB4035). The attenuation of paw thermal hypoalgesia persisted throughout the study (*P < 0.05, **P < 0.01, ***P < 0.001, for diabetic +vehicle vs. diabetic +XIB4035 mice: one-way ANOVA with Newman–Keuls post hoc test; error bars indicate SEM).
Topical Application of XIB4035 Curtails Progression of SFN in Two Animal Models.
Transgenic GDNF overexpression served as a proof-of-concept that increasing skin GDNF levels could prevent progressive SFN in line-D mice. However, K14-GDNF mice overexpress GDNF during embryogenesis, altering sensory neuron development, which could contribute to the apparent therapeutic effects. Also, when considering clinical use, GDNF overexpression in the skin would require gene therapy, with its drawbacks and complications, and dermal GDNF application would not work, as peptides do not readily penetrate the skin. Therefore, we tested whether topical application of XIB4035, a nonpeptidyl small molecule reported to be an agonist for GFRα1 (20), could mimic the effects of GDNF overexpression in the skin. We applied a cream containing XIB4035 (1.5 mM) to the hindpaws of line-D and wild-type mice starting at weaning (P21), when mutant mice present with mild neuropathic symptoms (Fig. 1B) (7). For control, mice of both genotypes were also treated with the base cream containing no drug (vehicle). Thermonociception was tested using a hot plate before treatment and every 7 d thereafter. The behavior of wild-type mice remained normal, independent of treatment (Fig. 1B), whereas vehicle-treated line-D mice exhibited disease progression as reported for untreated line-D animals (7) (Fig. 1B). In contrast, reaction times of XIB4035-treated line-D mice were indistinguishable from wild types 1 wk after treatment began and for the duration of the experiment (Fig. 1B). Importantly, response thresholds to mechanical stimuli were normal in all groups after the treatment period, indicating XIB4035 treatment had no effect on mechanoreception. As with GDNF treatment, topical XIB4035 prevented IENF loss (Fig. S3) and degeneration of Remak bundles and C-fiber axons (Fig. 1C and Table 1). Thus, topical treatment with XIB4035 is as effective at preventing progressive SFN in line-D mice as GDNF skin overexpression.
Table 1.
Treatment with XIB4035 preserves Remak bundle structure in line-D mice
| C-fiber area† | C-fibers/Remak bundle‡ | |
| WT +vehicle | 1.78 ± 0.10 mm2 | 9.90 ± 0.66 |
| WT +XIB4035 | 1.81 ± 0.06 mm2 | 8.80 ± 0.52 |
| Line-D +vehicle | 1.01 ± 0.07 mm2* | 4.71 ± 0.42* |
| Line-D +XIB4035 | 1.64 ± 0.07 mm2 | 8.40 ± 0.83 |
Animals were treated with XIB4035 or vehicle from P21 to P49. Sciatic nerves were prepared for EM, and the cross-sectional area of individual unmyelinated axons and the number of axons pre-Remak bundles were quantified.
P < 0.001; ANOVA Newman–Keuls post hoc.
n = 3 animals per genotype; ≥50 axons per animal.
n = 3 animals per genotype; ≥16 bundles per animal.
To explore the utility of XIB4035 in a more clinically relevant model, we focused on diabetic peripheral neuropathy, as more than 50% of diabetic patients develop some form of peripheral neuropathy, particularly SFN (www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001713/). We chose the streptozotocin (STZ)-induced diabetes model, in which STZ-induced pancreatic beta cell death causes diabetes (24) and produces SFN symptoms, including loss of thermal sensation (23). We initiated treatment early in the disease, i.e., after mice became hyperglycemic (>15 mmol/L) and continued it for 16 wk. Behavioral testing was initiated at week 8, a point at which diabetic mice showed significant hypoalgesia compared with normoglycemic mice (Fig. 1D). Remarkably, XIB4035-treated diabetic mice had better thermonociception than diabetic mice exposed to vehicle, and this improvement persisted throughout the experiment (Fig. 1D). Interestingly, diabetes induced a significant reduction in IENF density, which was not improved by XIB4035 treatment (Fig. S4), indicating that the behavioral improvement was not due to an effect on IENFs.
XIB4035 Improves Sensory Function in Animals with Advanced Neuropathy.
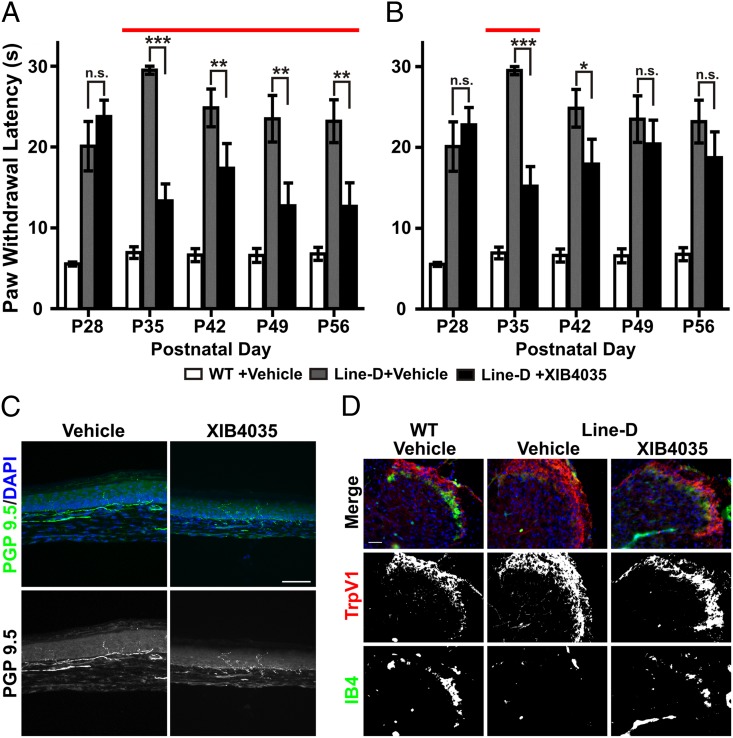
Our results demonstrated that topically applied XIB4035 early in disease progression eliminates or attenuates SFN symptoms in the line-D and diabetic models, respectively. However, many patients may begin treatment when they already present with advanced SFN symptoms. Therefore, we tested whether XIB4035 can act on line-D mice when treatment begins after animals have a more advanced SFN symptomatology (P28). Remarkably, neuropathic animals showed a significant reduction in symptoms 1 wk after treatment initiation (P35), and the improvement persisted as long as treatment was continued (Fig. 2A). However, if treatment was interrupted at P35, neuropathic symptoms reappeared 2 wk later and persisted for the duration of the experiment (Fig. 2B), indicating that chronic XIB4035 treatment is necessary to maintain sensory recovery.
Fig. 2.
XIB4035 treats line-D mice with advanced SFN symptoms. (A) Hot-plate (54 °C) test shows XIB4035 improves the SFN symptoms of line-D mice when treatment is initiated after severe symptoms are apparent (P28). Mutant mice showed significant improvement in thermal nociception after 1 wk of treatment (P35), and this was maintained as long as the treatment continued [red line indicates treatment duration; one-way ANOVA Newman–Keuls post hoc test, n ≥ 7; line-D +vehicle vs. line-D +XIB4035; n.s., not statistically significant; **P < 0.01, ***P < 0.001; WT +vehicle vs. line-D +vehicle, P < 0.001, WT +vehicle vs. line-D +XIB4035, P < 0.001 (P28, P35, P42), P < 0.01 (P49), P > 0.05 (P56); error bars indicate SEM]. (B) Chronic XIB4035 treatment is necessary to maintain gains. If mice were treated beginning at P28 (as in A), but treatment was interrupted at P35, neuropathic symptoms returned [red line indicates treatment duration; one-way ANOVA Newman–Keuls post hoc test, n ≥ 7; line-D +vehicle vs. line-D +XIB4035; n.s., not statistically significant; *P < 0.05, ***P < 0.001; WT +vehicle vs. line-D +vehicle, P < 0.001; WT +vehicle vs. line-D +XIB4035, P < 0.001 (P28, P35, P42, and P49), P < 0.01 (P56); error bars indicate SEM]. (C) If XIB4035 treatment is initiated at P28, the sensory recovery at P35 is not accompanied by recovery of IENF density. Representative images of PGP9.5-positive IENF staining at P35 in line-D hindpaw skin. (Scale bar: 100 μm.) (D) XIB4035 treatment initiated at P28 results in improved IB4 staining in the dorsal spinal cord. (Left) Representative images of spinal cord sections from wild-type mice treated with vehicle from P28 to P35 show the normal appearance of TrpV1+ (red) and IB4+ (green) nerve terminals. (Center) Images of line-D mice treated with vehicle show complete absence of IB4 staining. (Right) Images from line-D mice treated with XIB4035-containing cream show clear presence of IB4+ fibers. No obvious difference in TrpV1 labeling was observed between wild-type and mutant animals regardless of treatment. (Scale bar: 50 μm.)
Given the difference in the IENF density results between XIB4035-treated line-D and diabetic mice, we felt it necessary to explore the anatomical basis for behavioral recovery in advanced neuropathy in more than one way. Therefore, we examined the structure of both peripheral and central C-fiber axons. These neurons have their sensory terminals in the skin and project axons into laminae I and II of the spinal cord. XIB4035 had no effect on IENF density by P35 if treatment of line-D mice was initiated at P28 (Fig. 2C; vehicle: 1.7 ± 0.5 IENF/100 µm; XIB4035: 1.8 ± 0.3 IENF/100 µm), similar to the IENF results from the diabetic mice. In contrast, XIB4035 had a positive effect on the central projections of some C fibers. To visualize central projections of peptidergic C fibers, considered the principal heat nociceptive cells, we stained for vanilloid receptor 1 (TrpV1) (25, 26). For lamina II terminals, we used isolectin-B4 (IB4) labeling (10), a marker for nonpeptidergic fibers, which is down-regulated after injury (27). The IB4+ group of fibers includes those from GFRα2+ neurons that respond to nociceptive thermal stimuli (28–30). We found no discernible change in TrpV1+ lamina I terminals between wild-type and line-D mice (Fig. 2D), which was not unexpected as we previously showed that, at this age, line-D mice have no loss of dorsal root ganglion (DRG) peptidergic neurons (7). In contrast, IB4 labeling was absent in mutant mice treated with vehicle, but was clearly present in XIB4035-treated mutant mice, albeit at lower levels than wild types [Fig. 2D; 83% of XIB4035-treated mice (n = 6) with IB4+ lamina II terminals vs. 0% (n = 4) in vehicle-treated mice]. These data demonstrate that XIB4035 is an effective therapy for SFN and suggest functional recovery involves the improved health and structure of nonpeptidergic C-fiber central projections.
Finally, given the potential need for chronic application of XIB4035 for treatment, we examined whether XIB4035 produces side effects in mutant and wild-type mice. XIB4035 did not induce thermal hyperalgesia in wild types (Fig. S5), and we did not detect any changes in appearance, or other outward negative signs in animals treated twice a day for 7 wk. Together, these results demonstrate that topical XIB4035 can be used to treat SFN arising from diverse neurological insults without any overt side effects.
Mechanism of XIB4035 Action: It Is Not an Agonist for GFRα/RET Receptors.
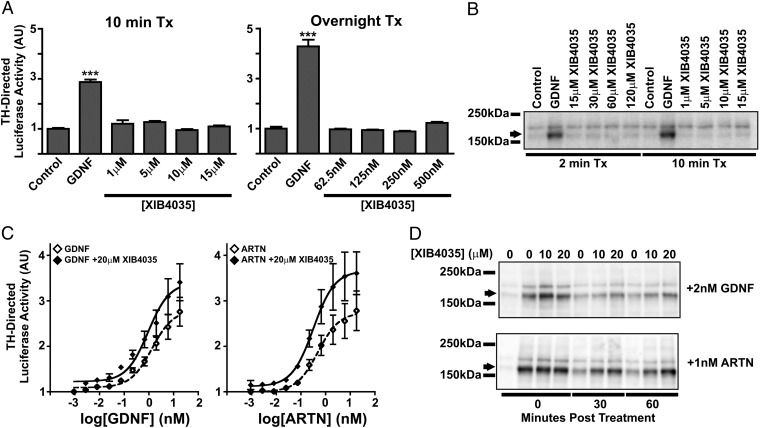
Tokugawa et al. (20) reported XIB4035 as a competitive agonist for the GDNF receptor GFRα1 in the Neuro2A (N2A) murine neuroblastoma cell line. Considering the ultimate goal is to use XIB4035 for treating SFN in humans but all data regarding XIB4035 have been obtained using murine models and cells, we measured the effects of XIB4035 on GFRα/RET signaling in the human neuroblastoma cell line SH-SY5Y. We performed two types of assays; immunoblots measuring RET phosphorylation and a reporter assay using the TH promoter to drive luciferase expression. For the latter, we used two paradigms, either overnight treatment immediately followed by luciferase activity measurements or 10-min treatments followed by washout and overnight incubation before measurements. As positive controls, we tested GDNF, NRTN, and ARTN, which induced TH-luciferase activity in a dose-dependent manner (Fig. S6). Contrary to expectations of a GFRα1 agonist, XIB4035 had no effect in the TH-luciferase assay in either the overnight (6.25–500 nM) or 10 min (1–15 µM) treatment paradigms (Fig. 3A) and failed to induce RET phosphorylation in these cells (Fig. 3B).
Fig. 3.
XIB4035 potentiates ligand-induced RET signaling but does not act as a GFRα/RET receptor agonist. (A) XIB4035 does not activate a TH-Luciferase reporter in SH-SY5Y cells. SH-SY5Y cells transfected with the reporter construct were treated with either 2 nM GDNF or increasing concentrations of XIB4035 either for 10 min and then incubated overnight in growth media or exposed to treatments overnight. Measurements of luciferase activity after the treatments show that GDNF treatment increases TH promoter activity in both conditions, but XIB4035 has no effect (one-way ANOVA Newman–Keuls post hoc test, n = 3; vs. control, ***P < 0.001; error bars indicate SEM). (B) XIB4035 does not induce RET phosphorylation. SH-SY5Y cells were treated with either 2 nM GDNF or various concentrations of XIB4035 for 2 or 10 min, and cells were lysed immediately. Anti-phosphotyrosine Western blot analysis shows that RET phosphorylation (arrow) is induced by GDNF but not by XIB4035. (C) XIB4035 enhances GFL-induced RET signaling. SH-SY5Y-THpGL3 stable cells were treated with increasing concentrations of GDNF or ARTN with or without 20 µM XIB4035 for 10 min. Treatments were replaced with growth media, and cells were maintained overnight before being assayed for luciferase activity. For both ligands, XIB4035 cotreatment caused a shift in the nonlinear regression of the dose–response curve (F test: GDNF vs. GDNF + 20 µM XIB4035, P = 0.00006; ARTN vs. ARTN + 20 µM XIB4035, P = 0.000005), reduced minimal ligand dose necessary to induce luciferase activity above control [Student t test vs. control: GDNF = 75 pM (P = 0.0063), GDNF + 20 µM XIB4035 = 2.7 pM (P = 0.038), ARTN = 75 pM (P = 0.0271), ARTN + 20 µM XIB4035 = 2.7 pM (P = 0.0124)], and increased maximal effect (Student t test: fold over control: 18 nM GDNF = 2.76 ± 0.32 vs. 18 nM GDNF + 20 µM XIB4035 = 3.41 ± 0.41, P = 0.0189; 18 nM ARTN = 2.78 ± 0.44 vs. 18 nM ARTN + 20 µM XIB4035 = 3.61 ± 0.47, P = 0.0241). (D) XIB4035 prolongs GFL-induced RET phosphorylation. SH-SY5Y cells were treated with 2 nM GDNF or 1 nM ARTN with or without 10 or 20 µM XIB4035 for 10 min. Cell lysates were either collected immediately or treatment was washed out and replaced with growth media for 30 or 60 min. Cell lysates were subjected to phosphotyrosine Western blot. RET phosphorylation (arrow) in the GDNF- or ARTN-treated samples returns to baseline by 60 min but not with addition of XIB4035.
XIB4035 Is a Positive Modulator of Ligand-Induced GFRα/RET Signaling.
Because the original report argued that XIB4035 displaces GDNF binding to GFRα1-expressing cells (Fig. 2 in ref. 20), we anticipated that cotreatment would reduce the effects of GDNF or other GFLs on TH-luciferase activity and Ret phosphorylation. We tested this hypothesis on GDNF and ARTN, which induced strong responses in these cells (Fig. S6). Surprisingly, XIB4035 cotreatment significantly potentiated the effects of both ligands on TH-luciferase activity over a range of doses (Fig. 3C), resulting in a significant shift in the nonlinear regression of the dose–response curve, reduced minimal ligand dose necessary to induce luciferase activity above control, and increased maximal effect. Moreover, Western blot assays revealed that cotreatment with XIB4035 prolongs the GDNF-induced RET phosphorylation. In this experiment, SH-SY5Y cells were treated with GDNF with or without XIB4035 for 10 min and cell lysates were either collected immediately (0 min) or treatment was washed out and replaced with growth media for 30 or 60 min before lysates were collected. Both treatments produced equal levels of RET phosphorylation at 0 min, but p-RET signals were higher in the cotreated cells at 30 and 60 min posttreatment (Fig. 3D and Fig. S7). XIB4035 had the same effect on ARTN-induced RET phosphorylation (Fig. 3D).
Because SH-SY5Y cells express GFRα1, 2, and 3, and there is significant cross talk between the different GFLs and GFRα receptors, the experiments described above did not define the specific ligand–receptor pairs for which XIB4035 serve as a positive modulator. This is an important issue because different C-fiber neuron populations express different GFRα receptors (31, 32). Therefore, we tested cells expressing only one GFRα using either N2A cells (which express RET) transfected with GFRα1 or GFRα3 expression constructs, or B(E)2-C cells, which express only GFRα2 together with RET. First, to verify the lack of endogenous GFRα activity in the parental N2A line, we treated control transfected (mGFP) cells with either GDNF or ARTN, and demonstrated that neither ligand induced RET phosphorylation (Fig. S8A). In contrast, GDNF and ARTN induced RET phosphorylation when GFRα1 or GFRα3 receptors were expressed, respectively (Fig. S8A). We then treated the GFRα1- or GFRα3-transfected N2A cells with XIB4035 alone, the appropriate ligand for the expressed receptor alone, or XIB4035 and ligand in combination for 10 min, and collected lysates immediately after treatment or 60 min after washout. XIB4035 alone did not induce RET phosphorylation in either GFRα1- or GFRα3-transfected cells (Fig. S8 B and C), but RET phosphorylation was clearly prolonged at the 60-min time point when cotreated with XIB4035 and ligand compared with ligand alone (Fig. S8 B and C). Similar results were obtained with GFRα2 and NRTN using B(E)2-C cells (Fig. S8D). Importantly, XIB4035 did not influence the activity of another receptor tyrosine kinase pathway, NGF-induced activation of TrkA signaling in PC12 cells (Fig. S9), suggesting that XIB4035 is a specific signaling modulator of GDNF family ligands. Thus, the data indicate that XIB4035 is a specific, positive modulator of signaling by GFLs and their receptors, not an agonist for GFRα1 as previously reported (20).
Discussion
Delivery of neurotrophic factors has been considered a potential strategy for the treatment of a variety of neurological disorders, including neuropathic pain (33). GFLs exhibited great promise in animal models, but have yet to yield any approved human therapies (34). Two major barriers for moving these therapies to the clinic have been the delivery methods and the high systemic doses necessary for efficacy. Previous studies demonstrated effectiveness of neurotrophic factors delivered via systemic or intrathecal injection in animal models (35–37). Nevertheless, these delivery routes proved ineffective and/or caused severe side effects in human patients. For example, intracerebroventricular administration of GDNF to Parkinson disease patients resulted in weight loss, anorexia, and nausea with little clinical benefit (38), and trials examining the efficacy of NGF treatment in diabetic patients with peripheral neuropathy or patients with HIV neuropathy have shown some improvement in the patient’s perception of symptom severity, but side effects including myalgia, peripheral edema, and hyperalgesia were also observed (39–41). Our finding that topical application of XIB4035 mitigates symptomatic pathology in two models of progressive SFN, acting even after SFN symptoms are severe, indicates that local directed delivery of small molecules that enhance GFL/GFRα/RET signaling could be an effective treatment for SFN without the side effects associated with generalized delivery. Furthermore, given that XIB4035 shows effectiveness in two models of SFN with different pathogenic mechanisms, it may be useful across a broad spectrum of SFNs.
Using cell-based assays, we show that XIB4035 is not a GFRα1 agonist as previously reported (20), but functions by specifically augmenting ligand-stimulated GFRα/RET signaling. Furthermore, the remarkable similarity between the effects of topical XIB4035 treatment and GDNF skin overexpression on SFN pathologies indicates that XIB4035 acts as an enhancer of GFL/GFRα/RET signaling in vivo. These findings introduce a unique paradigm for therapeutic intervention for SFN, i.e., use of a topical receptor tyrosine kinase signaling modulator rather than systemic delivery of an agonist. This therapeutic approach has the potential to minimize the side effects observed with systemic neurotrophic factor delivery; i.e., by not being an agonist, the drug will not activate GFRα/RET signaling in cells that express the receptors but are not normally exposed to the ligand; rather it will only enhance signaling where all of the endogenous components (ligands and receptors) are present. These findings also raise the possibility that small-molecule positive modulators of other receptor tyrosine kinase signaling pathways could be used as therapeutics for other neurological disorders.
We found that XIB4035-induced improvement of thermal sensation in diabetic mice and line-D mice with advanced symptoms is not accompanied by recovery of IENF density, a common clinical indicator of neuropathy severity (22). However, the absence of a simple linear association between IENF density and thermal sensitivity has been noted previously (23, 42). This phenomenon may reflect the specificity of sensory neuron subpopulations for heat sensation and growth factors (43) and/or a step function between IENF loss and behavioral response to noxious thermal stimuli (44). We also did not detect differences in Trpv1+ peptidergic terminals between mutant and wild-type animals; however, we cannot rule out the possibility that TrpV1+ cell function is disrupted. In contrast, IB4 labeling of nonpeptidergic C-fiber projections was lost in line-D mice and partially rescued by XIB4035 treatment. Because line-D mice have no DRG loss at the age analyzed (7), the recovery of IB4 labeling is likely an indicator that XIB4035 treatment improves the health of nonpeptidergic neurons (27). Further studies will be necessary to define which neuronal populations are responsible for the observed behavioral recovery and the more detailed mechanisms by which it occurs. The finding that application of the therapeutic to the skin, where sensory nerve terminals are located, improves overall sensory neuron structure, including projections into the spinal cord, shows that, in this disorder, neurons improve with very limited exposure to a drug that enhances trophic support, rather than requiring systemic exposure, as it has been done previously. This approach could have implications for the treatment of other neurological disorders as well.
Finally, our results concerning the molecular mechanism of XIB4035 action differ from the only published study pertaining to this molecule and the GFRα/RET complex. We found that XIB4035 functions as a positive modulator of GFL signaling by prolonging ligand-induced RET receptor activation and enhancing downstream effects of this receptor, in both rodent and human cells. We propose that XIB4035 acts by enhancing signaling of endogenous GFLs, e.g., NRTN produced by basal keratinocytes (45). Taken together, our data suggest that small-molecule modulators of GFL receptors and their coreceptor, RET, can be used to treat SFNs of different origins, and that XIB4035 has potential as a therapeutic molecule for treating SFN.
Materials and Methods
Animals.
Transgenic mouse lines were used as previously described (7, 11). Paw withdrawal latency was measured using hot-plate analgesia meters: Columbus Instruments for Fig. 1 A and B; IITC for Fig. 2 A and B and Fig. S4. Mechanical sensitivity was tested by simulation of the plantar surface of the hindpaw with a series of von Frey filaments. The use of animals was approved by the Animal Care and Use Committee of Boston Children’s Hospital. Detailed methods are provided in SI Materials and Methods.
Diabetic Neuropathy.
Adult female mice were made diabetic and paw thermal response latency was measured using a modified Hargreaves test, as described (23). Detailed methods are provided in SI Materials and Methods.
Preparation and Use of XIB4035.
The cream containing XIB4035 (1.5 mM; ZereneX Molecular) consisted of N-methyl-pyrrolidone (6.25%), isopropyl myristate (6.25%), and petroleum jelly (87.5%) (vol/vol). Control cream had the same ingredients without XIB4035. Cream was applied twice daily to the hindpaws of mice. The molecular structure of the compound was confirmed by NMR spectroscopy and mass spectrometry.
Immunohistochemistry.
Footpad skin was dissected from hindpaws and stained as floating sections with PGP9.5 rabbit polyclonal antibody (Ultraclone; 1:1,000). Spinal cord sections were stained, mounted on slides, and incubated with TrpV1 rabbit polyclonal antibody (Abcam; 1:2,000) and Alexa 488-conjugated isolectin-B4. Nuclei were stained with DAPI. Detailed methods are provided in SI Materials and Methods.
Immunohistochemistry Analysis.
Skin section images were acquired as 30-µm Z stacks and processed as maximum-intensity projections using a Zeiss LSM 700 microscope and ZEN software. Spinal cord images were acquired using a Zeiss Axioscope microscope. Detailed methods are provided in SI Materials and Methods.
Promoter Cloning and Stable Cell Generation.
The TH promoter was cloned into the pGL3 basic vector (Promega), as previously described (14). Detailed methods are provided in SI Materials and Methods.
Cell Assays.
For luciferase assays, SH-SY5Y-THpGL3 cells were treated for indicated times and assayed for firefly luciferase 16–24 h later (Promega). For phosphorylated Ret immunoblot experiments, cells were treated as indicated and lysates were subjected to Western blot analysis using anti-phosphotyrosine (4G10) mouse monoclonal antibody (EMD Millipore; 1:4,000). N2A and PC-12 cells were starved in basal media containing 1% FBS overnight before treatments. GDNF, NRTN, and ARTN were purchased from PeproTech. β-NGF (R&D Systems, Inc.) at 50 µg/mL was used to stimulate TrkA receptor phosphorylation in PC-12 cells. Detailed methods are provided in SI Materials and Methods.
Statistical Analyses.
All statistical analyses were performed using Prism 4 (GraphPad Software). SEM was used to indicate error in all analyses as n ≥ 3. For Fig. 3C, comparison of the nonlinear curve regression was performed using an F test. Analysis of the minimal ligand dose necessary to induce luciferase activity (Fig. 3C) was performed by Student t test comparing the average fold luciferase induction from three experiments to control (nontreated).
Supplementary Material
Acknowledgments
We thank Dr. Larry Brown for his advice on cream preparation, Dr. Qiufu Ma for advice on immunostaining, Dr. Jefferey Milbrandt for the GFRα constructs, and Dr. Marcin Liana for assistance with electron microscopy. We also thank John McInnis, Katie Frizzi, and Alison Quach for excellent technical support. This research was supported in part by National Institute of Neurological Disorders and Stroke (NINDS) Grant R01 NS35884 (to G.C.), Development Disability Research Center National Institutes of Health Grant P30-HD 18655 (to G.C.), Boston Children's Hospital Development Fund (to G.C.), National Multiple Sclerosis Society Postdoctoral Fellowship (to J.C.M.), Developmental Neurology Training Grant T32NS007473 (to K.L.H.), NINDS Grant R01 NS033730 (to K.A.), and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK057629 (to N.A.C.).
Footnotes
Conflict of interest statement: G.C. and J.C.M. are inventors on patent US7863295B2 associated with this work and owned by Children's Medical Center Corporation.
*This Direct Submission article had a prearranged editor.
See Commentary on page 2060.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308889111/-/DCSupplemental.
References
- 1.Hoitsma E, et al. Small fiber neuropathy: A common and important clinical disorder. J Neurol Sci. 2004;227(1):119–130. doi: 10.1016/j.jns.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Holland NR, et al. Small-fiber sensory neuropathies: Clinical course and neuropathology of idiopathic cases. Ann Neurol. 1998;44(1):47–59. doi: 10.1002/ana.410440111. [DOI] [PubMed] [Google Scholar]
- 3.Lacomis D. Small-fiber neuropathy. Muscle Nerve. 2002;26(2):173–188. doi: 10.1002/mus.10181. [DOI] [PubMed] [Google Scholar]
- 4.Devigili G, et al. The diagnostic criteria for small fibre neuropathy: From symptoms to neuropathology. Brain. 2008;131(Pt 7):1912–1925. doi: 10.1093/brain/awn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bednarik J, et al. Etiology of small-fiber neuropathy. J Peripher Nerv Syst. 2009;14(3):177–183. doi: 10.1111/j.1529-8027.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- 6.Cornblath DR, Hoke A. Recent advances in HIV neuropathy. Curr Opin Neurol. 2006;19(5):446–450. doi: 10.1097/01.wco.0000245366.59446.57. [DOI] [PubMed] [Google Scholar]
- 7.Chen S, et al. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat Neurosci. 2003;6(11):1186–1193. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- 8.Hellweg R, Raivich G, Hartung HD, Hock C, Kreutzberg GW. Axonal transport of endogenous nerve growth factor (NGF) and NGF receptor in experimental diabetic neuropathy. Exp Neurol. 1994;130(1):24–30. doi: 10.1006/exnr.1994.1181. [DOI] [PubMed] [Google Scholar]
- 9.Luo W, et al. A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron. 2007;54(5):739–754. doi: 10.1016/j.neuron.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 10.Molliver DC, et al. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19(4):849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 11.Zwick M, et al. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;22(10):4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Airaksinen MS, Saarma M. The GDNF family: Signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3(5):383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 13.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 14.Xiao H, Hirata Y, Isobe K, Kiuchi K. Glial cell line-derived neurotrophic factor up-regulates the expression of tyrosine hydroxylase gene in human neuroblastoma cell lines. J Neurochem. 2002;82(4):801–808. doi: 10.1046/j.1471-4159.2002.00993.x. [DOI] [PubMed] [Google Scholar]
- 15.Boucher TJ, McMahon SB. Neurotrophic factors and neuropathic pain. Curr Opin Pharmacol. 2001;1(1):66–72. doi: 10.1016/s1471-4892(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 16.Boucher TJ, et al. Potent analgesic effects of GDNF in neuropathic pain states. Science. 2000;290(5489):124–127. doi: 10.1126/science.290.5489.124. [DOI] [PubMed] [Google Scholar]
- 17.Magill CK, et al. The differential effects of pathway- versus target-derived glial cell line-derived neurotrophic factor on peripheral nerve regeneration. J Neurosurg. 2010;113(1):102–109. doi: 10.3171/2009.10.JNS091092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi JY, et al. Glial cell line-derived neurotrophic factor gene transfer exerts protective effect on axons in sciatic nerve following constriction-induced peripheral nerve injury. Hum Gene Ther. 2011;22(6):721–731. doi: 10.1089/hum.2010.036. [DOI] [PubMed] [Google Scholar]
- 19.Wang R, et al. Persistent restoration of sensory function by immediate or delayed systemic artemin after dorsal root injury. Nat Neurosci. 2008;11(4):488–496. doi: 10.1038/nn2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tokugawa K, et al. XIB4035, a novel nonpeptidyl small molecule agonist for GFRalpha-1. Neurochem Int. 2003;42(1):81–86. doi: 10.1016/s0197-0186(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 21.Prevot V, et al. Normal female sexual development requires neuregulin-erbB receptor signaling in hypothalamic astrocytes. J Neurosci. 2003;23(1):230–239. doi: 10.1523/JNEUROSCI.23-01-00230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology. 1999;53(8):1634–1640. doi: 10.1212/wnl.53.8.1634. [DOI] [PubMed] [Google Scholar]
- 23.Beiswenger KK, Calcutt NA, Mizisin AP. Dissociation of thermal hypoalgesia and epidermal denervation in streptozotocin-diabetic mice. Neurosci Lett. 2008;442(3):267–272. doi: 10.1016/j.neulet.2008.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson GL, Leiter EH. Streptozotocin interactions with pancreatic beta cells and the induction of insulin-dependent diabetes. Curr Top Microbiol Immunol. 1990;156:27–54. doi: 10.1007/978-3-642-75239-1_3. [DOI] [PubMed] [Google Scholar]
- 25.Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: The molecular logic of thermosensation. Curr Opin Neurobiol. 2003;13(4):487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 26.Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445(7130):858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- 27.Bennett DL, et al. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18(8):3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stucky CL, Lewin GR. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19(15):6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stucky CL, Rossi J, Airaksinen MS, Lewin GR. GFR alpha2/neurturin signalling regulates noxious heat transduction in isolectin B4-binding mouse sensory neurons. J Physiol. 2002;545(Pt 1):43–50. doi: 10.1113/jphysiol.2002.027656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodbury CJ, et al. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24(28):6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albers KM, Davis BM. The skin as a neurotrophic organ. Neuroscientist. 2007;13(4):371–382. doi: 10.1177/10738584070130040901. [DOI] [PubMed] [Google Scholar]
- 32.Ernsberger U. The role of GDNF family ligand signalling in the differentiation of sympathetic and dorsal root ganglion neurons. Cell Tissue Res. 2008;333(3):353–371. doi: 10.1007/s00441-008-0634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ossipov MH. Growth factors and neuropathic pain. Curr Pain Headache Rep. 2011;15(3):185–192. doi: 10.1007/s11916-011-0183-5. [DOI] [PubMed] [Google Scholar]
- 34.Ruozi B, et al. Neurotrophic factors and neurodegenerative diseases: A delivery issue. Int Rev Neurobiol. 2012;102:207–247. doi: 10.1016/B978-0-12-386986-9.00009-0. [DOI] [PubMed] [Google Scholar]
- 35.Christianson JA, Riekhof JT, Wright DE. Restorative effects of neurotrophin treatment on diabetes-induced cutaneous axon loss in mice. Exp Neurol. 2003;179(2):188–199. doi: 10.1016/s0014-4886(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 36.Hirata A, Masaki T, Motoyoshi K, Kamakura K. Intrathecal administration of nerve growth factor delays GAP 43 expression and early phase regeneration of adult rat peripheral nerve. Brain Res. 2002;944(1-2):146–156. doi: 10.1016/s0006-8993(02)02739-7. [DOI] [PubMed] [Google Scholar]
- 37.Wilson-Gerwing TD, Verge VM. Neurotrophin-3 attenuates galanin expression in the chronic constriction injury model of neuropathic pain. Neuroscience. 2006;141(4):2075–2085. doi: 10.1016/j.neuroscience.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 38.Nutt JG, et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60(1):69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- 39.Apfel SC, et al. Recombinant human nerve growth factor in the treatment of diabetic polyneuropathy. Neurology. 1998;51(3):695–702. doi: 10.1212/wnl.51.3.695. [DOI] [PubMed] [Google Scholar]
- 40.McArthur JC, et al. A phase II trial of nerve growth factor for sensory neuropathy associated with HIV infection. AIDS Clinical Trials Group Team 291. Neurology. 2000;54(5):1080–1088. doi: 10.1212/wnl.54.5.1080. [DOI] [PubMed] [Google Scholar]
- 41.Schifitto G, et al. Long-term treatment with recombinant nerve growth factor for HIV-associated sensory neuropathy. Neurology. 2001;57(7):1313–1316. doi: 10.1212/wnl.57.7.1313. [DOI] [PubMed] [Google Scholar]
- 42.Schley M, et al. Skin innervation at different depths correlates with small fibre function but not with pain in neuropathic pain patients. Eur J Pain. 2012;16(10):1414–1425. doi: 10.1002/j.1532-2149.2012.00157.x. [DOI] [PubMed] [Google Scholar]
- 43.Kiasalari Z, et al. Identification of perineal sensory neurons activated by innocuous heat. J Comp Neurol. 2010;518(2):137–162. doi: 10.1002/cne.22187. [DOI] [PubMed] [Google Scholar]
- 44.Malmberg AB, et al. Reduced heat sensitivity and epidermal nerve fiber immunostaining following single applications of a high-concentration capsaicin patch. Pain. 2004;111(3):360–367. doi: 10.1016/j.pain.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Golden JP, DeMaro JA, Osborne PA, Milbrandt J, Johnson EM., Jr Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158(2):504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.