Significance
Skin tanning is a protective response of epidermal cells involving increased melanin formation. Overexposure to sun can cause sunburn and even skin cancer, and such conditions are partly attributable to the accumulation of toxic side products of melanin and its intermediates. In this study, we reveal the importance of key immune cytokine IFN-γ in pigmentation biology by studying cultured human melanocyte cells as well as mice and human disease models. We show that IFN-γ signaling regulates enzymes involved in melanin biosynthesis through a transcription factor IFN regulatory factor-1. Our study identifies a new mechanism of skin pigmentation homeostasis and proposes that strength and durability of local skin immune response may be decisive factors to delineate outcome between skin tanning and cancer.
Keywords: interferon, melanin, gene regulation, detanning
Abstract
Cellular homeostasis is an outcome of complex interacting processes with nonlinear feedbacks that can span distinct spatial and temporal dimensions. Skin tanning is one such dynamic response that maintains genome integrity of epidermal cells. Although pathways underlying hyperpigmentation cascade are recognized, negative feedback regulatory loops that can dampen the activated melanogenesis process are not completely understood. In this study, we delineate a regulatory role of IFN-γ in skin pigmentation biology. We show that IFN-γ signaling impedes maturation of the key organelle melanosome by concerted regulation of several pigmentation genes. Withdrawal of IFN-γ signal spontaneously restores normal cellular programming. This effect in melanocytes is mediated by IFN regulatory factor-1 and is not dependent on the central regulator microphthalmia-associated transcription factor. Chronic IFN-γ signaling shows a clear hypopigmentation phenotype in both mouse and human skin. Interestingly, IFN-γ KO mice display a delayed recovery response to restore basal state of epidermal pigmentation after UV-induced tanning. Together, our studies delineate a new spatiotemporal role of the IFN-γ signaling network in skin pigmentation homeostasis, which could have implications in various cutaneous depigmentary and malignant disorders.
In response to external stimuli, biological systems exhibit a variety of complex behaviors attributed to interplay between signaling, metabolic, and regulatory pathways that function over a broad range of timescales. Coordinated functionality of these events is critical in maintaining physiological homeostasis (1). Epidermal pigmentation represents one such central mechanism operative in the body’s largest organ, skin, that protects the genome from external damage (2, 3). Pigmentation is an outcome of the interplay between two epidermal cell types: melanocytes and keratinocytes. Several paracrine and autocrine factors are known to regulate this intricate process (4). Although immune cells recruited to the skin protect this organ from various infections, it is not clear whether these cells could also influence skin pigmentation (5).
Melanocytes produce melanin sequestered within a lysosome-related organelle (LRO) called melanosome. The process of pigmentation involves concerted biogenesis, maturation, and transfer of melanosomes to keratinocytes (6, 7). During the irreversible melanosome maturation process, several proteins support formation of core melanosome assembly, and the organelle is progressively filled with melanin polymer. Pmel17/gp100 is among the early structural proteins recruited to the melanosome. Tyrosinase (Tyr), dopachrome tautomerase (Dct), and Tyr-related protein-1 (Tyrp1) are the core enzymes involved in melanin synthesis (8, 9). Microphthalmia-associated transcription factor (MITF) is suggested to be the master regulator that governs melanocyte development, melanogenesis, and survival (10). The MITF promoter is further regulated by other transcription factors through an array of intrinsic regulators derived from fibroblasts, as well as endocrine, neural, and inflammatory factors (11, 12). The dynamic regulatory aspects of pigmentation have been difficult to establish in physiological models because of the inherent intricacies of dissecting complex interactions along with the fragility of this complex trait (13). To identify regulatory networks underlying melanogenesis, we designed a pigmentation oscillator that can reveal simple negative feedback loops.
Based on mechanistic, physiological, and pathophysiological studies, we show that IFN-γ signaling plays an important role in fine tuning the melanosome maturation process. This effect is mediated through the classical IFN-γ pathway by IFN regulatory factor-1 (IRF1). Interestingly, withdrawal of IFN-γ rapidly restores cellular programming, indicating a crucial role for this inflammatory cytokine in melanocyte biology.
Results
Design of a Pigmentation–Depigmentation Oscillator.
Several studies highlight the essential requirement of negative feedback loops in sustaining rhythmic oscillations (14). Mathematical analysis of periodic information derived from such oscillators provides a robust unbiased approach to delineate regulatory cascades underlying biological processes. We decided to exploit the known unstable melanotic behavior of B16 cells to develop an oscillatory model of pigmentation and depigmentation (15). Passaging of pigmented B16 cells obtained from the tumor grown in C57BL/6 mice results in progressive loss of visible pigmentation in vitro [passage 1 (P1) to P4] (Fig. S1A); s.c. injection of depigmented P4 cells in mice generated dark-colored tumors. By using a stable tumor cell line expressing GFP (TNV2), we confirmed the P4 origin of the tumor cells in vivo (Fig. S1B), thus showing that depigmented cells (P4) possess capability to repigment under the favorable conditions. While exploring several conditions, we identified that low-density (LD) plating (102 cells/cm2) results in reproducible pigmentation in the absence of any extraneous factor (Fig. S2A). Visual pigmentation could be observed over several days during the time course study, and the process of pigmentation could be reversed by replating these cells at higher cell density (104 cells/cm2) in fresh medium. Cellular phenotype could also be confirmed by quantitative melanin estimation (Fig. S2B). One complete cycle of pigmentation and depigmentation could be completed in 20 days, and we confirmed this oscillatory process for several cycles (Fig. 1A).
Fig. 1.
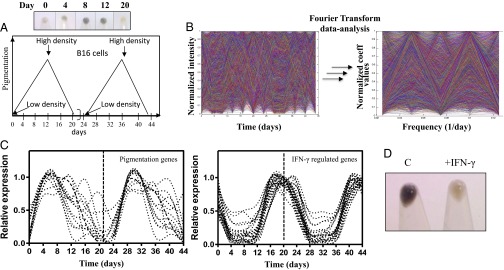
Analysis of the pigmentation oscillator reveals a dominant IFN-γ signature in depigmented cells. (A) Schematic representation of the in vitro biological oscillator showing two cycles of pigmentation and depigmentation of cultured cells at different densities (arrows indicate the time of plating). (Upper) Cell pellets of B16 cells on different days for cycle 1. (B, Left) Time-dependent changes in the relative expression of all genes in the oscillator model. (B, Right) Normalized coefficient value obtained by FT analysis of all genes as a function of their respective frequencies. (C, Left) Relative expression level of known pigmentation genes through two cycles of pigmentation and depigmentation. (C, Right) Relative expression level of known IFN-γ–regulated genes identified by FT analysis across the two cycles. (D) Cell pellets of control (C) and IFN-γ–treated B16 cells at day 12.
Periodogram Analysis Reveals Dominant IFN-γ Signature.
To identify regulatory features underlying the oscillations, we performed transcriptional analysis over two consecutive pigmentation–depigmentation cycles. The comparison between two microarray datasets showed good correlation (R2 = 0.84). Many genes associated with melanin synthesis and melanosome maturation showed periodic changes (Fig. S3). The plot between normalized gene expression changes and time for all regulated genes showed a complex pattern (Fig. 1B, Left). Pigmentation genes showed similar slope with peak at day 8; however, the downward trend exhibited differential decay rates (Fig. 1C, Left). Fourier transform (FT) analyses of the high-throughput data provided a means to identify periodicity in gene regulation. Periodogram analysis sorted out gene profiles with a 20-d period (dominant frequency = 1/20 d−1) (Fig. 1B, Right). For every profile, we checked the contribution of the frequency = 1/20 d−1 component by evaluating the ratio of the FT coefficient corresponding to this frequency to the sum of all FT coefficients. The enriched genes consist of a set of profiles where this ratio is greater than 70%.
Of all trajectories that were inversely associated with pigmentation, surprisingly, 40% of the down-regulated genes were associated with IFN-γ signaling (Fig. 1C, Right and Table S1). This observation prompted us to speculate that IFN-γ could possibly be one of the negative factors responsible for mediating depigmentation in the B16 oscillator model. Earlier studies had indicated a hypopigmentating effect for IFN-γ. Analyses of culture supernatant as well cellular mRNA did not detect IFN-γ during the entire pigmentation–depigmentation cycle (SI Text). However, the addition of a physiologically relevant low dose of IFN-γ (100 U/mL) to both B16 cells and primary human melanocytes suppressed pigmentation. Whereas depigmentation was complete in the B16 model, substantial hypopigmentation could be reproducibly achieved in foreskin-derived primary melanocyte cells from diverse individuals within 4–5 d (Fig. 1D and Fig. S4).
IFN-γ Causes Cellular Hypopigmentation by Arresting Melanosome Maturation.
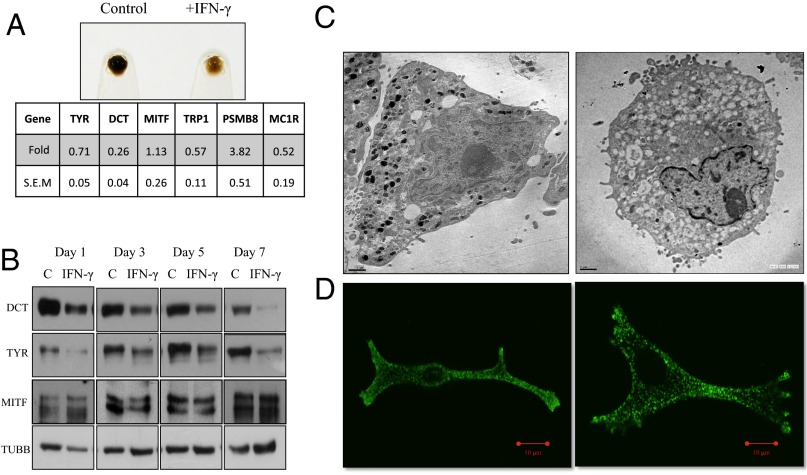
Because IFN-γ is known to have pleiotropic effect on various cell types (16), we decided to perform global transcriptional analysis to delineate the mechanism behind the hypopigmenting effect of IFN-γ on cultured primary human melanocytes. Microarray data analysis showed classical IFN-γ signatures with up-regulation of MHC genes and PSMB8, PSMB9 immunoproteasome subunits. Both the IFN-γ receptor subunits (IFN-γRI and IFN-γRII) also showed substantial up-regulation. Of the pigmentation genes, DCT showed a drastic decrease of around 80%. Similar observations were also reported during the course of another study (17, 18). TYRP1 and TYR were decreased to a lesser extent across different primary cultures treated with IFN-γ (Fig. 2A). Time course of Western blot analysis revealed a temporal decrease in the levels of TYR and DCT proteins after IFN-γ treatment (Fig. 2B). Interestingly, whereas the effect of IFN-γ on melanogenic proteins was evident as early as day 1 of treatment, the visible phenotypic changes were slow and occurred over a period of 4–5 d.
Fig. 2.
IFN-γ signaling mediates hypopigmentation of primary human melanocytes by arresting melanosome maturation. (A, Upper) Cell pellets of control and IFN-γ–treated primary human epidermal melanocytes after 7 d of treatment. (A, Lower) Fold change in gene expression levels of IFN-γ–treated melanocyte cultures expressed as mean fold change ± SEM across three independent cultures. (B) Western blot analysis of DCT, TYR, and MITF proteins after IFN-γ treatment as a function of time. C, control. (C) TEM images of (Left) control and (Right) IFN-γ–treated melanocytes. (D) Corresponding confocal images of melanocytes treated with IFN-γ stained with HMB45 (green). (Scale bar: C, 2 μm; D, 10 μm.)
Rapid decrease in the level of key melanosomal proteins is expected to have an impact on overall maturation of melanosomes. Microarray analysis of the oscillatory model indeed showed a downward trend for the genes involved in melanosome biogenesis and maturation (Fig. S3). Ultrastructural studies with transmission electron microscopy (TEM) confirmed drastic decrease in the number of darkly pigmented stage III and IV melanosomes in IFN-γ–treated cells (Fig. 2C). These cells instead accumulated electron-lucent LROs characteristic of early stage I and II melanosomes. Confocal microscopy with melanosome-specific HMB45 antibody showed comparable numbers of melanosomal structures in control and IFN-γ–treated cells (Fig. 2D). Cellular investigations, thus, suggest that IFN-γ treatment arrests melanosomes early in the process of maturation by decreasing flux of proteins being trafficked to early melanosomes.
Hypopigmentation Mediated by IFN-γ Is Reversible and Independent of MITF.
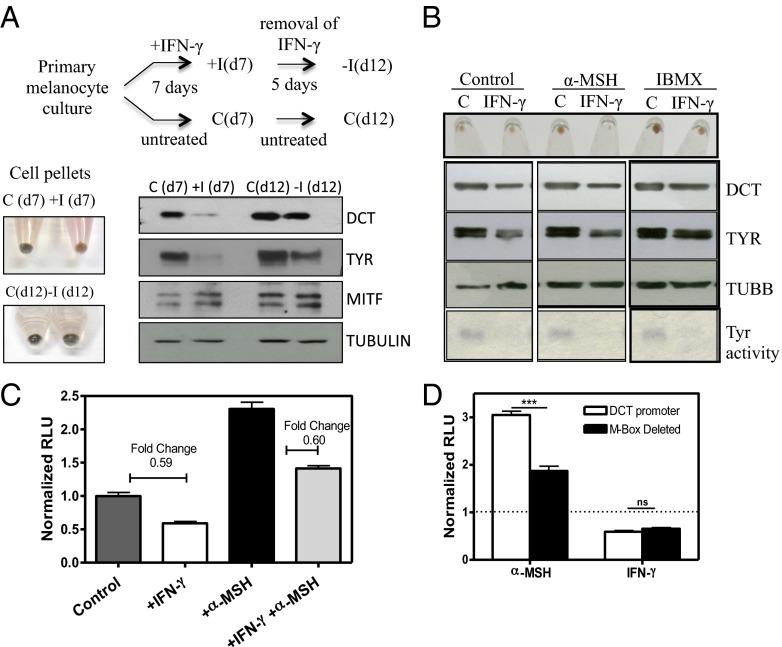
To examine whether IFN-γ–mediated hypopigmentation of melanocytes could be reversed, treated cells were replated in fresh medium (Fig. 3A, scheme). Removal of IFN-γ reinitiated the pigmentation process and distinct recovery, as evident from the cell pellets and levels of the melanogenesis proteins, was observed (Fig. 3A). Concomitant with repigmentation, an increased number of mature melanosomes could be visualized by TEM (Fig. S5). These observations reveal that IFN-γ–induced changes in melanogenesis are completely reversible and that normal cellular programming is reinstated on withdrawal of this cytokine. The IFN-γ concentration used (100 units/mL) showed no apparent changes in cell number and viability. Examination of different phases of the cell cycle by FACS analysis also showed no significant differences (Fig. S6A).
Fig. 3.
IFN-γ signaling mediates reversible hypopigmentation of melanocytes independent of MITF. (A) Experimental setup for studying reversibility of IFN-γ effect on melanocytes. Cell pellet of primary human melanocytes treated with IFN-γ for 7 d; the cells were allowed to recover for 5 d (day 12) by the removal of IFN-γ. Western blot analysis of DCT, TYR, and MITF proteins of untreated and IFN-γ–treated cells (day 7) and day 12 untreated and recovered cells. C, control; I, IFN. (B) Cell pellets of melanocytes. The levels of proteins by Western blot analysis and Tyr activity determined by an in-gel L-dopa assay of cells treated with 600 nM α-MSH and 100 μM isobutyl methyl xanthine (IBMX) for 5 d in the presence and absence of IFN-γ are shown. (C) Dct promoter (3.7 kb) activity in the presence of 600 nM α-MSH and 100 U/mL IFN-γ (alone or combined) as measured by dual luciferase assay. RLU, relative light units. (D) Promoter activity of a 3.7-kb WT Dct promoter and M-Box–deleted construct in the presence of α-MSH and IFN-γ. ***P < 0.001; ns, not significant.
Western blot studies showed that MITF protein levels did not change significantly during the hypopigmentation–repigmentation cycle (Figs. 2B and 3A). These data were surprising, since almost all known melanogenesis regulatory pathways converge to MITF. We investigated whether the known downstream MITF target genes were expressed on treatment of melanocytes with IFN-γ (19). More than 80% of the known genes showed expression in the transcriptome data, indicating that MITF is present in the functional form (Fig. S6B). Addition of known MITF-activating factors, such as α-melanocyte stimulating hormone (α-MSH) and isobutyl methyl xanthine (IBMX), to melanocytes could not prevail over IFN-γ–induced hypopigmentation (including reduction in TYR and DCT levels) (Fig. 3B). Because DCT is one of the important biosynthetic proteins in melanin synthesis and showed robust transcriptional regulation by IFN-γ, additional studies were carried out with Dct gene to understand the mechanism of MITF-independent hypopigmentation effect.
We cloned 3.7 kb of mouse Dct promoter upstream of firefly luciferase reporter and transfected it with Renilla luciferase plasmid on day 3 in the B16 LD model cells (20). The ratio of firefly to Renilla luciferase units measured on day 5 provided an estimate of Dct promoter activity. Competition assay between α-MSH and IFN-γ showed clear suppression of Dct promoter activity (Fig. 3C). Importantly, deletion of the MITF binding site (M-Box) in Dct promoter construct still showed IFN-γ–mediated repression, whereas M-Box deletion, as expected, led to reduction in α-MSH–stimulated Dct expression (Fig. 3D), indicating the noninvolvement of MITF in the repression of Dct expression. Together, our studies establish an MITF-independent mechanism for IFN-γ in melanocyte hypopigmentation.
IRF1-Mediated Transcriptional Regulation.
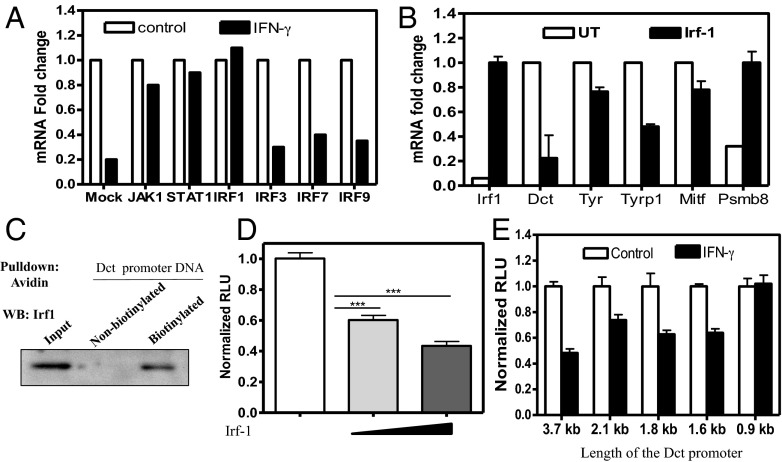
IFN-γ mediates its effect through canonical and noncanonical signaling routes (21). Microarray analysis showed elevation of transcriptional signatures for the canonical pathway (IFNGR-I, IFNGR-II, JAK-1, and STAT-1). This activation could also be seen by the increased phosphorylation status of STAT-1 protein (Fig. S7A). siRNA-mediated silencing of JAK-1 and STAT-1 rescued the suppression of DCT expression by IFN-γ in primary human melanocytes (Fig. 4A and Fig. S7B). The IRF family of transcription factors is among the key downstream effector molecules of IFN-γ signaling (22). Microarray data revealed significant up-regulation of IRF1, IRF3, IRF7, and IRF9 in melanocytes (Fig. S7C). We used siRNA-mediated silencing to investigate the requirement of relevant pathway. Of the four factors, IRF1 suppression could rescue DCT expression in IFN-γ–treated cells, indicating the significance of this signaling arm in mediating hypopigmentation (Fig. 4A and Fig. S7B). Overexpression of Irf1 during the LD phase of B16 cells (day 3) replicated transcriptional regulation observed on IFN-γ treatment, including the suppression of Dct (Fig. 4B). We then probed whether Irf1 could directly bind the Dct promoter. The avidin-immobilized 3.7-kb biotinylated promoter could specifically pull down Irf1 (Fig. 4C). Dct promoter activity could be repressed by overexpression of Irf1 in a dose-dependent manner (Fig. 4D). To identify the Irf1-responsive sites in Dct promoter, we performed systematic deletions to generate 2.1-kb, 1.8-kb, 1.6-kb, and 900-bp Dct reporter cassettes. Bioinformatic analysis using the PROMO transcription factor binding site predicted the presence of 13 putative Irf1 binding sites in the 3.7-kb fragment (23) (Fig. S8A). Although Irf1 binding activity could be observed for 2.1-, 1.8-, and 1.6-kb fragments in luciferase assays, the smaller 900-bp fragment showed no binding to Irf1 (Fig. 4E and Fig. S8B). Our data show that Irf1 is the crucial factor through which IFN-γ mediates its hypopigmentation effects.
Fig. 4.
Mechanism of Irf1-mediated down-regulation of Dct. (A) Levels of DCT mRNA as measured by real-time PCR in mock vs. JAK-1, STAT-1, IRF1, IRF3, IRF7, and IRF9 siRNA-transfected melanocytes in the absence or presence of IFN-γ. (B) mRNA levels of transcripts in cells transfected with Irf1 expression construct compared with mock-transfected B16 cells. UT, untransfected. (C) Binding of Irf1 to biotinylated Dct promoter in avidin pull down from IFN-γ–treated B16 cell lysates was detected by Western blot (WB). (D) Dct promoter activity in the absence and presence of varying concentrations of Irf1 expression plasmid (Dct-luciferase: Irf1 plasmid DNA, 1:0, 1:0.5, and 1:1). Bars represent mean ± SD across replicates. ***P < 0.001. (E) Dual luciferase reporter assay carried out with several deletion constructs of Dct promoter in the presence and absence of IFN-γ. Bars represent mean ± SD across replicates.
Relevance of IFN-γ on Physiological and Pathological Hypopigmentation.
Earlier studies on skin pigmentation dynamics had indicated a complex outcome of ultraviolet B radiation (UVB) on skin (24). Multiple cytokines secreted by infiltrating immune cells complicate the dissection of individual contributions. The physiological role of IFN-γ in altering the dynamic changes was examined in the mouse model by comparing C57BL/6 IFN-γ null (IFNG−/−) mice with the corresponding littermate controls (IFNG+/+). Careful observation of ear and tail regions, where epidermal melanocytes are present, showed small but significant differences (Fig. 5A). Quantitative differences could be established by mexameter across several experiments. Assessment of Dct and Tyr protein levels from the epidermal lysate revealed higher levels of Dct in IFN-γ KO animals, whereas no change was observed for Tyr levels (Fig. 5B). The difference in the two strains could be clearly noted by irradiating these mice with UVB, a stimuli that has been shown to enhance localized IFN-γ secretion by recruiting immune cells to skin (5). Although the WT mice regained basal pigmentation levels by day 11 post-UVB exposure, KO mice showed a hyperpigmentation phenotype (Fig. 5C). The sustained tanning response in the absence of IFN-γ, together with our in vitro studies, suggests a crucial role of this cytokine in dampening UVB-activated melanogenic cascade. Because UV radiation induces powerful changes to the immune landscape, the involvement of other factors cannot be ruled out. However, such modulations are likely to intersect with the IFN-γ signaling network.
Fig. 5.
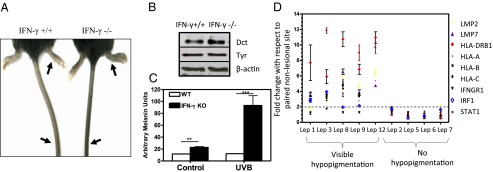
Physiological and pathophysiological roles of IFN-γ in skin pigmentation. (A) Images of IFNG+/+ and IFNG−/− mice in C57BL/6 background at 6 wk. Arrows indicate pigmentation at the sites where epidermal melanocytes are present. (B) Western blot analysis of Dct and Tyr proteins from the tail epidermis in IFNG+/+ and IFNG−/− mice. (C) Image analysis for quantitating levels of pigmentation. Bar graphs indicate average ± SEM across 22 measurements across the two ear lobes. **P < 0.005; ***P < 0.001. (D) Comparative real-time PCR analysis for a panel of known IFN-γ–modulated genes in nonlesional vs. lesional epidermis of patients with different manifestations of leprosy (Lep). Dashed line represents a cutoff of twofold considered as significant for up-regulation.
In humans, tuberculoid leprosy patients are known to have a dominant Th1 response, whereas the lepromatous form shows a Th2 cytokine predominance (25). Interestingly, tuberculoid leprosy patients are also known to possess hypopigmented patches on their skin. Our analysis of skin samples from leprosy patients showed a strong IFN-γ signature in the visibly hypopigmented lesions compared with matched unaffected skin (Fig. 5D). No apparent pigmentation changes were seen in patients in whom IFN-γ signatures were not observed. However, other clinical features, such as loss of sensation, were reported in these patients. Skin sections from the hypopigmented lesions were positive for melanocytes as determined by S100 immunostaining (Fig. S9A). TEM studies showed reduced numbers of pigmented type III and IV melanosomes within melanocytes of hypopigmented lesions (Fig. S9 B and C). Our studies, thus, suggest that increased levels of IFN-γ may be a determinant of skin hypopigmentation in certain pathological conditions.
Discussion
Skin, with its own immune system and ability to induce pigmentation, provides an efficient barrier against external stressors. The hyperpigmentation response is crucial for maintaining genome integrity of epidermal cells (26). Secreted cytokines, such as IFN-γ, IL-1, and IL-4, provide protection from biological insults and are critical for tissue remodeling. In this study, we provide strong evidence for the physiological relevance of IFN-γ in skin pigmentation homeostasis. We show that IFN-γ signaling mediates its hypopigmenting effect through the transcription factor IRF1, which in turn, controls melanosome maturation in melanocytes. The MITF-independent mechanism of IRF1 regulation of melanin biosynthetic genes, thus, adds another dimension to the study of melanogenesis.
Although several factors are known to dynamically alter pigmentation of human skin, precise mechanisms that maintain basal state have been difficult to elucidate. We, therefore, developed a cell autonomous cyclical oscillator model of pigmentation and depigmentation that is known to sustain its oscillations through a feedback loop to identify negative regulatory pathways involved in melanogenesis. Unbiased genome-wide transcriptional profiling of this pigmentation oscillator revealed a dominant IFN-γ signature inversely correlated with pigmentation. Although addition of IFN-γ to depigmented B16 melanoma cells prevents cells from pigmentation in the LD state, cultured primary melanocytes show distinct hypopigmentation in 4–5 d of IFN-γ treatment. The density-dependent oscillator model does not function in primary melanocytes, because cell–cell contact is important for culturing these cells. Moreover, proliferation and melanogenesis are tightly coupled in primary cells through MITF (27). Effect of IFN-γ on melanogenic genes and proteins is, however, evident as early as day 1 of the treatment. The delay observed in hypopigmentation of melanocytes is because of the presence of preformed mature melanosomes, which get diluted on melanocyte proliferation and/or secreted out of the cell. Although IFN-γ signatures were identified in the LD pigmentation oscillator model, at present, it is unclear which cognate ligand is involved in mediating density-dependent IFN-γ signaling. Similar instances of activated signaling without identification of factors have been noted in several earlier studies (28, 29).
IFN-γ is a common secreted cytokine in the skin; therefore, we wanted to explore whether the hypopigmentation mechanism is also operative in the physiological milieu. Interestingly, IFN-γ–overexpressing transgenic mice developed to study eczema have been reported to have a complete penetrance of hypopigmentation phenotype (30). Our studies with IFN-γ KO mice likewise show consistent differences in epidermal pigmentation. Furthermore, we show a clear association of increased IFN-γ signaling to a decreased number of stage III and IV melanosomes in the lesional skin of leprosy patients. Although other immune factors can also perturb skin pigmentation, the identification of a specific IRF1-mediated regulation of pigmentation genes provides confidence that IFN-γ must have a significant role in controlling melanosome maturation and thus, skin pigmentation. We show that chronic overexpression of IFN-γ in both mouse and human skin can result in hypopigmentation phenotype. On UV exposure, a clear delay in regaining the basal pigmentation of skin can be observed for the IFN-γ KO mice. Recent studies have, indeed, shown that UV radiation of skin elicits a delayed recruitment of IFN-γ–secreting immune cells to the epidermis (5). Together, our studies support the conjecture that the strength, durability, and temporal response of IFN-γ response could be crucial factors for maintaining epidermal pigmentation homeostasis, and this mechanism may have important implications in the detanning of human skin.
Two of the crucial mediators of skin pigmentation, α-MSH and TGF-β, are known to be involved in the protective tanning response and maintenance of melanocytes in an immature state, respectively (27, 31). While α-MSH is secreted by adjacent keratinocytes in response to UV and TGF-β by follicular bulge area cells, the source of IFN-γ could be the immune cells recruited during inflammation or UV exposure. We, thus, propose an αβγ-cytokine regulatory model of melanogenesis, where all three cytokines act in a concerted manner to modulate melanocyte biology. It is likely that all these factors function in an interlinked manner to bring about the spatiotemporal regulation of human skin color. Future studies would dissect detailed pathways associated with IFN-γ–mediated hypopigmentation cross-talk in delicately balancing skin pigmentation and its implications in disease pathophysiology.
Methods
Culture of B16 Cells and Pigmentation Model Setup.
B16 cells were maintained at subconfluence with 5% (vol/vol) CO2 levels in DMEM supplemented with 10% FCS (Invitrogen). Clonal population was isolated by limited dilution, and B16 TNV1 (referred to as B16) was derived. The pigmentation oscillator was set up by plating ∼100 cells/cm2, and cells were harvested at days 4, 8, and 12. For depigmentation, cells were replated at 10,000 cells/cm2 on day 12 and harvested after 4 and 8 d to complete the 20-d cycle. The second cycle was initiated from the depigmented cells obtained after a gap of 4 d to allow for all changes to stabilize.
Calculation of Correlation Coefficient for the Microarray Analysis.
The time profiles for the two cycles were treated as replicate measurements and a linear least squares fit, and the regression coefficient (R2) for that fit was calculated. Genes with very low expression (average expression score <100) were omitted from this analysis. Reported R2 value (0.84) is, thus, based on the profiles for 10,937 genes.
Identification of Genes Associated with the Pigmentation–Depigmentation Cycle.
Analyses of periodic data (32, 33) and time course microarray data (34) have been used to distinguish truly periodic genes from genes that show oscillatory behavior because of random effects. Identification of genes with frequency of 1/20 d−1 was performed using the Matlab function fft (an implementation of the fast FT). Genes for which the contribution corresponding to the frequency of 1/20 day-1 is more than 70% of the total contribution from all frequencies were selected. For every series of expression values at n time points for every gene i, the discrete FT values yij = fft(xi1, xi2, …, xin) were calculated at various frequencies, including one j = j′ corresponding to a frequency of 1/20 d−1. The criterion used for classifying a particular gene as correlated with the pigmentation–depigmentation cycle was yij′/sumj(yij) > 0.7.
Clinical Material.
Human ethical review committee approved this study, and it is in agreement with Declaration of Helsinki principles. Skin punch biopsies (2.5 mm) from involved and normal regions from patients with hypopigmentation were obtained after informed consent. RNA isolation and downstream processing were performed as indicated earlier (35).
Supplementary Material
Acknowledgments
We thank Dr. David E. Fisher for providing microphthalmia-associated transcription factor antibody, Dr. Nicole Clarke for providing the IFN regulatory factor-1 expression construct, and Dr. Satyajit Rath for fruitful discussions and providing IFN-γ KO mice. We also thank Dr. Alok Bhattacharya for providing access to the instrumentation facility. This work is supported by Department of Biotechnology Program Support Grant BT/01/COE/07/07 and Council for Scientific and Industrial Research, India Grants MLP6201 and BSC0302 (TOUCH). The imaging facility is supported through Infrastructure Project BSC0403. P.G. and K.K. are Senior Research Fellows and S.Y. is a Junior Research Fellow supported by the Council for Scientific and Industrial Research, India. S.P. is a Department of Biotechnology–Bioinformatics National Certification Fellow.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE54359).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1304988111/-/DCSupplemental.
References
- 1.Blanpain C, Fuchs E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10(3):207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyamura Y, et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 2007;20(1):2–13. doi: 10.1111/j.1600-0749.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- 3.Tadokoro T, et al. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB J. 2003;17(9):1177–1179. doi: 10.1096/fj.02-0865fje. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. Biofactors. 2009;35(2):193–199. doi: 10.1002/biof.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaidi MR, et al. Interferon-γ links ultraviolet radiation to melanomagenesis in mice. Nature. 2011;469(7331):548–553. doi: 10.1038/nature09666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ando H, et al. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J Invest Dermatol. 2012;132(4):1222–1229. doi: 10.1038/jid.2011.413. [DOI] [PubMed] [Google Scholar]
- 7.Raposo G, Marks MS. Melanosomes—dark organelles enlighten endosomal membrane transport. Nat Rev Mol Cell Biol. 2007;8(10):786–797. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445(7130):843–850. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- 9.Hearing VJ. Biogenesis of pigment granules: A sensitive way to regulate melanocyte function. J Dermatol Sci. 2005;37(1):3–14. doi: 10.1016/j.jdermsci.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Levy C, Fisher DE. Dual roles of lineage restricted transcription factors: The case of MITF in melanocytes. Transcription. 2011;2(1):19–22. doi: 10.4161/trns.2.1.13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou L, Pavan WJ. Transcriptional and signaling regulation in neural crest stem cell-derived melanocyte development: Do all roads lead to Mitf? Cell Res. 2008;18(12):1163–1176. doi: 10.1038/cr.2008.303. [DOI] [PubMed] [Google Scholar]
- 12.Liu JJ, Fisher DE. Lighting a path to pigmentation: Mechanisms of MITF induction by UV. Pigment Cell Melanoma Res. 2010;23(6):741–745. doi: 10.1111/j.1755-148X.2010.00775.x. [DOI] [PubMed] [Google Scholar]
- 13.Bennett DC, Lamoreux ML. The color loci of mice—a genetic century. Pigment Cell Res. 2003;16(4):333–344. doi: 10.1034/j.1600-0749.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 14.Novák B, Tyson JJ. Design principles of biochemical oscillators. Nat Rev Mol Cell Biol. 2008;9(12):981–991. doi: 10.1038/nrm2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett DC. Mechanisms of differentiation in melanoma cells and melanocytes. Environ Health Perspect. 1989;80:49–59. doi: 10.1289/ehp.898049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Boxel-Dezaire AH, Stark GR. Cell type-specific signaling in response to interferon-gamma. Curr Top Microbiol Immunol. 2007;316:119–154. doi: 10.1007/978-3-540-71329-6_7. [DOI] [PubMed] [Google Scholar]
- 17.Choi H, et al. IL-4 inhibits the melanogenesis of normal human melanocytes through the JAK2-STAT6 signaling pathway. J Invest Dermatol. 2013;133(2):528–536. doi: 10.1038/jid.2012.331. [DOI] [PubMed] [Google Scholar]
- 18.Le Poole IC, et al. Interferon-gamma reduces melanosomal antigen expression and recognition of melanoma cells by cytotoxic T cells. Am J Pathol. 2002;160(2):521–528. doi: 10.1016/s0002-9440(10)64871-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoek KS, et al. Novel MITF targets identified using a two-step DNA microarray strategy. Pigment Cell Melanoma Res. 2008;21(6):665–676. doi: 10.1111/j.1755-148X.2008.00505.x. [DOI] [PubMed] [Google Scholar]
- 20.Lang D, et al. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature. 2005;433(7028):884–887. doi: 10.1038/nature03292. [DOI] [PubMed] [Google Scholar]
- 21.Gough DJ, Levy DE, Johnstone RW, Clarke CJ. IFNgamma signaling-does it mean JAK-STAT? Cytokine Growth Factor Rev. 2008;19(5–6):383–394. doi: 10.1016/j.cytogfr.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Enesa K, et al. Pellino1 is required for interferon production by viral double-stranded RNA. J Biol Chem. 2012;287(41):34825–34835. doi: 10.1074/jbc.M112.367557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messeguer X, et al. PROMO: Detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002;18(2):333–334. doi: 10.1093/bioinformatics/18.2.333. [DOI] [PubMed] [Google Scholar]
- 24.Aoki H, Moro O. Upregulation of the IFN-gamma-stimulated genes in the development of delayed pigmented spots on the dorsal skin of F1 mice of HR-1 x HR/De. J Invest Dermatol. 2005;124(5):1053–1061. doi: 10.1111/j.0022-202X.2005.23721.x. [DOI] [PubMed] [Google Scholar]
- 25.Modlin RL. Th1-Th2 paradigm: Insights from leprosy. J Invest Dermatol. 1994;102(6):828–832. doi: 10.1111/1523-1747.ep12381958. [DOI] [PubMed] [Google Scholar]
- 26.Cui R, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128(5):853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura EK, et al. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell. 2010;6(2):130–140. doi: 10.1016/j.stem.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subramaniam S, et al. Distinct transcriptional networks in quiescent myoblasts: A role for Wnt signaling in reversible vs. irreversible arrest. PLoS One. 2013;8(6):e65097. doi: 10.1371/journal.pone.0065097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao B. Wnt regulation of planar cell polarity (PCP) Curr Top Dev Biol. 2012;101:263–295. doi: 10.1016/B978-0-12-394592-1.00008-9. [DOI] [PubMed] [Google Scholar]
- 30.Carroll JM, Crompton T, Seery JP, Watt FM. Transgenic mice expressing IFN-gamma in the epidermis have eczema, hair hypopigmentation, and hair loss. J Invest Dermatol. 1997;108(4):412–422. doi: 10.1111/1523-1747.ep12289702. [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Malek Z, et al. The melanocortin-1 receptor is a key regulator of human cutaneous pigmentation. Pigment Cell Res. 2000;13(Suppl 8):156–162. doi: 10.1034/j.1600-0749.13.s8.28.x. [DOI] [PubMed] [Google Scholar]
- 32.Wichert S, Fokianos K, Strimmer K. Identifying periodically expressed transcripts in microarray time series data. Bioinformatics. 2004;20(1):5–20. doi: 10.1093/bioinformatics/btg364. [DOI] [PubMed] [Google Scholar]
- 33.de Lichtenberg U, et al. Comparison of computational methods for the identification of cell cycle-regulated genes. Bioinformatics. 2005;21(7):1164–1171. doi: 10.1093/bioinformatics/bti093. [DOI] [PubMed] [Google Scholar]
- 34.Vijayan V, Deshpande P, Gadgil C, Gadgil M. Comparison of methods for identifying periodically varying genes. Int J Bioinform Res Appl. 2013;9(1):53–70. doi: 10.1504/IJBRA.2013.050653. [DOI] [PubMed] [Google Scholar]
- 35.Natarajan VT, et al. Transcriptional upregulation of Nrf2-dependent phase II detoxification genes in the involved epidermis of vitiligo vulgaris. J Invest Dermatol. 2010;130(12):2781–2789. doi: 10.1038/jid.2010.201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.