Significance
Plexins, a family of transmembrane receptors for semaphorins, control diverse biological processes during mouse development. However, it is largely unknown through which signaling pathways they exert their functions in vivo. Using an allelic series of transgenic mice, we show that the GTPase activating protein domain of plexins constitutes their key signaling module during development, which is required for proper formation of the nervous, cardiovascular, and skeletal system. In contrast, development of the liver vasculature specifically depends on the activation of the small GTPase RhoA by the plexin family member Plexin-B2. This study uncovers the in vivo context-dependence and functional specificity of individual plexin-mediated signaling pathways during mouse development.
Keywords: neural tube, cerebellum, outflow tract
Abstract
Mammalian plexins constitute a family of transmembrane receptors for semaphorins and represent critical regulators of various processes during development of the nervous, cardiovascular, skeletal, and renal system. In vitro studies have shown that plexins exert their effects via an intracellular R-Ras/M-Ras GTPase-activating protein (GAP) domain or by activation of RhoA through interaction with Rho guanine nucleotide exchange factor proteins. However, which of these signaling pathways are relevant for plexin functions in vivo is largely unknown. Using an allelic series of transgenic mice, we show that the GAP domain of plexins constitutes their key signaling module during development. Mice in which endogenous Plexin-B2 or Plexin-D1 is replaced by transgenic versions harboring mutations in the GAP domain recapitulate the phenotypes of the respective null mutants in the developing nervous, vascular, and skeletal system. We further provide genetic evidence that, unexpectedly, the GAP domain-mediated developmental functions of plexins are not brought about via R-Ras and M-Ras inactivation. In contrast to the GAP domain mutants, Plexin-B2 transgenic mice defective in Rho guanine nucleotide exchange factor binding are viable and fertile but exhibit abnormal development of the liver vasculature. Our genetic analyses uncover the in vivo context-dependence and functional specificity of individual plexin-mediated signaling pathways during development.
Plexins constitute a family of transmembrane proteins that serve as receptors for semaphorins (1). They function as key regulators of a multitude of developmental processes, including axon guidance, pattern and synapse formation in the nervous system (2), vasculogenesis and angiogenesis (3, 4), and morphogenesis of the heart, kidney, and skeletal system (5). In the adult organism, plexins play crucial roles in the physiology and pathophysiology of the immune and cardiovascular system, as well as in bone homeostasis and in cancer (6–9). Nine plexins have been identified in the mammalian system, which are grouped into four subfamilies, A–D, according to sequence homologies.
The activation of plexins by their semaphorin ligands triggers several intracellular signaling cascades, most of which modulate the activity of small GTPases (10). The intracellular domain of all plexins shares homology with GTPase-activating proteins (GAPs) and confers the deactivation of R-Ras, M-Ras, and Rap1 (11–17). The GAP activity toward R-Ras and M-Ras, but not toward Rap1, requires binding of Rnd GTPases to the plexin receptor (11, 12, 15, 16). Plexins of the B-subfamily differ from all other plexins in that they carry a C-terminal PDZ domain interaction motif that mediates a stable interaction with the Rho guanine nucleotide exchange factor (RhoGEF) proteins PDZ-RhoGEF and LARG (18–20). Activation of B-plexins by semaphorin ligands results in activation of the RhoGEF proteins and subsequent activation of RhoA and RhoC (18–21). This process and the GAP function of B-plexins are independent of each other, because mutations in the GAP domain do not interfere with the activation of RhoA, and deletion of the PDZ domain interaction motif does not influence GAP activity (11).
The plexin-mediated signal transduction pathways have been identified and characterized mainly in in vitro systems using recombinant proteins, purified membranes, and cell culture models. These in vitro approaches have often yielded conflicting results with respect to the functional relevance of individual plexin-mediated signaling pathways. For example, the axonal growth cone collapse of primary neurons has been suggested to be caused by plexin-mediated deactivation of R-Ras (11, 12), activation of RhoA (20, 22), and deactivation of Rap1 (15). Which plexin signaling pathways are functionally relevant in vivo is largely unknown.
Here we report on the generation of an allelic series of BAC transgenic mice carrying subtle mutations in the Plexin-B2 and Plexin-D1 gene, which specifically affect particular downstream signaling functions of these plexins. Our genetic analyses reveal a key function for the GAP domain of plexins during mouse development, which is independent of R-Ras and M-Ras inactivation. Furthermore, we identify a requirement of Plexin-B2–mediated RhoA activation for the development of the liver vasculature.
Results
Generation of Allelic Series of Plexin-B2 and Plexin-D1 BAC Transgenic Mice.
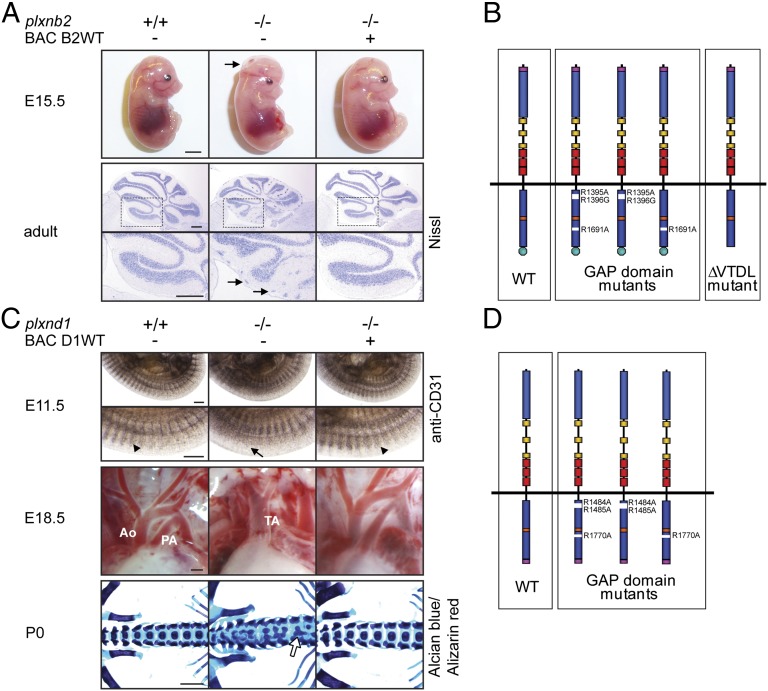
Plxnb2 knockout mice (plxnb2−/−) display neural tube closure defects, which result in exencephaly and perinatal lethality (23, 24). A small proportion of mice (∼5%) bypasses this neural tube closure phenotype and demonstrates abnormalities in cerebellar granule cell migration and defects in corticogenesis and migration of neuroblasts in the subventricular zone (23–26). In our effort to understand which signaling functions of Plexin-B2 are relevant in vivo, we first generated BAC transgenic mice that express triple-myc-tagged wild-type Plexin-B2 (BAC B2WT). This mouse line was crossed twice with mice heterozygous for the plxnb2 knockout allele (plxnb2+/−) to obtain animals in which the transgenic BAC-encoded plxnb2 allele was the only functional copy of the plxnb2 gene (BAC B2WT; plxnb2−/−). These mice were fully rescued from phenotypes observed in plxnb2−/− mice, were viable and fertile, and produced offspring with the expected Mendelian frequencies (Fig. 1A). On the basis of the BAC encoding wild-type Plexin-B2, we generated an allelic series of mice expressing Plexin-B2 mutants (Fig. 1B). These mutants included Plexin-B2 versions that carry mutations in all three arginines critical for GAP activity (BAC R1395A/R1396G/R1691A) (11), in the two N-terminal arginines (BAC R1395A/R1396G), or in the C-terminal arginine of the GAP domain (BAC R1691A), and a version that lacks the PDZ domain-binding motif (the four most C-terminal amino acids, VTDL) critical for interaction with RhoGEF proteins (20) (SI Appendix, Figs. S1 and S2A). In contrast to the Plexin-B2 versions carrying mutations of all three arginines or of the two N-terminal arginines of the GAP domain, the Plexin-B2 version with a mutation of the C-terminal arginine retained the ability to bind R-Ras (SI Appendix, Fig. S2 B–D). We extended our analysis of plexin-dependent signaling to the prototypic member of another plexin subfamily, Plexin-D1, which is mainly expressed in endothelial cells and neurons during embryonic development (27). Plexin-D1–deficient mice exhibit defects in vascular patterning, angiogenesis and outflow tract septation of the heart, skeletal malformations, errors in axonal projections and synapse formation, and defects in thymocyte migration (28–36). Mice lacking Plexin-D1 have only one great vessel arising from the heart (persistent truncus arteriosus) instead of two (aorta and pulmonary artery) and therefore die shortly after birth. In analogy to the approach with Plexin-B2, we first generated a BAC transgenic mouse line expressing triple-myc-tagged wild-type Plexin-D1 (BAC D1WT), which fully rescued the developmental defects observed in plxnd1−/− mice: the intersomitic vessels, the outflow tract of the heart, and the skeleton appeared indistinguishable from wild-type mice (Fig. 1C), and the mice were viable and fertile and produced offspring with the expected Mendelian frequencies. On the basis of the BAC encoding wild-type Plexin-D1, we established mouse lines harboring mutations of the three arginines required for the GAP function of Plexin-D1 (BAC R1484A/R1485A/R1770A, BAC R1484A/R1485A, BAC R1770A) (Fig. 1D).
Fig. 1.
Generation of BAC transgenic mice expressing triple-myc-tagged versions of Plexin-B2 and Plexin-D1. (A) (Upper) Pictures of E15.5 embryos; arrow points to the open cephalic neural tube (exencephaly). (Lower) Nissl-stained adult cerebella; boxed areas are magnified below, arrows point to clusters of ectopic granule cells at the cerebellar surface. (Scale bars, 3 mm in Upper, 300 µm in Lower.) (B) Schematic illustration of the allelic series of plxnb2 BAC transgenes. Purple, triple-myc-tag; blue, Sema domain; yellow, PSI domains; red, IPT/TIG domains; dark blue, split GAP domain; orange, Rnd/RhoD/Rac1 binding site; green, PDZ domain interaction motif. (C) (Top) WhoIe-mount immunohistochemistry on E11.5 embryos using an anti-CD31 antibody. Arrowheads point to intersomitic vessels, and arrow points to disorganized intersomitic vessels. (Middle) Pictures of the cardiac outflow tract of E18.5 embryos. Ao, Aorta; PA, pulmonary artery; TA, truncus arteriosus. (Bottom) Alcian blue/Alizarin red staining of P0 skeletons. Arrow points to fusion of vertebral bodies. (Scale bars, 300 µm in Top and Middle, 1 mm in Bottom.) (D) Schematic illustration of the allelic series of plxnd1 BAC transgenes. Purple, triple-myc-tag; blue, Sema domain; yellow, PSI domains; red, IPT/TIG domains; dark blue, split GAP domain; orange, Rnd/RhoD/Rac1 binding site.
The expression levels and the expression pattern of all BAC-encoded plexin proteins were comparable to the endogenous wild-type proteins (SI Appendix, Supporting Information and Figs. S3–S7).
The GAP Domain of Plexin-B2 Is Required for Neural Tube Closure and Cerebellar Granule Cell Migration.
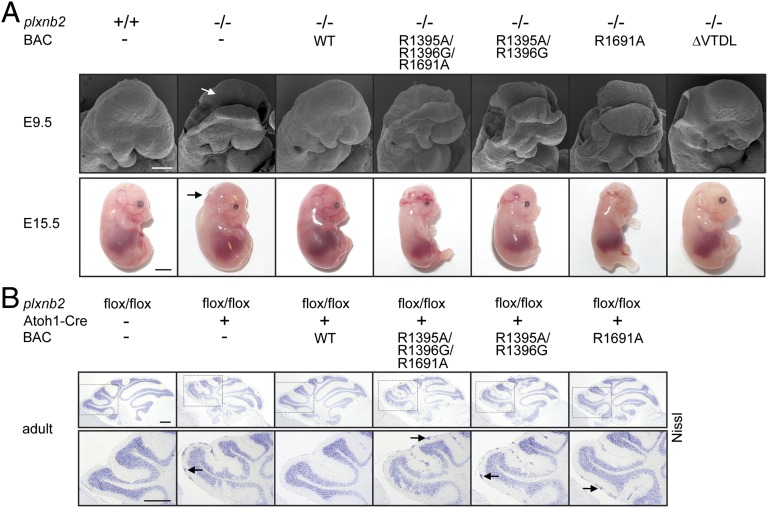
To assess the biological relevance of individual Plexin-B2–dependent signaling pathways during development, we analyzed embryos of the Plexin-B2 mutant mouse lines at embryonic day (E) 9.5 and 15.5. The neural tube closure defect observed in plxnb2 knockout mice was fully rescued by expression of a mutant Plexin-B2 version lacking the PDZ domain-binding motif required for interaction with RhoGEF proteins (Fig. 2A). In contrast, mice in which the endogenous Plexin-B2 was replaced by a Plexin-B2 version with mutations in the three arginines required for GAP activity phenocopied the plxnb2 knockout and displayed an open neural tube at E9.5 and exencephaly at E15.5 (Fig. 2A). Similarly, expression of Plexin-B2 versions with mutations in either the N-terminal or the C-terminal arginines of the split GAP domain of Plexin-B2 also failed to rescue the neural tube closure defects (Fig. 2A). To test the functional significance of individual Plexin-B2–mediated signaling pathways in the developing cerebellum, we crossed mice carrying floxed alleles of Plexin-B2 (plxnb2flox/flox) with mice expressing Cre specifically in cerebellar granule cell precursor cells under the control of the Atoh1 promoter (Atoh1-Cre;plxnb2flox/flox) (23, 37). These mice phenocopied the cerebellar abnormalities observed in surviving plxnb2 global knockout mice (Fig. 2B). Whereas expression of transgenic wild-type Plexin-B2 fully rescued the cerebellar defects of Atoh1-Cre;plxnb2flox/flox mice, expression of mutant Plexin-B2 versions defective in GAP activity failed to do so (Fig. 2B). Mice expressing Plexin-B2 defective in RhoGEF binding showed a normal development of the cerebellum (SI Appendix, Fig. S8). These data indicate that neural tube closure and migration of cerebellar granule cell precursors require a functional GAP domain of Plexin-B2 and are independent of its ability to interact with RhoGEF proteins.
Fig. 2.
Neural tube closure and cerebellar granule cell migration depend on the GAP domain of Plexin-B2. (A) (Upper) Scanning electron microscopy pictures of E9.5 embryos. Arrow points to nonfused neural head folds. (Lower) Pictures of E15.5 embryos. Arrow points to the open cephalic neural tube (exencephaly). (Scale bars, 200 µm in Upper, 3 mm in Lower.) (B) (Upper) Nissl staining of adult cerebella. (Lower) Magnification of the boxed areas in Upper. Arrows point to clusters of ectopic granule cells. (Scale bar, 300 µm.)
Outflow Tract Septation and Skeletal Morphogenesis Require the GAP Domain of Plexin-D1.
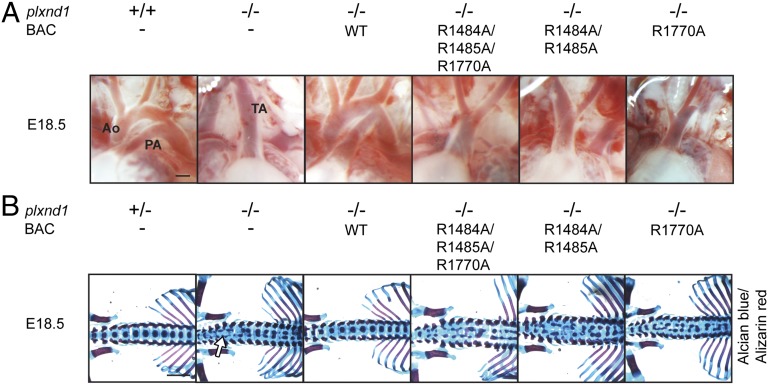
To evaluate the specific contribution of Plexin-D1–dependent signaling events during development, we examined mouse lines at E18.5 with respect to the heart and the skeletal system. Plexin-D1 mutant mouse lines, in which the endogenous Plexin-D1 was replaced by mutant Plexin-D1 proteins defective in GAP activity, displayed defects in outflow tract septation, with a persistent truncus arteriosus as observed in Plexin-D1–deficient animals (Fig. 3A). These animals also phenocopied the axial skeletal morphogenesis defects observed in Plexin-D1 knockout mice (Fig. 3B) and became cyanotic and died shortly after birth. These results indicate that outflow tract septation of the heart and axial skeletal patterning depend on the GAP domain of Plexin-D1.
Fig. 3.
Outflow tract septation and skeletal development depend on the GAP domain of Plexin-D1. (A) Pictures of the cardiac outflow tract of E18.5 embryos. Ao, Aorta; PA, pulmonary artery; TA, truncus arteriosus. (Scale bar, 300 µm.) (B) Alcian blue/Alizarin red staining of E18.5 skeletons. Arrow points to splitting of vertebral bodies. (Scale bar, 1 mm.)
The GAP Domain-Dependent Developmental Functions of Plexin-B2 and Plexin-D1 Are Independent of R-Ras and M-Ras Inactivation.
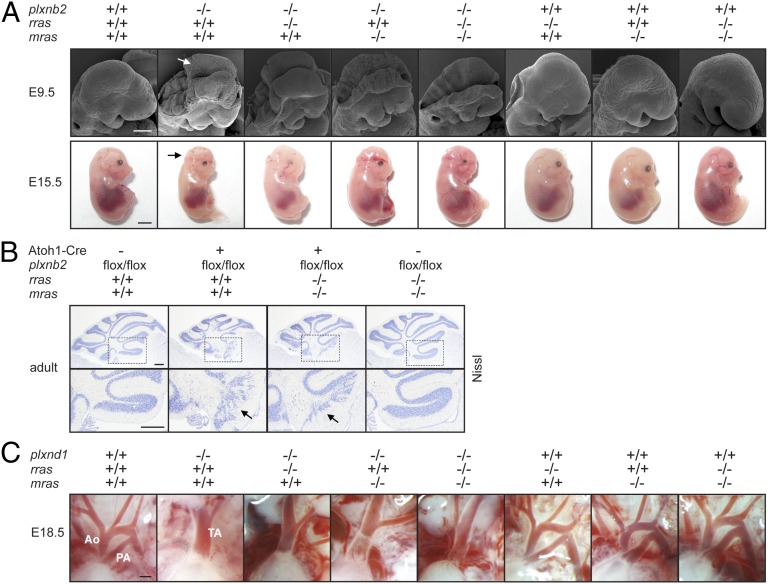
In in vitro assays, the GAP domain of plexins has been shown to exert its effects on cellular functions through inactivation of R-Ras (11, 12, 38). If the GAP domain-dependent developmental functions of plexins in vivo also rely on their ability to inactivate R-Ras, the developmental defects observed in plexin knockout mice should result from the failure to inactivate R-Ras. We reasoned that in plexin knockout mice this failure to inactivate R-Ras could be compensated for by genetic inactivation of R-Ras, which should at least partially rescue the developmental defects. To test this hypothesis, we crossed Plexin-B2- and Plexin-D1–deficient mice with R-Ras–deficient mice, which are viable and fertile and devoid of morphological abnormalities (39) (Fig. 4). Surprisingly, genetic inactivation of R-Ras ameliorated neither neural tube closure failure (Fig. 4A) nor cerebellar granule cell migration defects observed in Plexin-B2 knockout mice (Fig. 4B); furthermore, it failed to rescue the outflow tract septation defects in Plexin-D1 knockout mice (Fig. 4C). Given that plexins have been reported to influence cellular behavior also by inactivation of M-Ras (14), we also crossed Plexin-B2- and Plexin-D1–deficient mice with mice carrying a genetic inactivation of M-Ras, which are viable and develop normally (40). However, neither M-Ras deficiency nor R-Ras-/M-Ras double deficiency protected from defects in neural tube closure (Fig. 4A) or cerebellar (Fig. 4B) or heart development (Fig. 4C) in Plexin-B2 or Plexin-D1 knockout mice, respectively. A biochemical analysis revealed that R-Ras-GTP levels are unchanged in Plexin-B2 transgenic mice harboring mutations in the GAP domain (SI Appendix, Fig. S9). These results strongly suggest that the GAP domain-dependent developmental functions of Plexin-B2 and Plexin-D1 are independent of their ability to inactivate R-Ras and M-Ras.
Fig. 4.
R-Ras and M-Ras deficiency do not rescue Plexin-B2 and Plexin-D1 knockout phenotypes. (A) (Upper) Scanning electron microscopy pictures of E9.5 embryos. Arrow points to nonfused neural head folds. (Lower) Pictures of E15.5 embryos. Arrow points to the open cephalic neural tube (exencephaly). (Scale bars, 200 µm in Upper, 3 mm in Lower.) (B) (Upper) Nissl staining of adult cerebella. (Lower) Magnification of Insets in Upper. Arrows point to disorganized cerebellar folia. (Scale bars, 300 µm.) (C) Pictures of the cardiac outflow tract of E18.5 embryos. Ao, Aorta; PA, pulmonary artery; TA, truncus arteriosus. (Scale bar, 300 µm.)
Plexin-B2–Mediated RhoA Activation Is Dispensable for Neocortical Development.
During development of the nervous system, Plexin-B2 is strongly expressed in the telencephalic ventricular and subventricular zone, as well as in the neuroepithelium of the medial and lateral ganglionic eminences, and mice lacking Plexin-B2 that bypass the neural tube closure defect display severe abnormalities in corticogenesis, including abnormal cortical layering and defective migration and differentiation of several subtypes of cortical neurons (25, 41). The small GTPase RhoA plays a key role in the development of the nervous system by regulating a multitude of cellular functions, including cell migration, polarity, and survival (42, 43), and regulation of RhoA activity is required for corticogenesis (44, 45). To test whether Plexin-B2 exerts its functions in corticogenesis via activation of RhoA, we examined mice in which the endogenous Plexin-B2 was replaced by a Plexin-B2 version defective in RhoGEF binding. A detailed analysis of neocortical development over critical embryonic stages using typical marker proteins for cortical layering, neuronal processes, and glia cells did not reveal abnormalities in the morphology, number, and positioning of neurons. Tbr1-positive early-born neurons, Brn1-positive later-born neurons, CSPG-positive preplate neurons, MAP2-positive neuronal processes, Cajal-Retzius cells (Reelin-positive), and glia (GFAP-positive astrocytes and RC2-positive radial glia) at embryonic stages E15.5 (SI Appendix, Fig. S10A) and E17.5 (SI Appendix, Fig. S10B) appeared indistinguishable between mutants and controls, indicating that Plexin-B2–mediated RhoA activation is not essential for corticogenesis.
Development of Liver Vasculature Requires Plexin-B2–Mediated RhoA Activation.
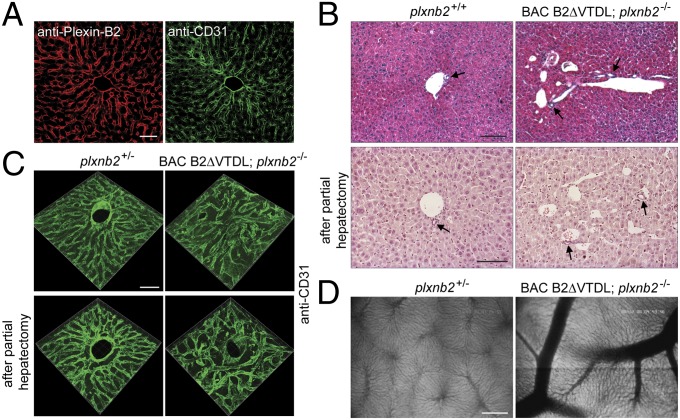
Plexin-B2 is strongly expressed in the endothelial cells of the hepatic sinusoids and portal veins (Fig. 5A) (46). We observed that mutant mice defective in RhoGEF binding exhibited malformations in the liver vasculature. These abnormalities included venous ectasias and misorganization of the vascular architecture (Fig. 5 B and C). Albeit not fully penetrant, this phenotype was found both under physiological conditions as well as in regenerating liver tissue after partial hepatectomy (Fig. 5 B and C). The vascular malformations were predominantly but not exclusively located at or in the vicinity of portal veins (Fig. 5 B and C). Consistent with this, intravital fluorescence microscopy revealed that sinusoidal blood flow and perfusion was largely unaffected (SI Appendix, Fig. S11 A–C), and signs of hepatocyte damage were not detectable (SI Appendix, Fig. S11 D and E). In addition to the venous ectasias, the livers of some mutant mice exhibited blood vessels with abnormally wide diameter, which were already visible macroscopically (Fig. 5D). These results show that Plexin-B2–mediated activation of RhoA is involved in the formation of the liver vasculature.
Fig. 5.
Plexin-B2–mediated RhoA signaling is required for development of the liver vasculature. (A) Immunofluorescence staining of adult liver using anti-Plexin-B2 and anti-CD31 antibodies. (Scale bar, 50 µm.) (B) H&E-stained sections derived from untreated liver (Upper) or regenerating liver 7 d after 2/3 partial hepatectomy (Lower). Arrows point to bile ducts. (Scale bars, 100 µm.) (C) 3D reconstructions of Z stacks of CD31-stained sections derived from untreated liver (Upper) or regenerating liver 7 d after 2/3 partial hepatectomy (Lower). (Scale bar, 50 µm.) (D) Intravital fluorescence microscopy pictures of adult livers. (Scale bar, 200 µm.)
Discussion
Plexins have been shown to regulate a number of different signaling pathways in diverse types of cells in vitro (10). Despite a wealth of literature on the important roles of plexins in development and disease, the signaling events downstream of plexins mediating their effects in vivo have been largely unknown. Here we directly addressed this fundamental question by generating an allelic series of BAC transgenic mice carrying subtle mutations in the Plexin-B2 and Plexin-D1 genes at sites that encode residues critical for distinct signaling pathways. Given that mouse null mutants of most genes are available, our BAC transgenic rescue approach represents an efficient alternative to conventional genetic approaches relying on the knockin of mutations by homologous recombination in embryonic stem cells, and is widely applicable to the analysis of other signaling pathways in vivo.
A common feature of all plexins is their intracellular GAP domain, which catalyzes the inactivation of R-Ras, M-Ras, and Rap1 upon binding of semaphorin ligands (11, 14, 15). Surprisingly, although plexins have been shown to regulate several independent signaling pathways in vitro, mutations in the GAP domain were sufficient to fully phenocopy the null mutants in vivo with respect to development of the nervous, the vascular, and the skeletal system. This crucial importance of the GAP domain of plexins during mouse development is in line with findings in Drosophila and Caenorhabditis elegans, where the GAP domains of PlexA and PLX-1 have been shown to be critical for development of the nervous system (47, 48). Owing to the perinatal lethality of Plexin-B2 and Plexin-D1 GAP domain mutant mice, we could not systematically address the functional significance of the GAP domain at postnatal stages of development or in physiological and pathophysiological processes in the adult [e.g., for Plexin-B2 in the postnatal migration and proliferation of neuroblasts (26) and in wound healing (49), or for Plexin-D1 in postnatal development of the nervous system (34, 35) and in angiogenesis (36, 50)]. Further studies using mice with floxed alleles crossed to the BAC transgenic and specific Cre mice will be required to address these open questions.
In addition to uncovering the crucial role of the plexin GAP domain in vivo, this study also revealed considerable insights into the mechanism of GAP domain function during development. The functional significance of the enzymatic activity of the plexin GAP domain has not been resolved hitherto in studies performed in vitro, owing to controversial observations: whereas several reports have shown that GAP domain-mediated inactivation of R-Ras and M-Ras regulates cellular behavior, including migration, axonal growth cone collapse, and dendrite morphology (11, 14, 38), others suggest that binding and sequestration of active R-Ras, rather than R-Ras inactivation, is required for the biological effects of plexins (15, 51). We found that mutations in all three critical arginines or the two N-terminal arginines of the Plexin-B2 GAP domain block both R-Ras GAP activity as well as R-Ras binding, whereas mutation of the C-terminal arginine results in the loss of GAP activity toward R-Ras while retaining the ability to bind R-Ras, thereby allowing a differentiation between these two scenarios. Our in vivo data on the phenotypes of transgenic mice expressing these different Plexin-B2 mutant versions favor a model whereby the enzymatic inactivation of small GTPases, rather than binding and sequestration, is the mechanism by which the plexin GAP domain exerts its biological effects.
To further test the functional relevance of plexin-mediated R-Ras and M-Ras inactivation in vivo, we performed a rescue experiment by genetically inactivating R-Ras and M-Ras in Plexin-B2- and Plexin-D1–deficient mice. Surprisingly, even the combined inactivation of R-Ras and M-Ras did not ameliorate the defects observed in the Plexin-B2- and Plexin-D1 null mutants, strongly suggesting that R-Ras and M-Ras are not the major effectors of Plexin-B2 and Plexin-D1 during development. Of note, plexins exert GAP activity toward R-Ras and M-Ras only when Rnd proteins are bound to their intracellular moieties (11, 14, 16). Interestingly, in vitro data show that the GAP domain of plexins can regulate cellular effects even in the absence of Rnd expression (30, 51). Recently, it has been shown that Rap1 proteins are alternative substrates of the plexin GAP domain and that GAP activity toward Rap1 does not require Rnd binding to plexins (15). Rap1 proteins are well-established regulators of inside-out signaling to integrins, cell adhesion, cell proliferation, and cell junction formation (52), and it is tempting to speculate that they could represent the critical substrates of the plexin GAP domain during development. Alternatively, the combinatorial inactivation of R-Ras, M-Ras, and Rap1 or the inactivation of an as yet unidentified GTPase could account for the developmental functions of plexins. The GTPase activity of plexins toward R-Ras and M-Ras might also be important at later developmental stages of neuronal development, including dendrite remodeling (14), or under pathophysiological conditions such as tumor angiogenesis, in which active R-Ras has been shown to improve vessel integrity and blood vessel perfusion (39, 53).
In addition to the GAP function common to all plexins, B-family plexins can activate the small GTPase RhoA, a central regulator of cytoskeletal dynamics and cell migration that is required for neocortical development (44, 45). Our data indicate that Plexin-B2–mediated RhoA activation is dispensable for development of the neocortex. Of note, the semaphorin ligand for Plexin-B2 in the embryonic cortex, Sema4D, also binds to the close Plexin-B2 homolog, Plexin-B1, with high affinity (1, 25). Although Plexin-B1 is strongly expressed in the developing neocortex, the nervous system of Plexin-B1–deficient mice develops normally (23, 41). It is plausible that the loss of Plexin-B1- or Plexin-B2–mediated regulation of RhoA activity could mutually be compensated for by the presence of the respective other family member and that the combined inhibition of this pathway could reveal its biological role in the developing neocortex.
In contrast to its redundant role in corticogenesis, we identified an involvement of Plexin-B2–mediated RhoA activation in the formation of the liver vasculature, indicating that the requirement for RhoA activation varies depending upon the tissue. The observed abnormalities are in line with the expression pattern of Plexin-B2 in the highly specialized discontinuous endothelium of the liver. During both development and liver regeneration in the adult, the hepatic portal veins and sinusoids derive from preexisting vessels through angiogenesis (54, 55). There is evidence that RhoA activation downstream of Plexin-B1 stimulates cellular processes in endothelial cells that are crucial for angiogenesis, including chemotactic migration and tubulogenesis (56). Of note, in liver sinusoidal endothelial cells, a critical role for RhoA in the regulation of the actin cytoskeleton has been described (57).
Apart from its role in the liver vasculature, Plexin-B2–mediated activation of RhoA may also be important under certain pathophysiological conditions. Indeed, there is evidence that RhoA signaling via the Plexin-B2 homolog, Plexin-B1, is crucially involved in the suppression of osteoblast differentiation and in the metastatic dissemination of ErbB-2–positive cancer cells (21, 58).
In summary, we demonstrate that Plexin-B2 and Plexin-D1 exert their developmental functions through the GAP domain independently of the inactivation of R-Ras and M-Ras. In addition, we show that Plexin-B2–mediated RhoA activation is required for development of the liver vasculature. This study clarifies signaling pathways that account for the diverse biological functions of plexins in mouse development in vivo and underscores the context- and tissue-dependence of the recruitment of specific pathways downstream of an activated plexin.
Materials and Methods
Generation of BAC Transgenic Mice.
The following BAC clones were obtained from the BACPAC Resource Center (Children’s Hospital Oakland Research Institute, Oakland, CA): RP24-245O13 carrying the murine plxnb2 gene, and RP23-396M22 carrying the murine plxnd1 gene. All BAC modifications were performed using the Counter Selection BAC Modification Kit (Gene Bridges). Further details are provided in SI Appendix, SI Materials and Methods.
RNA Extraction and RT-PCR.
RNA extraction was performed using an RNeasy Kit (Qiagen) according to the manufacturer's instructions. RT-PCR was done using standard reagents and protocols (Fermentas).
Supplementary Material
Acknowledgments
Rras−/− mice were kindly provided by Erkki Ruoslahti.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308418111/-/DCSupplemental.
References
- 1.Tamagnone L, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99(1):71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- 2.Pasterkamp RJ. Getting neural circuits into shape with semaphorins. Nat Rev Neurosci. 2012;13(9):605–618. doi: 10.1038/nrn3302. [DOI] [PubMed] [Google Scholar]
- 3.Bussolino F, Valdembri D, Caccavari F, Serini G. Semaphoring vascular morphogenesis. Endothelium. 2006;13(2):81–91. doi: 10.1080/10623320600698003. [DOI] [PubMed] [Google Scholar]
- 4.Sakurai A, Doçi CL, Gutkind JS. Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer. Cell Res. 2012;22(1):23–32. doi: 10.1038/cr.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perälä N, Sariola H, Immonen T. More than nervous: The emerging roles of plexins. Differentiation. 2012;83(1):77–91. doi: 10.1016/j.diff.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Kang S, Kumanogoh A. Semaphorins in bone development, homeostasis, and disease. Semin Cell Dev Biol. 2013;24(3):163–171. doi: 10.1016/j.semcdb.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Takamatsu H, Kumanogoh A. Diverse roles for semaphorin-plexin signaling in the immune system. Trends Immunol. 2012;33(3):127–135. doi: 10.1016/j.it.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Tamagnone L. Emerging role of semaphorins as major regulatory signals and potential therapeutic targets in cancer. Cancer Cell. 2012;22(2):145–152. doi: 10.1016/j.ccr.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 9.Wannemacher KM, Wang L, Zhu L, Brass LF. The role of semaphorins and their receptors in platelets: Lessons learned from neuronal and immune synapses. Platelets. 2011;22(6):461–465. doi: 10.3109/09537104.2011.561891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hota PK, Buck M. Plexin structures are coming: Opportunities for multilevel investigations of semaphorin guidance receptors, their cell signaling mechanisms, and functions. Cell Mol Life Sci. 2012;69(22):3765–3805. doi: 10.1007/s00018-012-1019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305(5685):862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- 12.Oinuma I, Katoh H, Negishi M. Molecular dissection of the semaphorin 4D receptor plexin-B1-stimulated R-Ras GTPase-activating protein activity and neurite remodeling in hippocampal neurons. J Neurosci. 2004;24(50):11473–11480. doi: 10.1523/JNEUROSCI.3257-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rohm B, Rahim B, Kleiber B, Hovatta I, Püschel AW. The semaphorin 3A receptor may directly regulate the activity of small GTPases. FEBS Lett. 2000;486(1):68–72. doi: 10.1016/s0014-5793(00)02240-7. [DOI] [PubMed] [Google Scholar]
- 14.Saito Y, Oinuma I, Fujimoto S, Negishi M. Plexin-B1 is a GTPase activating protein for M-Ras, remodelling dendrite morphology. EMBO Rep. 2009;10(6):614–621. doi: 10.1038/embor.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, et al. Plexins are GTPase-activating proteins for Rap and are activated by induced dimerization. Sci Signal. 2012;5(207):ra6. doi: 10.1126/scisignal.2002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uesugi K, Oinuma I, Katoh H, Negishi M. Different requirement for Rnd GTPases of R-Ras GAP activity of Plexin-C1 and Plexin-D1. J Biol Chem. 2009;284(11):6743–6751. doi: 10.1074/jbc.M805213200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyofuku T, et al. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat Neurosci. 2005;8(12):1712–1719. doi: 10.1038/nn1596. [DOI] [PubMed] [Google Scholar]
- 18.Aurandt J, Vikis HG, Gutkind JS, Ahn N, Guan KL. The semaphorin receptor plexin-B1 signals through a direct interaction with the Rho-specific nucleotide exchange factor, LARG. Proc Natl Acad Sci USA. 2002;99(19):12085–12090. doi: 10.1073/pnas.142433199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrot V, Vazquez-Prado J, Gutkind JS. Plexin B regulates Rho through the guanine nucleotide exchange factors leukemia-associated Rho GEF (LARG) and PDZ-RhoGEF. J Biol Chem. 2002;277(45):43115–43120. doi: 10.1074/jbc.M206005200. [DOI] [PubMed] [Google Scholar]
- 20.Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35(1):51–63. doi: 10.1016/s0896-6273(02)00750-x. [DOI] [PubMed] [Google Scholar]
- 21.Worzfeld T, et al. ErbB-2 signals through Plexin-B1 to promote breast cancer metastasis. J Clin Invest. 2012;122(4):1296–1305. doi: 10.1172/JCI60568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swiercz JM, Worzfeld T, Offermanns S. Semaphorin 4D signaling requires the recruitment of phospholipase C gamma into the plexin-B1 receptor complex. Mol Cell Biol. 2009;29(23):6321–6334. doi: 10.1128/MCB.00103-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng S, et al. Plexin-B2, but not Plexin-B1, critically modulates neuronal migration and patterning of the developing nervous system in vivo. J Neurosci. 2007;27(23):6333–6347. doi: 10.1523/JNEUROSCI.5381-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedel RH, et al. Plexin-B2 controls the development of cerebellar granule cells. J Neurosci. 2007;27(14):3921–3932. doi: 10.1523/JNEUROSCI.4710-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirschberg A, et al. Gene deletion mutants reveal a role for semaphorin receptors of the plexin-B family in mechanisms underlying corticogenesis. Mol Cell Biol. 2010;30(3):764–780. doi: 10.1128/MCB.01458-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saha B, Ypsilanti AR, Boutin C, Cremer H, Chédotal A. Plexin-B2 regulates the proliferation and migration of neuroblasts in the postnatal and adult subventricular zone. J Neurosci. 2012;32(47):16892–16905. doi: 10.1523/JNEUROSCI.0344-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Zwaag B, et al. PLEXIN-D1, a novel plexin family member, is expressed in vascular endothelium and the central nervous system during mouse embryogenesis. Dev Dyn. 2002;225(3):336–343. doi: 10.1002/dvdy.10159. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Oh WJ, Gaiano N, Yoshida Y, Gu C. Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes Dev. 2011;25(13):1399–1411. doi: 10.1101/gad.2042011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell. 2004;7(1):107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Gu C, et al. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307(5707):265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 31.Torres-Vázquez J, et al. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004;7(1):117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Chauvet S, et al. Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron. 2007;56(5):807–822. doi: 10.1016/j.neuron.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi YI, et al. PlexinD1 glycoprotein controls migration of positively selected thymocytes into the medulla. Immunity. 2008;29(6):888–898. doi: 10.1016/j.immuni.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding JB, Oh WJ, Sabatini BL, Gu C. Semaphorin 3E-Plexin-D1 signaling controls pathway-specific synapse formation in the striatum. Nat Neurosci. 2012;15(2):215–223. doi: 10.1038/nn.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, Arber S. Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature. 2009;459(7248):842–846. doi: 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukushima Y, et al. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest. 2011;121(5):1974–1985. doi: 10.1172/JCI44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujiyama T, et al. Inhibitory and excitatory subtypes of cochlear nucleus neurons are defined by distinct bHLH transcription factors, Ptf1a and Atoh1. Development. 2009;136(12):2049–2058. doi: 10.1242/dev.033480. [DOI] [PubMed] [Google Scholar]
- 38.Oinuma I, Katoh H, Negishi M. Semaphorin 4D/Plexin-B1-mediated R-Ras GAP activity inhibits cell migration by regulating beta(1) integrin activity. J Cell Biol. 2006;173(4):601–613. doi: 10.1083/jcb.200508204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komatsu M, Ruoslahti E. R-Ras is a global regulator of vascular regeneration that suppresses intimal hyperplasia and tumor angiogenesis. Nat Med. 2005;11(12):1346–1350. doi: 10.1038/nm1324. [DOI] [PubMed] [Google Scholar]
- 40.Nuñez Rodriguez N, et al. Characterization of R-ras3/m-ras null mice reveals a potential role in trophic factor signaling. Mol Cell Biol. 2006;26(19):7145–7154. doi: 10.1128/MCB.00476-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Worzfeld T, Püschel AW, Offermanns S, Kuner R. Plexin-B family members demonstrate non-redundant expression patterns in the developing mouse nervous system: An anatomical basis for morphogenetic effects of Sema4D during development. Eur J Neurosci. 2004;19(10):2622–2632. doi: 10.1111/j.0953-816X.2004.03401.x. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Billault C, et al. The role of small GTPases in neuronal morphogenesis and polarity. Cytoskeleton (Hoboken) 2012;69(7):464–485. doi: 10.1002/cm.21034. [DOI] [PubMed] [Google Scholar]
- 43.Linseman DA, Loucks FA. Diverse roles of Rho family GTPases in neuronal development, survival, and death. Front Biosci. 2008;13:657–676. doi: 10.2741/2710. [DOI] [PubMed] [Google Scholar]
- 44.Cappello S, et al. A radial glia-specific role of RhoA in double cortex formation. Neuron. 2012;73(5):911–924. doi: 10.1016/j.neuron.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 45.Govek EE, Hatten ME, Van Aelst L. The role of Rho GTPase proteins in CNS neuronal migration. Dev Neurobiol. 2011;71(6):528–553. doi: 10.1002/dneu.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zielonka M, Xia J, Friedel RH, Offermanns S, Worzfeld T. A systematic expression analysis implicates Plexin-B2 and its ligand Sema4C in the regulation of the vascular and endocrine system. Exp Cell Res. 2010;316(15):2477–2486. doi: 10.1016/j.yexcr.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Mizumoto K, Shen K. Interaxonal interaction defines tiled presynaptic innervation in C. elegans. Neuron. 2013;77(4):655–666. doi: 10.1016/j.neuron.2012.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang T, Terman JR. 14-3-3ε couples protein kinase A to semaphorin signaling and silences plexin RasGAP-mediated axonal repulsion. Neuron. 2012;74(1):108–121. doi: 10.1016/j.neuron.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Witherden DA, et al. The CD100 receptor interacts with its plexin B2 ligand to regulate epidermal γδ T cell function. Immunity. 2012;37(2):314–325. doi: 10.1016/j.immuni.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casazza A, et al. Sema3E-Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J Clin Invest. 2010;120(8):2684–2698. doi: 10.1172/JCI42118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakurai A, et al. Semaphorin 3E initiates antiangiogenic signaling through plexin D1 by regulating Arf6 and R-Ras. Mol Cell Biol. 2010;30(12):3086–3098. doi: 10.1128/MCB.01652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frische EW, Zwartkruis FJ. Rap1, a mercenary among the Ras-like GTPases. Dev Biol. 2010;340(1):1–9. doi: 10.1016/j.ydbio.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 53.Sawada J, et al. Small GTPase R-Ras regulates integrity and functionality of tumor blood vessels. Cancer Cell. 2012;22(2):235–249. doi: 10.1016/j.ccr.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collardeau-Frachon S, Scoazec JY. Vascular development and differentiation during human liver organogenesis. Anat Rec (Hoboken) 2008;291(6):614–627. doi: 10.1002/ar.20679. [DOI] [PubMed] [Google Scholar]
- 55.Ding BS, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468(7321):310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 2004;64(15):5212–5224. doi: 10.1158/0008-5472.CAN-04-0126. [DOI] [PubMed] [Google Scholar]
- 57.Yokomori H, et al. Rho modulates hepatic sinusoidal endothelial fenestrae via regulation of the actin cytoskeleton in rat endothelial cells. Lab Invest. 2004;84(7):857–864. doi: 10.1038/labinvest.3700114. [DOI] [PubMed] [Google Scholar]
- 58.Negishi-Koga T, et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med. 2011;17(11):1473–1480. doi: 10.1038/nm.2489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.