Significance
Nanoliposomal packaging of chemotherapeutics can increase efficacy while reducing toxicity, but its use is currently limited due to inefficient loading strategies. We developed an active loading procedure by designing a transient chemical handle for ferrying such agents into preformed liposomes. We illustrate its use with two candidates that recently failed phase II studies. We were able to load both these drugs into liposomes with high efficiency and administer them to animals at substantially higher doses than previously possible, thereby simultaneously enhancing efficacy and reducing toxicity. This strategy should be applicable to many agents that are currently being developed to treat cancer or other diseases, and can “rescue” drugs that would otherwise fail at the final stages of the drug discovery process.
Keywords: nanoliposomes, PLK-1 inhibitor, MEK-1 inhibitor
Abstract
Loading drugs into carriers such as liposomes can increase the therapeutic ratio by reducing drug concentrations in normal tissues and raising their concentrations in tumors. Although this strategy has proven advantageous in certain circumstances, many drugs are highly hydrophobic and nonionizable and cannot be loaded into liposomes through conventional means. We hypothesized that such drugs could be actively loaded into liposomes by encapsulating them into specially designed cyclodextrins. To test this hypothesis, two hydrophobic drugs that had failed phase II clinical trials because of excess toxicity at deliverable doses were evaluated. In both cases, the drugs could be remotely loaded into liposomes after their encapsulation (preloading) into cyclodextrins and administered to mice at higher doses and with greater efficacy than possible with the free drugs.
There is currently wide interest in the development of nanoparticles for drug delivery (1–7). This area of research is particularly relevant to cancer drugs, wherein the therapeutic ratio (dose required for effectiveness to dose causing toxicity) is often low. Nanoparticles carrying drugs can increase this therapeutic ratio over that achieved with the free drug through several mechanisms. In particular, drugs delivered by nanoparticles are thought to selectively enhance the concentration of the drugs in tumors as a result of the enhanced permeability and retention (EPR) effect (8–18). The enhanced permeability results from a leaky tumor vascular system, whereas the enhanced retention results from the disorganized lymphatic system that is characteristic of malignant tumors.
Much current work in this field is devoted to designing novel materials for nanoparticle generation. This new generation of nanoparticles can carry drugs—particularly those that are insoluble in aqueous medium—that are difficult to incorporate into conventional nanoparticles such as liposomes. However, the older generation of nanoparticles has a major practical advantage in that they have been extensively tested in humans and approved by regulatory agencies such as the Food and Drug Administration in the United States and the European Medicines Agency in Europe. Unfortunately, many drugs cannot be easily or effectively loaded into liposomes, thereby compromising their general use.
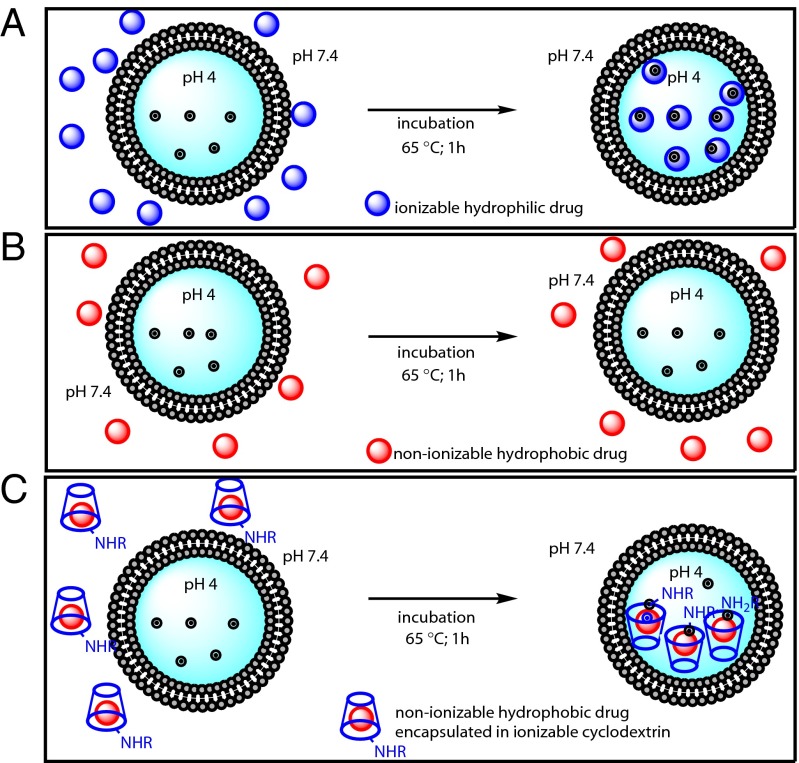
In general, liposomal drug loading is achieved by either passive or active methods (9, 19–22). Passive loading involves dissolution of dried lipid films in aqueous solutions containing the drug of interest. This approach can only be used for water-soluble drugs, and the efficiency of loading is often low. In contrast, active loading can be extremely efficient, resulting in high intraliposomal concentrations and minimal wastage of precious chemotherapeutic agents (9, 23, 24). In active loading, drug internalization into preformed liposomes is typically driven by a transmembrane pH gradient. The pH outside the liposome allows some of the drug to exist in an unionized form, able to migrate across the lipid bilayer. Once inside the liposome, the drug becomes ionized due to the differing pH and becomes trapped there (Fig. 1A). Many reports have emphasized the dependence of liposome loading on the nature of the transmembrane pH gradient, membrane–water partitioning, internal buffering capacity, aqueous solubility of the drug, lipid composition, and other factors (25–28). As described in a recent model (28), the aqueous solubility of the drug is one of the requirements for efficient active loading. Another key element for the success of remote loading is the presence of weakly basic functional groups on the small molecule.
Fig. 1.
Schematic representation of active loading of a liposome. (A) Remote loading of an ionizable hydrophilic drug using a transmembrane pH results in efficient incorporation. (B) A poorly soluble hydrophobic drug results in meager incorporation into preformed liposomes under similar conditions. (C) Encapsulation of a poorly soluble drug into an ionizable cyclodextrin (R = H, ionizable alkyl or aryl groups) enhances its water solubility and permits efficient liposomal loading via a pH gradient.
Only a small fraction of chemotherapeutic agents possesses the features required for active loading with established techniques. Attempts at active loading of such nonionizable drugs into preformed liposomes result in poor loading efficiencies (Fig. 1B). One potential solution to this problem is the addition of weakly basic functional groups to otherwise unloadable drugs, an addition that would provide the charge necessary to drive these drugs across the pH gradient. However, covalent modification of drugs often alters their biological and chemical properties, and is not desirable in many circumstances. We therefore attempted to develop a general strategy that would allow loading of unmodified hydrophobic chemotherapeutics devoid of weakly basic functional groups. For this purpose, we used modified β-cyclodextrins to facilitate the loading of such drugs into liposomes (Fig. 1C).
Cyclodextrins are a family of cyclic sugars that are commonly used to solubilize hydrophobic drugs (29–32). They possess a hydrophobic cavity and a hydrophilic surface and are known to stably encapsulate a large variety of hydrophobic organic molecules in aqueous media. Cyclodextrins bind to their cargos strongly enough to form relatively stable complexes, but allow a slow efflux of the entrapped drug and consequent steady concentrations of free drug once administered in vivo (33, 34). Cyclodextrins are nontoxic and biologically inert, and several have been approved for use in humans (35–38).
In this work, we synthesized cyclodextrins with multiple weakly basic or weakly acidic functional groups on their solvent-exposed surfaces. We hypothesized that these modified cyclodextrins would still be able to encapsulate chemotherapeutic agents and that the cyclodextrin–drug complexes could then be remotely loaded into liposomes using pH gradients that exploited ionizable groups on the cyclodextrins rather than on the drugs themselves.
Results
Chemically Modified β-Cyclodextrins Can Be Remotely Loaded into Liposomes.
We first designed and synthesized a set of modified cyclodextrins bearing ionizable groups at their 6′ position (Scheme S1). In analogs II–V, the 6′ primary hydroxyl moiety was modified to an amino group, an ethylenediamino group, or a fluorescent version of either, whereas analog VIII involved introduction of a succinyl group in that position. The rest of the analogs (VI, VII, and IX) had all seven primary hydroxyls replaced by amino, ethylenediamino, or succinyl moieties. All analogs were purified by HPLC and characterized by MS and NMR spectra. Appropriate negative controls were synthesized by introducing similarly sized chemical modifications not containing ionizable groups.
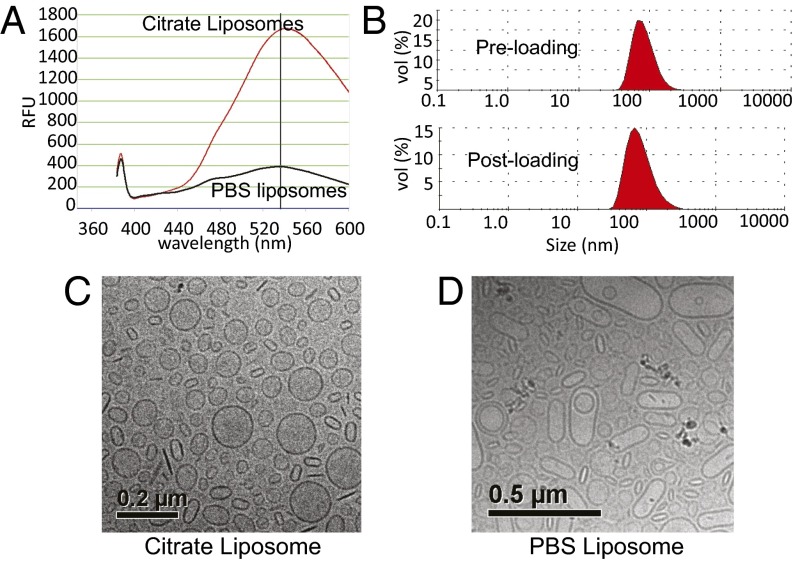
We then tested two fluorescent (dansylated) cyclodextrins (compounds IV and V) for their ability to be loaded into liposomes. The liposomes were generated by hydrating lipid films with 200 mM citrate buffer, so that their internal pH was 4.0. These liposomes were then dialyzed in PBS (pH 7.4) to remove the citrate from outside the liposomes and incubated at 65 °C for 1 h with cyclodextrins that had been dissolved in PBS. As a control, PBS-loaded liposomes were generated by rehydration of the lipid film with PBS instead of citrate. The incubation at relatively high temperature (65 °C) enhanced the fluidity of the lipid bilayer, thus allowing the cyclodextrins to cross it. The suspensions were then dialyzed overnight in PBS to remove cyclodextrins that had not been incorporated into liposomes, and analyzed for dansyl fluorescence. These experiments showed that >90% of each of these cyclodextrins were entrapped in the liposomes (example in Fig. 2A and Fig. S1). In the absence of a pH gradient, there was little incorporation of the same compounds into the liposomes (Fig. 2A). Light scattering showed that the preformed “empty” liposomes had a mean diameter of 98 nm with a narrow polydispersity index (PDI) (<0.10) (Fig. 2B). Incorporation of the cyclodextrins just slightly increased the mean diameter to 105 nm, without changing the PDI (Fig. 2B). Cryo-transmission electron microscopy revealed that the structure of the liposomes following incorporation of cyclodextrins was unchanged except for an increased density within the liposomes, presumably reflecting the high concentration of cyclodextrins within them (Fig. 2C). In contrast, cyclodextrins incubated with control, PBS-containing liposomes with no transmembrane pH gradient resulted in irregularly shaped, large vesicles, with no evidence of cyclodextrin incorporation within them (Fig. 2D). The change in shape observed with the control liposomes was presumably due to association of cyclodextrins with the lipid bilayer, leading to destabilization and “bloating” of the liposomal structure.
Fig. 2.
Active loading of modified β-cyclodextrin using a transmembrane pH gradient. (A) Fluorescence of β-cyclodextrin V in relative fluorescence units loaded into liposomes with a pH gradient (citrate liposomes) compared with that of the same compound loaded into liposomes in the absence of a pH gradient (PBS liposomes). (B) Dynamic light-scattering measurements show a marginal increase in hydrodynamic radius but no change in the PDI of liposomes remotely loaded with cyclodextrin V. (C and D) Cryo-transmission electron microscopy images of dansylated β-cyclodextrin V loaded with a pH gradient (C) (citrate liposomes) or without a pH gradient (D) (PBS liposomes).
Chemically Modified β-Cyclodextrins Can Encapsulate Small Hydrophobic Compounds and Ferry Them into Liposomes.
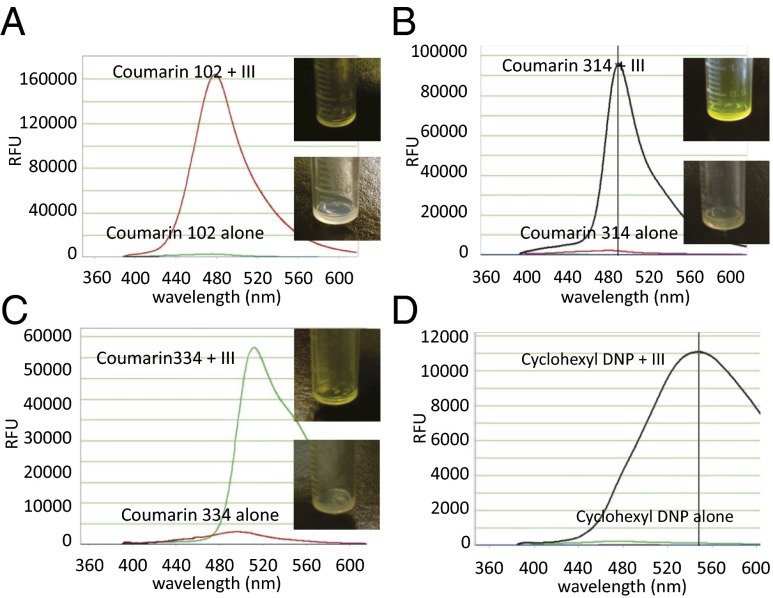
We then used organic dyes (coumarins) to determine whether the modified cyclodextrins could encapsulate and transport hydrophobic compounds across the liposome bilayer. Coumarins are very hydrophobic, and a dramatic improvement in aqueous solubility was observed after they were encapsulated into 6′-monoethylenediamino-6′-deoxy-cyclodextrin (compound III). We found that the most efficient and convenient way to encapsulate the coumarins was by freeze drying (39, 40). The solubility of the coumarins increased at least 10- to 20-fold (from 100 μg/mL to 1–2 mg/mL) through this procedure. Once dissolved, the cyclodextrin–coumarin complexes were incubated with preformed liposomes exactly as described above, using a pH gradient to drive the compounds across the bilayer. Following overnight dialysis to remove unincorporated complexes, the liposomes were subsequently disrupted with methanol and the coumarin fluorescence was measured. As shown by fluorescence spectroscopy, all cyclodextrin–dye complexes were incorporated into liposomes with high efficiency (>95%; Fig. 3). To ascertain that this highly efficient loading was indeed due to active transportation of the complex across the lipid membrane and not due to enhanced water solubility only, we evaluated the loading efficiencies of coumarin 314 in the absence of cyclodextrins and found it to be poorly incorporated into liposomes under the identical conditions (Fig. S2). Importantly, coumarin 314 encapsulated in unionizable native β-cyclodextrin only marginally improved the loading efficiency, despite substantially increased aqueous solubility of the dye. The incorporation of coumarin dyes into liposomes was easily discerned by the naked eye: The coumarin–cyclodextrin liposomes were bright yellow, whereas control liposomes (made without cyclodextrins, for example) were colorless (Fig. 3 A–C).
Fig. 3.
Remote loading of insoluble hydrophobic dyes into liposomes using modified β-cyclodextrins. Fluorescence intensity of remotely loaded coumarin 102 (A), coumarin 314 (B), coumarin 334 (C), and cyclohexyl 2,4-dinitrophenylhydrazone (DNP) (D). (Insets) Photos of vials containing liposomes incubated with the cyclodextrin-encapsulated dye (Upper) or free dye (Lower).
Chemotherapeutic Agents Can Be Loaded into Liposomes After Cyclodextrin Encapsulation.
We next investigated the ability of these amino-functionalized cyclodextrins to engender a liposomal formulation of BI-2536 (Fig. S3). BI-2536, developed by Boehringer Ingelheim, is a highly selective inhibitor of polo-like kinase-1 (PLK1), an enzyme required for the proper execution of mitosis (41–43). It has been shown that BI-2536 has potent tumoricidal activity against cancer cells, particularly those harboring mutations in TP53 (44–47). BI-2536 was the subject of several clinical trials in patients with cancers of the lung, breast, ovaries, and uterus (48–53). Although it showed evidence of efficacy in cancer patients, its development was abandoned after phase II trials revealed unacceptable toxicity (grade 4 neutropenia) at subtherapeutic doses.
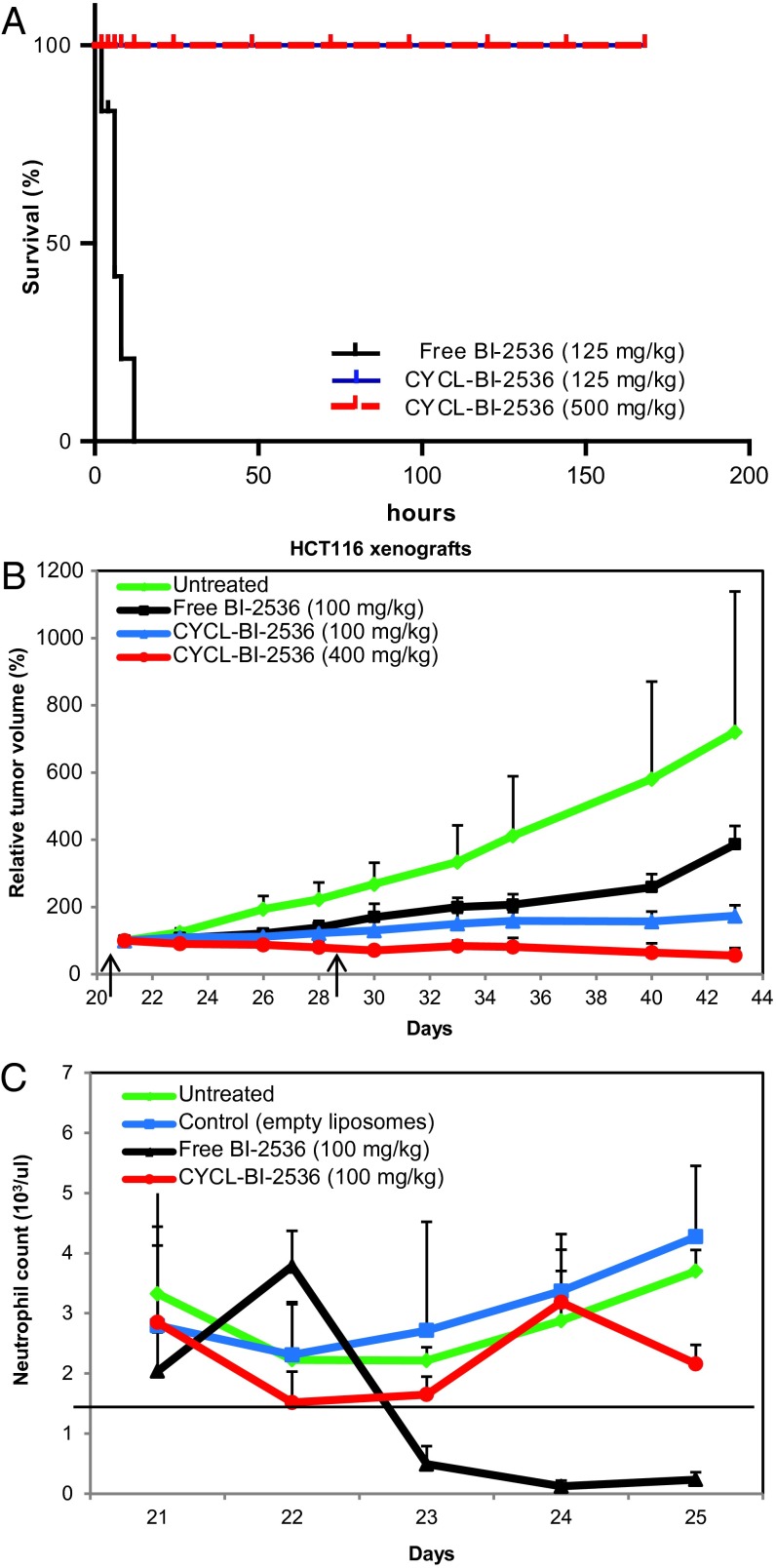
We found that aminated cyclodextrins VI and VII dramatically improved the aqueous solubility of BI-2536. As with the coumarins, we were able to reproducibly load the BI-2536 complexes into liposomes using compound VI, achieving stable aqueous solutions containing 10 mg/mL of drug. By comparison, the maximum aqueous solubility of free BI-2536 was determined to be 0.5 mg/mL. To assess the activity of cyclodextrin-encapsulated, liposomal (CYCL) forms of BI-2536, we assessed their effects in nude mice bearing s.c. xenografts of human HCT116 colorectal cancer cells. Three weeks after HCT116 cells were s.c. injected into the mice they were treated with empty liposomes, free BI-2536, or CYCL-BI-2536. At the initiation of treatment, the tumors were already relatively large, averaging ∼300 mm3 and more closely mimicking clinical situations than small tumors. Severe acute toxicity was evident when the free drug was administered intravenously (i.v.) at 125 mg/kg: The mice became lethargic within minutes, their eyes turned glassy, they exhibited ruffled fur, and died a few hours later (Fig. 4A). Mice treated with a slightly lower dose of free BI-2536 (100 mg/kg) were somewhat lethargic immediately after drug administration, but survived. However, delayed toxicity, manifest as a drastic decrease in peripheral WBCs, was evident within 24–36 h after free drug administration. This type of toxicity was identical to that observed in human clinical trials (53–55). Although toxic to bone marrow, the free BI-2536 was able to induce a significant antitumor response, slowing tumor growth by ∼30% after two doses at its maximum tolerated dose (MTD) (Fig. 4B). This efficacy was previously observed in other murine models (41, 47, 56–59), and provided the rationale for the clinical trials.
Fig. 4.
Loading and activity of the PLK1 inhibitor BI-2536. (A) Survival of animals injected with BI-2536 and CYCL-BI-2536. All treated animals (n = 5) succumbed overnight to a single i.v. dose (125 mg/kg) of BI-2536 in its free form, whereas a single i.v. dose of CYCL-BI-2536 did not elicit any signs of acute toxicity at similar (125 mg/kg; n = 5) or much higher (500 mg/kg; n = 5) doses. (B) Nude mice (n = 4 per arm) bearing HCT116 xenografts were treated with two i.v. doses (on days indicated by arrows) of (i) empty liposomes, (ii) free BI-2536 (100 mg/kg), (iii) CYCL-BI-2536 (100 mg/kg), and (iv) CYCL-BI-2536 (400 mg/kg). Liposomal formulations have been reported as equivalents of free drug; relative tumor volumes and SD of each experimental arm are shown. (C) Nude mice bearing HCT116 xenografts (n = 5 per arm) were treated with a single i.v. dose of (i) empty liposomes, (ii) BI-2536 (100 mg/kg), or (iii) CYCL-BI-2536 (100 mg/kg). Neutrophils were counted before any drug treatment and every 24 h thereafter. Means and SD of the neutrophil counts of five mice in each treatment arm are shown.
CYCL-BI-2536 proved far superior to the free form, with respect to both toxicity and efficacy. CYCL-BI-2536, even at a dose of 500 mg/kg, did not cause any noticeable adverse reactions; this dose was fourfold higher than the dose of free drug, which killed every animal (Fig. 4A). At a dose of 100 mg/kg (equivalent to the MTD of the free drug), CYCL-BI-2536 induced a significantly improved tumor response, slowing tumor growth by nearly 80% after only two doses (Fig. 4B). At a dose of 400 mg/kg, CYCL-BI-2536 resulted not only in slower growth but also in partial regressions of tumors (Fig. 4B). The equivalent dose of free CYCL-BI-2536 could not be administered, because the mice could not survive a dose even close to this amount (Fig. 4A). Moreover, relatively little bone marrow toxicity resulted from treatment with CYCL-BI-2536, as the WBC decrease was much less and did not pose a risk to the animals (Fig. 4C). Finally, we found that CYCL-BI-2536 had much greater efficacy than the free drug against a second human colorectal cancer model (HCT116 cells with genetically inactivated TP53 alleles). In both cases, significant regressions were observed with the CYCL form of the drug, but not with free drug.
To establish biodistribution and pharmacokinetics of the CYCL liposomes, we used liposomes loaded with coumarin 334 encapsulated in cyclodextrin VI and treated HCT116-bearing mice by i.v. injection. Samples from major tissues harvested at 2, 24, and 48 h posttreatment were analyzed for their fluorescence. As expected, coumarin 334 was cleared from most of the tissues examined at 48 h after treatment. Importantly, the agent encapsulated in liposomes persisted in the blood and tumor, which is consistent with the typical pharmacokinetics of PEGylated liposomes (Fig. S4).
We then compared our cyclodextrin-based loading method with the most common approaches to entrapping hydrophobic and insoluble agents in liposomes. First, we attempted to directly entrap BI-2536 in the lipid bilayer. BI-2536 was coevaporated with hydrogenated egg phosphatidylcholine–cholesterol–1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] to prepare a thin film, which was subsequently hydrated with 1 mL PBS and extruded through a 100-nm polycarbonate membrane at 700 psi to prepare small, unilamellar vesicles [average particle size (Zavg) 126 nm; PDI 0.09]. Upon overnight dialysis against PBS to remove unentrapped drug, the drug-containing liposomes rapidly swelled (Zavg 539 nm; PDI 0.49) and released nearly 90% of the entrapped drug. Second, we hydrated the lipid film with an aqueous formulation of BI-2536 (passive loading). Hydration of the lipid film, followed by extrusion and dialyses, led to stable liposomes. However, the encapsulation efficiency was <10%, 20-fold less than achievable with our modified cyclodextrins.
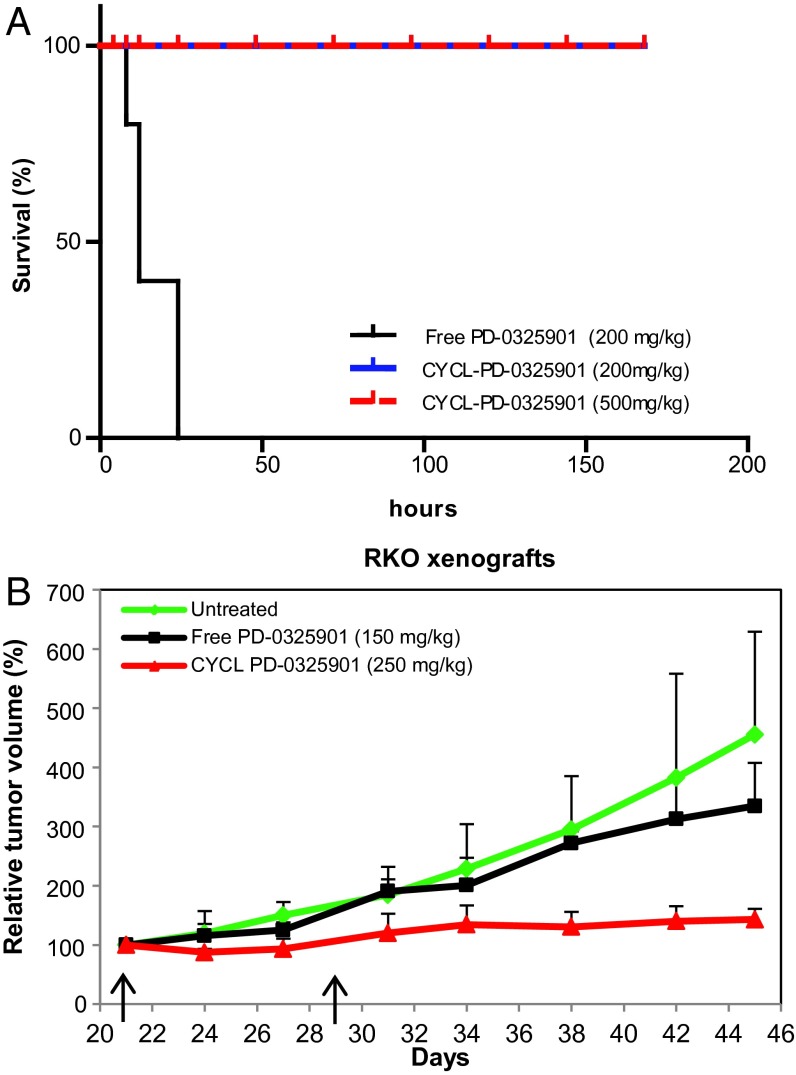
To determine whether other hydrophobic drugs could be encapsulated using our approach, we evaluated PD-0325901 (Fig. S3) (60, 61), a mitogen-activated protein kinase kinase 1 (MEK1) inhibitor developed by Pfizer that was abandoned because it caused retinal vein occlusion in phase II trials (62–64). We tested aminated (Scheme S1, compounds VI and VII) as well as succinylated (Scheme S1, compounds VIII and IX) cyclodextrins for their ability to encapsulate PD-0325901 and load them into acidic or basic liposomes, respectively. The free drug was exceedingly insoluble (0.1 mg/mL in water), and its solubility increased by nearly 40-fold after encapsulation into cyclodextrins. The best liposome loading was achieved with succinylated cyclodextrin IX, and this formulation was tested in animals bearing human tumor xenografts as described for BI-2536 above. As with BI-2536, we were able to reproducibly load the PD-0325901 complexes into liposomes, achieving stable solutions containing 5 mg/mL of drug. To assess the activity of CYCL forms of PD-0325901, we examined their effects in the RKO xenograft model. With the free drug, severe acute toxicity was evident. In previous studies, the free drug was administered by oral gavage, because its solubility is not sufficient for i.v. dosing. After oral gavage at 200 mg/kg, the treated animals were lethargic within minutes and over the next few hours they appeared hunched, always dying within 24 h (Fig. 5A). Mice treated with a slightly lower dose of free PD-0325901 (150 mg/kg) exhibited similar symptoms immediately after dosing but recovered over 24 h. However, a single dose of free PD-0325901 was unable to induce a dramatic antitumor response, slowing tumor growth only by ∼5% (Fig. 5B). This result was consistent with those previously reported in other murine models; higher efficacy of the free drug required multiple doses.
Fig. 5.
Loading and activity of the MEK1 inhibitor PD-0325901. (A) Survival curves of animals treated with a single dose of PD-0325901 and CYCL-PD-0325901. Nude mice bearing RKO xenografts were treated with a single dose (200 mg/kg; n = 5) of PD-0325901 in its free form, a single i.v. dose of CYCL-PD-0325901 at a low dose (200 mg/kg; n = 5), or a higher dose (500 mg/kg; n = 5). (B) Nude mice bearing RKO xenografts (n = 4 per arm) were treated with two i.v. doses (on days indicated by arrows) of (i) blank liposomes, (ii) free PD-0325901 (150 mg/kg), or (iii) CYCL-PD-0325901 (250 mg/kg). Liposomal formulations have been reported as equivalents of free drug; relative tumor volumes and SD of each experimental arm are shown.
CYCL-PD-0325901 proved far superior to the free drug. Even at a dose of 500 mg/kg, CYCL-PD-0325901 did not cause any noticeable adverse reactions; this dose was 2.5-fold higher than the dose of free drug that killed every animal (Fig. 5A). At a single dose of 250 mg/kg, CYCL-PD-0325901 resulted not only in slower growth but also in partial regressions of tumors (Fig. 5B). Finally, we evaluated CYCL-PD-0325901 against the two other human colorectal cancer models (HCT116 and its isogenic counterpart with genetically inactivated TP53 alleles) and similarly observed higher efficacy and lower toxicity compared with the free drug (Fig. S5).
Discussion
The results described above suggest a general strategy for loading hydrophobic drugs into liposomes. This strategy employs modified cyclodextrins with ionizable groups on their external surfaces (Scheme S1). The “pockets” of these cyclodextrins can encapsulate hydrophobic drugs and ferry them across the bilayer membrane of conventional liposomes using simple pH gradients. We have successfully encapsulated several types of compounds into the modified cyclodextrins, including coumarin dyes and drugs of potential clinical importance (Figs. 2–5). This incorporation not only dramatically increased the aqueous solubility of all these compounds but also allowed them to be remotely loaded into liposomes. And, most importantly, the loaded liposomes exhibited substantially less toxicity (Fig. 4) and greater activity (Figs. 4 and 5) when tested in mouse models of cancer.
Previous attempts to combine cyclodextrin inclusion complexes with liposomes were limited to passive loading of insoluble drugs (65–71) or active loading of soluble drugs (72). Passive loading often leads to undesirable membrane incorporation, lowering liposome stability, and is much less efficient than active loading. For example, the drug:lipid ratios achieved through the unique approach described here ranged from 0.4 to 0.6, which is more than 1,000-fold higher than the drug:lipid ratios commonly achieved through passive loading (65–69, 72).
Because many of the most promising drugs developed today and in the past are relatively insoluble, the approach described here may be broadly applicable. It not only increases water solubility but also enhances the selectivity of drug delivery to tumors through an EPR effect, as noted in the introduction. The experiments with BI-2536 provide a striking example of the benefits of these attributes—simultaneously increasing solubility and selective tumor delivery, resulting in less toxicity and higher efficacy. Our strategy therefore has the capacity to “rescue” drugs that fail at one of the last steps in the laborious and expensive process of drug development, allowing administration at higher doses and with less toxicity than otherwise obtainable.
Although we believe our strategy can be applied to many insoluble compounds, we caution that it cannot be applied to all. Previous experiments with unmodified β-cyclodextrins have shown broad, but not universal, potential for encapsulation of hydrophobic compounds (29, 35, 36). However, other forms of cyclodextrins, such as α- or γ-forms, have pockets of different sizes, in theory affording encapsulation of additional compounds. Moreover, the basic idea proposed here—the use of weakly ionizable groups on carriers to remotely load hydrophobic compounds—could be extended to excipients other than cyclodextrins.
Materials and Methods
Detailed descriptions of the materials and methods used in the synthesis of ionizable cyclodextrins as well as preparation of liposomes for active and passive loading are provided in SI Materials and Methods. Preparation and loading of encapsulated complexes and in vivo evaluation performed with them are described in detail in this section. Other techniques used in this work can also be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Honggong Cui for cryo-transmission electron microscopy images, Evangeline Watson for expert technical assistance with animal experiments, Nadine Forbes for assistance with complete blood count analyses on a Hemavet 950, and Kibem Kim, Margaret Hoang, William Hendricks, Nicholas Roberts, and Stephen Eacker for helpful comments and discussions. This project was supported by The Virginia and D. K. Ludwig Fund for Cancer Research and Grants CA062924 and CA043460 from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1324135111/-/DCSupplemental.
References
- 1.Heidel JD, Davis ME. Clinical developments in nanotechnology for cancer therapy. Pharm Res. 2011;28(2):187–199. doi: 10.1007/s11095-010-0178-7. [DOI] [PubMed] [Google Scholar]
- 2.Davis ME, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi CH, Alabi CA, Webster P, Davis ME. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc Natl Acad Sci USA. 2010;107(3):1235–1240. doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chertok B, Webber MJ, Succi MD, Langer R. Drug delivery interfaces in the 21st century: From science fiction ideas to viable technologies. Mol Pharm. 2013;10(10):3531–3543. doi: 10.1021/mp4003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubbell JA, Langer R. Translating materials design to the clinic. Nat Mater. 2013;12(11):963–966. doi: 10.1038/nmat3788. [DOI] [PubMed] [Google Scholar]
- 6.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 7.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12(11):967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 8.Peer D, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 9.Gubernator J. Active methods of drug loading into liposomes: Recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin Drug Deliv. 2011;8(5):565–580. doi: 10.1517/17425247.2011.566552. [DOI] [PubMed] [Google Scholar]
- 10.Huwyler J, Drewe J, Krähenbuhl S. Tumor targeting using liposomal antineoplastic drugs. Int J Nanomedicine. 2008;3(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- 11.Maruyama K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv Drug Deliv Rev. 2011;63(3):161–169. doi: 10.1016/j.addr.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Musacchio T, Torchilin VP. Recent developments in lipid-based pharmaceutical nanocarriers. Front Biosci (Landmark Ed) 2011;16:1388–1412. doi: 10.2741/3795. [DOI] [PubMed] [Google Scholar]
- 13.Baryshnikov AY. [Nanostructured liposomal systems as transport agents for anticancer drugs.] Vestn Ross Akad Med Nauk. 2012;(3):23–31. Russian. [PubMed] [Google Scholar]
- 14.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 15.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. [PubMed] [Google Scholar]
- 16.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65(1):71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Fang J, Sawa T, Maeda H. Factors and mechanism of “EPR” effect and the enhanced antitumor effects of macromolecular drugs including SMANCS. Adv Exp Med Biol. 2003;519:29–49. doi: 10.1007/0-306-47932-X_2. [DOI] [PubMed] [Google Scholar]
- 18.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Kita K, Dittrich C. Drug delivery vehicles with improved encapsulation efficiency: Taking advantage of specific drug-carrier interactions. Expert Opin Drug Deliv. 2011;8(3):329–342. doi: 10.1517/17425247.2011.553216. [DOI] [PubMed] [Google Scholar]
- 20.Schwendener RA, Schott H. Liposome formulations of hydrophobic drugs. Methods Mol Biol. 2010;605:129–138. doi: 10.1007/978-1-60327-360-2_8. [DOI] [PubMed] [Google Scholar]
- 21.Fahr A, Liu X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin Drug Deliv. 2007;4(4):403–416. doi: 10.1517/17425247.4.4.403. [DOI] [PubMed] [Google Scholar]
- 22.Chandran S, Roy A, Mishra B. Recent trends in drug delivery systems: Liposomal drug delivery system—Preparation and characterisation. Indian J Exp Biol. 1997;35(8):801–809. [PubMed] [Google Scholar]
- 23.Fenske DB, Cullis PR. Liposomal nanomedicines. Expert Opin Drug Deliv. 2008;5(1):25–44. doi: 10.1517/17425247.5.1.25. [DOI] [PubMed] [Google Scholar]
- 24.Barenholz Y. Relevancy of drug loading to liposomal formulation therapeutic efficacy. J Liposome Res. 2003;13(1):1–8. doi: 10.1081/lpr-120017482. [DOI] [PubMed] [Google Scholar]
- 25.Abraham SA, et al. An evaluation of transmembrane ion gradient-mediated encapsulation of topotecan within liposomes. J Control Release. 2004;96(3):449–461. doi: 10.1016/j.jconrel.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim Biophys Acta. 1993;1151(2):201–215. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 27.Madden TD, et al. The accumulation of drugs within large unilamellar vesicles exhibiting a proton gradient: A survey. Chem Phys Lipids. 1990;53(1):37–46. doi: 10.1016/0009-3084(90)90131-a. [DOI] [PubMed] [Google Scholar]
- 28.Zucker D, Marcus D, Barenholz Y, Goldblum A. Liposome drugs’ loading efficiency: A working model based on loading conditions and drug’s physicochemical properties. J Control Release. 2009;139(1):73–80. doi: 10.1016/j.jconrel.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 29.Albers E, Müller BW. Cyclodextrin derivatives in pharmaceutics. Crit Rev Ther Drug Carrier Syst. 1995;12(4):311–337. doi: 10.1615/critrevtherdrugcarriersyst.v12.i4.20. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Ma PX. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv Drug Deliv Rev. 2013;65(9):1215–1233. doi: 10.1016/j.addr.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laza-Knoerr AL, Gref R, Couvreur P. Cyclodextrins for drug delivery. J Drug Target. 2010;18(9):645–656. doi: 10.3109/10611861003622552. [DOI] [PubMed] [Google Scholar]
- 32.Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech. 2005;6(2):E329–E357. doi: 10.1208/pt060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uekama K, Hirayama F, Irie T. Cyclodextrin drug carrier systems. Chem Rev. 1998;98(5):2045–2076. doi: 10.1021/cr970025p. [DOI] [PubMed] [Google Scholar]
- 34.Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem Rev. 1998;98(5):1743–1754. doi: 10.1021/cr970022c. [DOI] [PubMed] [Google Scholar]
- 35.Stella VJ, He Q. Cyclodextrins. Toxicol Pathol. 2008;36(1):30–42. doi: 10.1177/0192623307310945. [DOI] [PubMed] [Google Scholar]
- 36.Rajewski RA, Stella VJ. Pharmaceutical applications of cyclodextrins. 2. In vivo drug delivery. J Pharm Sci. 1996;85(11):1142–1169. doi: 10.1021/js960075u. [DOI] [PubMed] [Google Scholar]
- 37.Thompson DO. Cyclodextrins—Enabling excipients: Their present and future use in pharmaceuticals. Crit Rev Ther Drug Carrier Syst. 1997;14(1):1–104. [PubMed] [Google Scholar]
- 38.Irie T, Uekama K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J Pharm Sci. 1997;86(2):147–162. doi: 10.1021/js960213f. [DOI] [PubMed] [Google Scholar]
- 39.Cao F, Guo J, Ping Q. The physicochemical characteristics of freeze-dried scutellarin-cyclodextrin tetracomponent complexes. Drug Dev Ind Pharm. 2005;31(8):747–756. doi: 10.1080/03639040500216220. [DOI] [PubMed] [Google Scholar]
- 40.Badr-Eldin SM, Elkheshen SA, Ghorab MM. Inclusion complexes of tadalafil with natural and chemically modified beta-cyclodextrins. I: Preparation and in-vitro evaluation. Eur J Pharm Biopharm. 2008;70(3):819–827. doi: 10.1016/j.ejpb.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 41.Steegmaier M, et al. BI 2536, a potent and selective inhibitor of Polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol. 2007;17(4):316–322. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 42.Lénárt P, et al. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of Polo-like kinase 1. Curr Biol. 2007;17(4):304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 43.Stewart HJ, Kishikova L, Powell FL, Wheatley SP, Chevassut TJ. The Polo-like kinase inhibitor BI 2536 exhibits potent activity against malignant plasma cells and represents a novel therapy in multiple myeloma. Exp Hematol. 2011;39(3):330–338. doi: 10.1016/j.exphem.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Sur S, et al. A panel of isogenic human cancer cells suggests a therapeutic approach for cancers with inactivated p53. Proc Natl Acad Sci USA. 2009;106(10):3964–3969. doi: 10.1073/pnas.0813333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanhaji M, et al. Polo-like kinase 1 inhibitors, mitotic stress and the tumor suppressor p53. Cell Cycle. 2013;12(9):1340–1351. doi: 10.4161/cc.24573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meng X, et al. Induction of mitotic cell death by overriding G2/M checkpoint in endometrial cancer cells with non-functional p53. Gynecol Oncol. 2013;128(3):461–469. doi: 10.1016/j.ygyno.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nappi TC, et al. Identification of Polo-like kinase 1 as a potential therapeutic target in anaplastic thyroid carcinoma. Cancer Res. 2009;69(5):1916–1923. doi: 10.1158/0008-5472.CAN-08-1693. [DOI] [PubMed] [Google Scholar]
- 48.Mross K, et al. A randomised phase II trial of the Polo-like kinase inhibitor BI 2536 in chemo-naïve patients with unresectable exocrine adenocarcinoma of the pancreas—A study within the Central European Society Anticancer Drug Research (CESAR) collaborative network. Br J Cancer. 2012;107(2):280–286. doi: 10.1038/bjc.2012.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellis PM, et al. A phase I open-label dose-escalation study of intravenous BI 2536 together with pemetrexed in previously treated patients with non-small-cell lung cancer. Clin Lung Cancer. 2013;14(1):19–27. doi: 10.1016/j.cllc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Hofheinz RD, et al. An open-label, phase I study of the polo-like kinase-1 inhibitor, BI 2536, in patients with advanced solid tumors. Clin Cancer Res. 2010;16(18):4666–4674. doi: 10.1158/1078-0432.CCR-10-0318. [DOI] [PubMed] [Google Scholar]
- 51.Sebastian M, et al. The efficacy and safety of BI 2536, a novel Plk-1 inhibitor, in patients with stage IIIB/IV non-small cell lung cancer who had relapsed after, or failed, chemotherapy: Results from an open-label, randomized phase II clinical trial. J Thorac Oncol. 2010;5(7):1060–1067. doi: 10.1097/JTO.0b013e3181d95dd4. [DOI] [PubMed] [Google Scholar]
- 52.Schöffski P, et al. Multicentric parallel phase II trial of the polo-like kinase 1 inhibitor BI 2536 in patients with advanced head and neck cancer, breast cancer, ovarian cancer, soft tissue sarcoma and melanoma. The first protocol of the European Organization for Research and Treatment of Cancer (EORTC) Network Of Core Institutes (NOCI) Eur J Cancer. 2010;46(12):2206–2215. doi: 10.1016/j.ejca.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 53.Mross K, et al. Phase I dose escalation and pharmacokinetic study of BI 2536, a novel Polo-like kinase 1 inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2008;26(34):5511–5517. doi: 10.1200/JCO.2008.16.1547. [DOI] [PubMed] [Google Scholar]
- 54.Frost A, et al. Phase I study of the Plk1 inhibitor BI 2536 administered intravenously on three consecutive days in advanced solid tumours. Curr Oncol. 2012;19(1):e28–e35. doi: 10.3747/co.19.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vose JM, et al. The Plk1 inhibitor BI 2536 in patients with refractory or relapsed non-Hodgkin lymphoma: A phase I, open-label, single dose-escalation study. Leuk Lymphoma. 2013;54(4):708–713. doi: 10.3109/10428194.2012.729833. [DOI] [PubMed] [Google Scholar]
- 56.Ackermann S, et al. Polo-like kinase 1 is a therapeutic target in high-risk neuroblastoma. Clin Cancer Res. 2011;17(4):731–741. doi: 10.1158/1078-0432.CCR-10-1129. [DOI] [PubMed] [Google Scholar]
- 57.Grinshtein N, et al. Small molecule kinase inhibitor screen identifies Polo-like kinase 1 as a target for neuroblastoma tumor-initiating cells. Cancer Res. 2011;71(4):1385–1395. doi: 10.1158/0008-5472.CAN-10-2484. [DOI] [PubMed] [Google Scholar]
- 58.Liu X, et al. Inhibition of polo-like kinase 1 leads to the suppression of osteosarcoma cell growth in vitro and in vivo. Anticancer Drugs. 2011;22(5):444–453. doi: 10.1097/CAD.0b013e32834513f4. [DOI] [PubMed] [Google Scholar]
- 59.Ding Y, et al. Combined gene expression profiling and RNAi screening in clear cell renal cell carcinoma identify PLK1 and other therapeutic kinase targets. Cancer Res. 2011;71(15):5225–5234. doi: 10.1158/0008-5472.CAN-11-0076. [DOI] [PubMed] [Google Scholar]
- 60.Frémin C, Meloche S. From basic research to clinical development of MEK1/2 inhibitors for cancer therapy. J Hematol Oncol. 2010;3:8. doi: 10.1186/1756-8722-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown AP, Carlson TC, Loi CM, Graziano MJ. Pharmacodynamic and toxicokinetic evaluation of the novel MEK inhibitor, PD0325901, in the rat following oral and intravenous administration. Cancer Chemother Pharmacol. 2007;59(5):671–679. doi: 10.1007/s00280-006-0323-5. [DOI] [PubMed] [Google Scholar]
- 62.LoRusso PM, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral MAPK/ERK kinase inhibitor PD-0325901 in patients with advanced cancers. Clin Cancer Res. 2010;16(6):1924–1937. doi: 10.1158/1078-0432.CCR-09-1883. [DOI] [PubMed] [Google Scholar]
- 63.Haura EB, et al. A phase II study of PD-0325901, an oral MEK inhibitor, in previously treated patients with advanced non-small cell lung cancer. Clin Cancer Res. 2010;16(8):2450–2457. doi: 10.1158/1078-0432.CCR-09-1920. [DOI] [PubMed] [Google Scholar]
- 64.Huang W, et al. PD0325901, a mitogen-activated protein kinase kinase inhibitor, produces ocular toxicity in a rabbit animal model of retinal vein occlusion. J Ocul Pharmacol Ther. 2009;25(6):519–530. doi: 10.1089/jop.2009.0060. [DOI] [PubMed] [Google Scholar]
- 65.Zhu Q, et al. Pluronic F127-modified liposome-containing tacrolimus-cyclodextrin inclusion complexes: Improved solubility, cellular uptake and intestinal penetration. J Pharm Pharmacol. 2013;65(8):1107–1117. doi: 10.1111/jphp.12074. [DOI] [PubMed] [Google Scholar]
- 66.Malaekeh-Nikouei B, Davies N. Double loading of cyclosporine A in liposomes using cyclodextrin complexes. PDA J Pharm Sci Technol. 2009;63(2):139–148. [PubMed] [Google Scholar]
- 67.Rahman S, Cao S, Steadman KJ, Wei M, Parekh HS. Native and β-cyclodextrin-enclosed curcumin: Entrapment within liposomes and their in vitro cytotoxicity in lung and colon cancer. Drug Deliv. 2012;19(7):346–353. doi: 10.3109/10717544.2012.721143. [DOI] [PubMed] [Google Scholar]
- 68.Ascenso A, et al. Novel tretinoin formulations: A drug-in-cyclodextrin-in-liposome approach. J Liposome Res. 2013;23(3):211–219. doi: 10.3109/08982104.2013.788026. [DOI] [PubMed] [Google Scholar]
- 69.Dhule SS, et al. Curcumin-loaded γ-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomedicine. 2012;8(4):440–451. doi: 10.1016/j.nano.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lapenda TL, et al. Encapsulation of trans-dehydrocrotonin in liposomes: An enhancement of the antitumor activity. J Biomed Nanotechnol. 2013;9(3):499–510. doi: 10.1166/jbn.2013.1554. [DOI] [PubMed] [Google Scholar]
- 71.Mendonça EA, et al. Enhanced antiproliferative activity of the new anticancer candidate LPSF/AC04 in cyclodextrin inclusion complexes encapsulated into liposomes. AAPS PharmSciTech. 2012;13(4):1355–1366. doi: 10.1208/s12249-012-9853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Modi S, Xiang TX, Anderson BD. Enhanced active liposomal loading of a poorly soluble ionizable drug using supersaturated drug solutions. J Control Release. 2012;162(2):330–339. doi: 10.1016/j.jconrel.2012.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.