Significance
Our laboratory has identified a critical role of IL-9 in promoting endogenous tumor-specific cytotoxic T lymphocyte response. In this study, we found that differentiation of CD8+ T cells under T helper 9-polarizing conditions induces the development of an IL-9–producing less cytolytic IL-9–skewed CD8+ T (Tc9) cell subset. Noticeably, adoptive transfer of tumor-reactive Tc9 cells elicited greater antitumor responses against large established tumors than classic type-I CD8+ cytotoxic T cells that are used in clinical protocols. Importantly, Tc9 cells have substantially enhanced persistence potential and possess the capacity to acquire/maintain effector function after transfer. Our results also revealed that Tc9-mediated therapeutic effect critically depended on IL-9 production in vivo. The ability of Tc9 cells to confer sustained antitumor responses might open an avenue for the advances of cancer immunotherapy.
Keywords: adoptive cell therapy, less-exhausted T cells, T-cell lineage plasticity
Abstract
Because cytokine-priming signals direct CD8+ T cells to acquire unique profiles that affect their ability to mediate specific immune responses, here we generated IL-9–skewed CD8+ T (Tc9) cells by priming with Th9-polarized condition. Compared with type-I CD8+ cytotoxic T (Tc1) cells, Tc9 secreted different cytokines and were less cytolytic in vitro but surprisingly elicited greater antitumor responses against advanced tumors in OT-I/B16-OVA and Pmel-1/B16 melanoma models. After adoptive transfer, Tc9 cells persisted longer and differentiated into IFN-γ– and granzyme-B (GrzB)–producing cytolytic Tc1-like effector cells. Phenotypic analysis revealed that adoptively transferred Tc9 cells secreted IL-2 and were KLRG-1low and IL-7Rαhigh, suggesting that they acquired a signature of “younger” phenotype or became long-term lived cells with capacity of self-renewal. Our results also revealed that Tc9-mediated therapeutic effect critically depended on IL-9 production in vivo. These findings have clinical implications for the improvement of CD8+ T-cell-based adoptive immunotherapy of cancers.
Adoptive cell therapy (ACT) using ex vivo differentiated type-I CD8+ cytotoxic T (Tc1) cells has shown significant clinical promises for the treatment of established cancers (1). Recent clinical trials using ACT combined with lymphodepletion have resulted in objective responses in 50–70% of patients with advanced melanoma (2). However, complete responses remain infrequent with most patients, and improvements to this approach are needed (2). Current understanding of determinants of successful CD8+ T-cell adoptive therapy includes, but is not limited to, persistence of transferred T cells (3), differentiation status of transferred T cells (4), telomere length (5), and lymphodepleting condition (6). In particular, administration of naïve or early effector T cells in combination with active immunization and IL-2 can result in eradication of large established tumors (4).
Cytokine priming signals direct CD8+ T cells to acquire unique profiles that affect their ability to mediate specific immune responses (7, 8). CD8+ T cells can acquire cytokine secreting phenotypes that require transcription factors similar to those of T helper (Th) cells (9). Tc1 cells secrete IFN-γ and kill tumor targets by releasing cytotoxic molecules such as GrzB and Perforin (9). The contribution of adoptively transferred Tc1 cells in antitumor responses has been clearly established, and Tc1 has stronger therapeutic effect than Tc2 and regulatory CD8+ T cells (10). In addition, naïve CD8+ T cells can be differentiated into Tc17 cells in Th17 polarizing conditions (8). The effect of Tc17-mediated antitumor responses remains controversial with one group describing enhanced antitumor immunity displayed by Tc17 (8) and two more recent studies demonstrating that Tc1 cells are superior to Tc17 cells in mediating tumor regression after transfer (11, 12). However, IL-17 and IL-17–producing T cells are tumor-promoting factors and can mediate IL-6-induced Stat3 activation to generate protumorigenic environment (13), which may limit the application of Tc17 for adoptive therapy. Nevertheless, identification of CD8+ T-cell subsets with optimal therapeutic potential remains a critical challenge for the advances of cancer immunotherapy.
IL-9 has attracted renewed interest recently owing to the identification of the most consistent IL-9–producing Th9 cells. IL-9 is a pleiotropic cytokine that has direct and indirect effects on multiple cell types (14). More recently, we and others reported that Th9 cell-derived IL-9 not only inhibited tumor progression, but also promoted greater tumor clearance than Th1 cells that have traditionally been considered as the most efficient CD4+ T-cell subset to generate antitumor immunity (15–17). It would be interesting to test under Th9-polarizing conditions whether naïve CD8+ T cells could also differentiate into IL-9–producing Tc9 cells, a potential CD8+ T-cell subset for ACT. In this study, we first analyzed the effect of Th9-polarizing medium on the priming of Tc9 cells. We found that differentiation of CD8+ T cells under Th9-polarizing conditions induced very low amounts of typical cytotoxic T lymphocyte (CTL)-expressing GrzB, Eomes, T-bet (encoded by Tbx21), and IFN-γ, while instead, promoted the development of IL-9–positive Tc9 cells. Surprisingly, adoptive transfer of tumor-reactive Tc9 cells elicited greater antitumor responses against large established tumors by differentiating into long-lasting IFN-γ–producing Tc1-like cells in tumor-bearing mice. These results demonstrated the potential advantages of differentiation of tumor-reactive CD8+ T cells into Tc9 cells that may exert superior antitumor efficacy upon adoptive transfer.
Results
IL-9–Producing Tc9 Cells Are Skewed Away from IFN-γ Production and Cytolytic Phenotype.
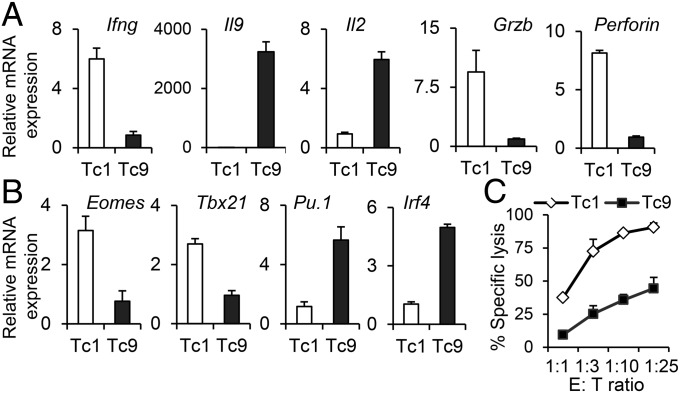
We first investigated the effect of Th9-polarization conditions on the antigen-driven acquisition of an IL-9–producing Tc9-cell phenotype. Similar to the cytokine profile of Th9 cells, IL-9–skewed CD8+ T cells demonstrated diminished mRNA expression of Ifng but enhanced expression of Il9 and Il2, which were associated with diminished expression of cytolytic molecules, such as GrzB and Perforin (Fig. 1A). The expression of Eomes and Tbx21, the transcriptional master regulators that confer cytolytic lymphocyte lineage characteristics (18), were substantially suppressed in IL-9–skewed CD8+ T cells (Fig. 1B). However, the expression of Irf4 and Pu.1, two transcription factors governing Th9-cell lineage development (19), was significantly up-regulated in Tc9 cells (Fig. 1B). The suppressed expression of CTL hallmark molecules, such as GrzB, Perforin, Eomes, and Tbx21, suggested the impaired CTL development of Tc9 cells and possibly diminished cytotoxicity. Indeed, OT-I Tc1 cells showed strong cytotoxicity against tumor target cells, whereas OT-I Tc9 cells exhibited minimal specific cytolytic activity (Fig. 1C and SI Appendix, Fig. S1). Therefore, compared with IL-2–primed IFN-γ–producing cytolytic Tc1 cells, IL-9–skewed Tc9 cells were diverted from the classic cytolytic phenotype.
Fig. 1.
Tc9 cells produced IL-9 and were diverted from cytolytic differentiation. (A and B) Real-time PCR analysis of relative mRNA expression of cytokines and cytolytic-related molecules (A) or transcription factors (B) in OT-I Tc1 and Tc9 cells. Expression relative to Gapdh is displayed. (C) T cells were added at the indicated ratios to CFSEhi B16-OVA target cells or CFSElo B16 nontarget cells in duplicate. Percent of specific lysis was determined after 8 h. Representative results from one of two performed experiments are shown.
Characterization of Tc9 Cytokine Expression Profiles.
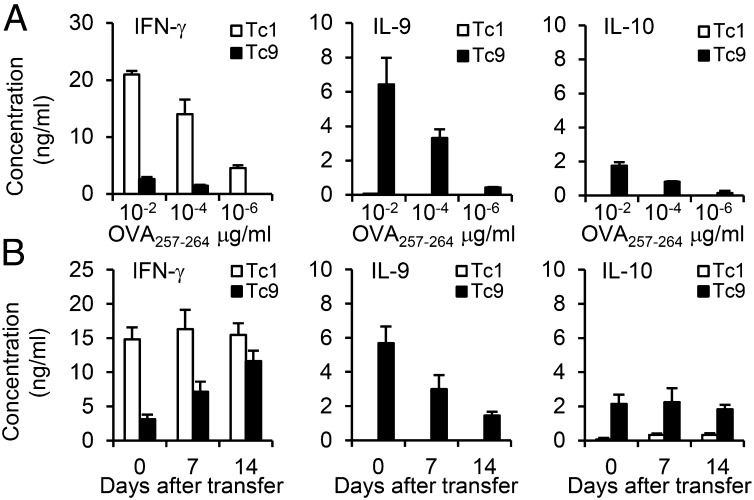
To determine the specific cytokine expression patterns of IL-9–skewed Tc9 cells, we measured by ELISA the cytokine production after restimulation. As expected, OT-I Tc1 cells produced a large amount of IFN-γ, whereas Tc9 cells released a minimal amount of IFN-γ but secreted a large amount of IL-9 (Fig. 2A). In addition, these Tc9 cells also produced some IL-4, IL-10, and IL-17 after in vitro restimulation (Fig. 2A and SI Appendix, Fig. S2A). In contrast, production of IL-9, IL-4, IL-10, or IL-17 from Tc1 cells was undetectable, suggesting distinct expression patterns between Tc1 and Tc9 cells.
Fig. 2.
Cytokine expression profile of Tc9 cells. (A) OT-I Tc1 or Tc9 cells were primed in polarized conditions and expanded with IL-2. The cells were then restimulated with splenocytes pulsed with OVA257–264 at indicated concentrations for 24 h. Production of indicated cytokines was determined by ELISA. (B) OT-I Tc1 or Tc9 cells (2 × 106) were adoptively transferred into CD45.1-transgenic mice, followed by i.v. injection of 5 × 105 OVA257–264-pulsed DCs and i.p. injection of four doses of exogenous IL-2. CD45.2+ transferred cells were sorted from splenocytes at days 7 and 14. Day 0 represents T cells before transfer. The cells were then restimulated with splenocytes pulsed with 0.01 µg/mL OVA257–264 in triplicate for 24 h. Production of indicated cytokines was determined by ELISA. Representative results from one of two repeated experiments are shown.
To further test the cytokine expression profile of Tc9 cells, we transferred CD45.2+ OT-I Tc1 or Tc9 cells into CD45.1 transgenic mice followed by OVA peptide-pulsed dentritic cell (DC) vaccination and four daily doses of rhIL-2 to boost the antitumor responses of ACT in vivo (20). CD45.2+CD8+ T cells were sorted from splenocytes 7 and 14 d after transfer, and cytokine production was measured after in vitro restimulation. Compared with cells before transfer, Tc1 cells continued to produce similar amounts of IFN-γ without other cytokines examined. Noticeably, IL-9 production from Tc9 cells was decreased over time after transfer, and these cells were converted to IFN-γ–producing Tc1-like cells especially at day 14 after transfer (Fig. 2B). In addition, Tc9 cells kept IL-10 production in vivo, whereas IL-4 and IL-17 secretion was reduced over time (Fig. 2B and SI Appendix, Fig. S2B). These results revealed that Tc9 cells might be unstable and flexible in cytokine production in vivo, and suggested that Tc9 cells could gain the ability to produce IFN-γ and might further differentiate into fully cytolytic effector cells after transfer.
OVA-Specific Tc9 Cells Eradicate Large Established B16-OVA Melanoma.
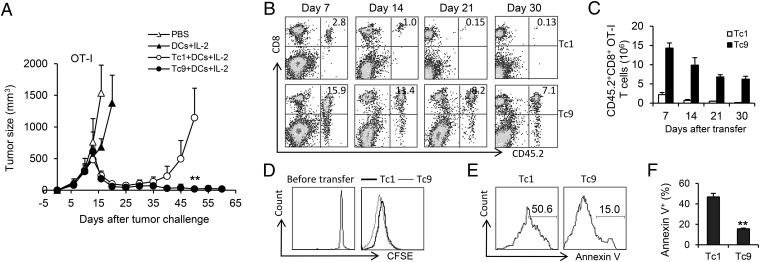
To assess whether the phenotypic differences between Tc1 and Tc9 cells would translate into different efficacy after transfer in vivo, we treated B16-OVA–bearing CD45.1-transgenic mice with equal numbers of adoptively transferred OT-I Tc1 or Tc9 cells, followed by DC vaccination and four daily doses of rhIL-2. To mimic a clinically relevant scenario, tumors were allowed to grow for 10 d before the treatment (SI Appendix, Fig. S3A). Surprisingly, only Tc9 cells mediated a significant tumor regression resulting in a complete cure and long-term survival, whereas Tc1 cell-treated mice relapsed 4 wk after T-cell infusion despite the initial tumor shrinkage within the first 3 wk (Fig. 3A). To better understand the differences between Tc1 and Tc9 cells in treatment outcomes, we analyzed spleens after T-cell infusion for the presence of adoptively transferred effector cells. Flow cytometry analysis revealed that the percentages and absolute numbers of CD45.2+CD8+ splenocytes from Tc9 cell-treated mice were consistently higher than Tc1 cell-treated mice over time (Fig. 3 B and C). Further evaluation by carboxyfluorescein succinimidyl ester (CFSE) dilution assay demonstrated that on the fourth day, CFSE intensity of Tc1 and Tc9 cells in spleens was similar, suggesting that both cells proliferated well in the mice (Fig. 3D). In contrast, Tc1 cells recovered on the fourth day had significantly higher percentages of Annexin V+ apoptotic cells in spleens compared with Tc9 counterparts (Fig. 3 E and F). These results suggested that the persistence of Tc9 cells, which might be one of the key reasons for the rejection of established tumor after transfer, may be the result of a survival advantage or resistance to apoptosis rather than increased proliferation of the cells.
Fig. 3.
OT-I Tc9 cells mediated enhanced antitumor response and displayed greater persistence. (A–C) Tc1 or Tc9 cells (2 × 106) were adoptively transferred into CD45.1-transgenic mice bearing 10-d large established B16-OVA melanoma. DC vaccination and IL-2 were administered to some group of mice as indicated. (A) Tumor responses (n = 5) to adoptive transfer of Tc1 or Tc9 were shown. (B and C) Persistence of transferred Tc1 or Tc9 cells in the spleens of treated tumor-bearing mice was analyzed by FACS. Numbers in histograms (B) represent the percentage of CD45.2+CD8+ OT-I T cells in splenocytes. (C) Total number of CD45.2+CD8+ OT-I T cells was calculated from B. The spleens of three mice per condition were examined at each time point. (D) CFSE-labeled Tc1 or Tc9 cells were transferred into tumor-bearing mice. Shown is CFSE dilution of gated CD45.2+CD8+ splenocytes 4 d after transfer. (E and F) Annexin V expression was measured in Tc1 and Tc9 cells 4 d after transfer. (E) Numbers in histograms represent the percentage of Annexin V+ apoptotic Tc1 or Tc9 cells in splenocytes. Summarized (n = 3) percentages of apoptotic transferred cells were shown in F. Representative results from one of two repeated experiments are shown. **P < 0.01.
Tc9 Cells Are Less Exhausted and Developed into Full Effector Cells in Vivo.
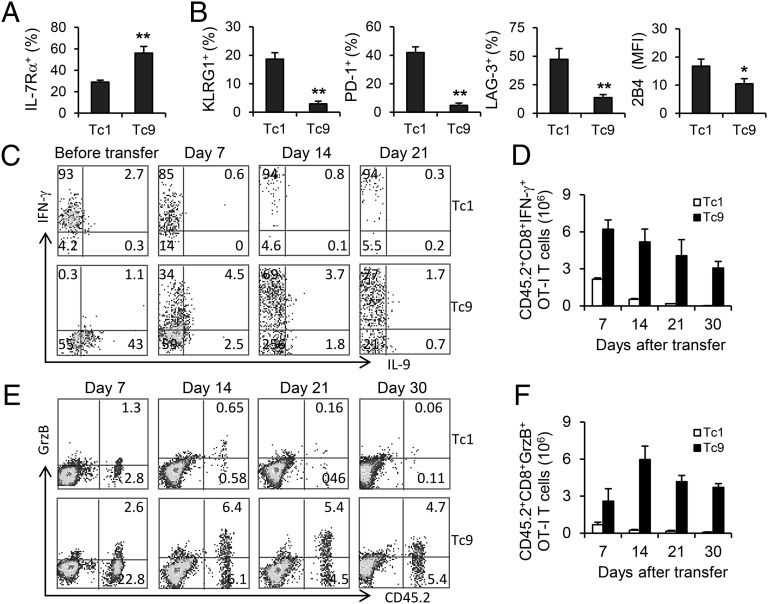
Driven by elevated levels of T-bet, Tc1 cells become terminally differentiated short-lived effector cells with KLRG-1high and IL-7Rαlow phenotype (21). However, compared with Tc1 counterparts, the tumor-specific Tc9 cells expressed significantly higher levels of IL-7Rα, a prosurvival cytokine receptor, suggesting a possible mechanism of the increased persistence of these cells (Fig. 4A). Furthermore, Tc9 cells had significantly down-regulated expression of the exhaustion markers, such as KLRG-1, PD-1, LAG3, and 2B4 (Fig. 4B), demonstrating that Tc9 cells were less exhausted T cells even upon repeated activation in vivo. Reciprocally, Tc1 cells acquired a signature of terminal differentiation with high expression of exhaustion-phenotypic markers, leading to the failure of homeostatic proliferation, dysfunction, and apoptosis of these cells (Fig. 4B). As less exhausted T cells, the long-term survival of Tc9 cells also allowed them to convert to IFN-γ–positive Tc1-like cells especially 14 d after transfer, which was accompanied by a decrease of IL-9–producing CD8+ T cells (Fig. 4C). By calculating the total number of IFN-γ–positive Tc1-like transferred cells, we found that Tc9 cell-transferred mice had already developed more than twofold Tc1-like cells compared with those in Tc1 cell-transferred mice 7 d after the transfer and this ratio kept increasing over time (Fig. 4D). Because production of IFN-γ is a quintessential characterization of cytolytic CD8+ T cells, we analyzed and calculated GrzB-producing CD8+ T cells from tumor-bearing mice. We enumerated significantly more GrzB-positive Tc9-derived cells than those in Tc1-cell transferred mice (Fig. 4 E and F). These results collectively suggested that Tc9 cells could evolve in vivo into distinct Tc1-like effector cells, which might be responsible for the Tc9 cell-mediated tumor destruction.
Fig. 4.
Tc9 cells display less exhausted phenotype and switch to Tc1-like cells in tumor-bearing mice. Tumor-bearing mice (n = 3) were transferred with OT-I Tc1 or Tc9 cells and treated the same as described in Fig. 1. Splenocytes were harvested and analyzed. (A) Expression of IL-7Rα by transferred cells 7 d after transfer. (B) Expression of indicated exhaustion markers by transferred cells 7 d after transfer. (C) FACS determination of intracellular cytokine production by Tc1 or Tc9 cells before and after transfer. (D) Total number of IFN-γ–producing Tc1 or Tc9 cells after transfer was calculated from FACS analysis. (E) FACS determination of GrzB-producing Tc1 or Tc9 cells after transfer. (F) Total number of GrzB-producing Tc1 or Tc9 cells after transfer was calculated from FACS analysis. Representative results from one of two performed experiments are shown. *P < 0.05; **P < 0.01.
Cyclophosphamide Synergizes with Pmel-1–Derived Tc9 Cells To Mediate Enhanced Antitumor Immunity.
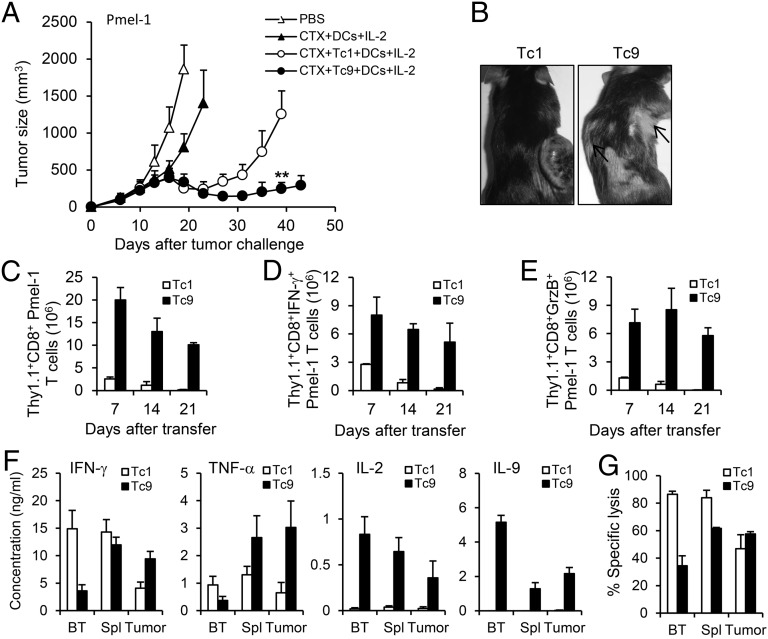
Because OT-I cells target artificial antigen, we next used the Pmel-1 model of adoptive immunotherapy, which reproduces the clinical challenge of targeting gp100 tumor/self-antigen in the poorly immunogenic B16 melanoma (22). One day before T-cell adoptive transfer, mice were given one dose of cyclophosphamide (CTX; 250 mg/kg), which can induce lymphopenia, sensitize tumor cells to immune destruction, and promote homeostatic proliferation of transferred T cells (23, 24). Tc1 or Tc9 cells were transferred into mice bearing large established B16 melanoma in conjunction with DC vaccination and four daily doses of rhIL-2 (SI Appendix, Fig. S3B). Noticeably, Tc9-cell transfer mediated sustained antitumor responses throughout the experiment, whereas Tc1 cells only induced temporary tumor regression, which was followed by relapse of aggressive tumor growth (Fig. 5A). In addition, the development of autoimmune vitiligo was apparent 4 wk after transfer of Tc9 cells but was not observed in any of Tc1 cell-treated mice (Fig. 5B).
Fig. 5.
CTX synergizes with Pmel-1 Tc9 cells to control large established B16 melanoma in vivo. Pmel-1 Tc1 or Tc9 cells were primed in polarized conditions and expanded with IL-2. Tc1 or Tc9 cells (2 × 106) were adoptively transferred into C57BL/6 mice bearing 10-d large established B16 melanoma. One dose of CTX was given 1 d before T-cell transfer. DC vaccination and IL-2 were administered to some group of mice after T-cell transfer. (A) Tumor responses (n = 5) to adoptive transfer of Tc1 or Tc9 were shown. (B) Representative autoimmune vitiligo of tumor-bearing mice 25 d after T-cell transfer. Arrows indicated the presence of vitiligo. (C) Persistence of transferred Tc1 or Tc9 cells in the spleens of treated tumor-bearing mice was analyzed by FACS. (D and E) Total numbers of IFN-γ–producing (D) or GrzB-producing (E) Thy1.1+CD8+ cells after transfer were calculated from FACS analysis. (F) Transferred Tc1 or Tc9 cells were sorted from the spleens or tumor tissues at day 14 after transfer. The cells were then restimulated with splenocytes pulsed with 0.01 µg/mL hgp10025–33 peptide in triplicate for 24 h. Production of indicated cytokines was determined by ELISA. BT represents cells before transfer. (G). Transferred Tc1 or Tc9 cells were sorted from the spleens or tumor tissues at day 14 after transfer. Cytolytic function of T cells was tested by in vitro cytotoxicity assay at 10 to 1 effector to target ratios with CFSEhi B16 target cells and CFSElo MC38 nontarget cells in duplicate. Percentage of specific lysis was determined overnight. Representative results from one of two performed experiments are shown. **P < 0.01.
This Pmel-1 Tc9 cell-mediated sustained antitumor response was also associated with superbly improved in vivo expansion and persistence of the transferred cells examined in the spleen of the tumor-bearing mice (Fig. 5C). Intracellular staining revealed that, similar to OT-I Tc9 cells, transfer of Pmel-1 Tc9 cells also developed into large numbers of IFN-γ–positive Tc1-like cells and GrzB-positive cytolytic effector cells (Fig. 5 D and E). We further measured the cytokine production by transferred cells isolated from the spleens and tumor tissues of tumor-bearing mice. ELISA results indicated that Tc1 cells maintained IFN-γ and TNF-α production in the spleens, but the production of these cytokines was significantly decreased in tumor tissues (Fig. 5F). In contrast, Tc9 cells gained the ability to produce IFN-γ and TNF-α in vivo, and tumor-infiltrating Tc9 cells maintained the production of these cytokines compared with Tc9 cells in the spleens. Notably, only Tc9 cells produced significant amounts of IL-9 and IL-2, which indicated a less differentiated phenotype of Tc9 cells (25). By comparing the cytotoxicity of these sorted cells, we found that tumor-infiltrating Tc1 and Tc9 cells were similar in their ability to lyse target tumor cells, although splenic Tc1 cells had slightly higher cytotoxicity than Tc9 cells (Fig. 5G). Collectively, Pmel-1 Tc9-cell transfer could confer effective antitumor response against large B16 melanoma, and the failure of Tc1 cells to control the disease might be due to the inability of these cells to expand and persist despite the higher cytotoxicity and ability to secrete IFN-γ in vitro and in vivo.
Therapeutic Effect of Tc9 Cells Critically Depends on IL-9.
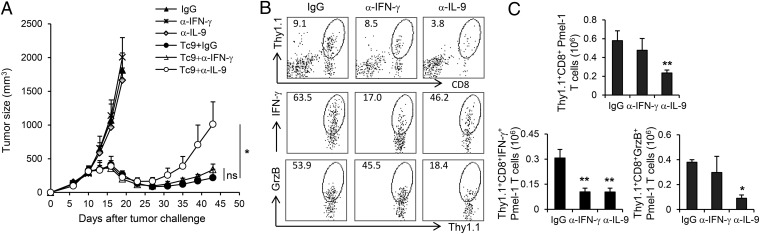
Because Tc9 cells acquired the ability to secrete IFN-γ in tumor-bearing mice, we next determined the importance of Tc9-derived IFN-γ and IL-9 in mediating tumor rejection in MC38-gp100 tumor model. In this model, transfer of Pmel-1 Tc9 cells could mediate significantly enhanced antitumor response than that of Pmel-1 Tc1 or naïve CD8+ T cells, which was associated with a superior persistence of Tc9 cells in recipient mouse spleens (SI Appendix, Fig. S4). We treated MC38-gp100 tumor-bearing mice with isotype controls, IL-9–neutralizing antibodies or IFN-γ–neutralizing antibodies and subsequently transferred them with Pmel-1 Tc9 cells. Unexpectedly, tumor rejection was abrogated only by anti–IL-9 treatment, whereas neutralizing IFN-γ did not reach statistical significance compared with isotype control (Fig. 6A). By calculating the absolute numbers of splenic Thy1.1+CD8+ cells in treated mice, we found that IL-9 neutralization did not impair the persistence of Tc9 cells or their ability to produce IFN-γ and GrzB (SI Appendix, Fig. S5). However, in IL-9–neutralized mice, the number of tumor-infiltrating Thy1.1+CD8+ T cells were substantially reduced compared with those in mice receiving isotype control or anti–IFN-γ antibodies. This impaired Thy1.1+CD8+ T-cell infiltration could also be demonstrated by the sharply decreased numbers of IFN-γ−producing and GrzB-producing Thy1.1+CD8+ cells recovered from tumor sites (Fig. 6 B and C). On the other side, anti–IFN-γ treatment only ablated the production of IFN-γ from transferred Tc9 cells, whereas the homing of cytolytic Tc9 cells to tumor tissues was not affected (Fig. 6 B and C). In addition, by examining the leukocyte subsets in tumor microenvironment, we also observed significantly increased IL-9–dependent tumor-infiltrating host Thy1.1–CD8+ T cells in Tc9 cell-transferred mice (SI Appendix, Fig. S6). Because Tc9-cell transfer mediated a sustained antitumor response in C57BL/6 Rag-1−/− mice similar to that in wild-type mice, host CTL responses may have contributed, but were not required, for the antitumor efficacy of Tc9 cells in vivo (SI Appendix, Fig. S7). Taken all together, these results thus far suggested that Tc9 cells may kill tumor cells independent of their secreted IFN-γ, possibly by using the cytolytic enzymes, and IL-9 provided critical help to their migration into tumor sites to exert effector function, which is an effect of IL-9 that has been extensively elucidated in our previous studies (15, 17).
Fig. 6.
IL-9 contributes to Tc9 cell-mediated tumor rejection. Pmel-1 Tc9 cells (2 × 106) were adoptively transferred into C57BL/6 mice bearing 10-d large established MC38-gp100 tumor. One dose of CTX was given 1 d before T-cell transfer. DC vaccination and IL-2 were administered to the mice that received T-cell transfer. mAbs neutralizing IL-9 or IFN-γ or control IgG were i.p. injected to mice as indicated. (A) Tumor responses (n = 5) to adoptive transfer of Tc9 and antibody treatment were shown. (B) FACS determination of the percentage of tumor-infiltrating, adoptively transferred Thy1.1+CD8+ T cells, IFN-γ–producing or GrzB-producing tumor-infiltrating, adoptively transferred Thy1.1+CD8+ T cells in the leukocyte fraction. Tumor tissues were harvested 3 wk after transfer. (C) Total number of tumor-infiltrating, IFN-γ–producing or GrzB-producing Thy1.1+CD8+ T cells 3 wk after transfer was calculated from FACS analysis. Cell number was normalized to 500-mg tumor tissues. Representative results from one of two performed experiments are shown. *P < 0.05; **P < 0.01.
Discussion
CD8+ CTLs are thought to play a crucial role in tumor rejection, and extensive focus has been devoted to the study of CD8+ T cells in adoptive transfer protocols. Nevertheless, complete and durable tumor regression or cure rates remains to be archived (2). In the current study, we identified unique IL-9–skewed CD8+ T cells, termed Tc9 cells, by priming with Th9-polarized condition. Apart from the differences in cytokine secretion, Tc9 cells differed from Tc1 cells in that they were less cytotoxic in vitro. In line with our observation, a recent publication also confirmed the existence of a Tc9 cell subset in both cultured system and in vivo (26). However, the role of this CD8+ T-cell subset has not been tested in cancer immunotherapy settings. In this study, we evaluated the efficacy of Tc9 cell transfer in both OT-I/B16-OVA and Pmel-1/B16 mouse models in comparison with the classic Tc1 cells. Our study demonstrated that transfer of Tc9 cells displayed superior efficacy to mediate regression of large established tumors by converting to IFN-γ–producing cytolytic effector cells in vivo. These findings are highly relevant to the improvement of CD8+ T-cell–based adoptive cancer immunotherapy.
IL-2 can augment antigen-drive acquisition of CD8+ effector T-cell phenotype with cytolytic function, and therefore, the generation of these tumor-specific Tc1 cells ex vivo involves using high levels of IL-2 in current clinic ACT protocols (27). However, ACT has met with only modest success in humans, possibly due to the fact that IL-2–induced T-bethigh Tc1 cells display end-effector features and short lifespan (21). It is increasingly evident that ACT can be improved by the transfer of less-differentiated CD8+ T cells that possess greater persistence potential (28). We found that tumor-specific Tc9 cells could mediate superior antitumor responses that were associated with superb persistence of the transferred cells. Compared with Tc1 cells, Tc9 cells did not display full-mature phenotype as determined by reduced expression of IFN-γ and cytotoxic effector molecules, but instead acquired a signature of “younger” phenotypes by IL-2–producing, KLRG-1low and IL-7Rαhigh, which are characteristic for long-term lived cells with capacity of self-renewal.
Our results also revealed that the robust persistence of Tc9 cells resulted from a survival advantage of resistance to apoptosis rather than increased proliferation. It is possible that TGF-β1 from Th9-conditioned medium contributed to the arrest of effector differentiation of Tc9 cells and the conversion of apoptotic stimuli into the signal for IL-9 production (29). In contrast, IL-2–primed Tc1 cells have increased susceptibility to apoptosis under repeated activation in vivo (30). In addition to persistence potential, several studies have indicated that effective cells for ACT must possess the capacity to acquire/maintain effector function after transfer (25). Although TGF-β1 from Th9-conditioned medium may play a crucial role of rapid expansion of CD8+ T cells (31), it is insufficient because CD8+ T cells primed with TGF-β1 alone display immunosuppressive regulatory CD8+ T-cell phenotype. Noticeably, Tc9 cells could efficiently switch to Tc1-like cells with the production of IFN-γ, which is well established as a key factor linked to tumor rejection and is crucial for cytolytic function of CD8+ T cells (32). In fact, we enumerated more than twofold Tc1-like cells in Tc9 cell-treated mice as early as 7 d after transfer, and 25∼30-fold of these cells after 3 wk compared with Tc1-cell transferred mice. Further evaluation revealed that anti–IFN-γ treatment did not significantly influence the protective effect against tumor, which was associated with little impact on the number and function of transferred Tc9 cells in both spleens and tumor tissues. Interestingly, when mice were treated with IL-9–neutralizing antibodies, Tc9 cells largely failed to migrate into tumor tissues to exert long-lasting antitumor therapeutic effect. Given the important role of IL-9 in provoking inflammation in tumor sites and recruitment of lymphocytes (15, 17), our results suggested that IL-9 secreted by Tc9 cells played an indispensible role for the homing of these cytolytic Tc1-like cells to tumor tissues and killing of tumor cells. These findings thus indicated that IL-9 rather than IFN-γ might be the critical cytokine contributed to the tumor destruction mediated by Tc9 cells.
Furthermore, it is well known that tumor-derived factors could provide the necessary signals/conditions for effector T cells to become functionally exhausted in tumor microenvironment (33). Upon adoptive transfer, splenic Tc1 cells displayed the characteristic features of exhausted CD8+ T cells by up-regulated expression of KLRG-1, PD-1, LAG3, and 2B4 (34), whereas Tc9 cells were less exhausted. Compared with splenic Tc1 cells, tumor-infiltrating Tc1 cells demonstrated significantly reduced cytolytic function as well as reduced ability to produce IFN-γ and TNF-α. Interestingly, tumor-infiltrating Tc9 cells maintained their capacity to produce IL-2, IFN-γ, and TNF-α and killed efficiently tumor cells compared with their counterparts in the spleens. This promising aspect of Tc9 cells is possibly, in part, owing to the extremely low surface expression of PD-1, a key T-cell exhaustion/inhibition pathway (35), thus rescuing T-cell effector functions of Tc9 cells in vivo.
Overall, our study highlights the clinical potential of tumor-reactive Tc9 cells for adoptive cancer immunotherapy. With the ability to confer tumor specificity by genetic engineering, selection of optimal T-cell subsets with enhanced antitumor potency is of great importance. As less-exhausted younger cells, the sufficient lineage plasticity of Tc9 cells allows these cells subsequently to differentiate into long-lasting IFN-γ–producing Tc1-like effector cells upon transfer. The ability of tumor-reactive Tc9 cells to confer sustained antitumor responses against large established tumors might open an avenue to overcome the challenges encountered by current ACT protocols.
Materials and Methods
All mice were maintained in specific pathogen-free conditions, and animal study was approved by the Institutional Animal Care and Use Committee of Cleveland Clinic Foundation. The procedures of Tc1 and Tc9 cell differentiation, tumor models and adoptive transfer are described in SI Appendix, Materials and Methods. The detailed quantitative real-time PCR, flow cytometry, CFSE labeling, and cytotoxicity assay are also explained in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by a startup fund from Cleveland Clinic, National Cancer Institute Grants R01 CA138402, R01 CA138398, R01 CA163881, and P50 CA142509, the Leukemia and Lymphoma Society, Multiple Myeloma Research Foundation, and Commonwealth Foundation for Cancer Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1317431111/-/DCSupplemental.
References
- 1.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21(2):233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinrichs CS, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci USA. 2009;106(41):17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinrichs CS, et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117(3):808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gattinoni L, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202(7):907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinrichs CS, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111(11):5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinrichs CS, et al. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 2009;114(3):596–599. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrikant PA, et al. Regulating functional cell fates in CD8 T cells. Immunol Res. 2010;46(1-3):12–22. doi: 10.1007/s12026-009-8130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye Z, et al. Type 1 CD8+ T cells are superior to type 2 CD8+ T cells in tumor immunotherapy due to their efficient cytotoxicity, prolonged survival and type 1 immune modulation. Cell Mol Immunol. 2007;4(4):277–285. [PubMed] [Google Scholar]
- 11.Yu Y, et al. Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J Immunol. 2013;190(4):1873–1881. doi: 10.4049/jimmunol.1201989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Hernandez MdeL, et al. Adoptive transfer of tumor-specific Tc17 effector T cells controls the growth of B16 melanoma in mice. J Immunol. 2010;184(8):4215–4227. doi: 10.4049/jimmunol.0902995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, et al. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206(7):1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 2011;186(6):3283–3288. doi: 10.4049/jimmunol.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Y, et al. Th9 cells promote antitumor immune responses in vivo. J Clin Invest. 2012;122(11):4160–4171. doi: 10.1172/JCI65459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purwar R, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. 2012;18(8):1248–1253. doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y, Yi Q. Utilizing TH9 cells as a novel therapeutic strategy for malignancies. OncoImmunology. 2013;2(3):e23084. doi: 10.4161/onci.23084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6(12):1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan MH. Th9 cells: Differentiation and disease. Immunol Rev. 2013;252(1):104–115. doi: 10.1111/imr.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lou Y, et al. Dendritic cells strongly boost the antitumor activity of adoptively transferred T cells in vivo. Cancer Res. 2004;64(18):6783–6790. doi: 10.1158/0008-5472.CAN-04-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27(2):281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overwijk WW, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198(4):569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155(4):1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Most RG, et al. Cyclophosphamide chemotherapy sensitizes tumor cells to TRAIL-dependent CD8 T cell-mediated immune attack resulting in suppression of tumor growth. PLoS ONE. 2009;4(9):e6982. doi: 10.1371/journal.pone.0006982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinrichs CS, Gattinoni L, Restifo NP. Programming CD8+ T cells for effective immunotherapy. Curr Opin Immunol. 2006;18(3):363–370. doi: 10.1016/j.coi.2006.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Visekruna A, et al. Tc9 cells, a new subset of CD8(+) T cells, support Th2-mediated airway inflammation. Eur J Immunol. 2013;43(3):606–618. doi: 10.1002/eji.201242825. [DOI] [PubMed] [Google Scholar]
- 27.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117(6):1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gattinoni L, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115(6):1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takami M, Love RB, Iwashima M. TGF-β converts apoptotic stimuli into the signal for Th9 differentiation. J Immunol. 2012;188(9):4369–4375. doi: 10.4049/jimmunol.1102698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waldmann TA. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6(8):595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, et al. TGF-beta1 induces preferential rapid expansion and persistence of tumor antigen-specific CD8+ T cells for adoptive immunotherapy. J Immunother. 2010;33(4):371–381. doi: 10.1097/CJI.0b013e3181cd1180. [DOI] [PubMed] [Google Scholar]
- 32.Böhm W, et al. T cell-mediated, IFN-gamma-facilitated rejection of murine B16 melanomas. J Immunol. 1998;161(2):897–908. [PubMed] [Google Scholar]
- 33.Baitsch L, et al. Exhaustion of tumor-specific CD8+ T cells in metastates from melanoma patients. J Clin Invest. 2011;121(6):2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25(2):214–221. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.