Significance
Polycomb repressor proteins are recruited to the inactive X chromosome in mammals, and this has been attributed to a biochemical interaction between the non–protein-coding RNA X-inactive specific transcript (Xist), which initiates the X inactivation process, and core polycomb subunits. We have studied this using a combination of genome mapping analysis and 3D structured illumination microscopy (3D-SIM) that allows 3D imaging with eightfold volumetric resolution improvement compared with previous state-of-the-art confocal microscopy. Our findings reveal that Xist-mediated recruitment of polycomb repressors does not correlate well with gene silencing and, moreover, that using 3D-SIM, polycomb proteins and Xist RNA show significant spatial separation. These observations challenge prevailing models and prompt a reappraisal of the role of Xist RNA in polycomb recruitment.
Abstract
In female mammals, one of the two X chromosomes is transcriptionally silenced to equalize X-linked gene dosage relative to XY males, a process termed X chromosome inactivation. Mechanistically, this is thought to occur via directed recruitment of chromatin modifying factors by the master regulator, X-inactive specific transcript (Xist) RNA, which localizes in cis along the entire length of the chromosome. A well-studied example is the recruitment of polycomb repressive complex 2 (PRC2), for which there is evidence of a direct interaction involving the PRC2 proteins Enhancer of zeste 2 (Ezh2) and Supressor of zeste 12 (Suz12) and the A-repeat region located at the 5′ end of Xist RNA. In this study, we have analyzed Xist-mediated recruitment of PRC2 using two approaches, microarray-based epigenomic mapping and superresolution 3D structured illumination microscopy. Making use of an ES cell line carrying an inducible Xist transgene located on mouse chromosome 17, we show that 24 h after synchronous induction of Xist expression, acquired PRC2 binding sites map predominantly to gene-rich regions, notably within gene bodies. Paradoxically, these new sites of PRC2 deposition do not correlate with Xist-mediated gene silencing. The 3D structured illumination microscopy was performed to assess the relative localization of PRC2 proteins and Xist RNA. Unexpectedly, we observed significant spatial separation and absence of colocalization both in the inducible Xist transgene ES cell line and in normal XX somatic cells. Our observations argue against direct interaction between Xist RNA and PRC2 proteins and, as such, prompt a reappraisal of the mechanism for PRC2 recruitment in X chromosome inactivation.
X chromosome inactivation (XCI) is the dosage compensation mechanism used by mammals to equalize levels of X-linked genes in females relative to males. The process is triggered in early development by expression and in cis localization of the noncoding RNA (ncRNA), X-inactive specific transcript (Xist). Chromosome coating by Xist RNA sets in motion a cascade of chromatin modifications culminating in mitotically stable silencing of X-linked genes. Chromatin modifications on the inactive X chromosome (Xi) include gain or loss of specific histone tail posttranslational modifications, histone variants, proteins that modulate higher order chromosome structure, and gain of DNA methylation at promoter-associated CpG islands (CGIs) (1). Xist transgenes located on autosomes function similarly, triggering chromatin modification and gene silencing in cis on the transgene-bearing chromosome (2, 3).
The molecular mechanisms linking Xist RNA to the silencing machinery remain incompletely understood (4). Nevertheless, several factors have been identified as having a role in Xist-mediated chromosome silencing. Notably, the transcription factor YY1 (5); the nuclear matrix protein SAF-A/hnRNPU (6); and the polycomb repressive complex 2 (PRC2), which catalyzes trimethylation of histone H3 at lysine 27 (H3K27me3) (7), have been suggested to interact directly with Xist RNA.
Recruitment of PRC2 in XCI has been studied extensively. Initial evidence came from a genetic study (8), with subsequent cell biological work demonstrating enrichment of PRC2 proteins on Xi and a close overlap with sites of Xist RNA localization, both at metaphase and interphase (9–11). PRC2 recruitment was seen to occur coincidently with the onset of XCI (10, 11) and to be strictly dependent on continuous Xist RNA transcription (11, 12). Collectively, these observations point to PRC2 being recruited directly via interaction with Xist RNA, and this idea has been reinforced by biochemical evidence supporting direct interaction between the conserved A-repeat domain of Xist RNA and the PRC2 proteins Enhancer of zeste 2 (Ezh2) and/or Supressor of zeste 12 (Suz12) (7, 13–15). There are nevertheless some observations that are difficult to reconcile with this view. Notably, Xist expression in early mouse embryos precedes PRC2 recruitment to Xi (16, 17), and expression of Xist RNA transgenes lacking the A-repeat nevertheless recruits PRC2, albeit less efficiently (12).
A second major polycomb group (PcG) complex, PRC1, that mediates monoubiquitylation of histone H2A lysine 119 is also recruited in XCI (18). Until recently, this finding was attributed to binding of PRC1 to PRC2-mediated H3K27me3 via the chromodomain of the core PRC1 subunit, Cbx2/4/6/7/8. However, recent studies have revealed that noncanonical PRC1 complexes in which Cbx family proteins are not present are also recruited to Xi, via an H3K27me3-independent pathway (19, 20). It is not known, however, if this involves direct interaction of noncanonical PRC1 with Xist RNA. Studies on the XCI system have prompted suggestions of a wider role for ncRNA in PRC2 and PRC1 recruitment (recently reviewed in ref. 21). Notable examples are the Kcnqlot1 ncRNA that functions as a master regulator in parental imprinting (22); the ncRNA HOTAIR implicated in recruitment of PRC2 to Hox gene loci in trans (23); the COLDAIR ncRNA proposed to play a role in PRC2 recruitment to the FLC locus in Arabidopsis thaliana (24); and ANRIL ncRNA, which is implicated in recruitment of PRC1 complexes to the INK4a locus (25).
An important question regarding the mechanism of XCI is the identity of chromosomal sites to which Xist RNA binds. Cytogenetic analyses indicate that these sites are located discontinuously along the length of the chromosome, being concentrated in gene-rich domains (26). Xist RNA-mediated recruitment of PRC2 has been analyzed by ChIP sequencing both in differentiating XX ES cells (27, 28) and in trophoblast stem (TS) cells (29). These studies determined that sites of PRC2 enrichment on the Xi map broadly within gene-rich domains, consistent with cytogenetic analysis, and further suggested primary localization to preexisting sites of PRC2 occupancy and spreading to noncanonical genic and intergenic sites, short interspersed nuclear elements (SINEs), and simple repeats (27–29). In this study, we used a similar approach to map PRC2 binding sites at an early time point following synchronous induction of a Xist transgene located on an autosome, mouse chromosome 17. Using this system, we show that newly acquired sites of PRC2 activity map predominantly to gene-rich regions but that Xist-linked PRC2 recruitment does not correlate with gene silencing. Importantly, using superresolution microscopy, we find that Xist RNA and PRC2 proteins do not colocalize and, in fact, exhibit significant spatial separation. Our observations argue against direct interaction between the bulk of Xist RNA and PRC2 proteins and, as such, prompt a reexamination of the mechanism of PcG protein recruitment in X inactivation.
Results
PRC2 Is Recruited to Chromosome 17 Gene-Rich Domains in Response to Xist RNA Induction.
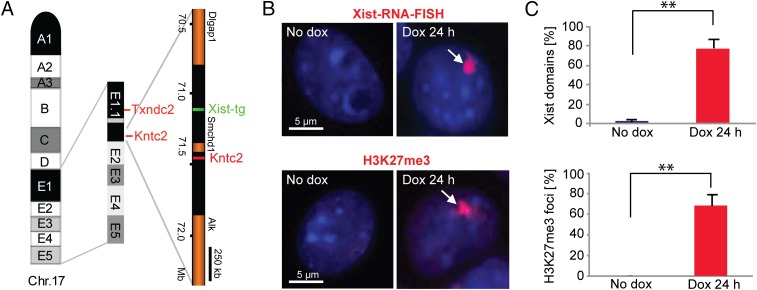
We set out to map Xist-mediated PRC2 binding sites at an early time point following the onset of Xist RNA expression. This required a system in which Xist RNA expression could be induced synchronously and in a high proportion of cells. We therefore made use of a previously characterized mouse ES cell line, 3E, that carries a doxycycline-inducible Xist transgene integrated on chromosome 17 (30) (Fig. 1A). We established 24 h postinduction as the earliest time point at which robust Xist RNA domains could be observed in a high proportion of interphase nuclei in undifferentiated 3E ES cells (Fig. 1 B and C). Similar kinetics were observed for PRC2-mediated H3K27me3 (Fig. 1 B and C), as seen previously (12).
Fig. 1.
Analysis of Xist expression and H3K27me3 localization in undifferentiated 3E ES cells. (A) Schematic representation of chromosome 17 (Chr. 17) and magnification of the cytological region encompassing the inducible Xist transgene (Xist-tg) as determined by DNA-FISH mapping of flanking loci Txndc2 and Kntc2 (30). Genomic coordinates are from mm8 assembly. (B) Representative images of Xist RNA-FISH and H3K37me3 IF stainings on 3E cells 24 h after Xist induction with doxycycline (dox). No Xist up-regulation is detected in noninduced cells. (C) Quantification of cells with Xist RNA and H3K27me3 foci (n ≥ 200). Error bars represent SD in three independent experiments. Two asterisks (**) indicate high statistical significance (P ≤ 0.001, t test).
To map sites of PRC2-mediated H3K27me3, we developed a high-density oligonucleotide-tiling array encompassing a 65.4-Mb span of mouse chromosome 17 [Mb 20–86, Mus musculus 8 (mm8) assembly]. Control regions included known ES cell PRC2 target genes, non-PRC2 target genes, and around 800 kb of randomly selected regions on chromosomes 12 and 18 (Table S1). To determine sites of PRC2 activity, we carried out ChIP using an antibody specific to H3K27me3. Immunoprecipitated material from multiple experiments was pooled and hybridized to tiling arrays to determine relative signal in doxycycline-induced and matched untreated control 3E ES cells. Data were obtained from at least three biological replicates. Strong enrichment of H3K27me3 was seen at positive control loci, for example, the HoxC cluster (Fig. S1A), and not at negative controls, for example, the Sox2 gene promoter (Fig. S1B).
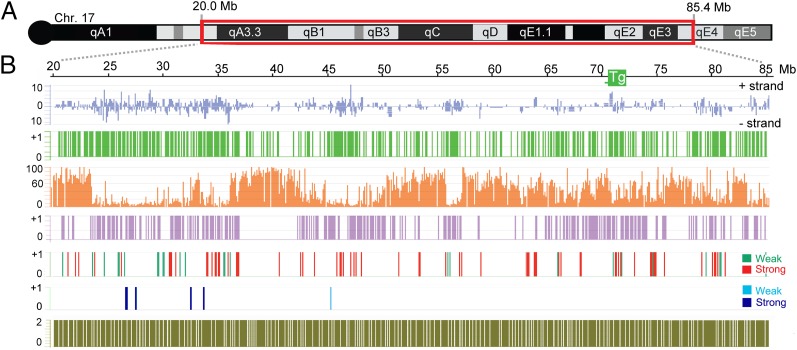
We defined H3K27me3 peaks using Tilemap software (http://jilab.biostat.jhsph.edu/software/tilemap/index.htm) (Materials and Methods) and determined peaks that are common to uninduced and doxycycline-induced samples, as well as new peaks present only in induced samples (H3K27me3 new peaks). As anticipated, chromosome 17 peaks present in both induced and uninduced 3E cells correspond to known ES cell PRC2 target genes (31) (Fig. S1C). An example, the Ptchd4 locus, is depicted in Fig. S1D. Peaks present only in induced 3E ES cells showed a highly significant association with chromosome 17 relative to regions on other chromosomes (P < 0.0005, χ2 test). Selected new peaks and nonpeak regions were verified at 24 h by ChIP-quantitative PCR (qPCR) assay for H3K27me3 and also for the core PRC2 subunit, Suz12 (Fig. S2 A and B). We also performed ChIP followed by qPCR at the same genomic locations at 72 h after Xist induction (Fig. S2C). Data show correlated enrichment of H3K27me3 and Suz12 at new peaks at both time points. The location of new peaks relative to gene density, CGIs, and LINE1 (L1) repeat density is summarized in Fig. 2. New peaks are located across chromosome 17 with no discernible clustering around the transgene location. There is a strong correlation with the location of genes (P = 1.97 * 10−62, χ2 test) and a reciprocal relationship relative to L1 repeats (P = 1.72 * 10−58, χ2 test). These observations are in keeping with cytogenetic analyses (26) and previous ChIP mapping data on the X chromosome (27–29).
Fig. 2.
Summary of epigenomic and transcriptional profiling of chromosome 17 in 3E ES cells following Xist-transgene induction. (A) Schematic showing tiled chromosome 17 region corresponding to Mb 20–85.4 (mm8). (B) Chromosome 17 features are as follows: gene density on + and − strands (blue), CGI density (green), L1 repeat density (%, orange), Xist-dependent new H3K27me3 peaks acquired 24 h after transgene induction (violet), Xist-mediated gene silencing 72 h after transgene induction indicating strongly (red) and weakly (green) down-regulated genes, strongly (dark blue) and weakly (light blue) down-regulated genes, and density of tiled probes (olive). 0/+1 indicates the absence or presence of a given feature (CGI, new H3K27me3 peaks, etc.).
Xist-Mediated H3K27me3 Deposition Occurs at Genic Sites but Is Not Linked to Transcriptional Silencing.
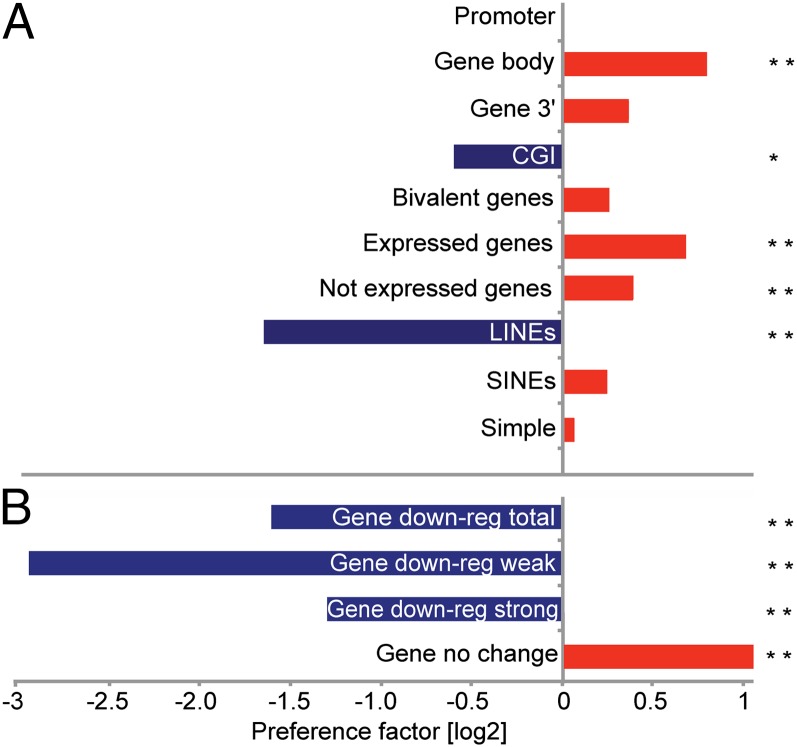
We went on to test the relationship of new (Xist-induced) H3K27me3 peaks and underlying chromosome features (Fig. 3A). We found a strong correlation with gene bodies for both highly expressed and poorly expressed genes, as defined in an analysis of uninduced 3E ES cells (30). There was a slight preference for active vs. inactive genes. Interestingly, new H3K27me3 peaks did not correlate with gene promoters and anticorrelated with CGIs, contrasting with canonical PRC2 target sites. Some new peaks occurred at preexisting PRC2 target genes (31), most likely reflecting an expansion of existing peaks in response to Xist-mediated silencing. There was also a small positive correlation with SINE repeats that are concentrated in gene-rich chromosomal domains, and an anticorrelation with L1 repeats, consistent with the chromosomal distribution of new H3K27me3 peaks (Fig. 3A).
Fig. 3.
Summary of associations (preference factor) for chromosome 17 Xist-dependent H3K27me3 new peaks. (A) Preference summary of genomic features associated with 24-h Xist-induced H3K27me3 new peaks. Preference was expressed as the log ratio of H3K27me3 density at the genomic features of interest (gene bodies, gene 3′ regions, etc.) compared with H3K27me3 density outside of these features (both computed for the area covered by our tiling microarray). (B) Preference summary for Xist-induced peaks vs. 72-h down-regulated genes. A single asterisk (*) and two asterisks (**) indicate statistical (F ≤ 0.05) and highly statistical (F ≤ 0.001) significance, respectively (Fisher test).
We anticipated that new genic peaks would indicate loci that are silenced by Xist RNA at an early time point. To investigate this, we assayed Xist-mediated gene silencing in undifferentiated 3E ES cells using expression microarrays. Down-regulated genes mapped predominantly to chromosome 17, as expected (Fig. S3A). Of a total of 119 significantly down-regulated genes, 85 were strongly repressed (21–45%), with the remainder showing relatively weak repression (5–20%) (Fig. S3B). For selected examples, changes in expression level were verified by quantitative RT-PCR assay (Fig. S3C). The total number of down-regulated genes was less than the 231 observed in a previous study, in which we analyzed differentiated 3E ES cells (30), indicating that Xist-mediated silencing is less efficient in undifferentiated ES cells. We next assessed the correlation of Xist-dependent H3K27me3 sites with down-regulated genes. As shown in Fig. 3B, there is a negative correlation for strongly and weakly down-regulated genes. This finding was unexpected, given that we anticipated that genes in the vicinity of Xist RNA binding sites would be inactivated relatively efficiently. In summary, results from epigenomic mapping of H3K27me3 following Xist induction in 3E ES cells confirm that early sites of PRC2 activity map to gene-rich chromosomal domains, with a significant proportion lying within gene bodies. However, there was no direct link between these sites and Xist-mediated silencing of associated genes.
Spatial Separation of PRC2 Proteins and Xist RNA Revealed by Superresolution Microscopy.
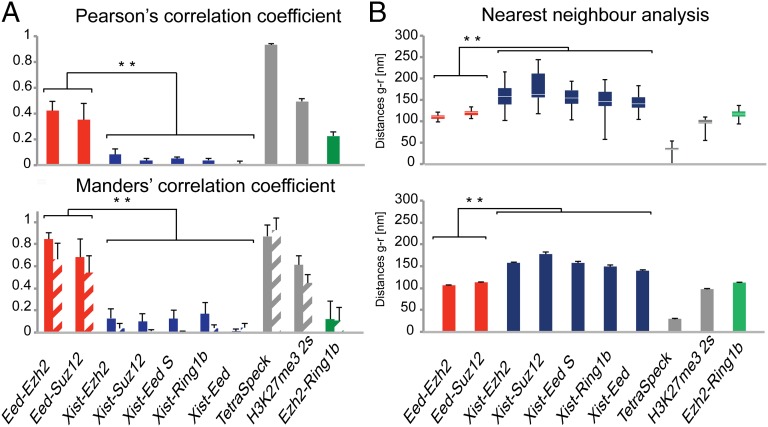
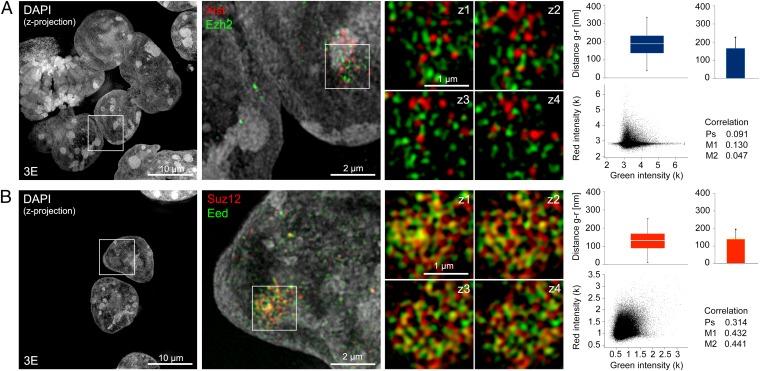
To explore the localization of PRC2 relative to Xist RNA further, we used immunofluorescence (IF) detection in combination with RNA FISH (immuno-RNA-FISH) and applied superresolution 3D structured illumination microscopy (3D-SIM) (32, 33). In comparison to conventional fluorescence light microscopy, 3D-SIM increases the optical resolution in both lateral and axial directions by a factor of 2 below the diffraction limit, allowing multicolor 3D optical sectioning of whole cells with an eightfold increase in volumetric resolution (32). We applied 3D-SIM to compare localization patterns of Xist RNA and core PRC2 proteins directly in the inducible Xist transgene ES cell line 3E. To assess spatial relationships of the detected signals quantitatively, we used three different complementary analysis methods: nearest neighbor centroid distance measurements of segmented spot signals (SI Materials and Methods and Fig. S4), scatter plots, and calculation of pixel intensity-based colocalization coefficients using both Pearson’s and Manders’ correlations (additional details are provided in Materials and Methods). To determine system-related inherent colocalization error between the respective color channels, we imaged 0.2-μm diameter TetraSpeck microspheres (Invitrogen) stained throughout with multiple fluorescent dyes and applied cross-correlation channel alignment. From this, we estimated the error distance to be about 25 nm (Fig. 4 A and B and Fig. S5A). Additionally, signal separation attributable to biologically detected particles (antibody detection) was estimated by imaging SNL mouse fibroblast cells stained with H3K27me3 primary antibody and then simultaneously labeled with Alexa 488- and Alexa 594-conjugated secondary antibodies (Fig. S5B). Quantitative colocalization analysis determined the two signals to be highly correlated and the nearest neighbor distances to be ∼100 nm (Fig. 4 A and B and Fig. S5B). These values represent the collective inaccuracy associated with dual-antibody/fluorophore detection. These include labeling specific variations (e.g., by antibody competition, accessibility, epitope density, quenching, epitope-to-fluorophore distance) but may also involve intrinsic errors in the spot segmentation and nearest neighbor centroid determination. In addition, imaging-specific error sources, such as wavelength-dependent variation in optical resolution and channel alignment mismatch along the optical axis by chromatic aberrations, may contribute to some extent. We therefore assume that spatially associated biological structures/proteins display the observed range of minimal distances (∼100 nm).
Fig. 4.
Summary of 3D-SIM experiments. (A) Graphs illustrate the averages of Pearson’s correlation coefficient (Upper) and Manders’ correlation coefficients 1 and 2 (Lower, hatched bars), respectively, for all 3D datasets analyzed for each indicated labeling pair. Error bars indicate SD. (B) Green-red distances shown as box plots (Upper, ±1.5 IQR) and mean distances (Lower, SEM) of all analyzed datasets. For each indicated labeling pair, quantifications were performed for at least 10 datasets comprising ∼15 z-sections per cell or a comparably sized region of beads. Red bars indicate PRC2-PRC2 pairs, and blue bars indicate Xist-PRC2 pairs. A PRC1-PRC2 pair is shown in green, and controls with H3K27me3 dual labeling (H3K27me3 2s) and TetraSpeck beads are shown in gray. **, difference with high statistical significance P > 0.001, t test.
Previous studies have proposed that Xist RNA interacts directly with the PRC2 subunit(s) Ezh2 and/or Suz12 (7, 13–15), and we therefore assessed their relative spatial distribution by 3D-SIM. We found that the two signals show significant separation. The median minimal green-red distance of segmented spots was ∼160 nm, and colocalization analyses indicated no correlation (exemplary cells are shown in Fig. 5A and Fig. S6A, and average values for 10 analyzed cells are shown in Fig. 4 A and B). We further analyzed colocalization of Xist RNA and the core PRC2 subunit Eed; again, we observed clear spatial separation of the signals (Fig. S6B). The estimated intersignal distances were in the range of 150–180 nm, and colocalization analyses indicated absence of correlation (Fig. 4 A and B and Fig. S6B). Similar results were obtained in an analysis of colocalization of Xist RNA and Eed in a female somatic cell line, C2C12 (Fig. 4 A and B and Fig. S7A). By way of comparison and to validate our approach, we imaged 3E cells with antibodies to two different PRC2 subunits, Suz12 and Eed. We observed a significant overlap with mean nearest neighbor distances of ∼110 nm and strong correlation of the two signals (Figs. 5B and 4 A and B). These results closely match expectations taking into account distances between core PRC2 subunits (34) and measurements done on the cells labeled with a single antibody against H3K27me3 but with two different secondary antibodies (∼100 nm, arbitrary colocalization baseline; Fig. S5). Comparable results were obtained analyzing colocalization of the PRC2 subunits Ezh2 and Eed (Fig. 4 A and B and Fig. S6C).
Fig. 5.
Spatial separation of Xist RNA and Ezh2 determined by 3D-SIM. (A) Example of immuno-RNA-FISH for Xist (red) and Ezh2 (green) illustrating a maximum intensity z-projection of DAPI-stained cell nuclei (Left and Center), a magnified region surrounding the Xist RNA-enriched domain in one of the cells (Center), and four further magnified serial z-sections (125-nm z-distance, Right) centered on the “Barr body.” Graphs illustrate distance and colocalization analysis from the entire Barr body region (∼15 z-sections). (Upper) Nearest neighbor distances for segmented green and red signals (Distance g-r) are displayed as box plots (median, Q1, Q3), with whiskers indicating the 1.5× interquartile range (1.5 IQR), and as averages plus SD. (Lower) Colocalization analysis based on pixel intensities displayed as a green-red scatter plot with Pearson’s (Ps), Manders’ 1 (M1), and Manders’ 2 (M2) correlation coefficients is indicated. Averaged values from multiple cells are shown in Fig. 4 A and B. (B) IF detection of PRC2 proteins Suz12 (red) and Eed (green) with panels arranged as in A.
In light of recent evidence demonstrating that noncanonical PRC1 complexes are recruited to the Xi via a PRC2-independent pathway (19, 20), we extended our 3D-SIM analyses to determine relative localization of Xist RNA and the core PRC1 protein Ring1b, common to both canonical and noncanonical PRC1 complexes. Quantitative colocalization analysis and distance measurements revealed spatial separation similar to that observed with PRC2 proteins (Fig. 4 A and B and Fig. S7B). We further analyzed the localization of PRC1 relative to PRC2 within the inactive X domain, labeling the catalytic components Ezh2 and Ring1b. In line with expectations, the determined intermediate average distance and correlation coefficient values were between the colocalization controls (Eed-Ezh2/Eed-Suz12) and Xist-PRC2/1 (Fig. 4 A and B and Fig. S7C). In summary, our 3D-SIM analyses indicate that there is little or no direct interaction between Xist RNA and core PcG proteins. This is at apparent odds with the prevailing view that PcG proteins interact directly with Xist RNA and suggests a need to consider and test alternative mechanisms for PcG protein recruitment in XCI.
Discussion
In this study, we analyzed Xist-mediated deposition of H3K27me3 in an ES cell line with an inducible Xist transgene located on mouse chromosome 17. This system offered the advantages of allowing assessment of H3K27me3 deposition at an early time point following synchronous induction of Xist RNA and a means to correlate our observations with Xist-mediated gene silencing in an autosomal context. At the chromosomal level, we found that Xist-mediated H3K27me3 deposition occurs preferentially within gene-rich domains, correlating well with prior studies using either cytogenetic (26) or high-throughput epigenomic mapping (27–29). A more detailed analysis of our data revealed that sites of H3K27me3 deposition occur predominantly within the body of genes rather than at CGI promoters, as in the case of other PRC2 target loci (31). We also observed some increase in H3K27me3 at preexisting PRC2 target gene promoters and additionally at SINEs.
There are both similarities and differences compared with previous epigenomic mapping studies that analyzed PRC2 localization on Xi. In differentiating XX ES cells and XX TS cells, increased PRC2 levels on Xi were seen at both genes and at transcription start sites (27–29). Moreover, a recent analysis of differentiating XX ES cells reported moderate PRC2 enrichment sites broadly distributed within genic and intergenic intervals and at SINE elements, also noting an approximate 40:1 ratio of noncanonical vs. canonical PRC2 sites in XCI (28). We consider that the absence of PRC2 enrichment at gene promoters in our study likely reflects the relatively inefficient gene silencing of chromosome 17 that we observe in response to Xist expression in undifferentiated 3E ES cells, contrasting with the complete and efficient silencing on Xi that occurs in differentiated XX ES cells and in TS cells (27–29). Extrapolating from this, our data indicate that H3K27me3 deposition in gene bodies and at SINE elements likely occurs early in response to Xist RNA expression, with subsequent expansion or spreading of H3K27me3 to promoters and other sites being linked to progressive gene silencing. Our initial assumption was that early PRC2 deposition sites would indicate primary Xist RNA binding sites, and that genes linked to these sites would therefore be inactivated relatively quickly. However, analysis of gene silencing in 3E ES cells revealed a negative correlation between Xist-mediated gene silencing and new sites of H3K27me3 deposition. This observation argues against a role for PRC2 in establishment of gene silencing in XCI, a conclusion that is consistent with the relatively mild XCI phenotypes observed in PRC2-null embryos (8, 10), and also with the observation that Xist-mediated PRC2 recruitment and gene silencing are separable (12). That early H3K27me3 deposition occurs in gene bodies rather than promoters is likely a factor in the lack of correlation with gene silencing.
Our epigenomic mapping studies prompted us to apply superresolution microscopy to reexamine the proposed direct relationship between Xist RNA and PRC2 recruitment. Using 3D-SIM, we found a clear spatial separation of Xist RNA and PRC2 proteins, estimated to be between 50 nm and 100 nm. This conclusion is reinforced by the observation that different PRC2 subunits show significant colocalization relative to one another, consistent with the size of the PRC2 complex as determined in a recent EM study (34). It should be noted that foci observed in 3D-SIM experiments might represent more than a single molecule/complex, although this does not have a bearing on our finding that Xist RNA and PcG proteins are spatially separated. One potential caveat to our conclusions is that the sample processing for immuno-RNA-FISH and IF detection alone is not identical. More generally, we cannot entirely rule out that cell fixation contributes to the observed spatial separation of Xist RNA and PRC2 proteins.
Although our 3D-SIM analyses argue against a direct interaction between Xist RNA and PcG complexes, this is in contrast to biochemical evidence (7, 13–15), as well as recent molecular mapping of Xist RNA binding sites (35, 36). At present, we cannot account for this apparent discrepancy, but it is possible that the biochemical assays, being more sensitive, detect a low level of Xist RNA/PRC2 binding. Such an interaction may be physiologically relevant or, alternatively, may reflect nonspecific binding of PRC2 proteins to RNA. Our findings indicate that it will be important to reappraise the proposed direct interactions of PRC2 with other ncRNAs, given that the Xist-PRC2 model has served as a paradigm in many cases. In some instances, it may be possible to apply 3D-SIM or other high-resolution approaches to address this issue further. In light of the evidence presented here indicating that the bulk of PRC2 and Xist RNA do not interact directly, it is interesting to consider alternative hypotheses to explain PcG recruitment in XCI. One possibility is that there is an RNA binding adaptor protein that forms a bridge between PRC2 and Xist RNA. We consider this to be unlikely, given the estimated additional 50–100 nm of spatial separation (beyond the ∼100-nm measured inter-PRC2 subunit distances), and also because no such candidates have been identified in biochemical purifications of PRC2 complexes. A second possibility, suggested recently (21), is that PRC2 recruitment occurs as an indirect consequence of changes in underlying chromatin configuration associated with Xist-RNA–mediated silencing. To investigate this idea, it will be important to define the underlying chromatin configuration at sites of Xist-dependent H3K27me3 deposition identified in this and other epigenomic mapping studies.
Materials and Methods
Cell Culture.
Growth, maintenance, and doxycycline induction of the Xist-inducible ES cell line 3E were as previously described (30). C2C12 cells were grown in DMEM supplemented with 10% (vol/vol) FBS, 2 mM glutamine, and 1% penicillin/streptomycin. All cells were incubated at 37 °C in a humidified 5% (vol/vol) CO2 incubator.
ChIP and DNA Microarrays.
H3K27me3 ChIP and hybridization to custom microarrays were performed essentially as described previously (37). Full details are provided in SI Materials and Methods.
Gene Expression Analysis.
Gene expression change after doxycycline treatment was monitored by Affymetrix microarray as previously described (30). Genes were classified as strongly and weakly down-regulated, corresponding to 21–45% and 5–20% down-regulation, respectively. Maximum allelic down-regulation is 50%.
IF and DNA/RNA FISH Analyses.
Preparation of cells for conventional IF detection, RNA-FISH, and immuno-RNA-FISH was as previously described (18, 38). IF staining and immuno-RNA-FISH for superresolution microscopy were performed using 3D-preserving conditions described elsewhere (32) and in SI Materials and Methods.
Image Acquisition and Analysis.
Conventional IF imaging was performed as previously described (30). Superresolution imaging is described in detail in SI Materials and Methods. Colocalization was assessed as in the study by Ronnemberg et al. (39). Full details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Haruhiko Koseki for Ring 1b antibody; Marion Cremer, Sarah Cooper, and Anca M. Farcas for critical reading of the manuscript; Nathan Rose and Alfredo Castello for RNA binding analysis PRC2 components; and Rob Klose and all members of N.B. and Klose laboratory for helpful comments and stimulating discussions. This study was funded by Wellcome Trust Grant 081385 (to N.B.) and Wellcome Trust Strategic Award 091911, supporting advanced microscopy at Micron Oxford (http://micronoxford.com). D.S. was supported by a fellowship of the German Academy Exchange Service, and F.K. was supported by an Erasmus Fellowship of the European Union.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312951111/-/DCSupplemental.
References
- 1.Morey C, Avner P. The demoiselle of X-inactivation: 50 years old and as trendy and mesmerising as ever. PLoS Genet. 2011;7(7):e1002212. doi: 10.1371/journal.pgen.1002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JT, Jaenisch R. Long-range cis effects of ectopic X-inactivation centres on a mouse autosome. Nature. 1997;386(6622):275–279. doi: 10.1038/386275a0. [DOI] [PubMed] [Google Scholar]
- 3.Wutz A, Jaenisch R. A shift from reversible to irreversible X inactivation is triggered during ES cell differentiation. Mol Cell. 2000;5(4):695–705. doi: 10.1016/s1097-2765(00)80248-8. [DOI] [PubMed] [Google Scholar]
- 4.Brockdorff N. Chromosome silencing mechanisms in X-chromosome inactivation: Unknown unknowns. Development. 2011;138(23):5057–5065. doi: 10.1242/dev.065276. [DOI] [PubMed] [Google Scholar]
- 5.Jeon Y, Lee JT. YY1 tethers Xist RNA to the inactive X nucleation center. Cell. 2011;146(1):119–133. doi: 10.1016/j.cell.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasegawa Y, et al. The matrix protein hnRNP U is required for chromosomal localization of Xist RNA. Dev Cell. 2010;19(3):469–476. doi: 10.1016/j.devcel.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322(5902):750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, et al. Imprinted X inactivation maintained by a mouse Polycomb group gene. Nat Genet. 2001;28(4):371–375. doi: 10.1038/ng574. [DOI] [PubMed] [Google Scholar]
- 9.Mak W, et al. Mitotically stable association of polycomb group proteins eed and enx1 with the inactive x chromosome in trophoblast stem cells. Curr Biol. 2002;12(12):1016–1020. doi: 10.1016/s0960-9822(02)00892-8. [DOI] [PubMed] [Google Scholar]
- 10.Silva J, et al. Establishment of histone h3 methylation on the inactive X chromosome requires transient recruitment of Eed-Enx1 polycomb group complexes. Dev Cell. 2003;4(4):481–495. doi: 10.1016/s1534-5807(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 11.Plath K, et al. Role of histone H3 lysine 27 methylation in X inactivation. Science. 2003;300(5616):131–135. doi: 10.1126/science.1084274. [DOI] [PubMed] [Google Scholar]
- 12.Kohlmaier A, et al. A chromosomal memory triggered by Xist regulates histone methylation in X inactivation. PLoS Biol. 2004;2(7):E171. doi: 10.1371/journal.pbio.0020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneko S, et al. Phosphorylation of the PRC2 component Ezh2 is cell cycle-regulated and up-regulates its binding to ncRNA. Genes Dev. 2010;24(23):2615–2620. doi: 10.1101/gad.1983810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maenner S, et al. 2-D structure of the A region of Xist RNA and its implication for PRC2 association. PLoS Biol. 2010;8(1):e1000276. doi: 10.1371/journal.pbio.1000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanhere A, et al. Short RNAs are transcribed from repressed polycomb target genes and interact with polycomb repressive complex-2. Mol Cell. 2010;38(5):675–688. doi: 10.1016/j.molcel.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mak W, et al. Reactivation of the paternal X chromosome in early mouse embryos. Science. 2004;303(5658):666–669. doi: 10.1126/science.1092674. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto I, Otte AP, Allis CD, Reinberg D, Heard E. Epigenetic dynamics of imprinted X inactivation during early mouse development. Science. 2004;303(5658):644–649. doi: 10.1126/science.1092727. [DOI] [PubMed] [Google Scholar]
- 18.de Napoles M, et al. Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev Cell. 2004;7(5):663–676. doi: 10.1016/j.devcel.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Leeb M, Wutz A. Ring1B is crucial for the regulation of developmental control genes and PRC1 proteins but not X inactivation in embryonic cells. J Cell Biol. 2007;178(2):219–229. doi: 10.1083/jcb.200612127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tavares L, et al. RYBP-PRC1 complexes mediate H2A ubiquitylation at polycomb target sites independently of PRC2 and H3K27me3. Cell. 2012;148(4):664–678. doi: 10.1016/j.cell.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brockdorff N. Noncoding RNA and Polycomb recruitment. RNA. 2013;19(4):429–442. doi: 10.1261/rna.037598.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandey RR, et al. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol Cell. 2008;32(2):232–246. doi: 10.1016/j.molcel.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 23.Rinn JL, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129(7):1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heo JB, Sung S. Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science. 2011;331(6013):76–79. doi: 10.1126/science.1197349. [DOI] [PubMed] [Google Scholar]
- 25.Yap KL, et al. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell. 2010;38(5):662–674. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duthie SM, et al. Xist RNA exhibits a banded localization on the inactive X chromosome and is excluded from autosomal material in cis. Hum Mol Genet. 1999;8(2):195–204. doi: 10.1093/hmg/8.2.195. [DOI] [PubMed] [Google Scholar]
- 27.Marks H, et al. High-resolution analysis of epigenetic changes associated with X inactivation. Genome Res. 2009;19(8):1361–1373. doi: 10.1101/gr.092643.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinter SF, et al. Spreading of X chromosome inactivation via a hierarchy of defined Polycomb stations. Genome Res. 2012;22(10):1864–1876. doi: 10.1101/gr.133751.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calabrese JM, et al. Site-specific silencing of regulatory elements as a mechanism of X inactivation. Cell. 2012;151(5):951–963. doi: 10.1016/j.cell.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang YA, et al. Efficiency of Xist-mediated silencing on autosomes is linked to chromosomal domain organisation. Epigenetics Chromatin. 2010;3(1):10. doi: 10.1186/1756-8935-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikkelsen TS, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448(7153):553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schermelleh L, et al. Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science. 2008;320(5881):1332–1336. doi: 10.1126/science.1156947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clemson CM, McNeil JA, Willard HF, Lawrence JB. XIST RNA paints the inactive X chromosome at interphase: Evidence for a novel RNA involved in nuclear/chromosome structure. J Cell Biol. 1996;132(3):259–275. doi: 10.1083/jcb.132.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciferri C, et al. Molecular architecture of human polycomb repressive complex 2. Elife. 2012;1:e00005. doi: 10.7554/eLife.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon MD, et al. High-resolution Xist binding maps reveal two-step spreading during X-chromosome inactivation. Nature. 2013;504(7480):465–469. doi: 10.1038/nature12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engreitz JM, et al. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science. 2013;341(6147):1237973. doi: 10.1126/science.1237973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Geen H, Nicolet CM, Blahnik K, Green R, Farnham PJ. Comparison of sample preparation methods for ChIP-chip assays. Biotechniques. 2006;41(5):577–580. doi: 10.2144/000112268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casanova M, et al. Polycomblike 2 facilitates the recruitment of PRC2 Polycomb group complexes to the inactive X chromosome and to target loci in embryonic stem cells. Development. 2011;138(8):1471–1482. doi: 10.1242/dev.053652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ronneberger O, et al. Spatial quantitative analysis of fluorescently labeled nuclear structures: Problems, methods, pitfalls. Chromosome Res. 2008;16(3):523–562. doi: 10.1007/s10577-008-1236-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.