Significance
Farnesoid X (FXR) receptor is a ligand-activated transcription factor that regulates bile acid, lipid and glucose metabolism. It can be activated by endogenous bile acids, including chenodeoxycholic acid. This study uncovers a unique role for FXR to regulate urine volume independently of antidiuretic hormone and identifies aquaporin 2 as a direct target gene of FXR in renal collecting ducts. These findings provide a unique mechanism involved in the regulation of urinary concentrating process. The present study may also help understand the pathogenesis of hepatorenal syndrome, a state with increased circulating bile acid levels and impaired renal function.
Keywords: water homeostasis, bile acid receptor
Abstract
The farnesoid X receptor (FXR) is a ligand-activated transcription factor belonging to the nuclear receptor superfamily. FXR is mainly expressed in liver and small intestine, where it plays an important role in bile acid, lipid, and glucose metabolism. The kidney also has a high FXR expression level, with its physiological function unknown. Here we demonstrate that FXR is ubiquitously distributed in renal tubules. FXR agonist treatment significantly lowered urine volume and increased urine osmolality, whereas FXR knockout mice exhibited an impaired urine concentrating ability, which led to a polyuria phenotype. We further found that treatment of C57BL/6 mice with chenodeoxycholic acid, an FXR endogenous ligand, significantly up-regulated renal aquaporin 2 (AQP2) expression, whereas FXR gene deficiency markedly reduced AQP2 expression levels in the kidney. In vitro studies showed that the AQP2 gene promoter contained a putative FXR response element site, which can be bound and activated by FXR, resulting in a significant increase of AQP2 transcription in cultured primary inner medullary collecting duct cells. In conclusion, the present study demonstrates that FXR plays a critical role in the regulation of urine volume, and its activation increases urinary concentrating capacity mainly via up-regulating its target gene AQP2 expression in the collecting ducts.
The farnesoid X receptor (FXR) is a member of the nuclear receptor superfamily, with the typical functional domains including the DNA binding domain and the ligand binding domain. Upon binding to its ligands, FXR forms a heterodimer with another nuclear receptor, retinoid X receptor, whereupon the receptor dimer binds to the FXR response element (FXRE) located in the promoter regions of FXR target genes, thereby regulating the transcription of these genes (1–3). The single FXR gene gives rise to two isoforms, designated as FXRα and FXRβ, as a result of alternative use of the promoters (4). In addition, each FXR isoform has two variants (FXRα1/FXRβ1 and FXRα2/β2), depending on the presence or absence of an insert of four amino acids (MYTG) immediately adjacent to the DNA binding domain in the hinge domain (5). Because FXRβ constitutes a pseudogene in humans and primates, all recent studies focus on FXRα (6).
FXR is highly expressed in the liver and intestine, where it functions as an intracellular sensor of bile acids and is required for the negative feedback regulation of bile acid biosynthesis and its enterohepatic cycle (7, 8). Most recently FXR has been found to be involved in the regulation of adrenal and vascular function, as well as hepatic glucose and lipid metabolism, which suggests an important role for FXR in many tissues beyond the hepatointestinal system (9–12). Among most tissues tested, the kidney exhibits the highest expression levels (13). However, the role of FXR in the kidney remains largely unknown. It has been previously reported that FXR may negatively regulate sterol regulatory element-binding proteins-1c activity and attenuate albuminuria levels in both type 1 and type 2 diabetes models (14–16), suggesting that FXR might represent a therapeutic target for the treatment of diabetic nephropathy. It is currently unclear whether FXR plays an important role in the regulation of renal physiology.
The kidney is a key organ in the maintenance of water and sodium homeostasis. It regulates urine output by a counter-current mechanism. Although almost all renal tubules are involved in urine volume regulation, renal collecting ducts are essential for urine concentration. Urine volume depends on the function of many aquaporin water channels (AQPs) located in the apical and basolateral membrane of epithelial cells along renal tubules. To date, at least seven AQPs are expressed in the kidney, four of which (AQP1–4) play a major role in renal water reabsorption (17). AQP1 mediates constitutive water reabsorption in the proximal tubules and thin descending limbs of Henle. In the principal cells of the collecting ducts, water enters the cell through apical aquaporin 2 (AQP2) and exits via basolateral AQP3 and AQP4. Unlike most other AQPs, AQP2 is under tight control by its major transcriptional activator, arginine vasopressin (AVP) or antidiuretic hormone. Increase of circulating AVP concentrations induces expression, phosphorylation, and translocation of AQP2, leading to urine concentration. In contrast, decrease of circulating AVP levels or dysfunction of AVP receptor V2 reduces AQP2 expression and its apical localization, resulting in polyuria or urine dilution. In addition to AVP, other factors, such as hypertonicity, inflammation, insulin, aldosterone, prostaglandins, and extracellular calcium levels are also involved in AQP2 transcription, translation, and posttranslational modifications (18).
Hepatorenal syndrome (HRS) is a life-threatening medical condition that consists of rapid deterioration in kidney function in individuals with cirrhosis or, less commonly, with fulminant liver failure. Patients with HRS or cholestasis usually exhibit ascites and peripheral edema with high plasma bile acid levels, impaired renal water and sodium excretion, but histologically normal kidney tissue (19, 20). It is estimated that 18% of individuals with cirrhosis and ascites will develop HRS within 1 y of their diagnosis with cirrhosis, and 39% of these individuals will develop HRS within 5 y of diagnosis (21). Although multiple factors, including nitric oxide, prostaglandin, and renin–angiotensin systems, have been found to be involved in the pathogenesis of deteriorated renal function, the precise mechanisms remain incompletely characterized. Because FXR is the receptor of bile acids and is highly abundant in the kidney, we anticipate that FXR may participate in the regulation of renal water and sodium homeostasis and is involved in the development of fluid retention in liver cirrhosis or cholestasis (22).
In the present study we show that FXR plays a critical role in the regulation of renal water reabsorption, mainly by controlling the transcription of AQP2 gene. We also provide evidence that AQP2 gene is a direct target gene of FXR. These findings uncover a previously unidentified role of FXR in whole-body water homeostasis.
Results
Expression of FXR in the Kidney.
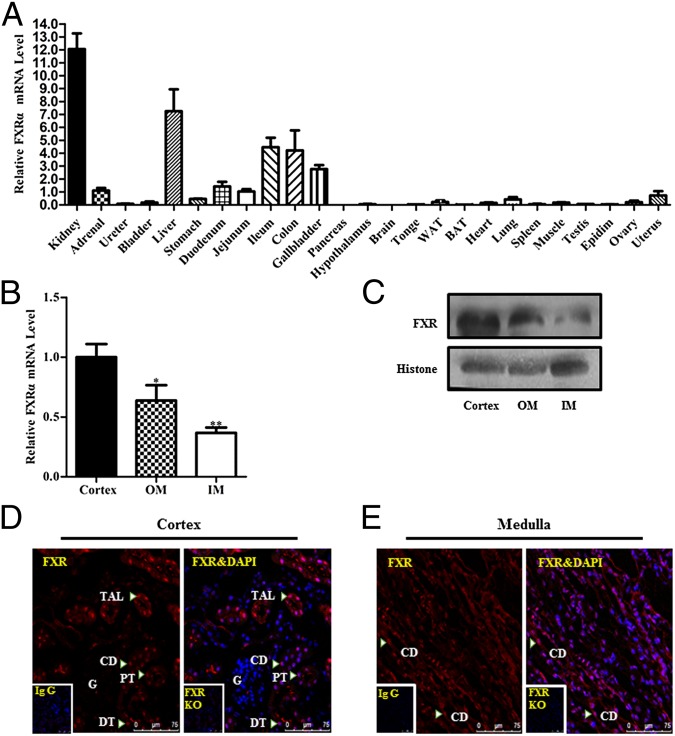
As previous studies reported, the kidney is the organ with the highest expression levels of FXR (Fig. 1A). In the kidney, FXR represents the nuclear receptor with the highest expression levels among all members of the nuclear receptor superfamily (13). In the present study we determined the intrarenal localization of FXR in mouse. FXR mRNA expression was highest in the cortex, followed by the outer medulla and inner medulla (Fig. 1B). Western blot analysis using the cell nuclear fraction of mouse kidney showed that FXR protein expression level in the cortex was much higher than that in the outer medulla and inner medulla (Fig. 1C). Immunofluorescence study further demonstrated that FXR expression was mainly localized in the nuclei of renal cells, with much lower expression in the cytoplasm (Fig. 1D). FXR exhibited a ubiquitous expression pattern in the kidney, with an exception in renal glomeruli, where very little FXR expression was evident (Fig. 1 D and E). Immunohistochemistry indicated that FXR was expressed in almost all of the segments of renal tubules, with relatively higher expression in the proximal tubules and thick ascending limbs, followed by the distal tubules, thin descending limbs, and collecting ducts (Fig. S1). There were also some FXR signals in interstitial fibroblasts, located between the collecting ducts in renal medulla (Fig. S1).
Fig. 1.
Intrarenal localization of FXR in normal mice. (A) Mouse tissue distribution of FXRα mRNA determined by real-time PCR. The results were expressed as relative expression levels after standardization by 18S. The FXRα mRNA levels were normalized as the average of jejunum mRNA levels = 1. WAT, white adipose tissue; BAT, brown adipose tissue. n = 6. Data are presented as mean ± SEM. (B) Real-time PCR analysis showing FXR mRNA levels in renal cortex, outer medullar (OM), and inner medulla (IM). Data are presented as mean ± SEM. *P < 0.05, **P < 0.01 vs. cortex. n = 9. (C) Western blot assay demonstrating FXR protein levels in renal cortex, OM, and IM. (D and E) Intrarenal localization of FXR protein in mouse cortex (D) and medulla (E) as assessed by immunofluorescence. Diffuse FXR immunoreactivity was observed in renal cortex and medulla. FXR signals (arrowheads) were mainly located in the nuclei (red) of epithelial cells of the proximal tubules (PT), thick ascending limbs (TAL), distal convoluted tubules (DT), and collecting ducts (CD). Faint FXR signals were evident in the glomeruli (G). (D and E, Insets) Negative immunostaining with the IgG (without the primary antibody against FXR) in FXR wild-type mice and with the FXR antibody in FXR gene knockout mice, respectively. No specific signal indicates high specificity of the FXR antibody used in the present study.
Effect of FXR on Urine Concentration.
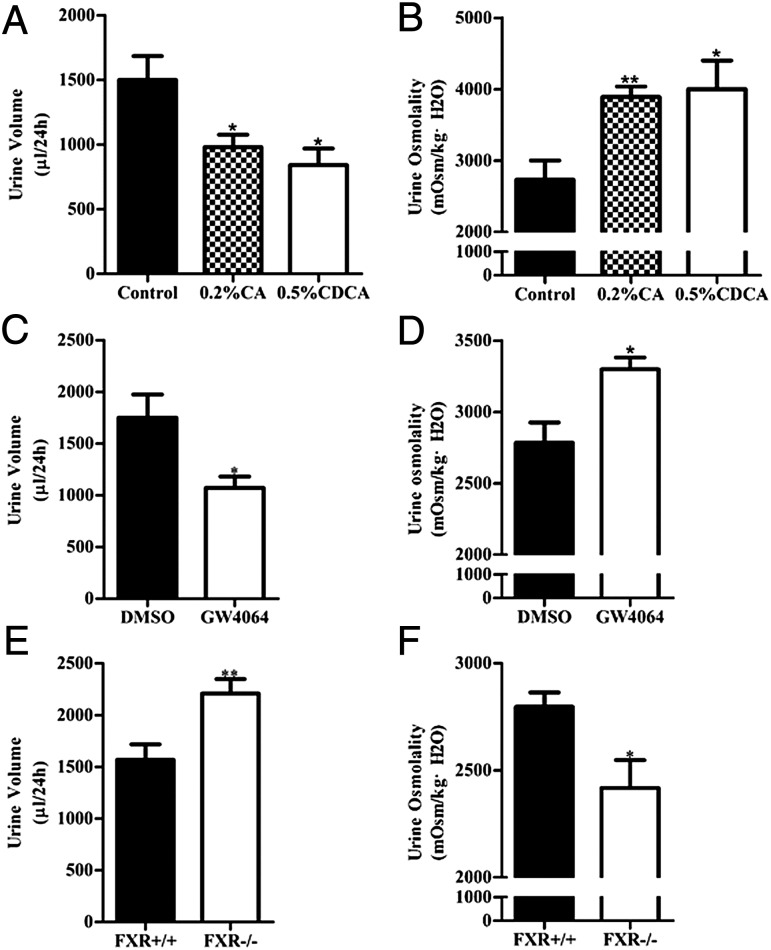
The kidney is a key organ regulating water and salt metabolism. To test whether FXR is involved in regulation of urine concentration, cholic acid (CA) and chenodeoxycholic acid (CDCA), two endogenous agonists for FXR (2, 23), were used to treat C57BL/6 mice (0.2% CA and 0.5% CDCA mixed in diet) for 5 d. Mice were housed in metabolic cages for 24 h with free access to water. Treatment of mice with CA and CDCA resulted in a significant decrease in urine volume and increase in urine osmolality (Fig. 2 A and B). Selective FXR agonist GW4064 treatment also resulted in a similar phenotype (Fig. 2 C and D). In contrast, FXR gene knockout mice exhibited much more daily urine output and much lower urine osmolality than wild-type mice. (Fig. 2 E and F). These findings suggest that FXR may play an important role in regulation of urine concentration. The polyuria phenotype observed in FXR−/− mice seems to be the result of the defect in the kidney. Urine concentrating response to water restriction and desmopressin (dDAVP) treatment was similar between FXR+/+ and FXR−/− mice (Fig. S2 A–C).
Fig. 2.
Role of FXR in the regulation of mouse urine volume and osmolality. (A and B) Urine volume (A) and osmolality (B) of control, 0.2% CA-, and 0.5% CDCA-fed mice, n = 5 in each group. (C and D) Twenty-four-hour urine volume (C) was significantly lower, whereas urine osmolality (D) was significantly higher, in GW4064-treated mice (n = 5) than in control mice (n = 6). (E and F) Twenty-four-hour urine volume (E) was significantly higher, whereas urine osmolality was significantly lower (F), in FXR gene knockout (FXR−/−) mice (n = 17) than in wild-type (FXR+/+) mice (n = 13). Data are presented as mean ± SEM. *P < 0.05, **P < 0.01 vs. control (A and B), DMSO (C and D), or FXR+/+ mice (E and F).
Effect of FXR on Renal AQP2 Expression.
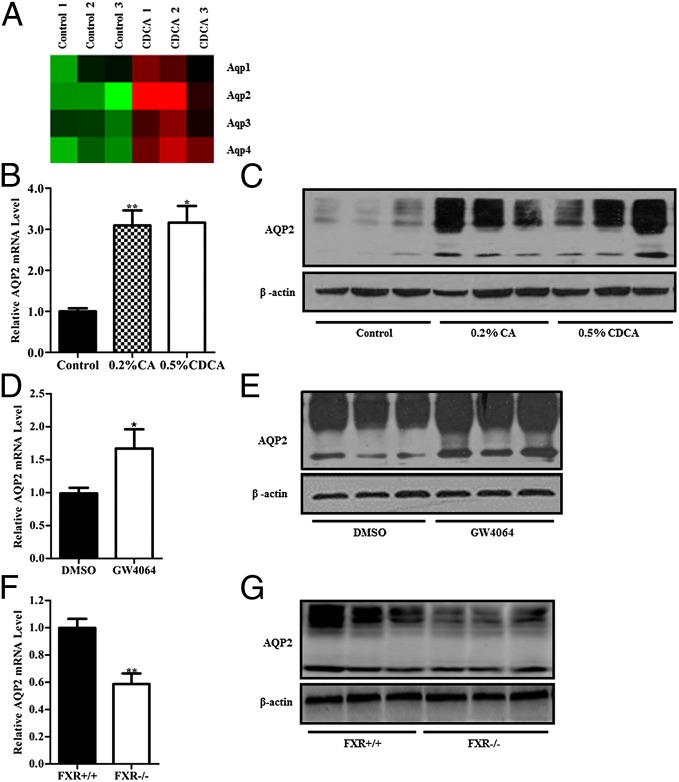
To explore the mechanism through which FXR influences the urine concentration, gene expression profiles were examined by using the Affymetrix chip. After treatment of wild-type mice with CDCA for 5 d, expression levels of a total of 242 genes were significantly changed (fold change ≥1.5, P ≤ 0.05). Among them, 173 genes were up-regulated and 69 genes were down-regulated, with some of these genes involved in regulation of water homeostasis (Table S1). As shown in Fig. 3A, CDCA treatment markedly up-regulated AQP2 gene expression in the kidney. Real-time PCR analysis demonstrated that renal AQP2 mRNA levels were significantly higher in CA- and CDCA-fed mice compared with the control mice (Fig. 3B). As previously reported, glycosylation is important for cell surface expression of AQP2 (24). Both CA and CDCA treatment resulted in a marked increase in total AQP2 protein levels, as well as the glycosylated (multiple bands with high molecular weights) and nonglycosylated (a sharp band with low molecular weight) forms of AQP2 protein (Fig. 3C). Immunohistochemical assay showed that AQP2 protein expression was mainly increased in medullary collecting ducts, with little change in cortical collecting ducts of CDCA-fed mice (Fig. S3A). Treatment of mice with GW4064 also significantly increased AQP2 expression levels both at mRNA and protein levels (Fig. 3 D and E). In sharp contrast, FXR knockout mice exhibited significantly reduced AQP2 mRNA and protein abundance (Fig. 3 F and G). Reduced AQP2 protein expression, especially apical expression, was further confirmed by the immunostaining assays (Fig. S3B). Consistent with the findings of urine volume and osmolarity, both AQP2 mRNA and protein levels were significantly attenuated under basal conditions and were slightly induced after water deprivation in FXR −/− mice (Fig. S4 A and B). These findings were further confirmed by the immunohistochemistry and immunofluorescence studies (Fig. S4 C and D). FXR−/− mice showed reduced AQP2 abundance and weak apical expression in the collecting ducts under both basal conditions and after water deprivation compared with wild-type mice. In addition, normal blood fasting glucose levels in FXR−/− mice and no change in blood glucose after bile acid and GW4064 treatment in wild-type mice excluded the possibility that hyperglycemia contributes to polyuria in FXR−/− mice (Fig. S5 A–C). Together, these results suggest that both total AQP2 level and its membrane translocation in renal collecting ducts were diminished in FXR gene knockout mice.
Fig. 3.
Effect of FXR on AQP2 expression in mouse kidney. (A) Heat map visualization of changes of four major aquaporins in the kidneys of CDCA-fed mice. AQP2 was the one with the most significantly induction. Fold change of each gene symbol was visualized by red–green color scale: green for down-regulation, black for insignificant change, and red for up-regulation. (B and C) Both mRNA (B) and protein (C) levels of AQP2 were increased in the kidneys of 0.2% CA- and 0.5% CDCA-fed mice. (D and E) Treatment of mice with a specific FXR agonist, GW4064, via i.p. injection for 3 d up-regulated AQP2 expression at both mRNA (D) and protein (E) levels in the kidneys. (F and G) FXR gene-deficient mice exhibited diminished AQP2 expression at both mRNA (F) and protein (G) levels. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01 vs. control (A and B), DMSO (C and D) or FXR +/+ mice (E and F). β-Actin was used as an internal control.
FXR Increases Expression of AQP2 in Vitro.
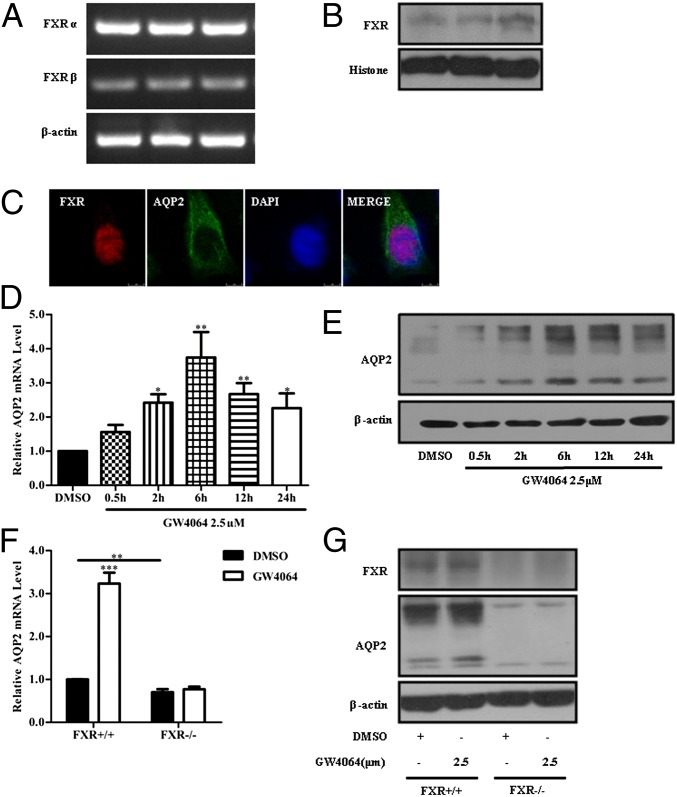
To further confirm the regulation of AQP2 expression by FXR, primary epithelial cells of inner medullary collecting ducts (IMCDs) were cultured. RT-PCR and Western assays showed that both FXR mRNA and protein were expressed in IMCD cells (Fig. 4 A and B). Immunofluorescence analysis further demonstrated that FXR was mainly expressed in the nucleus of IMCD cells (Fig. 4C). Treatment of IMCDs with GW4064 (2.5 μM) increased AQP2 mRNA and protein levels in a time-course-dependent manner, with the highest levels at 6-h and 12-h time points, respectively (Fig. 4 D and E). Treatment of the cells with CDCA (80 μM) resulted in similar time-course changes in AQP2 expression levels (Fig. S6 A and B). Furthermore, overexpression of FXRα in IMCDs by infecting with FXRα adenoviruses encoding either FXRα1 or FXRα2 results in an increase in AQP2 mRNA and protein levels (Fig. S6 C and D). FXR agonists CDCA and GW4064 also increased AQP2 expression and membrane localization in IMCDs, as assessed by immunofluorescence study (Fig. S6E). To exclude off-target effects of FXR agonists, primary IMCD cells of FXR knockout mice (FXR−/−) were cultured, and AQP2 expression was measured. Basal levels of AQP2 mRNA and protein expression in FXR−/− mouse IMCDs were markedly lower than that in wild-type mouse IMCDs. Treatment of IMCDs of FXR−/− mice with the FXR-specific agonist GW4064 failed to induce AQP2 expression (Fig. 4 F and G). Interestingly, although CDCA-induced AQP2 expression was diminished, this bile acid still slightly increased AQP2 expression levels in FXR−/− IMCD cells (Fig. S6 F and G), suggesting that other mechanisms than FXR activation may also participate in this regulation.
Fig. 4.
FXR activation with GW4064 induced AQP2 expression in cultured primary IMCD cells. (A) RT-PCR analysis of FXRα and FXRβ mRNA expression in three preparations of cultured primary IMCD cells. (B) Western blot assay showing FXR protein expression in the nuclei of primary IMCD cells. (C) Nuclear localization of FXR (red) and AQP2 (green) in a primary IMCD cell. The nucleus was visualized by staining the cell with DAPI (blue). (D and E) Primary IMCD cells were treated with 2.5 μM GW4064 for the indicated time points. Both AQP2 mRNA (D) and protein (E) levels were induced by the GW4064 treatment. (F and G) GW4064 treatment for 6 h failed to induce AQP2 expression at both mRNA (F) and protein (G) levels in primary IMCD cells isolated from FXR−/− mice. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01 vs. DMSO (D, n = 3) and FXR+/+ mice (F, n = 4).
AQP2 Is a Direct Target Gene of FXR.
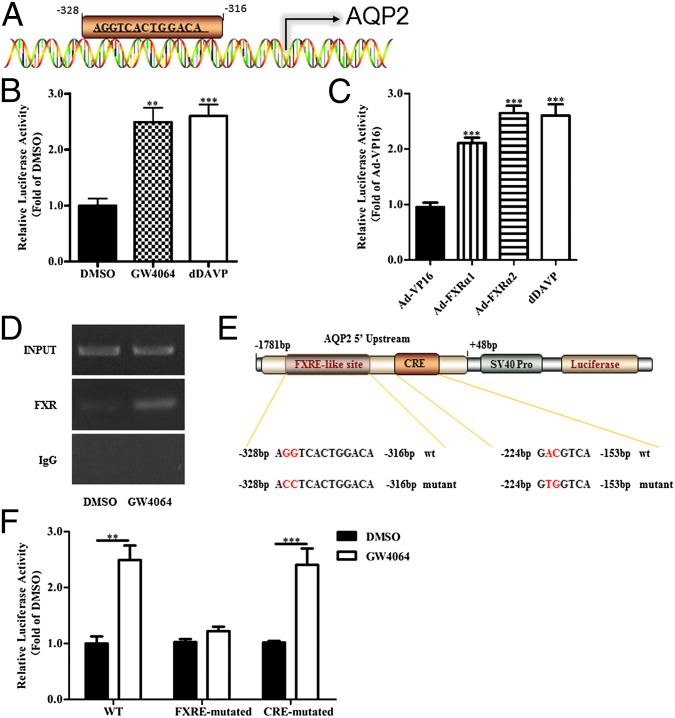
Sequence analysis of the promoter region of mouse AQP2 gene using PROMO 3.0 software [supervised by D. Farré (25, 26)] showed a putative FXRE sequence between −328 bp and −316 bp upstream from the transcription start site (Fig. 5A), suggesting that AQP2 gene might be directly regulated by FXR. A DNA fragment containing −1781 to +48 bp sequence of the 5′ untranslated region of AQP2 gene was cloned into the pGL3-basic vector, resulting in an AQP2 gene promoter-driven luciferase reporter plasmid (AQP2-Luc). AQP2-Luc plasmid was transfected into primary IMCD cells in the presence or absence of GW4064 or FXR adenovirus. Both GW4064 and FXR overexpression significantly increased transcription activity of mouse AQP2 gene promoter (Fig. 5 B and C). A previous study reported that dDAVP can increase cAMP levels and then the binding of cAMP to the cAMP-responsive element (CRE) in the AQP2 promoter, resulting in the transcriptional activation of the AQP2 gene (27). Thus, dDAVP was used as the positive control in the present study. To verify whether FXR could directly bind to the FXRE site, ChIP assay was performed. In the basal condition, FXR indeed bound to the FXRE site, which was further enhanced by the FXR agonist GW4064 and CDCA and FXR adenoviruses (Fig. 5D and Fig. S7 A and B). To further confirm that AQP2 gene is a direct target of FXR, the predicted FXRE sequence 5′AGGTCACTGGACA3′ in the AQP2 promoter region of AQP2-Luc plasmid was mutated to 5′ACCTCAcTGGACA3′, resulting in an FXRE-mutant AQP2 promoter-driven luciferase reporter (Fig. 5E). As a control, a CRE-mutant AQP2 promoter-driven luciferase reporter was constructed according to a previous study (27). As expected, the mutation of the FXRE site abolished GW4064-induced AQP2 gene transcriptional activity, but not in the CRE mutant (Fig. 5F).
Fig. 5.
FXR activation increased AQP2 transcription via the transactivation mechanism. (A) DNA sequence analysis revealed a potential FXRE-like site within the proximal promoter region of mouse AQP2 gene located between −328 bp and −316 bp upstream from the transcription start site. (B) GW4064 (n = 8) treatment for 6 h significantly increased the luciferase activity of the mouse AQP2-Luc reporter in primary IMCD cells. dDAVP (1 nM, n = 8) 0. **P < 0.01, ***P < 0.001 vs. DMSO (n = 6). (C) FXR overexpression significantly increased the AQP2-luc reporter activity. Cells were infected with Ad-FXRα1 or Ad-FXRα2 for 36 h. Adenovirus carrying cDNA encoding control VP16 alone (Ad-VP16) was used as the control for AdFXRα1 (n = 11) or AdFXRα2 (n = 11). ***P < 0.001 vs. Ad-VP16 (n = 9). (D) ChIP assay revealed that FXR can bind to the FXRE-like site in mouse AQP2 promoter. GW4064 could enhance the binding of FXR to the FXRE-like site. Input, positive control; FXR, anti-FXR antibody precipitated DNA; IgG, IgG precipitated DNA as negative control. (E) Schematic structure of the AQP2-Luc reporter. Site-directed mutagenesis of the putative FXRE from AGGTCACTGGACA to ACCTCACTGGACA, and CRE from GACGTCA to GTGGTCA was shown. (F) Mutation of the putative FXRE, but not the CRE, completely abolished the stimulatory effect of GW4064 on the luciferase activity of the AQP2-Luc reporter in IMCDs. **P < 0.01, ***P < 0.001 vs. DMSO, n = 8. Data are presented as mean ± SEM.
Discussion
The present study provides evidence that FXR plays an important role in regulation of renal water homeostasis. FXR activation significantly decreases, whereas FXR inactivation markedly increases, urine volume in mice. Both FXR endogenous agonists (CDCA and CA) and synthetic ligand (GW4064) induce AQP2 expression in the kidney and in cultured renal collecting duct cells. FXR is constitutively expressed in medullary collecting duct cells, where its activation up-regulates AQP2 transcription mainly via directly binding to the FXRE site in the promoter region of AQP2 gene. Our findings uncover a previously unidentified role for FXR in renal urine concentration and suggest that FXR overactivation may represent a potential underlying mechanism mediating fluid retention in clinical settings, including hepatic cirrhosis and cholestasis.
FXR, the bile acid nuclear receptor, is highly expressed in the liver and small intestine, where it is essential in the regulation of bile acid metabolism and enterohepatic circulation (28). As a member of the metabolic nuclear receptors, it also regulates expression of many target genes relevant for lipid and glucose metabolism at the transcription level (29–31). Increasing evidence suggests that FXR may play an important role in liver detoxification, regeneration, and carcinogenesis (6, 32, 33). FXR has also been found to be expressed in many “nonclassical” bile salt target tissues, including the vasculature and macrophages, where FXR influences vascular tension and regulates the unloading of cholesterol from foam cells, respectively (34). It has been previously reported that FXR is expressed in the adrenal glands and that its activation by GW4064 modulates adrenal cholesterol metabolism and glucocorticoid synthesis (35). The present study demonstrates that the kidney has constitutively high expression of FXR, with a ubiquitous expression pattern along renal tubules, suggesting that FXR may play an important role in renal physiology and pathophysiology. In support, recent studies by Levi Moshe’s group have demonstrated that FXR modulates renal lipid metabolism and is involved in the pathogenesis of diabetic nephropathy and represents an attractive therapeutic target for this disease (16, 36).
The present study provides evidence that FXR plays an important role in urine concentration. Treatment with endogenous FXR agonists (CA and CDCA) and synthetic FXR agonist GW4064 significantly decreased urine volume and increased urine osmolality in mice, indicative of enhanced urine concentration. In contrast, FXR gene deficiency mice exhibited increased urine output with reduced osmolality, suggesting an attenuated urine concentrating ability. Microarray analysis found that a group of genes involved in controlling water reabsorption was up-regulated, especially the aquaporin 2 water channel. Regulation of AQP2 expression by FXR was further confirmed by decreased AQP2 expression in FXR gene-deleted mice and increased AQP2 expression in FXR agonist-treated mice, as assessed by real-time PCR, Western blot, and immunohistochemistry assays. Collectively these findings indicate that FXR plays an important role in renal water homeostasis, possibly through regulating the expression of AQP2 in renal collecting ducts.
It was previously reported that the nuclear receptor liver X receptors (LXRs), which have a “yin-yang” relationship with FXR in regulation of bile acid metabolism, also participate in water homeostasis regulation. LXRβ deficiency results in central diabetes insipidus and impaired renal proximal tubule aquaporin-1 expression in mice (37). This finding suggests that LXR and FXR may act in concert in maintaining renal water homeostasis. In addition, FXR has been shown to play an important role in glucose metabolism. Although FXR−/− mice exhibit insulin resistance, there was no difference in fasting blood glucose levels between wild-type and FXR−/− mice (16). Therefore, it seems unlikely that hyperglycemia is responsible for polyuria in FXR−/− mice.
The present study also identifies AQP2 as a direct target gene of FXR. The kidney is the key organ in maintenance of water homeostasis. Normally, 99% of water in urine filtered by glomeruli is reabsorbed by renal tubules and collecting ducts. Among them, 70% is reabsorbed by the proximal tubules, 15% by the loops of Henle, and the rest by the distal tubules and collecting ducts (38). The collecting ducts play a determining role in final urine volume, depending on AQP2 expression and localization. Collecting duct AQP2 abundance is largely regulated by the AVP/V2 receptor system and to a less extent by prostaglandins and the transcription factors nuclear factor kappa B and tonicity-responsive enhancer binding protein (39). The present study has uncovered a unique mechanism by which AQP2 is regulated by FXR, independent of AVP and other known factors. Mouse AQP2 promoter contains a typical FXRE sequence and can be bound to FXR and activated by FXR agonists, resulting in a significant increase in AQP2 gene transcription. The identification of AQP2 as a direct target gene of FXR may not only help to understand the molecular mechanisms involved in maintaining normal urine concentrating capacity but also provide a possible explanation for increased fluid retention in clinical settings with elevated circulating bile acid levels, such as the hepatorenal syndrome and cholestasis. On the basis of our findings, we anticipate that in the state of severe liver damage elevated plasma bile acids can activate FXR in renal collecting ducts, resulting in impaired urine excretion through an FXR/AQP2-dependent mechanism.
It is interesting to note that, in cultured primary medullary collecting duct cells of FXR gene knockout mice, endogenous FXR agonist CDCA still slightly increased the transcription of AQP2 gene, indicating that other mechanisms may also be involved. It has been previously reported that in addition to the nuclear receptor FXR, bile acids can also activate a G protein-coupled receptor TGR5 and exert their biological effects through a few nongenomic signaling cascades (40). It is also known that other nuclear receptors, like pregnane X receptor, vitamin D receptor, and constitutive androstane receptor, may respond to bile acids, albeit to a more restricted set of bile acid species (41).
Taken together, our study demonstrates that FXR is constitutively expressed in the epithelial cells along renal tubules, especially renal collecting ducts. FXR activation enhances, whereas FXR gene deficiency lowers, urine concentrating capacity. FXR can directly bind to the FXRE site in the promoter region of the AQP2 gene, thereby increasing its gene expression in the collecting duct cells. These findings not only uncover a unique mechanism in the maintenance of renal water homeostasis but may also help to understand the pathogenesis of fluid retention frequently occurring in diseases like the hepatorenal syndrome and cholestasis.
Materials and Methods
Animals.
All experiments were reviewed and approved by the Animal Care and Use Review Committee of Peking University Health Science Center. FXR knockout mice, purchased from the Jackson Laboratory, were maintained on standard mouse chow and housed on a 12-h light/black cycle under controlled temperature (22–24 °C) and humidity (50–65%) in the animal facility of Peking University Health Science Center. Experiments were performed with male wild-type and FXR knockout mice, aged 4–6 mo.
Bile Acid and GW4064 Treatment.
Fifteen male mice (12 wk old) were divided into three groups randomly: one was placed on a control diet (n = 5), the other two on the same diet supplemented with 0.2% CA (n = 5) or 0.5% CDCA (n = 5), respectively. Five days later, 24-h urine samples were collected, and then the mice were killed. For GW4064 treatment, 11 male mice (12 wk old) were divided into two groups randomly. The mice in both groups received i.p. injection once daily for 3 d. One group of mice was injected with DMSO as vehicle (n = 6). The other group was injected with 30 mg/kg GW4064 dissolved in DMSO (n = 5). Three days later, 24-h urine samples and the kidneys of the mice were collected for further analysis.
Urine Collection and Osmolality Analysis.
Mice were housed in individual metabolic cages (Tecniplast) with free access to water and food for collection of 24-h urine. Body weight, urine excretion, and water consumption were measured. Mice were placed in individual metabolic cages for 24 h before measurement. Urine samples were centrifuged at 3,000 × g at 4 °C for 5 min, and the supernatants were saved for osmolality analysis using a freezing point depression osmometer (Micro-Osmometer 3300).
Construction of Mouse AQP2-Luc.
The DNA sequence of mouse AQP2 promoter region of 2,000 bp upstream of the transcription start site was analyzed using promoter 3.0 (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3). Mouse AQP2 promoter fragment between −1781 and +48 was amplified by PCR using mouse kidney genomic DNA as template and cloned in pGL3-luciferase reporter vector (Promega). The forward primer 5′-GCC CAA TCT TGA TAC-3′ and the reverse primer 5′-TCT TCC TCC CTC CCT CTC TCT-3′ were used for the PCR. Site-directed mutagenesis of the FXRE-like site at −327 and −326 and CREB site at −223 and −222 was accomplished using the Fast Mutagenesis System (FM111; TransGen Biotech) by using FXRE mutagenic primers (forward primer: 5′-CAG CCT TTT AGT CAA AGA GAA CCT CAC TGG ACA-3′; FXRE mutagenic reverse primer: 5′- GGT TCT CTT TGA CTA AAA GGC TGG CCA AGG AAG-3′) and CRE mutagenic primers (forward primer: 5′- AAC GAG GAA AAC AGA GTG GTC AAT CCT TAT-3′; CRE mutagenic reverse primer: 5′- CAC TCT GTT TTC CTC GTT TTT TCC TCA GTT-3′). The cloned mouse AQP2 promoter fragments were confirmed by DNA sequencing.
ChIP Assay.
ChIP assays were performed using a ChIP assay kit (CA92590; Millipore) according to the manufacturer’s instructions. Soluble chromatin was prepared from primary IMCD cells treated with 80 μmol/L CDCA or 2.5 μmol/L GW4064 for 6 h. Chromatin was immunoprecipitated with antibodies (2 μg) directed against FXR (sc13063; Santa Cruz Biotechnology). Final DNA extractions were sequenced at −395 to −137 in the AQP2 promoter, and the primers used were as follows: forward 5′-GCC TAT CAC CCC ATC TTA GCT-3′; antisense 5′-CCC ACA TTT CCT CAC AGT T-3′.
Chemicals and reagents, methods of real-time PCR, Western blot, immunostaining, microarray, dDAVP treatment, blood glucose measurement, primary culture of mouse IMCD cells, and luciferase assay are described in SI Materials and Methods.
Statistical Analysis.
The data were analyzed using the Prism software package (GraphPad Software). Data are presented as mean ± SEM. A two-sided Student t test was used to analyze individual differences. A value of P < 0.05 was considered to be statistically significant.
Supplementary Material
Acknowledgments
This work was supported by Ministry of Science and Technology Grant 2012CB517504/2010C8912500/2011ZX09102-011-12 (to Y.G.) and Natural Science Foundation Grants 81030003/81270275 (to Y.G.) and 81200511 (to X.Z.). J.-Å.G. is supported by the Swedish Research Council and by Robert A. Welch Foundation Grant E-0004.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323977111/-/DCSupplemental.
References
- 1.Laffitte BA, et al. Identification of the DNA binding specificity and potential target genes for the farnesoid X-activated receptor. J Biol Chem. 2000;275(14):10638–10647. doi: 10.1074/jbc.275.14.10638. [DOI] [PubMed] [Google Scholar]
- 2.Makishima M, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 3.Gustafsson JA. Seeking ligands for lonely orphan receptors. Science. 1999;284(5418):1285–1286. doi: 10.1126/science.284.5418.1285. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Kast-Woelbern HR, Edwards PA. Natural structural variants of the nuclear receptor farnesoid X receptor affect transcriptional activation. J Biol Chem. 2003;278(1):104–110. doi: 10.1074/jbc.M209505200. [DOI] [PubMed] [Google Scholar]
- 5.Huber RM, et al. Generation of multiple farnesoid-X-receptor isoforms through the use of alternative promoters. Gene. 2002;290(1-2):35–43. doi: 10.1016/s0378-1119(02)00557-7. [DOI] [PubMed] [Google Scholar]
- 6.Wang YD, Chen WD, Moore DD, Huang W. FXR: A metabolic regulator and cell protector. Cell Res. 2008;18(11):1087–1095. doi: 10.1038/cr.2008.289. [DOI] [PubMed] [Google Scholar]
- 7.Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem. 2001;276(31):28857–28865. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- 8.Chiang JY, Kimmel R, Weinberger C, Stroup D. Farnesoid X receptor responds to bile acids and represses cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J Biol Chem. 2000;275(15):10918–10924. doi: 10.1074/jbc.275.15.10918. [DOI] [PubMed] [Google Scholar]
- 9.Bishop-Bailey D, Walsh DT, Warner TD. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci USA. 2004;101(10):3668–3673. doi: 10.1073/pnas.0400046101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee H, et al. FXR regulates organic solute transporters alpha and beta in the adrenal gland, kidney, and intestine. J Lipid Res. 2006;47(1):201–214. doi: 10.1194/jlr.M500417-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Sinal CJ, et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102(6):731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 12.Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116(4):1102–1109. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanz RB, et al. Nuclear Receptor Signaling Atlas ( www.nursa.org): Hyperlinking the nuclear receptor signaling community. Nucleic Acids Res. 2006;34(Database issue):D221–D226. doi: 10.1093/nar/gkj029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang T, et al. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes. 2007;56(10):2485–2493. doi: 10.2337/db06-1642. [DOI] [PubMed] [Google Scholar]
- 15.Proctor G, et al. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes. 2006;55(9):2502–2509. doi: 10.2337/db05-0603. [DOI] [PubMed] [Google Scholar]
- 16.Wang XX, et al. Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes. 2010;59(11):2916–2927. doi: 10.2337/db10-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noda Y, Sohara E, Ohta E, Sasaki S. Aquaporins in kidney pathophysiology. Nat Rev Nephrol. 2010;6(3):168–178. doi: 10.1038/nrneph.2009.231. [DOI] [PubMed] [Google Scholar]
- 18.Hasler U, Leroy V, Martin PY, Féraille E. Aquaporin-2 abundance in the renal collecting duct: New insights from cultured cell models. Am J Physiol Renal Physiol. 2009;297(1):F10–F18. doi: 10.1152/ajprenal.00053.2009. [DOI] [PubMed] [Google Scholar]
- 19.Setchell KD, Matsui A. Serum bile acid analysis. Clin Chim Acta. 1983;127(1):1–17. doi: 10.1016/0009-8981(83)90070-0. [DOI] [PubMed] [Google Scholar]
- 20.Jonassen TE, Nielsen S, Christensen S, Petersen JS. Decreased vasopressin-mediated renal water reabsorption in rats with compensated liver cirrhosis. Am J Physiol. 1998;275(2 Pt 2):F216–F225. doi: 10.1152/ajprenal.1998.275.2.F216. [DOI] [PubMed] [Google Scholar]
- 21.Ginès A, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105(1):229–236. doi: 10.1016/0016-5085(93)90031-7. [DOI] [PubMed] [Google Scholar]
- 22.Tain Y-L, Hsieh C-S, Chen C-C, Huang L-T. Toward nitric oxide deficiency in hepatorenal syndrome: Is farnesoid X receptor the link? Biosci Hypotheses. 2008;1(3):145–146. [Google Scholar]
- 23.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3(5):543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 24.Baumgarten R, Van De Pol MH, Wetzels JF, Van Os CH, Deen PM. Glycosylation is not essential for vasopressin-dependent routing of aquaporin-2 in transfected Madin-Darby canine kidney cells. J Am Soc Nephrol. 1998;9(9):1553–1559. doi: 10.1681/ASN.V991553. [DOI] [PubMed] [Google Scholar]
- 25.Farré D, et al. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 2003;31(13):3651–3653. doi: 10.1093/nar/gkg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messeguer X, et al. PROMO: Detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002;18(2):333–334. doi: 10.1093/bioinformatics/18.2.333. [DOI] [PubMed] [Google Scholar]
- 27.Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F. Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol. 1997;8(6):861–867. doi: 10.1681/ASN.V86861. [DOI] [PubMed] [Google Scholar]
- 28.Inagaki T, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Han SI, et al. Bile acids enhance the activity of the insulin receptor and glycogen synthase in primary rodent hepatocytes. Hepatology. 2004;39(2):456–463. doi: 10.1002/hep.20043. [DOI] [PubMed] [Google Scholar]
- 30.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7(8):678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe M, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113(10):1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang W, et al. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312(5771):233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, et al. PARP1 promotes oxidative stress-induced liver cell death via suppressing FXR. Mol Cell Biol. 2013;33(22):4492–503. doi: 10.1128/MCB.00160-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hageman J, Herrema H, Groen AK, Kuipers F. A role of the bile salt receptor FXR in atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30(8):1519–1528. doi: 10.1161/ATVBAHA.109.197897. [DOI] [PubMed] [Google Scholar]
- 35.Hoekstra M, et al. FXR agonist GW4064 increases plasma glucocorticoid levels in C57BL/6 mice. Mol Cell Endocrinol. 2012;362(1-2):69–75. doi: 10.1016/j.mce.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Wang XX, et al. The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am J Physiol Renal Physiol. 2009;297(6):F1587–F1596. doi: 10.1152/ajprenal.00404.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabbi C, et al. Central diabetes insipidus associated with impaired renal aquaporin-1 expression in mice lacking liver X receptor β. Proc Natl Acad Sci USA. 2012;109(8):3030–3034. doi: 10.1073/pnas.1200588109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Callaghan CA. The Renal System at a Glance. 3rd Ed. Chichester, UK: Wiley-Blackwell; 2009. [Google Scholar]
- 39.Hasler U, et al. NF-kappaB modulates aquaporin-2 transcription in renal collecting duct principal cells. J Biol Chem. 2008;283(42):28095–28105. doi: 10.1074/jbc.M708350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe M, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 41.Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol. 2013 doi: 10.1038/nrgastro.2013.151. 10.1038/nrgastro.2013.151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.