Significance
Advances in organ transplantation and treatment of allergy and autoimmune disease hinge upon harnessing a physiological switch that allows T cells to decide between proliferating extensively or actively becoming tolerant. The experiments presented here illuminate a critical element of this natural switch, Ndfip1 (neural precursor cell expressed, developmentally down-regulated protein 4 family-interacting protein 1), a partner protein of ubiquitin ligases induced during the first several divisions after T cells encounter antigen. They define the cellular action of Ndfip1 in vivo, acting within dividing helper T cells that have responded to innocuous foreign or self-antigen that should normally be tolerated, to force their exit from cell cycle before they have divided so many times that they acquire tissue-damaging effector functions.
Keywords: immunological tolerance, allergy, T lymphocyte, Interleukin-4, Aire (Autoimmune Regulator)
Abstract
The NDFIP1 (neural precursor cell expressed, developmentally down-regulated protein 4 family-interacting protein 1) adapter for the ubiquitin ligase ITCH is genetically linked to human allergic and autoimmune disease, but the cellular mechanism by which these proteins enable foreign and self-antigens to be tolerated is unresolved. Here, we use two unique mouse strains—an Ndfip1-YFP reporter and an Ndfip1-deficient strain—to show that Ndfip1 is progressively induced during T-cell differentiation and activation in vivo and that its deficiency causes a cell-autonomous, Forkhead box P3-independent failure of peripheral CD4+ T-cell tolerance to self and exogenous antigen. In small cohorts of antigen-specific CD4+ cells responding in vivo, Ndfip1 was necessary for tolerogen-reactive T cells to exit cell cycle after one to five divisions and to abort Th2 effector differentiation, defining a step in peripheral tolerance that provides insights into the phenomenon of T-cell anergy in vivo and is distinct from the better understood process of Bcl2-interacting mediator of cell death-mediated apoptosis. Ndfip1 deficiency precipitated autoimmune pancreatic destruction and diabetes; however, this depended on a further accumulation of nontolerant anti-self T cells from strong stimulation by exogenous tolerogen. These findings illuminate a peripheral tolerance checkpoint that aborts T-cell clonal expansion against allergens and autoantigens and demonstrate how hypersensitive responses to environmental antigens may trigger autoimmunity.
In healthy individuals, mature T cells in peripheral lymphoid tissues proliferate and acquire effector functions in response to antigens from pathogenic microbes but remain tolerant to self-antigens and innocuous environmental antigens. Defects in this phenomenon of “peripheral T-cell tolerance” are thought to contribute to the burden of autoimmune and allergic disease, but there is only a fragmented understanding of its cellular basis, its connection to specific genetic circuits, and the interconnection between autoimmunity and hypersensitivity to exogenous antigens (1). This problem is exemplified by the genetic circuit encoding Ndfip1 [neural precursor cell expressed, developmentally down-regulated protein 4 (NEDD4) family-interacting protein 1], a transmembrane protein localized to the Golgi and intracellular vesicles that recruits and activates the HECT-type E3 ubiquitin ligase Itch (2–7). Human genetic studies have associated NDFIP1 and ITCH with allergic and autoimmune diseases. Inherited ITCH deficiency results in asthma-like chronic lung disease with nonfibrotic lymphocytic pneumonitis (90% cases) and organ-specific autoimmunity (60% cases) variably involving the thyroid, liver, intestine, or pancreatic islets (8). Inherited NDFIP1 polymorphisms are associated with inflammatory bowel disease (9, 10), asthma (11), rheumatoid arthritis (12), and multiple sclerosis (13). It remains unclear which cellular mechanisms of tolerance are disrupted by these genetic variants to result in allergic and autoimmune disease.
Ndfip1 and Itch were first revealed as important immune regulators in mouse genetic studies. Homozygous inactivating mutations in the Itchy strain cause dermatitis, lung mononuclear inflammation, lymphadenopathy with follicular hyperplasia, increased activated T cells (notably IL-4–producing Th2 cells), expansion of B1b cells in the peritoneal cavity, and early death (5, 6, 14, 15). Although the murine pathology has often been described as autoimmune because of its spontaneous development, there is currently little direct evidence of T-cell autoimmunity, and the predominant inflammation of skin and mucosal surfaces suggests an exaggerated response to innocuous environmental antigens. Indeed, elegant studies showed that Itch deficiency prevents high-zone tolerance in an experimental model of respiratory exposure to an egg protein allergen (16). An almost identical skin and lung inflammatory syndrome occurs in mice inheriting a homozygous gene-trap insertion that greatly reduces Ndfip1 mRNA and protein (2). Although much progress has been made elucidating diverse biochemical functions of Itch and Ndfip1 in many cell types (3, 17), the cellular basis for immune dysregulation in their absence is unresolved, and their role in T-cell tolerance to self-antigens has yet to be examined.
Defects in several different cellular mechanisms for peripheral T-cell tolerance have been implicated in the inflammatory disease caused by defects in the Itch-Ndfip1 genetic circuit. T-cell anergy is a mechanism defined initially in tissue culture that prevents initiation of T-cell proliferation when T cells are stimulated without a CD28 costimulus (18). Itch was required for T-cell anergy in cultured cells rendered anergic by prolonged in vitro treatment with ionomycin or harvested from TCR transgenic (Tg) mice 10 d after exposure to a high-tolerogenic dose of foreign antigen. An intact Itch gene was correlated with diminished TCR signaling and proteolytic degradation of protein kinase C (PKC)-θ, phospholipase C (PLC)-γ, JunB, and c-Jun proteins (16, 19). A role for Itch in nondegradative ubiquitination of the TCR CD3 ζ subunit to inhibit its phosphorylation and the activation of Zap-70 has also been shown (20). Likewise, Ndfip1 deficiency causes JunB accumulation (2) and allows T cells to make IL-2 for a sustained period in vitro without the need for CD28 costimulation (21). Itch-deficient T cells also display exaggerated NF-κB signaling in response to TNF-α, because Itch forms a complex that recruits Tnfaip3 to terminate NF-κB signaling (22). Itch has also been implicated in ubiquitination and degradation of Bcl-10, an adaptor protein with an essential role in TCR and CD28 signaling to NF-κB (23). TCR-activated T cells that lack Itch or Ndfip1 form a diminished percentage of Forkhead box P3 (Foxp3+) induced T-regulatory cells (iTregs) when cultured with TGF (24, 25).
Increased differentiation of Th2 effector cells is a prominent feature of Itch or Ndfip1 deficiency that is partly explained by their role in ubiquitination and degradation of JunB, an Il4 gene transcription factor preferentially expressed in Th2 cells (2, 14, 26). However, Tg mice that express equivalently high levels of JunB in T cells do not exhibit a similar spontaneous accumulation of Th2 effector cells or inflammatory disease (26, 27). Abnormal Notch signaling within activated T cells might also contribute (28–30) because the Itch ortholog in Drosophila, Suppressor of Deltex, was discovered as a negative regulator of Notch signaling, and the Drosophila Ndfip1 ortholog has similar genetic effects on this pathway (31, 32). Itch ubiquitinates and terminates ligand-independent Notch signaling in endosomes, requiring an adaptor protein that may be Ndfip1 based on Drosophila studies (31, 33, 34).
Although there are many possible cellular explanations for how allergy and autoimmunity may result from defects in the Itch-Ndfip1 genetic circuit, resolving these alternatives to place the biochemical circuit in its correct cellular context awaits a systematic comparison of the fates of mutant and wild-type T cells responding to normally tolerogenic antigens in vivo. Here, we perform this comparison and reveal that the critical function for Ndfip1 in peripheral tolerance is as an induced, cell-autonomous brake against effector CD4+ cell differentiation.
Results
Lethal Immune-Mediated Th2 Disease Caused by a Truncating Mutation of Ndfip1.
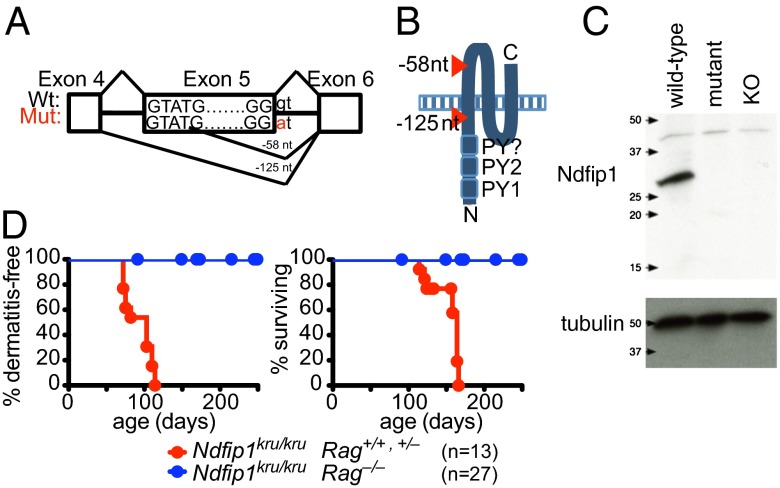
In a C57BL/6 × C57BL/10 mouse pedigree segregating point mutations induced by ethylnitrosourea, offspring exhibited a Mendelian recessive syndrome of spontaneous mast cell-rich dermatitis with median onset age 90 d, followed by weight loss and premature mortality at a median age of 160 d (Fig. 1 and Fig. S1 A and B). This was accompanied by lymphadenopathy, splenomegaly, increased CD86 and CD23 activation markers on B cells, expansion of the activated/memory subset of CD4+ and CD8+ T cells, greatly elevated serum IgE, and formation of a prominent population of IL-4+ and IFN-γ+ CD4+ cells (Fig. S1 C–E). The mutation was given the allele name “krusty” and mapped in a genome-wide scan of C57BL/6 × C57BL/10 SNPs to a chromosome 18 region containing a strong candidate gene, Ndfip1, based on a similar phenotype in a strain bearing a gene-trap insertion (2). Sequencing the Ndfip1 exons revealed a G-to-A substitution in the first nucleotide of the intron following exon 5, disrupting the mRNA splice donor sequence (Fig. 1A, allele denoted “Ndfip1kru”). Genotyping for this mutation showed perfect concordance between Ndfip1kru homozygosity and the lethal dermatitis phenotype in >100 affected offspring (>800 meioses) generated by successively backcrossing to wild-type C57BL/6 mice and intercrossing heterozygous progeny.
Fig. 1.
Immune-mediated lethal inflammatory syndrome in mice with a truncating Ndfip1 splice site mutation. (A) Schematic showing the location of the Ndfip1kru mutation within the Ndfip1 exon 5 splice donor sequence and the resulting two aberrant splice products. (B) Schematic showing the topology of the Ndfip1 protein and position of the truncating mutations. (C) Western blot of primary T-cell lysates from wild-type, Ndfip1kru/kru (mutant), and Ndfip1−/− (KO) mice, probed with an antibody raised to a conserved N-terminal peptide of Ndfip1, and then stripped and reprobed with antibody to tubulin to assess loading. (D) Dermatitis and survival of Ndfip1kru/kru mice with normal or null Rag1 genes, aged to 250 d or until moribund necessitating the animal to be killed.
Amplification and sequencing of cDNA from Ndfip1wt/wt versus Ndfip1kru/kru splenocytes revealed the mutation abolished normal splicing and resulted in two aberrantly spliced mRNA products, encoding frame shifts and premature stop codons truncating the Ndfip1 protein either immediately preceding or following the first of the three transmembrane domains (Fig. 1 A and B and Fig. S2 A and B). In transfected HEK293 cells, Ndfip1 protein was detected from only the aberrantly spliced cDNA retaining the first transmembrane sequence and from the wild-type cDNA (Fig. S2C). Western blotting of activated T-cell lysates from Ndfip1kru/kru mice with antibody to the Ndfip1 N terminus (35) confirmed the absence of the ∼27-kDa wild-type Ndfip1 protein and only trace amounts of ∼20-kDa protein corresponding to the aberrantly spliced mRNA retaining the first transmembrane sequence (Fig. 1C). This truncated residual protein included the N-terminal cytoplasmic PY motifs that bind and activate Itch and Nedd4 (3) but was evidently insufficient to suppress lethal inflammation.
Because Ndfip1 is expressed widely and has been implicated in functions outside of the adaptive immune system (36), we tested whether or not the dermatitis or premature lethality was suppressed by the selective elimination of T and B cells in Rag1−/− Ndfip1kru/kru mice. In contrast to lymphocyte-sufficient Ndfip1kru/kru littermate controls, dermatitis and mortality did not develop in Rag1−/− Ndfip1kru/kru mice (Fig. 1D), demonstrating that the spontaneous pathology absolutely requires T and/or B lymphocytes.
Ndfip1 Deficiency Causes Cell Autonomous Accumulation of CD4+ Effectors Not Corrected by Normal Foxp3+ Cells.
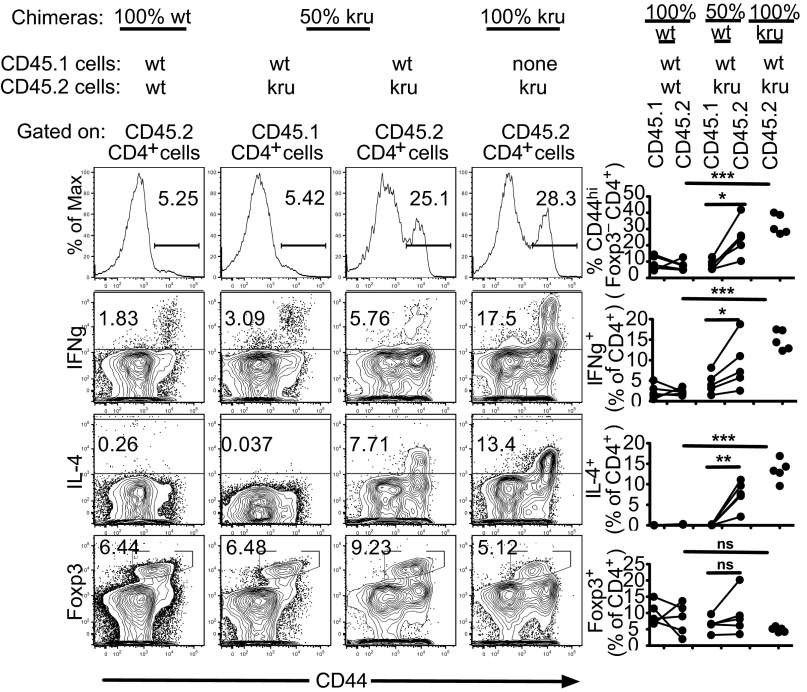
To determine which subsets of T or B cells are regulated by Ndfip1, 50:50 mixed hematopoietic chimeras were constructed by transplanting bone marrow from Ndfip1kru/kru or wild-type donors, distinguished by CD45.1 or CD45.2 allotypic markers, into irradiated Rag1−/− recipients. As observed in unmanipulated Ndfip1kru/kru mice, B cells and CD8 T cells were both dramatically altered in chimeras reconstituted with only 50% Ndfip1kru/kru hematopoietic cells (Fig. S3 A–C), but these effects were an indirect consequence of Ndfip1kru/kru in other lymphocytes because they occurred to an equal extent in the CD45.1 B and CD8 T cells bearing wild-type Ndfip1 developing alongside Ndfip1kru/kru lymphocytes. By contrast, there was a direct, cell-autonomous requirement for Ndfip1 in CD4+ T cells (Fig. 2). Ndfip1 deficiency resulted in greatly increased frequencies of CD44hi IFN-γ+ and IL-4+ cells among CD4+ cells in chimeras reconstituted with 100% Ndfip1kru/kru marrow. In mixed chimeras bearing 50% Ndfip1kru/kru marrow, expansion of IL-4+ cells, CD44hi cells, and IFN-γ+ CD4+ cells was limited to the CD45.2+ T cells lacking normal Ndfip1 and did not occur in the CD45.1+ wild-type CD4+ cells that developed in the same animals. Analysis of Foxp3+ CD4+ cells in the mixed chimeras showed that Ndfip1kru/kru neither increased nor decreased the frequency of Foxp3+ cells even under competitive reconstitution conditions where wild-type marrow was also present (Fig. 2). Despite the presence of large numbers of wild-type Foxp3+ cells in these 50:50 mixed chimeras, accumulation of mutant CD44hi Th2 and Th1 CD4+ effector cells was not corrected.
Fig. 2.
Ndfip1 deficiency causes cell-autonomous accumulation of activated/effector CD4+ T cells that is not corrected by the presence of wild-type Foxp3+ Tregs. Three groups of mixed bone marrow chimeras were generated by transplanting irradiated Rag1−/− mice: “100% kru,” transplanted with 100% Ndfip1kru/kru CD45.2 bone marrow; “50% kru,” transplanted with a 50:50 mixture of Ndfip1kru/kru CD45.2 bone marrow and Ndfip1+/+CD45.1 bone marrow; “100% wt,” transplanted with a 50:50 mixture of Ndfip1+/+CD45.2 bone marrow and Ndfip1+/+CD45.1 bone marrow. Flow cytometry was used to analyze lymph node CD4+ cells, gated into either CD45.2+ or CD45.1+ cells, measuring cell surface expression of CD44, intracellular IFN-γ or IL-4 after 3 h ex vivo phorbol myristate acetate (PMA)/ionomycin stimulation, and intracellular Foxp3. Representative plots and quantitation in multiple animals are shown. Lines link data points from the same recipient animal. *P < 0.05; **P < 0.01; ***P < 0.001 by Student t test.
Ndfip1-YFP Is Progressively Induced During T-Cell Maturation and Activation.
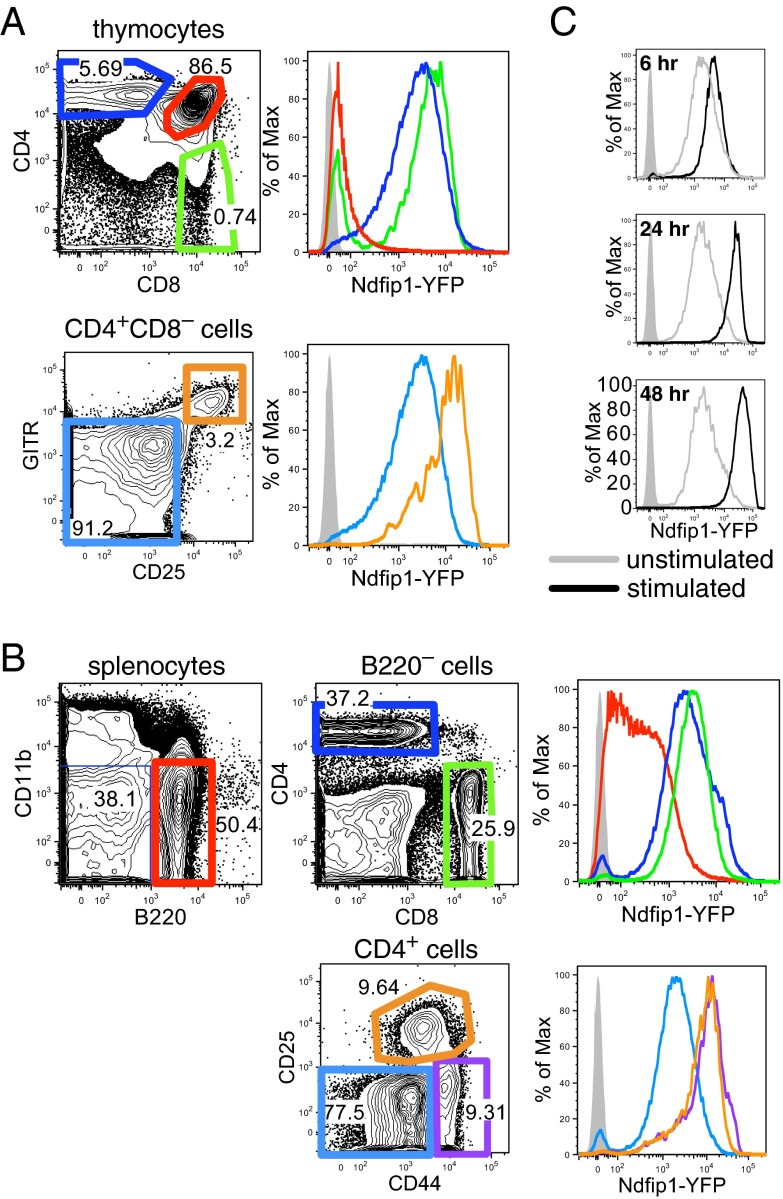
To understand the cell-autonomous requirement for Ndfip1 in preventing pathological accumulation of effector CD4+ T cells, Ndfip1-YFP Tg reporter mice were constructed by pronuclear injection of a mouse bacterial artificial chromosome (BAC) containing the Ndfip1 genomic locus with an eYFP cDNA inserted at the Ndfip1 translation start site (Ndfip1-YFP Tg mice). In the thymus, YFP fluorescence of CD4+CD8+ double-positive (DP) cells was only marginally higher than control thymocytes from non-Tg mice, but fluorescence was ∼100-fold higher in CD4+CD8– and CD4–CD8+ single-positive (SP) cells, corresponding to the stage of positive selection by TCR-pMHC ligation (Fig. 3A). A further ∼fivefold higher YFP fluorescence was observed in the CD25+GITR+ subset of CD4+ SP cells, which comprise cells receiving a stronger TCR signal, including nascent Foxp3+ regulatory T cells (Tregs) and cells undergoing negative selection (37). In the spleen, YFP fluorescence in mature CD4+ and CD8 T+ cells was comparable to their thymic SP counterparts, whereas lower heterogeneous levels were observed in B cells (Fig. 3B). Within splenic CD4+ cells, CD44hi CD25– effector/memory and CD44int CD25+ Treg fractions had ∼fivefold increased YFP above CD44lo CD25– naïve CD4+ T cells. These results, measured at single-cell resolution, mirror the pattern of Ndfip1 mRNA expression observed in sorted T-cell subsets in the Immgen database (38). When splenocytes from Ndfip1-YFP Tg animals were cultured with plate-bound antibodies against CD3 and CD28, YFP increased ∼30-fold above the level in resting CD4+ T cells over the course of 2 d, with ∼2-fold induction by 6 h and ∼10-fold induction by 24 h (Fig. 3C). Thus, Ndfip1 is progressively induced by antigen-receptor signaling as T cells mature and then become activated.
Fig. 3.
Progressive induction of an Ndfip1 reporter gene during T-cell selection and activation. Flow cytometric analysis of Tg Ndfip1 reporter (Ndfip1-YFP Tg) mice bearing a BAC with eYFP integrated in place of the Ndfip1 translation initiation codon. (A) YFP fluorescence of CD4+CD8+, CD4+CD8–, CD4–CD8+, CD4+CD8–CD25–GITR–, and CD4+CD8–CD25+GITR+ thymocyte populations from Ndfip1-YFP Tg mice, gated as indicated. (B) YFP fluorescence of CD4+, CD8+, B220+, CD4+CD44loCD25–, CD4+CD44hiCD25–, and CD4+CD44intCD25+ splenocyte populations from Ndfip1-YFP Tg mice, gated as indicated. (C) YFP fluorescence of gated CD4+ cells among splenocytes from Ndfip1-YFP Tg mice that were incubated with or without plate-bound antibodies against CD3 and CD28 for the indicated periods. In all cases, the shaded gray histogram shows the YFP fluorescence of cells from non-Tg control mice.
Ndfip1 Aborts Antigen-Induced Division and Differentiation of CD4+ T Cells After Several Days.
The findings above indicated that Ndfip1 may regulate either the initial activation of naive CD4+ cells by innocuous foreign- or self-antigens or their subsequent clonal accumulation as effector cells. To resolve these alternatives, the Ndfip1kru mutation was crossed into the 3A9 TCR Tg strain (37, 39), providing a source of naïve CD4+ T cells recognizing hen egg lysozyme (HEL) peptide presented by the MHC class II protein I-Ak. Equal numbers of naïve HEL-specific CD4+ T cells from wild-type 3A9 Tg donors (allotypically marked as heterozygous CD451/2) and Ndfip1kru/kru 3A9 Tg donors (allotypically marked as homozygous CD452/2) were mixed, labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE), and transferred into normal congenic B10.BR CD451/1 recipients. In these experiments, normal Ndfip1 was present in all cells other than the small subset of transferred CD452/2 CD4+ TCR Tg cells. To analyze the response to innocuous foreign antigen, the recipient mice were injected i.p. with 100 μg of HEL protein in saline without adjuvant, and clonal expansion of the transferred T cells monitored 3 or 6 d later using antibodies against the CD45 allotypes and by CFSE fluorescence (Fig. 4A). After 3 d, donor T cells in control recipients that did not receive HEL persisted in equal frequencies and had not diluted CFSE, indicating that absence of Ndfip1 did not result in spontaneous activation of naïve CD4+ T cells. Three days after HEL injection, however, both mutant and wild-type donor T cells had increased in frequency 8- to 10-fold and had undergone multiple rounds of cell division, reported by CFSE dilution (Fig. 4 A and B). At this initial stage of the response, there was little difference in the proliferation of mutant and wild-type TCR Tg CD4+ cells, although the Ndfip1kru/kru T cells were ∼1.5-fold as frequent.
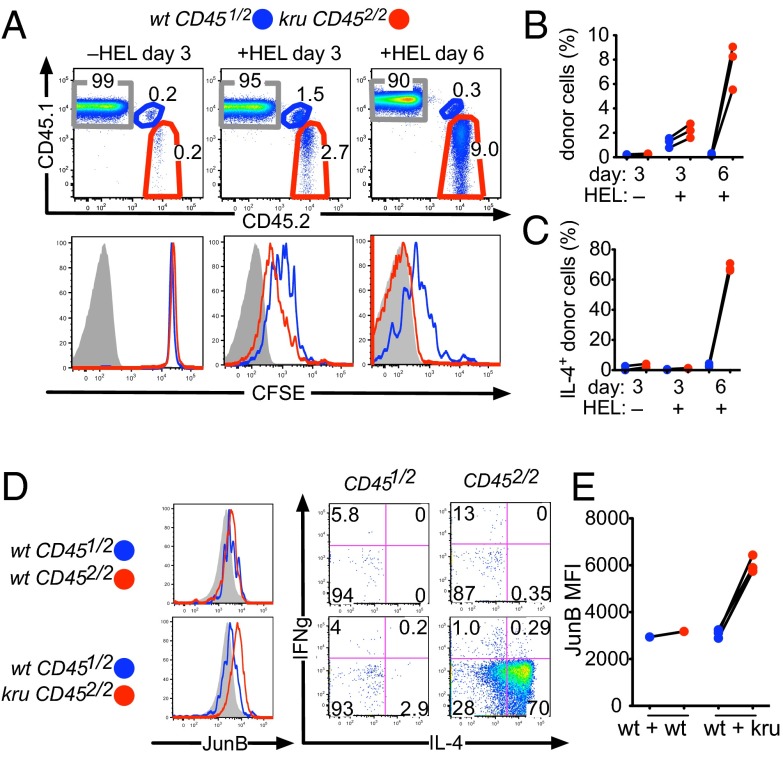
Fig. 4.
Ndfip1 is required in activated CD4+ T cells to prevent effector accumulation and differentiation in response to exogenous antigen without adjuvant. (A) An equal mixture of HEL-specific CD4+ T cells was prepared from 3A9 TCRHEL Tg mice that were Ndfip1kru/kru CD452/2 or wild-type CD451/2, labeled with CFSE, and 2 × 106 total CD4+ cells injected into the circulation of normal B10.BR CD451/1 recipient mice. A subset of the recipient mice was given 100 μg of HEL antigen in saline i.p. at the time of cell transfer. Shown is representative flow cytometric analysis of recipient spleen cells on the indicated day after T-cell transfer. Upper graphs are gated on CD4+ T cells and show the percentage derived from the two donors and the recipient. Lower graphs show CFSE fluorescence of the respective donor and recipient cells. (B) Percentage of donor cells in multiple recipients analyzed as in A. Lines link data points from the same recipient animal. (C) Percentage of donor T cells expressing IL-4 measured by intracellular staining and gated as in A after 3 h ex vivo PMA/ionomycin stimulation. (D) Intracellular staining for JunB, IL-4, and IFN-γ on gated CD451/2 or CD452/2 donor CD4+ cells at day 6 after transfer and injection of HEL in saline. (E) Quantitation of the JunB MFI of gated donor cells in multiple recipients analyzed as in D.
A remarkable divergence between wild-type and Ndfip1kru/kru CD4+ T cells developed by day 6 after exposure to HEL antigen in saline, when the frequency of wild-type Tg donor cells had decreased ∼fivefold compared with their peak at day 3, and the remaining cells retained appreciable CFSE (Fig. 4 A and B). In contrast, the Ndfip1kru/kru CD4+ T cells had continued to increase in frequency a further ∼fourfold from day 3 and had diluted their CFSE label beyond the limit of detection. Consequently, at day 6, the Ndfip1kru/kru CD4+ progeny were ∼30- to 50-fold more abundant than their cotransferred wild-type counterparts. Intracellular flow cytometric staining at this time point revealed more JunB in Ndfip1kru/kru CD4+ Tg cells than in coresident wild-type cells and that most Ndfip1kru/kru cells produced high levels of IL-4 upon brief restimulation ex vivo, whereas few of the wild-type Tg cells did (Fig. 4 C–E).
The continued expansion and effector differentiation of Ndfip1kru/kru CD4+ T cells observed at day 6 of the response to exogenous HEL “tolerogen” was not attributable to a deficiency in transacting suppression by Tregs, because it occurred cell-autonomously in recipient mice that had a normal T-cell repertoire. It was nevertheless possible that differences in Foxp3 induction within the transferred mutant and wild-type CD4+ T cells might explain their divergent behavior. To test this possibility, we repeated the cotransfer experiments with a parallel group of recipients that received a mixture of CD452/2 Foxp3-deficient 3A9 TCR Tg CD4 cells and CD451/1 wild-type Tg cells (Fig. 5). Six days after transfer and administration of HEL in saline, Ndfip1kru/kru CD4+ T cells had expanded dramatically as before, but the expansion of Foxp3-deficient CD4+ T cells was indistinguishable from their cotransferred wild-type CD4+ cells (Fig. 5A). Hence, Foxp3 is not required within tolerogen-responding CD4+ T cells to curtail their proliferative response in vivo, whereas Ndfip1 is essential.
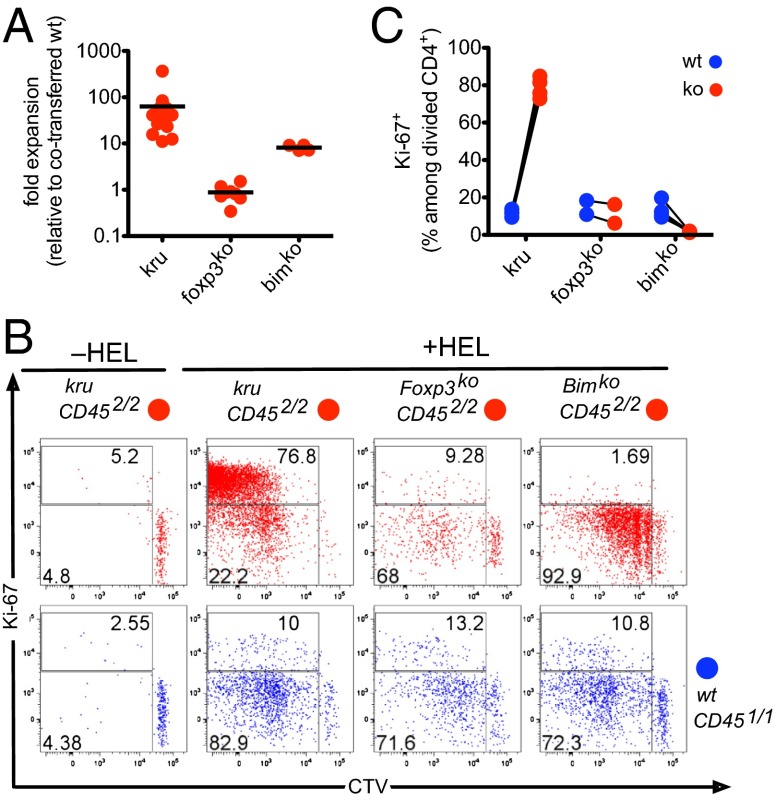
Fig. 5.
Ndfip1 mediates tolerance distinct from mitochondrial apoptosis or Foxp3, by aborting the proliferative response of CD4+ T cells. Cotransfer of allotypically distinguished 3A9 TCRHEL Tg cells was performed as in Fig. 4 but including parallel groups of recipients that received cell mixtures from wild-type and Foxp3-deficient, or wild-type and Bim-deficient mice (all mice 3A9 TCRHEL Tg), and using the cell division label CTV. (A) Quantitation of the fold-expansion (relative to cotransferred wild-type cells) of wild-type, Ndfip1kru/kru, Foxp3-deficient or Bim-deficient CD4+ cells in recipient mice at day 6 following injection of HEL in saline, compared with the cell inputs at day 0. (B) Representative Ki-67 and CTV data from CD451/2 recipients on day 6, gated on the cotransferred CD452/2 (red plots) or CD451/1 (blue plots) 3A9 TCRHEL CD4+ T cells of the indicated genotypes. (C) Percentage of Ki-67+ cells remaining in cell cycle among donor CD4+ cells that had previously divided (CTVlo) in response to 100ug HEL in saline, measured in individual recipients as in B.
Mitochondrial apoptosis regulated by the Bcl-2 and Bcl2-interacting mediator of cell death (Bim) proteins is a well-established pathway contributing to peripheral T-cell tolerance (1, 40). To test whether a defect in this pathway could replicate the failure of Ndfip1kru/kru cells to abort clonal expansion, we included a parallel group of recipients that received a mixture of CD452/2 Bim-deficient 3A9 TCR Tg cells and CD451/1 wild-type 3A9 Tg cells (Fig. 5). In contrast to the >50-fold greater expansion of Ndfip1kru/kru cells, Bim-deficient cells exposed to exogenous HEL in saline accumulated an average ∼eightfold higher than the wild-type internal controls (Fig. 5A).
To compare how defects in Ndfip1 or Bim affected the proliferative state of the responding cells, we labeled the transferred T cells with cell trace violet (CTV) and measured division-linked CTV dilution together with expression of the cell cycle marker Ki-67 at day 6 of the response (Fig. 5 B and C). Analysis of CTV dilution indicated that, like their wild-type counterparts and also the Foxp3-deficient cells, most of the Bim-deficient CD4+ cells had responded to exogenous HEL tolerogen by dividing one to five times. However, the resulting CTVlow progeny expressed little Ki-67 on day 6, indicating that they had exited cell cycle by this time point. By contrast, the transferred Ndfip1kru/kru cells remained predominantly Ki-67+, revealing a failure to exit cell cycle (Fig. 5 B and C). These results establish that peripheral tolerance to innocuous exogenous antigen involves two genetically separate cellular mechanisms within responding CD4+ T cells: (i) Bim-dependent apoptosis; and (ii) Ndfip1-dependent exit from cell cycle that occurs even when apoptosis is defective.
Ndfip1 Prevents Autoimmune Disease by Aborting CD4+ Cell Proliferation and Differentiation.
Peripheral tolerance mechanisms become critical backups when thymic tolerance fails (41); for example, because of inherited Aire mutations that disrupt thymic deletion of organ-specific T cells (39, 42). In B10.BR mice with intact peripheral tolerance mechanisms, Aire deficiency caused little clinical autoimmune disease on its own. We therefore combined Aire and Ndfip1 deficiency by transplanting wild-type or Aire−/− B10.BR mice with Ndfip1kru/kru bone marrow. Whereas Aire-deficient recipients of wild-type marrow showed no signs of disease or histological evidence of pancreatitis, recipients of Ndfip1kru/kru marrow lost weight and became moribund 30–40 d after transplant with extensive lymphocytic infiltration and autoimmune destruction of the exocrine pancreatic cells (Fig. S4). This genetic cooperation mirrored that observed between Aire and Cblb, the latter encoding another ubiquitin ligase with a role in peripheral tolerance (41).
To analyze peripheral tolerance to a defined self-antigen made in the pancreas, we crossed Ndfip1kru 3A9 TCRHEL Tg mice with mice expressing HEL protein controlled by the rat insulin gene promoter (insHEL Tg mice). Despite greatly increased thymic production of CD4+ T cells specific for the pancreatic islets, the majority of TCRHEL:insHEL double Tg mice with one or two wild-type Ndfip1 alleles were protected from diabetes (Fig. 6A). This protection has previously been shown to be attributable to Aire-dependent thymic deletion (39) and Cblb-dependent peripheral tolerance (43). By contrast, 100% of Ndfip1kru/kru double Tg animals developed diabetes by 60 d of age (Fig. 6A). Analysis of the thymi nevertheless showed no effect of the Ndfip1kru/kru mutation upon thymic deletion of 3A9 TCRHEL CD4+ cells in insHEL Tg animals. Thus, the enhanced progression to diabetes caused by Ndfip1 deficiency likely reflected the fact that some TCRHEL CD4+ cells escape thymic deletion and must be controlled by Ndfip1-mediated peripheral tolerance.
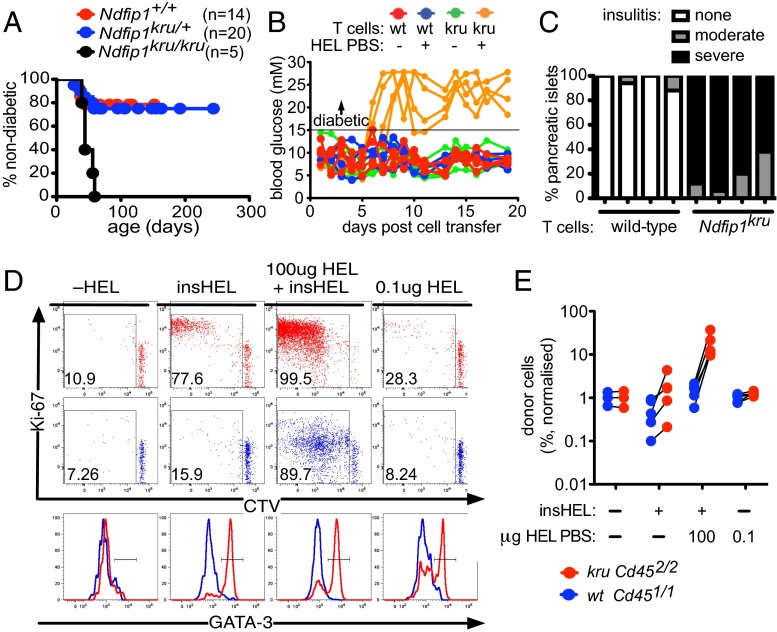
Fig. 6.
Ndfip1 deficiency disrupts peripheral T-cell tolerance to pancreatic islets. (A) Incidence of diabetes in a cohort of mice with the indicated Ndfip1 genotypes that were all doubly Tg for the 3A9 TCRHEL transgene and an insulin-promoter HEL transgene (insHEL Tg). (B) Daily blood glucose readings in singly insHEL Tg mice injected i.v. with 106 CD4+ T cells from 3A9 TCRHEL Tg donors that were either Ndfip1kru/kru or wild-type. Half of the recipients also received 100 μg of HEL in saline i.p. at the time of T-cell transfer. (C) Quantitation of insulitis in ≥10 individual islets in individual recipients (denoted by columns) 3 wk after transfer of wild-type or Ndfip1kru/kru T cells together with HEL in saline. (D) A mixture of HEL-specific CD4+ T cells from 3A9 TCRHEL Tg mice that were Ndfip1kru/kru CD452/2 or wild-type CD451/2 was CTV-labeled and injected into the circulation of non-Tg or insHEL Tg B10.BR CD451/1 recipient mice. One group of insHEL Tg recipients was given 100 μg of HEL in saline i.p. at the time of cell transfer, whereas another group of non-Tg recipients received 0.1 μg of HEL in saline i.p. The donor CD4+ cells were analyzed 6 d later in the spleen for CTV dilution and expression of Ki-67 and GATA-3. Shown are representative plots of CTV fluorescence versus Ki-67 staining (upper plots) or GATA-3 staining (lower plots) on the cotransferred kru CD452/2 CD4+ cells (red) and wt CD451/2 CD4+ cells (blue). (E) Frequency among CD4+ cells of wild-type and Ndfip1kru/kru donor cells in individual recipient mice described in D, normalized to frequencies in a non-Tg recipient that received no HEL. Lines link data points from the same recipient animal.
The role of Ndfip1 in peripheral tolerance to pancreatic islets was pinpointed by transferring mature naïve CD4+ T cells from 3A9 TCRHEL Tg wild-type or Ndfip1kru/kru mice into wild-type insHEL Tg recipients. This experimental design restricted Ndfip1 deficiency to the small population of transferred cells, which were admixed into a normal, diverse repertoire of T cells including Tregs. Neither the wild-type nor the Ndfip1kru/kru CD4+ T cells precipitated diabetes in insHEL Tg recipients unless 100 μg of HEL in saline was also given at the time of T-cell transfer (Fig. 6B). Within 8 d, all of the mice that had received Ndfip1kru/kru T cells and exogenous HEL became diabetic, whereas none of the mice that received wild-type T cells and exogenous HEL became diabetic. Diabetes in exogenous HEL-exposed mice receiving Ndfip1kru/kru T cells was accompanied by severe lymphocytic infiltration and destruction of most pancreatic islets (Fig. 6C).
To test whether Ndfip1 deficiency affected the peripheral response to islet-derived self-antigen alone, we tracked congenically marked mixtures of Ndfip1kru/kru and wild-type 3A9 TCRHEL Tg T cells in insHEL Tg recipients. In insHEL Tg recipients not given exogenous HEL, a subset of Ndfip1kru/kru and wild-type donor CD4+ T cells were stimulated to divide in response to low levels of self-HEL, but only the Ndfip1kru/kru divided extensively based on analysis of CTV dilution on day 6, remained Ki-67+, and had differentiated into GATA-3+ effector cells (Fig. 6D). In control recipients that lacked the insHEL transgene, neither mutant nor wild-type T cells were induced to divide or become GATA-3+. Thus, Ndfip1 is critical to abort CD4+ T-cell proliferation and differentiation induced by pancreas-derived self-antigen.
Although responding Ndfip1kru/kru T cells remained in cell cycle after stimulation by self-antigen alone, their net expansion was only ∼threefold more than the cotransferred wild-type counterparts (Fig. 6E). In insHEL Tg recipients that also received 100 μg of exogenous HEL in saline, however, the Ndfip1kru/kru T cells accumulated to >10-fold higher frequencies compared either to cotransferred wild-type T cells or to mutant T cells encountering self-antigen alone (Fig. 6 D and E). Because limited amounts of HEL are made and presented to 3A9 TCR Tg CD4+ cells in the pancreas-draining nodes of insHEL Tg recipients (44), we hypothesized that the amount of tolerogen presented (from either self or foreign source) also governed the net accumulation of Ndfip1kru/kru T cells. We tested this hypothesis by including a recipient group that lacked the insHEL transgene and was instead given a 1,000-fold lower dose of exogenous HEL (0.1 μg) in saline (Fig. 6 D and E). Like the cells exposed to tolerogenic HEL in other formats (insHEL and/or 100 μg of exogenous HEL), the Ndfip1kru/kru donor CD4+ cells in recipients given the low dose of exogenous HEL differentiated into GATA-3+ effectors and remained Ki-67+, but their accumulation was ∼10-fold less than in animals given the high dose.
Discussion
The experiments above illuminate cellular mechanisms for peripheral T-cell tolerance, which remain to be harnessed for allergy desensitization, transplantation tolerance, or autoimmune disease therapy. As reviewed in ref. 1, many in vivo studies have revealed that peripheral T-cell tolerance occurs by a burst of tolerogen-induced proliferation followed by Bim-mediated apoptotic elimination. The results here reveal a separate mechanism, showing that Ndfip1 acts within individual CD4+ T cells that have begun proliferating in response to innocuous self or foreign antigens, forcing them to exit cell cycle after one to five divisions in vivo. Because CD4+ cell differentiation into IL-4–producing cells is a function of the number of times they have divided (45, 46), the failure of tolerogen-responding Ndfip1-deficient T cells to exit cell cycle may explain their differentiation into GATA-3+ IL-4–producing Th2 effector cells. The failure to exit cycle without Ndfip1 contrasted with the peripheral tolerance defect caused by Bim deficiency, where responding progeny still exited cycle after dividing but resisted elimination by apoptosis. These results identify an activation-induced partner of HECT ubiquitin ligases, Ndfip1, to explain previous observations that Bim-deficient CD4+ T cells are still able to cease dividing in response to self-antigen in vivo despite their inability to undergo apoptosis (47). Our findings provide a cellular explanation for the association of NDFIP1 with both allergic and autoimmune disease (9–13), the co-occurrence of autoimmunity and asthma-like disease in humans with ITCH deficiency (8), and how cross-reactive environmental antigens may help to trigger autoimmune disease (48, 49). As discussed below, Ndfip1-mediated exit from cycle may identify a critical in vivo counterpart of T-cell anergy in vitro.
The findings here address the unresolved role of Ndfip1 in peripheral T-cell tolerance. Previous studies have established that Ndfip1 deficiency in mice causes a T-cell driven, lethal inflammatory disorder affecting particularly the skin, gastrointestinal tract, and lungs, which could be recapitulated by selective Ndfip1 deficiency in the T-cell lineage and suppressed by skewing the TCR repertoire to an innocuous ovalbumin OT-II TCR specificity (2, 21, 24, 25). In the earlier studies, mixed bone marrow chimera experiments found a higher fraction of Ndfip1-deficient CD4+ and CD8+ T cells were activated, but analysis was limited because the mutant animals were on a heterogeneous strain background derived variably from 129 and B6 strains, so that complicated cell sorting and permeabilization was needed to distinguish mutant cells from the homogeneous B6-strain controls. The findings here extend those mixed chimera results using matched congenic B6.CD45.2/CD45.1 strains, revealing that activation of many CD8+ cells and B cells is a reaction to Ndfip1 deficiency in other hematopoietic cells, whereas CD4+ cells lacking Ndfip1 undergo cell-autonomous expansion into Th1 and Th2 effector cells despite the presence of wild-type Tregs and other cells.
Foxp3+ Tregs that develop during negative selection in the thymus (nTregs) or during T-cell activation in the periphery (iTregs) mediate a non–cell-autonomous mechanism of peripheral tolerance, and previous studies established that Itch- or Ndfip1-deficient T cells are crippled in their differentiation into iTregs (24, 25). The experiments here showing that Ndfip1 mutation induced autoimmune and inflammatory disease in congenically matched mixed bone marrow chimeras and in cell transfer recipients despite numerous normal Foxp3+ cells, and failure of Ndfip1-deficient T cells to abort proliferation that was not recapitulated by Foxp3-deficient cells, excludes deficits in Foxp3-expressing cells or their function as a primary explanation.
The results here are consistent with Ndfip1 acting as a mechanism to steadily “raise the stakes” for each T cell to proceed into successive rounds of division, thereby forcing cells that have received a suboptimal (tolerogenic) stimulus to abort ongoing cell division several days into the response. Ndfip1-YFP Tg reporter mice revealed that TCR/CD28 stimulation causes a 30-fold increase in YFP fluorescence occurring gradually over the course of 2 d. This result is consistent with the Ndfip1 induction previously reported for TCR/CD28-stimulated T cells: at the protein level in cultured unfractionated T cells (2) and at the mRNA level in sorted naïve T cells cultured with TGF-β (25). The induction of Ndfip1-YFP in all CD44hi activated/memory T cells contrasts with models of T-cell anergy that would postulate selective induction of Ndfip1 by tolerizing stimuli. Instead, induction of Ndfip1 in actively dividing T cells may progressively raise the signaling threshold needed to keep the cells in cycle, and in the absence of sufficient microbial costimuli, this may force cell cycle exit. A feature of the “rising stakes” model of Ndfip1-mediated peripheral tolerance is that it defers the decision of whether to attack or disarm until after an initial proliferative response is made, providing more time for T cells to sample the environment without compromising their speed of mobilization.
Ndfip1-induced exit from cell cycle in CD4+ cells responding to tolerogens may be an unexpected in vivo manifestation of T-cell clonal anergy. T-cell anergy has been best defined in T cells stimulated in vitro through their TCR without CD28 costimulation, and is mostly viewed as a mechanism blocking initiation of T-cell IL-2 production and proliferation (18). Anergy has not been considered as a mechanism to trigger dividing T cells to exit cycle. T-cell responses to antigen in the absence of CD28 costimuli in vivo have nevertheless not recapitulated the in vitro models: instead, proliferation was initiated but followed by Bim-mediated apoptosis, although the remaining cells have been shown to be anergic to reinitiation of proliferation or IL-2 production provided sufficient tolerogen was present (1, 47). Itch has been shown to be required in T cells for in vitro and in vivo T-cell anergy, as measured by reinitiation of cytokine production and cell proliferation (16, 19). Ndfip1-deficient CD4+ cells stimulated in culture with antibodies to CD3 in the absence of CD28 have recently been shown to make more IL-2 for an extended period (21). This difference could nevertheless be secondary to a failure to exit cell cycle without Ndfip1, because IL-2 production normally increases on a per cell basis as a function of the number of TCR-induced cell divisions (45, 46), and the IL-2 measurements did not control for cell division history. Decreased production of IL-2 without apparent loss of proliferative response during in vitro restimulation has been observed in anergic DO11 TCR Tg CD4+ T cells that have exited cycle in response to tolerogenic self antigen but failed to undergo apoptosis because of Bim deficiency (47). The extent to which Ndfip1 forces cell cycle exit by down-regulation of IL-2 synthesis or by independent effects on TCR-induced cell proliferation will require cell division-based analysis with Il2/Ndfip1 double-deficient T cells in future studies.
Another future question raised by the findings here is which biochemical targets of Ndfip1 result in exit from cell cycle in CD4+ T cells stimulated by self- or foreign-antigen in the absence of adjuvant. Induction of Ndfip1 in actively dividing T cells may impose a sustained and elevated TCR-CD28 costimulatory requirement by downregulating TCR-ζ (20), PKC-θ, PLC-γ, JunB and c-Jun proteins (16, 19), Bcl-10, and NF-κB (22, 23). This is supported by the demonstration that Ndfip1-deficient T cells make more IL-2 than wild-type T cells even when CD28 is genetically ablated from both (21). However, normal T cells abort their proliferation to tolerogenic stimuli in vivo even when CD28 signals have been received (50–56). The presence of additional cytokines is also normally required to promote sustained rounds of T-cell division and effector differentiation, notably IL-12, IFN-α and -β, IL-1, and IL-4 (55, 57–59). These cytokines are typically produced extrinsically to the responding T cells in response to infection, adjuvants, or cell damage, although autocrine or paracrine sources arise if the T cells divide enough times to differentiate into effector cells that produce IFN-γ or IL-4. Ndfip1 may suppress the potential for autocrine production of IL-2 (21) or IL-4 in actively dividing CD4+ cells by degrading c-Jun and JunB (2, 14), and by inhibiting Notch (31, 32). Ndfip1 deficiency may also allow tolerogen-stimulated CD4+ T cells to remain in cycle by crippling the TGFβ signaling pathway (24, 25), which normally delivers an important anti-proliferative signal for T cells (60). The findings here provide the in vivo cellular context to understand the integration of these diverse biochemical pathways in future studies.
A key finding from the experiments is that large numbers of autoimmune effector T cells and autoimmune islet destruction only developed when an intrinsic peripheral tolerance defect was combined with a sufficiently large pool of organ-specific CD4+ cells that had escaped thymic deletion and also a large exogenous antigen trigger. The experiments highlight a third control mechanism—limiting amount of tolerogenic antigen stimulus—that has also frequently been observed as a key variable in peripheral T-cell tolerance (1). A high density of pMHC may simply drive more rapid progress through successive cell cycles or decrease apoptotic loss of daughter cells. One can envisage two situations in which self-reactive T cells that have evaded thymic deletion might be strongly stimulated: (i) when an exogenous food, environmental, or microbial protein happens to contain peptides of similar sequence to the self-antigen (48, 49); and (ii) where damage to an organ releases much more self-antigen for presentation in the draining lymph node. Given the association of NDFIP1 polymorphisms with a number of inflammatory diseases involving exogenous or self-antigens (9–13), it is not inconceivable that the cellular defect in peripheral tolerance defined here could arise through polygenic inheritance patterns involving NDFIP1, HLA, and other T-cell regulatory genes. The findings here imply that “high-zone tolerance” intervention with exogenous peptides or whole antigens, as practiced in some allergy-desensitization regimes, may in fact exacerbate disease in individuals where there is an intrinsic failure of tolerogen-reactive CD4+ cells to exit cycle and abort effector differentiation.
Materials and Methods
Mice.
The 3A9 TCR Tg, insHEL Tg, Aire−/−, Bim−/−, and Foxp3−/− mice were as described previously (37, 39, 61). Mice were either on the C57BL/6 background, the congenic C57BL/6.SJL-PtprcCD45.1 background, or the congenic B10.BR and B10.BR.SJL-PtprcCD45.1 strains, as indicated. All procedures were approved by the Australian National University Animal Ethics and Experimentation Committee.
Ndfip1 cDNA Sequencing and Western Blotting.
TRIzol-isolated RNA from splenocytes was reverse transcribed with oligo-dT, amplified using primers flanking the Ndfip1 coding region, separated by agarose gel electrophoresis, cloned into pMXs-IRES-GFP retroviral vector, and sequenced on an ABI3730 capillary sequencer. Naïve T cells (CD4+CD44loCD62LhiCD25–) were sorted from wild-type, Ndfip1kru/kru, and Ndfip1null/null mice, stimulated 48 h with 5 μg/mL plate-bound anti-CD3 (145-2C11) and 2 μg/mL soluble anti-CD28 (37.51), and lysed in 1× SDS sample buffer with sonication and boiling, and lysates were resolved on a polyacrylamide gel. Proteins were blotted to PVDF membrane, blocked with 5% (wt/vol) milk, probed with rat monoclonal antibody against an N-terminal peptide of mouse Ndfip1 (35), and developed with HRP-conjugated anti-rat IgG (Millipore) and Enhanced Chemiluminescence Substrate (Perkin-Elmer).
Flow Cytometry.
In vitro stimulation and staining for cell surface and intracellular proteins and CFSE dilution was performed as previously described (61). CTV labeling was done at room temperature as described (62) with slight modifications as described in SI Materials and Methods.
Bone Marrow Chimeras.
RAG1-deficient mice were X-irradiated with 1 × 500 cGy, and wild-type or Aire-deficient mice were given 2 × 500 cGy 4 h apart, before i.v. injection of 2 × 106 T cell-depleted bone marrow cells per mouse. Recipients were maintained on polymixin B and neomycin for 6 wk.
T-Cell Responses to HEL Protein in Vivo.
CD45 and CFSE/CTV-labeled splenocyte suspensions containing 0.5–2 × 106 TCR3A9-expressing CD4+ T cells were transferred by i.v. injection to B10.BR-congenic recipients. Where indicated, recipients received 0.1 or 100 μg of HEL in PBS by i.p. injection on the same day. For cellular analysis, spleens were collected on days 3 or 6 for flow cytometry. For analysis of pathology, recipients were monitored daily for hyperglycemia using a blood glucose meter (Medisense Optium), and at 3 wk posttransfer, pancreata were formalin-fixed, paraffin-embedded, and stained with hematoxylin/eosin, and the extent of lymphocytic infiltration of individual islets was scored blinded to the sample identity.
Supplementary Material
Acknowledgments
We thank the Genotyping Team of the Australian Phenomics Facility (APF) for expert mapping, sequencing, and genotyping; the staff of the APF for husbandry; Debbie Howard for expert technical assistance; and Dr. Ian Parish for advice on the manuscript. This work was supported by National Institutes of Health Grants AI100627 and AI054523, Wellcome Trust Grant 082030/B/07/Z, and National Health and Medical Research Council Grants 585490, 1016953, 427620, 1009190, and 1002863.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322739111/-/DCSupplemental.
References
- 1.Goodnow CC, Ohashi PS. 2013. Immunologic Tolerance. Fundamental Immunology, ed Paul WE, 7th Ed (Wolters Kluwer Health/Lippincott Williams and Wilkins, Philadelphia), pp 765–794.
- 2.Oliver PM, et al. Ndfip1 protein promotes the function of itch ubiquitin ligase to prevent T cell activation and T helper 2 cell-mediated inflammation. Immunity. 2006;25(6):929–940. doi: 10.1016/j.immuni.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mund T, Pelham HR. Control of the activity of WW-HECT domain E3 ubiquitin ligases by NDFIP proteins. EMBO Rep. 2009;10(5):501–507. doi: 10.1038/embor.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvey KF, Shearwin-Whyatt LM, Fotia A, Parton RG, Kumar S. N4WBP5, a potential target for ubiquitination by the Nedd4 family of proteins, is a novel Golgi-associated protein. J Biol Chem. 2002;277(11):9307–9317. doi: 10.1074/jbc.M110443200. [DOI] [PubMed] [Google Scholar]
- 5.Hustad CM, et al. Molecular genetic characterization of six recessive viable alleles of the mouse agouti locus. Genetics. 1995;140(1):255–265. doi: 10.1093/genetics/140.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perry WL, et al. The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18H mice. Nat Genet. 1998;18(2):143–146. doi: 10.1038/ng0298-143. [DOI] [PubMed] [Google Scholar]
- 7.Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol. 2009;10(6):398–409. doi: 10.1038/nrm2690. [DOI] [PubMed] [Google Scholar]
- 8.Lohr NJ, et al. Human ITCH E3 ubiquitin ligase deficiency causes syndromic multisystem autoimmune disease. Am J Hum Genet. 2010;86(3):447–453. doi: 10.1016/j.ajhg.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramon HE, et al. The ubiquitin ligase adaptor Ndfip1 regulates T cell-mediated gastrointestinal inflammation and inflammatory bowel disease susceptibility. Mucosal Immunol. 2011;4(3):314–324. doi: 10.1038/mi.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franke A, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42(12):1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira MA, et al. Australian Asthma Genetics Consortium Identification of IL6R and chromosome 11q13.5 as risk loci for asthma. Lancet. 2011;378(9795):1006–1014. doi: 10.1016/S0140-6736(11)60874-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu X, et al. Integrating autoimmune risk loci with gene-expression data identifies specific pathogenic immune cell subsets. Am J Hum Genet. 2011;89(4):496–506. doi: 10.1016/j.ajhg.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawcer S, et al. International Multiple Sclerosis Genetics Consortium Wellcome Trust Case Control Consortium 2 Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476(7359):214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang D, et al. Dysregulation of T lymphocyte function in itchy mice: A role for Itch in TH2 differentiation. Nat Immunol. 2002;3(3):281–287. doi: 10.1038/ni763. [DOI] [PubMed] [Google Scholar]
- 15.Parravicini V, Field AC, Tomlinson PD, Basson MA, Zamoyska R. Itch-/- alphabeta and gammadelta T cells independently contribute to autoimmunity in Itchy mice. Blood. 2008;111(8):4273–7282. doi: 10.1182/blood-2007-10-115667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venuprasad K, et al. Convergence of Itch-induced ubiquitination with MEKK1-JNK signaling in Th2 tolerance and airway inflammation. J Clin Invest. 2006;116(4):1117–1126. doi: 10.1172/JCI26858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu YC. The E3 ubiquitin ligase Itch in T cell activation, differentiation, and tolerance. Semin Immunol. 2007;19(3):197–205. doi: 10.1016/j.smim.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: A costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 19.Heissmeyer V, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5(3):255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 20.Huang H, et al. K33-linked polyubiquitination of T cell receptor-zeta regulates proteolysis-independent T cell signaling. Immunity. 2010;33(1):60–70. doi: 10.1016/j.immuni.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramos-Hernandez N, et al. Ndfip1 enforces a requirement for CD28 costimulation by limiting IL-2 production. J Immunol. 2013;191(4):1536–1546. doi: 10.4049/jimmunol.1203571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shembade N, et al. The E3 ligase Itch negatively regulates inflammatory signaling pathways by controlling the function of the ubiquitin-editing enzyme A20. Nat Immunol. 2008;9(3):254–262. doi: 10.1038/ni1563. [DOI] [PubMed] [Google Scholar]
- 23.Scharschmidt E, Wegener E, Heissmeyer V, Rao A, Krappmann D. Degradation of Bcl10 induced by T-cell activation negatively regulates NF-kappa B signaling. Mol Cell Biol. 2004;24(9):3860–3873. doi: 10.1128/MCB.24.9.3860-3873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venuprasad K, et al. The E3 ubiquitin ligase Itch regulates expression of transcription factor Foxp3 and airway inflammation by enhancing the function of transcription factor TIEG1. Nat Immunol. 2008;9(3):245–253. doi: 10.1038/niXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beal AM, Ramos-Hernández N, Riling CR, Nowelsky EA, Oliver PM. TGF-β induces the expression of the adaptor Ndfip1 to silence IL-4 production during iTreg cell differentiation. Nat Immunol. 2012;13(1):77–85. doi: 10.1038/ni.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li B, Tournier C, Davis RJ, Flavell RA. Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. EMBO J. 1999;18(2):420–432. doi: 10.1093/emboj/18.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szremska AP, et al. JunB inhibits proliferation and transformation in B-lymphoid cells. Blood. 2003;102(12):4159–4165. doi: 10.1182/blood-2003-03-0915. [DOI] [PubMed] [Google Scholar]
- 28.Fang TC, et al. Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity. 2007;27(1):100–110. doi: 10.1016/j.immuni.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amsen D, et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27(1):89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tu L, et al. Notch signaling is an important regulator of type 2 immunity. J Exp Med. 2005;202(8):1037–1042. doi: 10.1084/jem.20050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalton HE, et al. Drosophila Ndfip is a novel regulator of Notch signaling. Cell Death Differ. 2011;18(7):1150–1160. doi: 10.1038/cdd.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornell M, et al. The Drosophila melanogaster Suppressor of deltex gene, a regulator of the Notch receptor signaling pathway, is an E3 class ubiquitin ligase. Genetics. 1999;152(2):567–576. doi: 10.1093/genetics/152.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkin MB, et al. Regulation of notch endosomal sorting and signaling by Drosophila Nedd4 family proteins. Curr Biol. 2004;14(24):2237–2244. doi: 10.1016/j.cub.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 34.Chastagner P, Israël A, Brou C. AIP4/Itch regulates Notch receptor degradation in the absence of ligand. PLoS ONE. 2008;3(7):e2735. doi: 10.1371/journal.pone.0002735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howitt J, et al. Ndfip1 regulates nuclear Pten import in vivo to promote neuronal survival following cerebral ischemia. J Cell Biol. 2012;196(1):29–36. doi: 10.1083/jcb.201105009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sang Q, et al. Nedd4-WW domain-binding protein 5 (Ndfip1) is associated with neuronal survival after acute cortical brain injury. J Neurosci. 2006;26(27):7234–7244. doi: 10.1523/JNEUROSCI.1398-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daley SR, Hu DY, Goodnow CC. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD-1 or NF-κB. J Exp Med. 2013;210(2):269–285. doi: 10.1084/jem.20121458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heng TS, Painter MW. Immunological Genome Project Consortium The Immunological Genome Project: Networks of gene expression in immune cells. Nat Immunol. 2008;9(10):1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 39.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4(4):350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 40.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5(3):189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 41.Teh CE, Daley SR, Enders A, Goodnow CC. T-cell regulation by casitas B-lineage lymphoma (Cblb) is a critical failsafe against autoimmune disease due to autoimmune regulator (Aire) deficiency. Proc Natl Acad Sci USA. 2010;107(33):14709–14714. doi: 10.1073/pnas.1009209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson MS, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298(5597):1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 43.Hoyne GF, et al. Visualizing the role of Cbl-b in control of islet-reactive CD4 T cells and susceptibility to type 1 diabetes. J Immunol. 2011;186(4):2024–2032. doi: 10.4049/jimmunol.1002296. [DOI] [PubMed] [Google Scholar]
- 44.Silva DG, et al. Anti-islet autoantibodies trigger autoimmune diabetes in the presence of an increased frequency of islet-reactive CD4 T cells. Diabetes. 2011;60(8):2102–2111. doi: 10.2337/db10-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gett AV, Hodgkin PD. Cell division regulates the T cell cytokine repertoire, revealing a mechanism underlying immune class regulation. Proc Natl Acad Sci USA. 1998;95(16):9488–9493. doi: 10.1073/pnas.95.16.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gudmundsdottir H, Wells AD, Turka LA. Dynamics and requirements of T cell clonal expansion in vivo at the single-cell level: Effector function is linked to proliferative capacity. J Immunol. 1999;162(9):5212–5223. [PubMed] [Google Scholar]
- 47.Barron L, Knoechel B, Lohr J, Abbas AK. Cutting edge: Contributions of apoptosis and anergy to systemic T cell tolerance. J Immunol. 2008;180(5):2762–2766. doi: 10.4049/jimmunol.180.5.2762. [DOI] [PubMed] [Google Scholar]
- 48.Oldstone MB. Molecular mimicry and autoimmune disease. Cell. 1987;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- 49.Sollid LM, Jabri B. Celiac disease and transglutaminase 2: A model for posttranslational modification of antigens and HLA association in the pathogenesis of autoimmune disorders. Curr Opin Immunol. 2011;23(6):732–738. doi: 10.1016/j.coi.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wells AD, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5(11):1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 51.Mittrücker HW, Shahinian A, Bouchard D, Kündig TM, Mak TW. Induction of unresponsiveness and impaired T cell expansion by staphylococcal enterotoxin B in CD28-deficient mice. J Exp Med. 1996;183(6):2481–2488. doi: 10.1084/jem.183.6.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kündig TM, et al. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity. 1996;5(1):41–52. doi: 10.1016/s1074-7613(00)80308-8. [DOI] [PubMed] [Google Scholar]
- 53.Kearney ER, et al. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J Immunol. 1995;155(3):1032–1036. [PubMed] [Google Scholar]
- 54.Khoruts A, Mondino A, Pape KA, Reiner SL, Jenkins MK. A natural immunological adjuvant enhances T cell clonal expansion through a CD28-dependent, interleukin (IL)-2-independent mechanism. J Exp Med. 1998;187(2):225–236. doi: 10.1084/jem.187.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hernández J, Aung S, Marquardt K, Sherman LA. Uncoupling of proliferative potential and gain of effector function by CD8(+) T cells responding to self-antigens. J Exp Med. 2002;196(3):323–333. doi: 10.1084/jem.20011612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Townsend SE, Goodnow CC. Abortive proliferation of rare T cells induced by direct or indirect antigen presentation by rare B cells in vivo. J Exp Med. 1998;187(10):1611–1621. doi: 10.1084/jem.187.10.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: Dissociating proliferation and development of effector function. J Exp Med. 2003;197(9):1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ben-Sasson SZ, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci USA. 2009;106(17):7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95(7):3810–3815. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshimura A, Muto G. TGF-β function in immune suppression. Curr Top Microbiol Immunol. 2011;350:127–147. doi: 10.1007/82_2010_87. [DOI] [PubMed] [Google Scholar]
- 61.Altin JA, et al. Decreased T-cell receptor signaling through CARD11 differentially compromises forkhead box protein 3-positive regulatory versus T(H)2 effector cells to cause allergy. J Allergy Clin Immunol. 2011;127(5):1277–1285. doi: 10.1016/j.jaci.2010.12.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quah BJ, Parish CR. The use of carboxyfluorescein diacetate succinimidyl ester (CFSE) to monitor lymphocyte proliferation. J Vis Exp. 2010;(44):e2259. doi: 10.3791/2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.